Abstract
The response to respiratory syncytial virus (RSV), negative strand ssRNA virus, depends upon the ability to recognize specific pathogen associated targets. In the present study the role of TLR7 that recognizes ssRNA was examined. Using TLR7−/− mice we found that the response to RSV infection in the lung was more pathogenic as assessed by significant increases in inflammation and mucus hyper-secretion. While there appeared to be no effect of TLR7 deficiency on Type I IFN, the pathology was associated with an alteration in T cell responses with increases in mucogenic cytokines, IL-4, IL-13 and IL-17. Examination of DC from TLR7−/− animals indicated a preferential activation of IL-23 (a Th17 associated cytokine) and a decrease in IL-12 production. Neutralization of IL-17 in the TLR7−/− mice resulted in a significant decrease in the mucogenic response in the lungs of the RSV-infected mice. Thus, without TLR7-mediated responses an altered immune environment ensued with a significant effect on airway epithelial cell remodeling and goblet cell hyper/metaplasia leading to mucus overproduction.
Introduction
The role of innate immune responses for establishing the most appropriate and least pathologic responses for destruction and clearance of the infectious or deleterious agents is the first line of defense and is responsible for recognizing the vast array of pathogens with a limited set of fixed germ-line encoded receptors. To deal with this challenge with infectious agents, innate immunity relies on the detection of patterns or conserved molecular motifs unique to various classes of pathogens (1–3). The heterogeneity of viral glycoproteins along with their ability to genetically drift from season to season creates even greater challenges for innate immune recognition of viruses. To circumvent these obstacles, the innate immune system has evolved mechanisms to detect characteristics of viral nucleic acids that are either distinct in structure (dsRNA) or subcellular location (ssRNA). Recognition of viral nucleic acids triggers the induction of type I interferons that induce an anti-viral state in virally infected cells and activate immunoregulatory functions in nearby cells. Since RSV is a ssRNA negative sense virus, both ssRNA and dsRNA species are formed and provide targets for the innate immune system. A subset of pattern recognition receptors includes the toll-like receptors (TLR), which recognize different pathogen-associated molecular patterns (PAMPs) and activate NF-kB and other innate signaling pathways (1, 4, 5). In particular, TLR3 and TLR7/8 recognize pathogen associated dsRNA and ssRNA species, respectively, and initiate important cytokine mediator pathways for the initiation of the immune responses. These earliest responses are likely critical to establishing the proper immune response.
The RSV F protein has been reported to bind and activate TLR4, the receptor for lipopolysacharide (6), and distinct polymorphisms to TLR4 has been associated with disease severity in patient populations (7–11). Activation of specific chemokines during RSV infection of epithelial cells can be upregulated via TLR3. Our laboratory identified that while CCL5 and CxCL10 can be upregulated via a TLR3 dependent pathway, CxCL8 is dependent upon a MyD88-dependent pathway (12). Thus, multiple TLR pathways likely contribute to the generation of an effective anti-RSV response. Interestingly, our findings suggest that RSV infection of TLR3−/− mice (a MyD88-independent mechanism) leads to altered immune environment and promotes increased IL-13 that is associated with increases in mucus production (13). Thus, alteration of the dsRNA recognition pathway leads to a pathogenic phenotype. Likewise, when MyD88 pathways are deleted in mice an even more pathogenic environment is induced with increased eosinophilia, mucus overexpression and an overall induction of a Th2 cytokine environment (14). In the present study, we have examined one of the important MyD88-dependent pathways associated with viral RNA detection, TLR7. While the present perception is that pDC are the primary cell population that expresses TLR7 (15, 16), several other cell populations appear to be able to upregulate its expression, including mDC, B cells, and macrophages (17–19). In the present studies the deletion of TLR7 results in the generation of pathogenic responses during RSV infection and initiates an increase in a number of pathogenic cytokines, especially IL-17, which appears to be responsible for the increased mucus responses observed in TLR7−/− mice.
Methods
Mice
B6 wildtype mice were purchased from Jackson Laboratories. TLR7−/− mice on a B6 background were originally provided by Dr. S. Akira (Osaka, Japan) and a breeding colony subsequently established at the University of Michigan. All animal work was performed in accordance with the University of Michigan Committee on Use and Care of Animals policy.
Respiratory Syncytial Virus
Our laboratory utilizes the antigenic subgroup A strain of RSV, referred to as Line 19. This isolate was obtained from a sick infant at the University of Michigan (20), and has been demonstrated in animal models to mimic human infection by stimulating mucus production (21).
Real-time Taqman PCR
The smallest lobe was removed and homogenized in 1 ml of Trizol reagent (Invitrogen). RNA was isolated as described (Invitrogen), and 5μg was reverse-transcribed to assess gene expression. Detection of cytokine mRNA in lung samples was determined using pre-developed primer/probe sets (PE Biosystems, Foster City, CA) and analyzed using an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). GAPDH was analyzed as an internal control and gene expression was normalized to GAPDH. Fold changes in gene expression levels were calculated by comparison of the gene expression in unchallenged mice, which were assigned an arbitrary value of 1.
Lymph node restimulation
Mediastinal and cervical lymph nodes were harvested and single cell suspensions obtained by passing nodes though a 40μm nylon mesh filter. Samples were counted and plated in duplicate at 1×106 cells per well followed by restimulation with either 4×104 PFU RSV. Cells were incubated at 37°C for 24 hours and supernatants collected for analysis on the BioRad Bioplex 200 system according to the manufacturer’s protocol. Kits (Biorad) containing antibody beads to Th cytokines (IL-17, IFNγ, IL-4, IL-5, IL-13) were used to assay for cytokine production in each of the samples.
Generation of bone marrow dendritic cells
Bone marrow was harvested from B6 or TLR7−/− mice and seeded in tissue culture flasks in RPMI 1640 based complete media with 20 ng GM-CSF/ml (R&D Systems, Minneapolis, MN). Cells were fed every 3 days and loosely adherent cells were collected after 10 days. The majority of the cells, >85%, were CD11c+ BMDCs and subsequently were infected with RSV (moi=1.0) or stimulated with a specific TLR ligand. The cells were then assessed for gene and protein expression as well as used for flow cytometric analysis.
Flow cytometric analysis
Cells were stained with the indicated antibodies (BD Pharmingen, San Diego, CA) that were specific for costimulatory molecules and analyzed using a FACS Calibur and Cell Quest software (BD Biosciences, San Jose, CA). Isotype control antibodies were used to demonstrate specificity of our staining and to establish the criteria for our flow cytometry populations.
Statistics
Data was analyzed using Prism GraphPad software. Unless otherwise specified, data shown is representative of two or more experiments. Statistical significance in all experiments was determined by One-way ANOVA, followed by a Newman-Keuls post test. Significant differences were regarded as p < 0.05.
Results
Increased expression of TLR7 during RSV infection
The expression of TLR7 has been identified on multiple immune cell populations, but is thought to be most closely associated with APCs, such as DC and B cells. To determine if RSV infection upregulates TLR7 expression infected animals were assessed for TLR7 expression by quantitative PCR. The lungs (Figure 1A) demonstrated a time dependent increase in TLR7 expression that first appeared on day 4 and increased and plateaued on day 8 and 12, respectively. When cells from the RSV-infected mice (day 8 of infection) were assessed by intracellular staining for TLR7 by Flow cytometry, the primary cells that expressed TLR7 were B cells, plasmacytoid DC, and the strongest staining pattern for myeloid (conventional) DC based upon their median fluorescent intensity (MFI) in both the lung and lymph node (LALN) (Figure 1B). Thus, it appears that numerous immune cells express TLR7, primarily in CD11b+CD11C+ cDC, but there was no increase in TLR7 intensity on a per cell basis in RSV compared to uninfected animals. Rather the increase in mRNA expression levels is likely due to an increased recruitment of those cells in animals infected with RSV.
Figure 1.
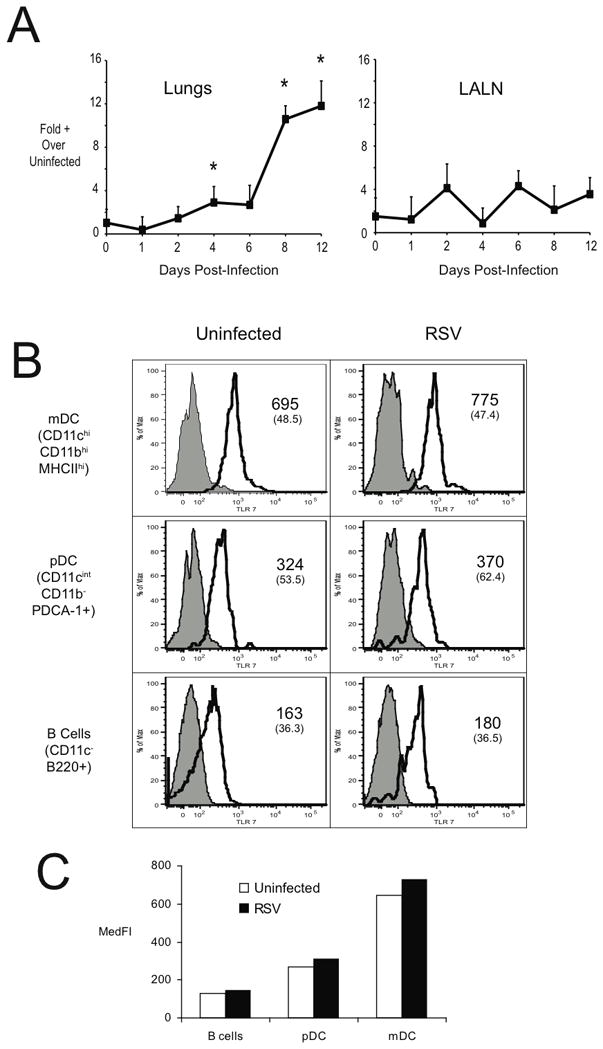
TLR 7 Expression during RSV infection. In (A), the expression of TLR7 in the lungs and lung draining lymph nodes at various times post RSV infection. Expression of TLR was assessed via quantitative PCR (Taqman) from whole lung/lymph node RNA. Each timepoint represents the mean of 4–5 mice, and error bars represent the standard error of the mean. * = p<0.05. In (B), the expression of TLR7 by various cell types (B cells, pDC, and mDC) was determined via flow cytometric analysis. Leukocytes from the lungs and LALN of control and RSV infected mice (day 8 post-infection) were isolated via enzymatic digests. The resulting cell suspensions were stained with antibodies to surface markers, then permeabilized to stain TLR7. Columns represent the median fluorescent intensity (MedFI) of TLR specific antibody staining in the various cell populations.
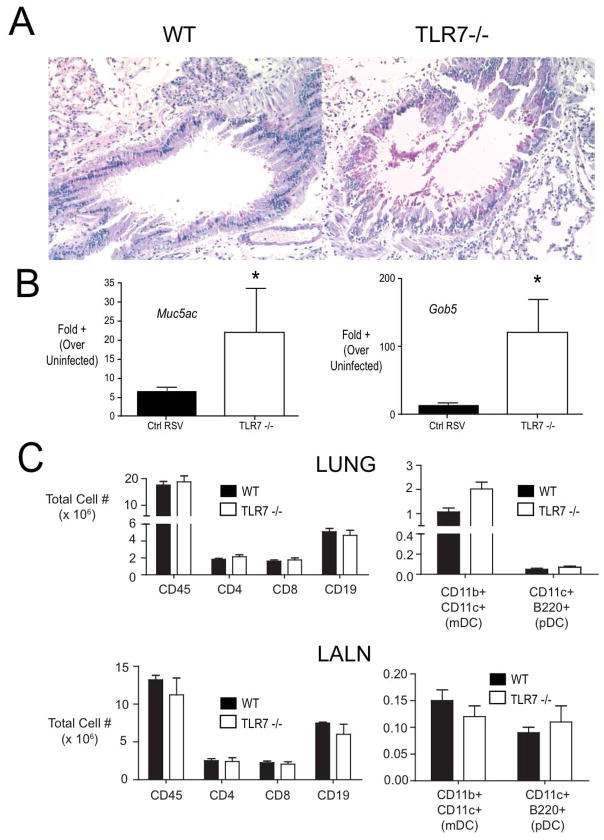
Deletion of TLR7 enhances RSV-induced goblet cell hyperplasia
The role of TLRs for the recognition of PAMPs, such as virus-associated ssRNA by TLR7, is thought to be critical in the recognition of an infectious process. TLR7 signaling is dependent upon MyD88 adapter mediated pathways and our previous studies have established that deletion of MyD88 results in a skewed response with heightened levels of Th2 cytokines, eosinophilia and mucus overproduction. In the present studies when TLR7−/− mice were infected with RSV there was also an increase in pathologic responses especially the expression of mucus and increased numbers of goblet cells in the airways (Figure 2A). When we examined the expression of mucus-associated genes Muc5AC and gob5, they also demonstrated a significant upregulated expression in the TLR7−/− compared to RSV infected wild type animals (Figure 2B). Interestingly, there was not a remarkable increase in inflammatory cells in the TLR7−/− mice as evidenced in the lung sections and by flow cytometry examining individual populations of cells including T cells and DC subsets in both the lung and draining lymph nodes (LALN) (Figure 2C). Thus, in the absence of TLR7 there was a pathogenic alteration of the RSV-induced responses that was primarily centered on airway pathology.
Figure 2.
Role of TLR7 in limiting RSV-induced mucus hypersecretion and goblet cell hyperplasia. (A) Periodic acid Schiff’s (PAS) staining of lung sections from control and TLR7−/− mice at day 9 post-infection. In (B), The expression of mucus associated genes Muc5ac and Gob5 was determined by real-time PCR of whole lung RNA (day 9 post-infection). Columns represent the mean +/− SEM of 5 mice per group. * = p <0.05. The experiment was performed three times with similar results.
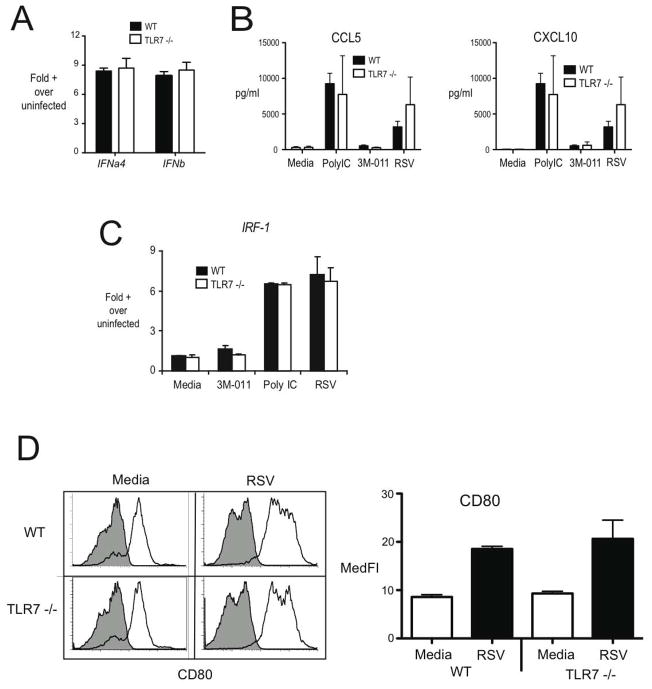
Alteration in cytokine profiles in TLR7−/− mice with RSV-induced disease
Several studies have identified that type I IFN is most often associated with an appropriate immune response and that TLR7 can mediate these effects (). Given the above data on cell population expression of TLR7 our studies focused on the myeloid DCs that appeared to have the highest expression level using bone marrow-derived DC as our model. When we examined type I IFN in DC after RSV infection there was no alteration of either IFNalpha4 or IFNbeta in the TLR7−/− compared to wildtype cell populations (Figure 3A). Furthermore, examining chemokines that are associated with type I IFN induction, CCL5 and CxCL10, demonstrated a similar induction with not only RSV but also with a TLR3/poly I:C stimulus (Figure 3B). In addition, when we examined the type I IFN dependent transcription factor, IRF-1, in RSV infected or PolyI:C stimulated DC it was similarly upregulated in WT and TLR7−/− dendritic cells (Figure 3C). Finally, several studies have demonstrated that DC maturation in general and specifically during RSV infection is dependent upon type I IFN and the DC were able to mature properly as demonstrated by examination of CD80 expression (Figure 3D). These data suggest that a defect in Type I IFN associated responses are unlikely to be the proximate cause for the dysregulated response to RSV observed in TLR7 −/− mice.
Figure 3.
TLR 7 limits early cytokine production in the lymph nodes during RSV infection. Control and TLR7 −/− mice were infected with RSV and lung draining lymph nodes were harvested at day 3 post-infection and single-cell suspensions were obtained via enzymatic digests. Lymph node cells were restimulated with RSV (MOI = ~0.5). Cytokine production in cultures of lymph node cells was assessed via Bioplex multiplex assay. * = p <0.05. Similar results were obtained in three independent experiments.
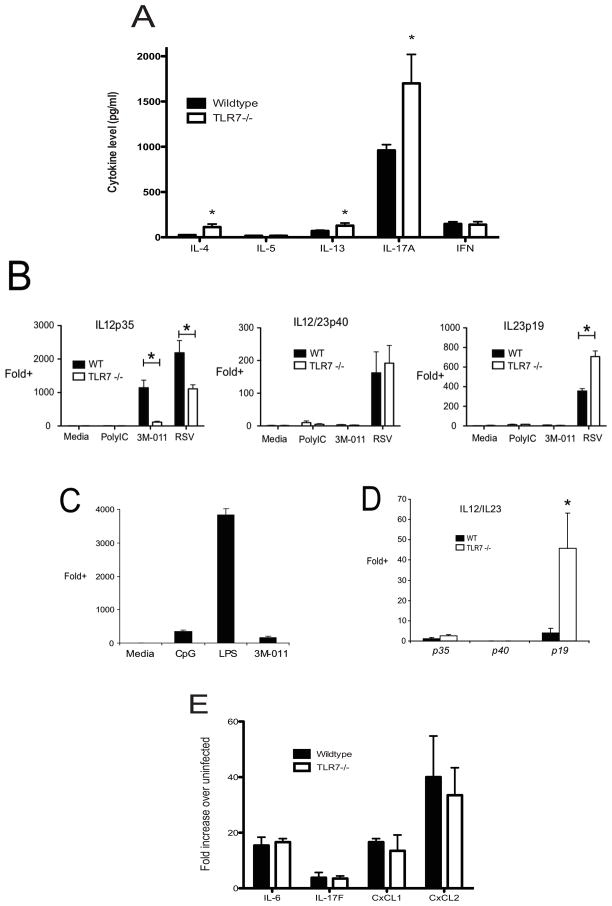
To begin to understand the changes in immune responses in the TLR7−/− animals that correspond to the pathologic alterations observed, draining lymph nodes from RSV infected mice were isolated and dispersed into single cell suspensions. The single cell suspensions were then given RSV (MOI-0.5) to initiate a recall response to RSV. The supernatants were assessed for cytokine levels using bioplex proteomics analysis after 24 hrs (Figure 4A). The data indicate that while there was no alteration in IFNγ production in the TLR7−/− mice compared to wildtype, a very significant increase in IL-17 production could be observed as well as significantly more Th2 cytokines IL-4 and IL-13, but not IL-5 (Fig. 4A). Thus, the increased production of IL-17, as well as Th2 cytokines IL-4 and IL-13 corresponded with associated increases in mucus gene expression and goblet cell hyperplasia.
Figure 4.
TLR7 mediates IL-12 versus IL-23 signaling in bone marrow derived dendritic cells. BMDC were isolated and cultured from wild-type and TLR7 −/− mice in the presence of media, the TLR7 agonist 3M-011, or RSV (MOI~0.5) for 24 hours. In (A), the production of IL12p35, IL-12/23p40, and IL-23p19 were determined via QPCR. In (B) and (C) the expression of CD86 was assessed via flow cytometry. In (B), representative histograms depict CD86 expression of uninfected (hollow) and RSV-infected (shaded) wild type and TLR7−/− BMDCs. In (C), CD86 expression is expressed quantitatively, with columns representing median fluoresence intensity of wild-type and TLR7−/− BMDC. Each column represents the mean of triplicate samples +/− SEM.
In order to further examine if there was an alteration in APC function in the TLR7−/− that would alter the subsequent production of cytokines, we isolated and grew bone marrow-derived dendritic cells (BMDC) from both wildtype and TLR7−/− mice. After ten days in growth media the BMDC were infected with RSV (MOI-0.5) or other TLR agonists and assessed for expression of IL-6, IL-12p40 (the common chain) as well as the IL-12 and IL-23 specific chains, p35 and p19, respectively. No differences in the expression of induction of IL-6 were found (data not shown). However, the data in Figure 4B illustrate that while IL-12p40 expression was similar between the wildtype and TLR7−/− BMDC there was a significant decrease in IL-12p35, and concomitant increase in IL-23p19 in TLR7−/− BMDC. These data indicate a skewing of the dendritic cell response to RSV, which could explain the increased level of IL-17 observed in the TLR7−/− mice following RSV infection. Together these data suggest a dysregulation in innate cytokine production in BMDC, away from Th1 promoting IL-12, and in favor of Th17-promoting IL-23, that correlates with the dysregulated immune responses in the lungs of TLR7−/− mice, resulting in increased pathology.
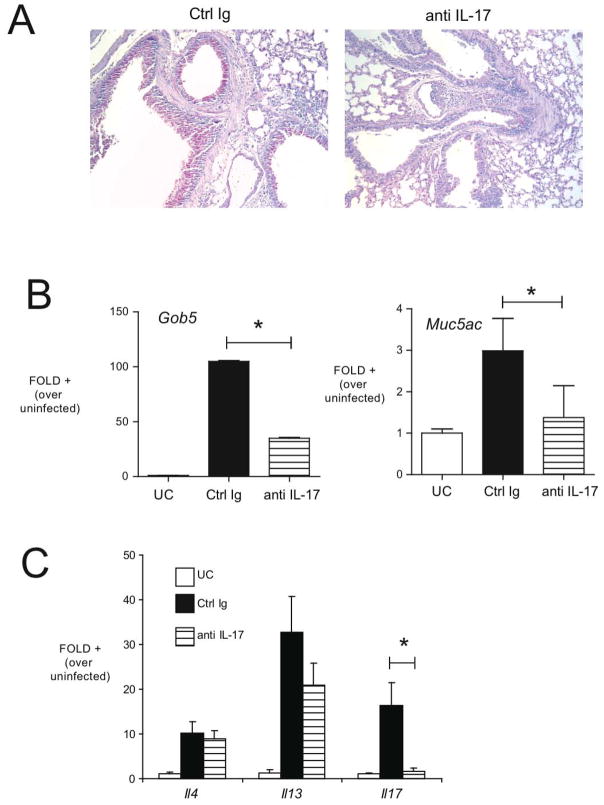
IL-17 overexpression is a dominant pathogenic signal in TLR7−/− animals
Our data indicated that the predominant cytokine that was upregulated in TLR7−/− animals was IL-17. Results have suggested that IL-17 may directly induce mucus hypersecretion from epithelial cell populations in the lung (22, 23). To address the role played by IL-17 in immunopathology observed in RSV infected TLR7−/− mice, we generated anti-IL-17 antibodies and treated TLR7−/− animals using passive immunization during RSV infection. Control or anti-IL17 antibodies were administered systemically (ip) beginning at day 4 post-infection. The levels of IL-17 in lung homogenates and bronchoalveolar lavage were significantly decreased in antibody-treated mice, demonstrating the effectiveness of in vivo neutralization (Table I). IL-17 neutralization of RSV-infected TLR7−/− mice had a clear decrease in the mucus hypersecretion, as assessed via the degree of PAS staining in lung sections (Figure 5A). As a more quantiitative measure, we assessed the expression of the mucus-associated genes muc5ac and gob5 in the lungs of control Ig-treated and anti-IL-17 treated TLR7−/− mice. Compared to control IgG treated mice, anti-IL-17 treated TLR7−/− exhibited significantly reduced expression of these mucus associated genes (Figure 5B). In addition, we also examined the cytokine profile (Figure 5C) and found that while anti-IL-17 strikingly reduced the expression of IL-17 in the lung, it had no significant effect on other cytokines, including IL-4 and IL-13 (although a trend toward reduction in IL-13 was also observed). Thus, our data overall suggest that the effect of TLR7 deletion was partially associated with an increase in mucus by an IL-17 associated mechanism.
Figure 5.
Neutralizing IL-17 beginning on day 4 of RSV infection of TLR7−/− mice attenuates mucus production. A, PAS staining of lung sections from control Ig or anti-IL-17-treated, RSV-infected TLR7−/− mice at day 8 postinfection (original magnification ×100). Expression of mucus-associated genes Muc5ac and Gob5 (B), IL-4, IL-13, and IL-17 (C) was assessed via QPCR of lung RNA in uninfected control (UC), control Ig RSV-infected mice, and in anti-IL-17-treated animals. Columns represent the mean fold increase over uninfected controls, and error bars represent SEM. *p < 0.05.
Discussion
The response to viral infections within the lung must proceed in a balanced and programmed manner to optimally clear the virus without damaging critical pulmonary function or promoting pathologic responses. This is particularly important in individuals with underlying disease, such as those with COPD, asthma, or end-stage fibrotic disease. It is clear that the determination of the direction of the immune responses relies on innate immune cell activation and the mediators that they produce. In the present study the innate response and subsequent T cell response to RSV, an ssRNA virus, depends upon the presence of ssRNA recognition through TLR7 in order to promote a relatively non-pathogenic response. Original studies described that TLR7 was necessary for recognition of ssRNA and elicited both IL-12 and type I IFN necessary for Th1 type immune responses (24–26). The response of the TLR7−/− BMDC in the present studies demonstrated that without TLR7 there was a change in balance of innate cytokines with increases in Th17 inducing IL-23 and a decrease in Th1 inducing IL-12. Interestingly, in the absence of TLR7 no alteration in RSV clearance could be detected. However, the immune response and subsequent pulmonary response was greatly affected, with a significant increase in cytokines and mucus hypersecretion, demonstrating that a link between viral clearance and immunopathology was not evident. Although several immune cytokines that were upregulated, IL-4, IL-13 and IL-17, have clearly been linked to the generation of pathogenic responses, IL-17 was the most noteworthy. Numerous studies have suggested that by targeting TLR activation in general and TLR7 specifically, a skewing of the immune response away from Th2 could be accomplished (27–33). In addition, a recent study demonstrated that type I IFN induced by TLR7 regulates IL-17 production (34). It may be the absence of this pathway that alters the response perhaps through regulation of IL-23. Thus, our findings support these previous findings using a clinically relevant animal model of pulmonary viral infection.
Several studies have examined the role of TLRs in RSV and other respiratory viral infections. TLR4 was first implicated in RSV infections (6, 35), and while initially controversial (36), numerous studies have identified a clear connection between TLR4 and RSV clearance (6, 35, 37, 38) as well as a link involving TLR4 polymorphisms and severe RSV-induced disease in infants (9, 39–41). The ability of RSV F protein to bind and induce activation of TLR4 suggests that even the initial binding of RSV to the cell surface may provide an early innate activation signal that is important to sensing the infection. Discrete amino acid changes in F protein appears to regulate the induction of IL-13 and mucus in different strains of RSV (42) and may be linked to TLR4 activation. A recent study also examined the role of TLR2/TLR6 in RSV infection and found that they had a role in innate cell cytokine and chemokine production along with efficacy of viral clearance (43). A most profound pathogenic effect was found when MyD88−/− mice were used with RSV infection and included increased Th2 cytokines, mucus hypersecretion and profound eosinophilia (14). This latter response was profound, likely due to the removal of multiple TLR pathways, including TLR2, TLR4 and TLR7. The response in MyD88−/− mice appeared to be of a similar intensity as that described in STAT1−/− mice (23, 44), perhaps linking it to a type I IFN response that appears to be important early in the anti-viral responses. In addition, when the only MyD88-independent sensing TLR was removed, TLR3, a pathogenic effect was also observed linked to alteration of IL-13 production leading to mucus hypersecretion (12). Recent studies have clearly linked TLR4 specifically with the increased expression of IL-23 via AP-1 activation, which can be especially enhanced by co-activation with IFNγ (45). Interestingly, IL-23 along with IL-1β induces IL-17 from γ/δ T cells and can subsequently enhance CD4 T cell-derived IL-17 (46). The latter mechanism may be critical in driving IL-17 in the absence of TLR7 signaling by shifting the balance away from IL-12 and toward IL-23. Thus, it is clear that the pathogen sensing system induced by multiple TLRs is critical at several levels for driving an appropriate anti-viral response.
In the context of innate cell activation, TLR2 and TLR4 are found predominantly on the surface of innate cells and allow the early recognition of the virus, subsequently followed by TLR3 and TLR7 that are found in the endosomic compartment and recognize ds- and ssRNA. These later TLRs appear to have a prominent impact on shaping the T cell immune response. In addition, since RSV infects predominantly via cytoplasmic entry, other important nonendosomic sensing systems are also important, such as RIG-I (47). A recent study that utilized mice deficient in the primary adapter molecule for RIG-I, MAVS, infected with RSV demonstrated a significant abrogation of early cytokine production, but pathogenesis later in disease was not investigated (48). Alteration of any of these pathogen-sensing pathways may lead to an alteration in either the clearance of the virus and/or the immunopathology of the response. Thus, there may be multiple associated mechanisms for induction of severe disease that may result from genetic or environmental alteration of PRR expression.
Establishing an altered immune environment of the lung early in development may have subsequent impact on later responses in specific susceptible individuals. While it is still controversial whether severe RSV disease predisposes children to subsequent pulmonary disease, clear epidemiology studies have linked early RSV disease with persistent pulmonary problems (49–54). Interestingly, several studies have now linked TLR7 polymorphisms with altered responses to HIV and HCV induced responses and most interesting, the TLR7 polymorphisms have been associated with development of asthma (55–58). Thus, similar to TLR4 polymorphism studies, a link may exist between TLR7 polymorphisms and RSV disease and associated persistent pulmonary problems. The changes in the ability of particular or multiple TLR systems may lead to multiple disease phenotypes in the lung including asthma and COPD, diseases that most severely manifest themselves through viral exacerbation responses.
The overall response generated during a viral response relies upon multiple means to sense the pathogen with an emphasis on generating the most successful clearance response that is not pathogenic. In these studies, the absence of TLR7 does not alter viral clearance, but does allow a more pathogenic response that includes more mucus via an IL-17 mediated mechanism. Thus, we can consider that a TLR7-mediated event allows for fining tuning of the immune responses during respiratory viral infections limiting pathology.
References
- 1.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill L. The Toll/interleukin-1 receptor domain: a molecular switch for inflammation and host defence. Biochem Soc Trans. 2000;28:557–563. doi: 10.1042/bst0280557. [DOI] [PubMed] [Google Scholar]
- 4.Sandor F, Buc M. Toll-like receptors. I. Structure, function and their ligands. Folia Biol (Praha) 2005;51:148–157. [PubMed] [Google Scholar]
- 5.Roeder A, Kirschning CJ, Rupec RA, Schaller M, Weindl G, Korting HC. Toll-like receptors as key mediators in innate antifungal immunity. Med Mycol. 2004;42:485–498. doi: 10.1080/13693780400011112. [DOI] [PubMed] [Google Scholar]
- 6.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagro A, Tominac M, Krsulovic-Hresic V, Bace A, Matic M, Drazenovic V, Mlinaric-Galinovic G, Kosor E, Gotovac K, Bolanca I, Batinica S, Rabatic S. Increased Toll-like receptor 4 expression in infants with respiratory syncytial virus bronchiolitis. Clin Exp Immunol. 2004;135:267–272. doi: 10.1111/j.1365-2249.2004.02364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puthothu B, Forster J, Heinzmann A, Krueger M. TLR-4 and CD14 polymorphisms in respiratory syncytial virus associated disease. Dis Markers. 2006;22:303–308. doi: 10.1155/2006/865890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awomoyi AA, Rallabhandi P, Pollin TI, Lorenz E, Sztein MB, Boukhvalova MS, Hemming VG, Blanco JC, Vogel SN. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007;179:3171–3177. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y, Shimojo N, Suzuki Y, Campos Alberto EJ, Yamaide A, Suzuki S, Arima T, Matsuura T, Tomiita M, Aoyagi M, Hoshioka A, Honda A, Hata A, Kohno Y. CD14 -550 C/T, which is related to the serum level of soluble CD14, is associated with the development of respiratory syncytial virus bronchiolitis in the Japanese population. J Infect Dis. 2007;195:1618–1624. doi: 10.1086/516790. [DOI] [PubMed] [Google Scholar]
- 11.Tulic MK, Hurrelbrink RJ, Prele CM, Laing IA, Upham JW, Le Souef P, Sly PD, Holt PG. TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide. J Immunol. 2007;179:132–140. doi: 10.4049/jimmunol.179.1.132. [DOI] [PubMed] [Google Scholar]
- 12.Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudd BD, Smit JJ, Flavell RA, Alexopoulou L, Schaller MA, Gruber A, Berlin AA, Lukacs NW. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 14.Rudd BD, Schaller MA, Smit JJ, Kunkel SL, Neupane R, Kelley L, Berlin AA, Lukacs NW. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J Immunol. 2007;178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol. 2005;26:221–229. doi: 10.1007/s00281-004-0180-4. [DOI] [PubMed] [Google Scholar]
- 16.Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gantier MP, Tong S, Behlke MA, Xu D, Phipps S, Foster PS, Williams BR. TLR7 is involved in sequence-specific sensing of single-stranded RNAs in human macrophages. J Immunol. 2008;180:2117–2124. doi: 10.4049/jimmunol.180.4.2117. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 20.Herlocher ML, Ewasyshyn M, Sambhara S, Gharaee-Kermani M, Cho D, Lai J, Klein M, Maassab HF. Immunological properties of plaque purified strains of live attenuated respiratory syncytial virus (RSV) for human vaccine. Vaccine. 1999;17:172–181. doi: 10.1016/s0264-410x(98)00155-8. [DOI] [PubMed] [Google Scholar]
- 21.Lukacs NW, Moore ML, Rudd BD, Berlin AA, Collins RD, Olson SJ, Ho SB, Peebles RS., Jr Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am J Pathol. 2006;169:977–986. doi: 10.2353/ajpath.2006.051055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto K, Durbin JE, Zhou W, Collins RD, Ho SB, Kolls JK, Dubin PJ, Sheller JR, Goleniewska K, O’Neal JF, Olson SJ, Mitchell D, Graham BS, Peebles RS., Jr Respiratory syncytial virus infection in the absence of STAT 1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J Allergy Clin Immunol. 2005;116:550–557. doi: 10.1016/j.jaci.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 24.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 25.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osterlund P, Veckman V, Siren J, Klucher KM, Hiscott J, Matikainen S, Julkunen I. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol. 2005;79:9608–9617. doi: 10.1128/JVI.79.15.9608-9617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 28.Watts C, Zaru R, Prescott AR, Wallin RP, West MA. Proximal effects of Toll-like receptor activation in dendritic cells. Curr Opin Immunol. 2007;19:73–78. doi: 10.1016/j.coi.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 31.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WW, Matlashewski G. Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infect Immun. 2008;76:3777–3783. doi: 10.1128/IAI.01527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fili L, Ferri S, Guarna F, Sampognaro S, Manuelli C, Liotta F, Cosmi L, Matucci A, Vultaggio A, Annunziato F, Maggi E, Guarna A, Romagnani S, Parronchi P. Redirection of allergen-specific TH2 responses by a modified adenine through Toll-like receptor 7 interaction and IL-12/IFN release. J Allergy Clin Immunol. 2006;118:511–517. doi: 10.1016/j.jaci.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Jin J, Tang Y, Speer D, Sujkowska D, Markovic-Plese S. IFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol. 2009;182:3928–3936. doi: 10.4049/jimmunol.0802226. [DOI] [PubMed] [Google Scholar]
- 35.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 36.Ehl S, Bischoff R, Ostler T, Vallbracht S, Schulte-Monting J, Poltorak A, Freudenberg M. The role of Toll-like receptor 4 versus interleukin-12 in immunity to respiratory syncytial virus. Eur J Immunol. 2004;34:1146–1153. doi: 10.1002/eji.200324449. [DOI] [PubMed] [Google Scholar]
- 37.Haeberle HA, Takizawa R, Casola A, Brasier AR, Dieterich HJ, Van Rooijen N, Gatalica Z, Garofalo RP. Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and toll-like receptor 4-dependent pathways. J Infect Dis. 2002;186:1199–1206. doi: 10.1086/344644. [DOI] [PubMed] [Google Scholar]
- 38.Cyr SL, Angers I, Guillot L, Stoica-Popescu I, Lussier M, Qureshi S, Burt DS, Ward BJ. TLR4 and MyD88 control protection and pulmonary granulocytic recruitment in a murine intranasal RSV immunization and challenge model. Vaccine. 2009;27:421–430. doi: 10.1016/j.vaccine.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 39.Paulus SC, Hirschfeld AF, Victor RE, Brunstein J, Thomas E, Turvey SE. Common human Toll-like receptor 4 polymorphisms--role in susceptibility to respiratory syncytial virus infection and functional immunological relevance. Clin Immunol. 2007;123:252–257. doi: 10.1016/j.clim.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Rallabhandi P, Bell J, Boukhvalova MS, Medvedev A, Lorenz E, Arditi M, Hemming VG, Blanco JC, Segal DM, Vogel SN. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol. 2006;177:322–332. doi: 10.4049/jimmunol.177.1.322. [DOI] [PubMed] [Google Scholar]
- 41.Tal G, Mandelberg A, Dalal I, Cesar K, Somekh E, Tal A, Oron A, Itskovich S, Ballin A, Houri S, Beigelman A, Lider O, Rechavi G, Amariglio N. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis. 2004;189:2057–2063. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 42.Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, Newcomb DC, Buchholz UJ, Crowe JE, Jr, Goleniewska K, Williams JV, Collins PL, Peebles RS., Jr A Chimeric A2 Strain Respiratory Syncytial Virus (RSV) with the Fusion Protein of RSV Strain Line 19 Exhibits Enhanced Viral Load, Mucus, and Airway Dysfunction. J Virol. 2009 doi: 10.1128/JVI.01853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murawski MR, Bowen GN, Cerny AM, Anderson LJ, Haynes LM, Tripp RA, Kurt-Jones EA, Finberg RW. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, Graham BS. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol. 2002;168:2944–2952. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- 45.Liu W, Ouyang X, Yang J, Liu J, Li Q, Gu Y, Fukata M, Lin T, He JC, Abreu M, Unkeless JC, Mayer L, Xiong H. AP-1 activated by toll-like receptors regulates expression of IL-23 p19. J Biol Chem. 2009;284:24006–24016. doi: 10.1074/jbc.M109.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres JP, Mejias A, Gomez AM, Jafri H, Ramilo O, Chen ZJ. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 50.Sigurs N. Clinical perspectives on the association between respiratory syncytial virus and reactive airway disease. Respir Res. 2002;3(Suppl 1):S8–14. doi: 10.1186/rr186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sigurs N. A cohort of children hospitalised with acute RSV bronchiolitis: impact on later respiratory disease. Paediatr Respir Rev. 2002;3:177–183. doi: 10.1016/s1526-0542(02)00191-4. [DOI] [PubMed] [Google Scholar]
- 52.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(Suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 53.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 54.Price JF. Acute and long-term effects of viral bronchiolitis in infancy. Lung. 1990;168(Suppl):414–421. doi: 10.1007/BF02718159. [DOI] [PubMed] [Google Scholar]
- 55.Oh DY, Baumann K, Hamouda O, Eckert JK, Neumann K, Kucherer C, Bartmeyer B, Poggensee G, Oh N, Pruss A, Jessen H, Schumann RR. A frequent functional toll-like receptor 7 polymorphism is associated with accelerated HIV-1 disease progression. AIDS. 2009;23:297–307. doi: 10.1097/QAD.0b013e32831fb540. [DOI] [PubMed] [Google Scholar]
- 56.Moller-Larsen S, Nyegaard M, Haagerup A, Vestbo J, Kruse TA, Borglum AD. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax. 2008;63:1064–1069. doi: 10.1136/thx.2007.094128. [DOI] [PubMed] [Google Scholar]
- 57.Prescott SL, Noakes P, Chow BW, Breckler L, Thornton CA, Hollams EM, Ali M, van den Biggelaar AH, Tulic MK. Presymptomatic differences in Toll-like receptor function in infants who have allergy. J Allergy Clin Immunol. 2008;122:391–399. 399, e391–395. doi: 10.1016/j.jaci.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 58.Schott E, Witt H, Neumann K, Bergk A, Halangk J, Weich V, Muller T, Puhl G, Wiedenmann B, Berg T. Association of TLR7 single nucleotide polymorphisms with chronic HCV-infection and response to interferon-a-based therapy. J Viral Hepat. 2008;15:71–78. doi: 10.1111/j.1365-2893.2007.00898.x. [DOI] [PubMed] [Google Scholar]