Abstract
Oscillatory contractile activity is an inherent property of blood vessels. Various cellular mechanisms have been proposed to contribute to oscillatory activity. Mouse small mesenteric arteries display a unique low frequency contractile oscillatory activity (1 cycle every 10-12 min) upon phenylephrine stimulation. Our objective was to identify mechanisms involved in this peculiar oscillatory activity. First-order mesenteric arteries were mounted in tissue baths for isometric force measurement. The oscillatory activity was observed only in vessels with endothelium, but it was not blocked by L-NAME (100 μM) or indomethacin (10 μM), ruling out the participation of nitric oxide and prostacyclin, respectively, in this phenomenon. Oscillatory activity was not observed in vessels contracted with K+ (90 mM) or after stimulation with phenylephrine plus 10 mM K+. Ouabain (1 to 10 μM, an Na+/K+-ATPase inhibitor), but not K+ channel antagonists [tetraethylammonium (100 μM, a nonselective K+ channel blocker), Tram-34 (10 μM, blocker of intermediate conductance K+ channels) or UCL-1684 (0.1 μM, a small conductance K+ channel blocker)], inhibited the oscillatory activity. The contractile activity was also abolished when experiments were performed at 20°C or in K+-free medium. Taken together, these results demonstrate that Na+/K+-ATPase is a potential source of these oscillations. The presence of α-1 and α-2 Na+/K+-ATPase isoforms was confirmed in murine mesenteric arteries by Western blot. Chronic infusion of mice with ouabain did not abolish oscillatory contraction, but up-regulated vascular Na+/K+-ATPase expression and increased blood pressure. Together, these observations suggest that the Na+/K+ pump plays a major role in the oscillatory activity of murine small mesenteric arteries.
Keywords: Oscillatory activity, Na+/K+-ATPase pump, Ouabain, Small mesenteric arteries
Introduction
Vasomotion is the oscillation of vascular tone or vascular diameter that can be detected in many vascular segments (1). These spontaneous or induced oscillations of blood vessel diameter occur independently of cardiac and respiratory cycles, or the propagation of pulse pressure (2).
Intrinsic oscillatory activity, or vasomotion, within the microcirculation has many potential functions, including modulation of vascular resistance (3), increased vascular conductance (4), reduced energy usage when compared to a constant contraction (1), tissue oxygenation (5), and capillary exchange function (6). Additionally, increased vasomotion correlates positively with increased blood pressure in some experimental models of hypertension (7,8). These findings, together with the observation that vasomotion seems to be less prevalent in hypertensive rats treated with ACE inhibitors (9,10), seem to indicate that high blood pressure evoked through several different mechanisms may induce the oscillations.
Various cellular mechanisms have been proposed to contribute to oscillatory activity, including action potentials generated by pacemaker cells, intercellular communication, activation of voltage-operated calcium channels, sarcoplasmic reticulum calcium pool, potassium (K+) channels, Na+/K+-ATPase pump and nitric oxide (NO) (11-13).
Whereas the great majority of oscillatory activities occur spontaneously and are characterized by contraction-relaxation cycles that occur in a time frame of 60-120 s for each cycle, we have observed that mouse small mesenteric arteries display a unique contractile oscillatory activity, characterized by a low frequency (1 cycle every 10-12 min) and which is observed with the use of agents that evoke contractile response, such as phenylephrine (PE), an α-1 adrenergic agonist, or U-46619, a thromboxane A2 (TXA2) mimetic. These contraction-relaxation cycles do not occur at basal tonus. Therefore, the objective of the present study was to determine the mechanisms involved in this peculiar oscillatory activity of murine small mesenteric arteries. We hypothesized that the oscillatory contraction reflects a contribution of Na+/K+-ATPase. Different approaches were used to inhibit Na+/K+-ATPase in vitro and in vivo to test this hypothesis. Additionally, the presence of the α-1 and α-2 isoforms of Na+/K+-ATPase was demonstrated in mouse resistance mesenteric arteries.
Material and Methods
Animals
Male C57BL/6 mice (16 weeks old, 25-30 g; Harlan, USA) were used in these studies. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Medical College of Georgia Committee on the Use of Animals in Research and Education. The animals were housed 4 per cage with a 12-h light/dark cycle and fed a standard chow diet and water ad libitum.
Preparation and study of mesenteric arteries
After euthanasia, the mesentery was rapidly excised and placed in an ice-cold physiological salt solution (PSS), pH 7.2, containing 130 mM NaCl, 14.9 mM NaHCO3, 5.5 mM dextrose, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4.7H2O, 1.6 mM CaCl2.2H2O, and 0.026 mM EDTA. PSS was maintained at 37°C and was continuously bubbled with a mixture of 5% CO2 and 95% O2. First-order mesenteric arteries were carefully dissected and mounted as ring preparations on two stainless steel wires (≅ 200 mm in length with an internal diameter ≅100 to 150 μm) in an isometric Mulvany-Halpern small-vessel myograph (Danish MyoTech, Denmark). In some vessels, endothelium was removed mechanically, by rubbing gently with metal wire. One wire was attached to a force transducer and the other to a micrometer. Both dissection and mounting of the vessels were carried out in cold (4°C) PSS. The segments were adjusted to maintain a passive force of 2.5 mN. Vessels were equilibrated for 45 min in PSS at 37°C. Arterial integrity was assessed first by stimulation of vessels with 120 mM KCl (in a solution where osmolarity was corrected) and, after washing and a new stabilization period, by contracting the segments with 3 μM PE, followed by stimulation with 10 μM acetylcholine (ACh).
Oscillatory activity was assessed by contracting the segments with 0.1 to 10 μM PE or with 0.01 μM U-46619, the stable analog of the prostaglandin H2 endoperoxide (PGH2) that serves as a TXA2 mimetic. To study the contribution of NO and prostaglandins to the PE-induced oscillatory activity, experiments were performed in the presence of 100 μM L-NAME and 10 μM indomethacin, respectively, or both. To study the role of calcium-activated potassium (KCa) channels in PE-induced oscillatory activity, experiments were performed in the presence of 100 μM tetraethylammonium (TEA), a nonselective K+ channel blocker, 0.1 μM UCL-1684, a selective small calcium-activated K+ channel (SKCa) inhibitor, or 10 μM TRAM-34, a selective intermediate calcium-activated K+ channel (IKCa) inhibitor. These drugs were chosen because previous studies from our laboratory have shown that KCa, SKCa and IKCa are the main K+ channels present in small mesenteric arteries (14). The vessels were incubated with 100 μM ouabain to test the role of the Na+/K+-ATPase pump in PE-induced oscillatory activity. Some experiments were performed in K+-free medium, obtained from modified PSS. When antagonists or inhibitors were used, drugs were incubated for 30-45 min. Time control experiments were performed to determine if force development of small mesenteric artery rings were related to effects other than those of each antagonist/inhibitor. Some results are presented as representative tracings of the vessels’ behavior in different experiments, and “n” represents the number of experiments performed. The oscillatory contractions were also quantified both in terms of their frequency and magnitude of force, the latter reported as percentage of PE-induced contraction.
Blood pressure measurement and ouabain-induced hypertension
Surgery was performed in control mice (N = 8; Jackson Laboratories C57BL/6J) to implant blood pressure radiotelemetry transmitters (Data Sciences PA-C20, International, USA). Briefly, after isoflurane anesthesia, the transmitter was implanted into the abdominal aorta via a 2.5 to 3 cm laparotomy under aseptic conditions, and the transmitter body was routed to a subcutaneous pocket in the midback region. The incision was infiltrated with bupivacaine and closed with sterile 6-0 Ethicon Ophthalmic suture. Following recovery, the mice were housed in individual cages in the laboratory animal facility and provided with standard laboratory chow and water ad libitum. Mean arterial pressure (MAP) was measured from 12:00 pm to 6:00 am (i.e., during a period of 18 h) every day, and studies were started only after normal circadian rhythm was reestablished (7 days post-surgery). After 1 week of baseline MAP measurement, animals were anesthetized with isoflurane and a mini-osmotic pump (Alzet, Durect Corporation, USA) was implanted to deliver ouabain subcutaneously for 14 days at a rate of 300 μg·kg-1·day-1.
Western blotting
Mesenteric arteries from untreated mice were used for α-1 subunit expression. Mesenteric arteries, aorta and heart from ouabain-treated animals were used for α-1 and α-2 subunit expression. Tissues were isolated, cleaned from fat, dissected and frozen in liquid nitrogen. Proteins were extracted from mesenteric bed, aorta or heart and immediately homogenized in ice-cold buffer containing 10 μL protease inhibitor cocktail (cat# P8340, Sigma, USA), 100 mM sodium ortovanadate, 100 mM PMFS, and 1 mL protein extraction reagent (Pierce, USA). The homogenate was kept on ice for 30 min (vortexed every 5 min - extraction period). The homogenate was centrifuged for 30 min at 8500 g and the supernatant was utilized. Protein quantification was performed by bicinchoninic acid assay (Pierce) using bovine serum albumin as standard. Laemmli sample buffer (Bio-Rad, USA) was added to the protein (1:1, v/v) and heated to 100°C for 5 min. The proteins (40 μg) were separated by electrophoresis on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5% skim med milk in Tris-buffered saline solution with Tween (0.1%) for 1 h at 24°C. Membranes were then incubated with antibodies overnight at 4°C. The following antibodies were used: Na+/K+ pump (1:250; Affinity BioReagents, USA), anti-HERED α-2 isoform of the Na+/K+-ATPase (1:500), anti-HERED α-1 isoform of the Na+/K+-ATPase (1:500), and β-actin (1:1000, Sigma-Aldrich, USA). Anti-HERED antibodies were provided by Dr. Deborah L. Carr and Dr. Thomas A. Presley, Texas Tech University Health Sciences Center.
After incubation with secondary antibodies, signals were developed by chemiluminescence, visualized by autoradiography, and quantified densitometrically. Results were normalized by β-actin.
Data analysis
Results are reported as means ± SEM. The Student t-test was used to compare experimental to control mice. In some experiments, ANOVA and Bonferroni’s post-test were used. Probability values <0.05 were considered to be significant.
Drugs
Acetylcholine chloride, bupivacaine, indomethacin, Nω-nitro-L-arginine methyl ester (L-NAME), ouabain octahydrate, phenylephrine chloride, tetraethylammonium chloride (TEA), and 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) were purchased from Sigma. 9,11-Dideoxy-9α,11α-methanoepoxyprostaglandin F2α (U-46619) was purchased from Calbiochem (USA). 6,12,19,20,25,26-Hexahydro-5,27:13,18:21,24-trietheno-11,7-metheno-7H-dibenzob,n [1,5,12,16] tetraazacyclotricosine-5,13-diium ditrifluoroacetate (UCL-1684) was purchased from Tocris (Ellisville, MI, USA).
Results
Profile of the oscillatory activity and effect of endothelium removal
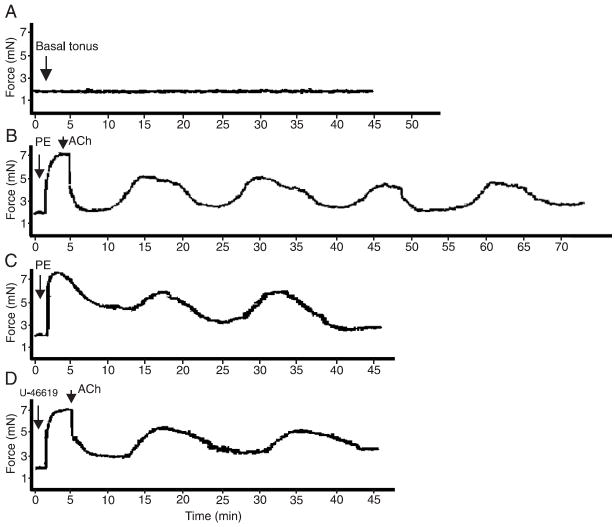
Oscillatory contractions in small mesenteric arteries were not observed at basal tension levels, as shown in Figure 1A. Upon stimulation with PE, a low frequency (1 cycle every 10-12 min) oscillatory activity was observed (Figure 1B). Oscillatory events were observed upon stimulation with PE at different concentrations (0.1 to 100 μM) and, consequently, at various levels of PE-induced tension (data not shown). The oscillatory activity in PE-contracted vessels was observed either upon stimulation with 10 μM ACh or in vessels contracted with PE in the absence of Ach (Figure 1C). Most of our figures report oscillatory activity upon stimulation with both PE and ACh to illustrate integrity or removal of the endothelium. Since the oscillatory activity only occurred in contracted vessels, we determined if this response was specific for PE. Contractile experiments were performed with 0.01 μM U-46619, the stable analog of PGH2 or a TXA2 mimetic, and the same profile of oscillatory activity was observed (Figure 1D). After PE-induced (3 μM) contraction, oscillatory activity was observed in small endothelium-intact mesenteric arteries (Figure 2A), but not in endothelium-denuded vessels (Figure 2B). In the next set of experiments, whose objective was to characterize the mechanisms involved in the contraction-relaxation cyclic response, oscillatory contractions were induced by adding 3 μM PE to endothelium-intact vessels.
Figure 1.
Role of tonus in oscillations of small mesenteric arteries. Representative tracings showing that oscillatory activity in murine mesenteric vessels only occurs after agonist stimulation. Vasomotion does not occur under basal tonus conditions (A; N = 10), but is observed upon stimulation with 3 μM PE followed by 10 μM ACh (B; N = 10), PE (3 μM; N = 5) in the absence of ACh (C), and U-46619 (D; 0.01 μM; N = 5). Contraction data are reported in millinewtons (mN). PE = phenylephrine; ACh = acetylcholine.
Figure 2.
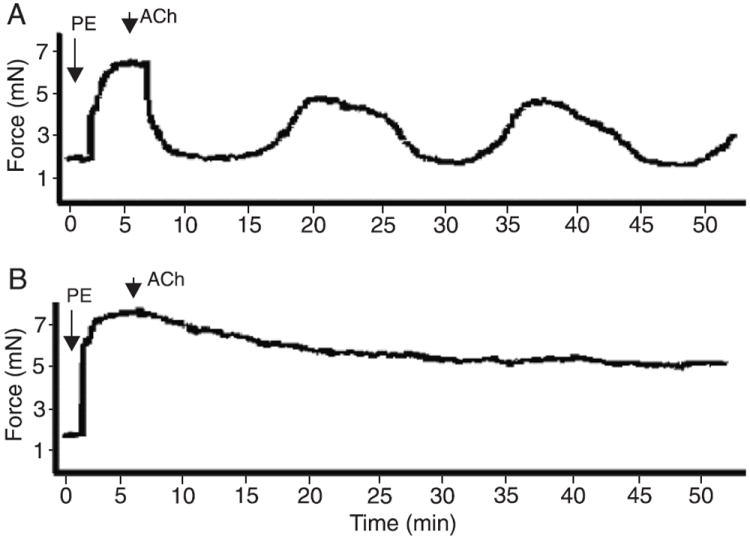
Role of the endothelium in oscillations of small mesenteric arteries. Representative tracings showing that oscillatory activity in murine mesenteric vessels depends on the endothelium. Vessels were contracted with 3 μM PE and the integrity of the endothelium was tested with 10 μM ACh. A) Endothelium-intact arteries exhibit vasomotion (N = 10). B) Endothelium-denuded arteries do not display oscillatory activity (N = 10). Contraction data are reported in millinewtons (mN). PE = phenylephrine; ACh = acetylcholine.
Role of endothelium-derived hyperpolarizing factor, nitric oxide and prostacyclin
As described before, the oscillatory activity developed by small mesenteric arteries is endothelium-dependent. Endothelium-dependent relaxations are achieved by a combination of endothelium-derived relaxing factors: prostacyclin (PGI2), NO, and endothelium-derived hyperpolarizing factor (EDHF), with the last playing an increased role in smaller or resistance vessels (15). The following experiments were performed in order to determine which of these components would also contribute to the oscillatory activity.
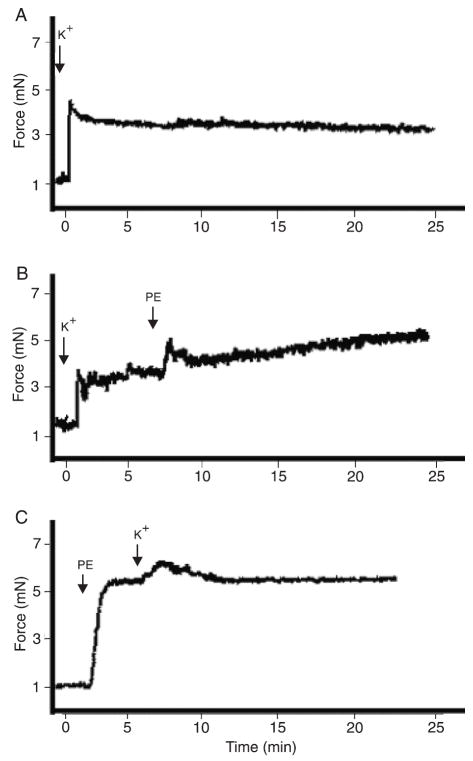
Increased extracellular K+ concentrations were used as an experimental approach to test the role of EDHF in the oscillatory activity. No contraction-relaxation cycles were observed in vessels stimulated with 90 mM K+, as shown in Figure 3A. The addition of 3 μM PE did not restore the oscillatory activity in these vessels (Figure 3B). Oscillatory activity was not observed when vessels were contracted with PE plus 10 mM K+ (Figure 3C).
Figure 3.
Effect of K+ in oscillations of small mesenteric arteries. Representative tracings showing that oscillatory activity in murine mesenteric vessels is not observed in the presence of high extracellular K+ concentrations. No oscillatory activity is observed in vessels contracted with A) 90 mM K+, B) 90 mM K+ plus 3 μM PE, or C) 3 μM PE plus 10 mM K+ (N = 5). Contraction data are reported in millinewtons (mN). PE = phenylephrine.
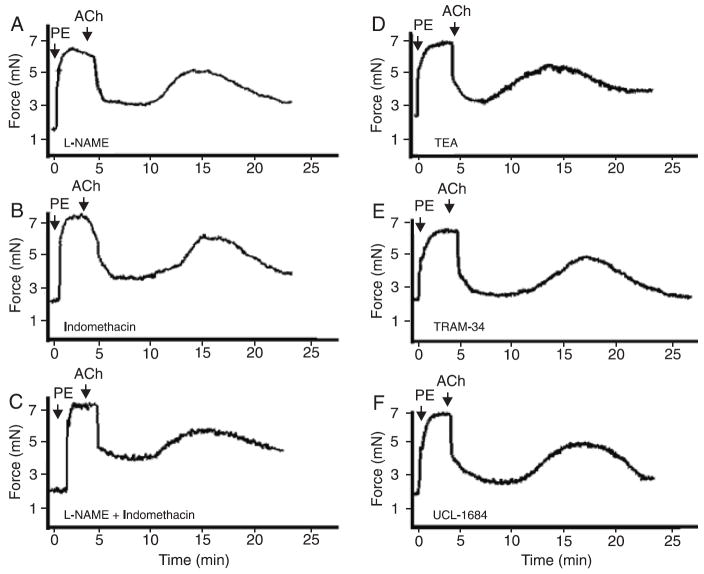
Small mesenteric arteries were incubated with 100 μM L-NAME, to block NO synthesis, with 10 μM indomethacin to block synthesis of PGI2 or cyclooxygenase (COX)-derived products, or both. The profile of oscillatory activity (magnitude of contractions) was not changed by these drugs, showing that NO and COX-derived products are not involved in this phenomenon (Figure 4A). Force development during initial PE contraction was slightly increased in the presence of 100 μM L-NAME (6.8 ± 0.2 mN) and 10 μM indomethacin (7.1 ± 0.2 mN) or both (7.2 ± 0.3 mN), compared to control conditions (6.5 ± 0.3 mN).
Figure 4.
Role of K+ channels, NO and COX-derived products in the magnitude of oscillations of small mesenteric arteries. Vessels were contracted with 3 μM PE and endothelium integrity was tested with 10 μM ACh. Oscillatory activity in murine mesenteric vessels occurs after blockade of NOS and COX. Blockade of (A) NO synthesis by incubation with 100 μM L-NAME, (B) inhibition of COX activity with 10 μM indomethacin, or (C) simultaneous incubation with 100 μM L-NAME and 10 μM indomethacin did not change the magnitude of the oscillatory activity (N = 5). B, Oscillatory activity in murine mesenteric vessels occurs after blockade of K+ channels. The blockade of (D) potassium channels with 100 μM TEA, a nonspecific blocker, (E) 10 μM TRAM-34, a selective inhibitor of IKCa channels, or (F) 0.1 µM UCL-1684, a selective inhibitor of SKCa channels, did not interfere with the magnitude of oscillation phenomena (N = 4 for each inhibitor). Average of 3 cycles were evaluated for each vessel. Data are reported as means ± SEM. NO = nitric oxide; COX = cyclooxygenase; PE = phenylephrine; ACh = acetylcholine; NOS = nitric oxide synthase; TEA = tetraethylammonium; TRAM-34 = tetraethylammonium-34; IKCa = intermediate calcium-activated K+ channel; SKCa = small calcium-activated K+ channel.
Endothelium-dependent relaxation in small or resistance vessels is still observed in the presence of COX and NO synthase inhibitors, but is usually blocked by increased extra-cellular K+ concentration or K+ channel inhibitors (14,15).
Role of K+ channels and Na+/K+-ATPase
Because IKCa and SKCa are especially important in EDHF-mediated relaxation and hyperpolarization of resistance-sized arteries (15-18), we performed experiments to determine the effects of K+ channel blockers [100 μM TEA (a nonselective blocker), 10 μM TRAM-34 (an IKCa channel blocker), and 0.1 μM UCL-1684 (an SKCa channel inhibitor)] on the oscillatory activity. The concentration of each inhibitor was chosen on the basis of other studies performed in our laboratory (14). None of these drugs changed the magnitude of oscillatory activity in small mesenteric arteries (Figure 4B). Force development during initial PE contraction was slightly increased in the presence of 100 μM TEA (6.9 ± 0.3 mN), 10 μM TRAM-34 (6.8 ± 0.3 mN), and 0.1 μM UCL-1684 (7.0 ± 0.2 mN), compared to control conditions (6.5 ± 0.3 mN).
Another protein that can contribute to changes in cellular K+ concentration is the Na+/K+-ATPase pump. Thus, our next goal was to determine if the Na+/K+-ATPase pump plays a role in oscillatory activity. Ouabain (10 μM), a Na+/K+-ATPase inhibitor, completely inhibited vasomotion in murine mesenteric arteries (Figure 5A,B).
Figure 5.
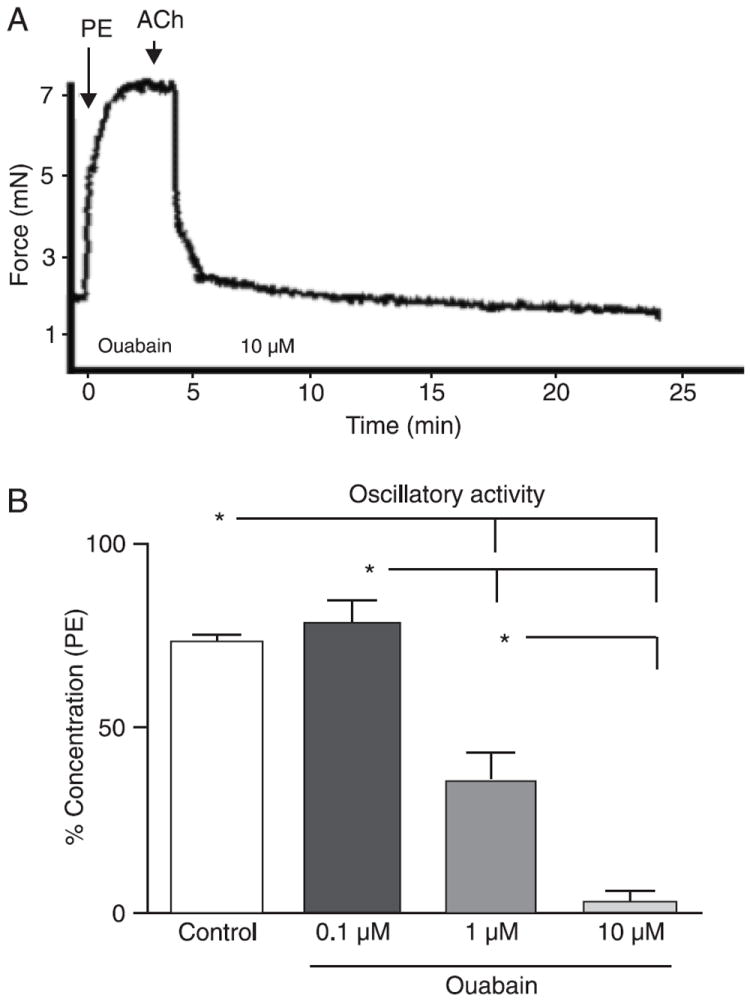
Role of Na+/K+-ATPase in oscillations of small mesenteric arteries. Representative tracings showing that oscillatory activity in murine mesenteric vessels relies on Na+/K+-ATPase activity. Vessels were contracted with 3 μM PE and endothelium was tested with 10 μM ACh. A, The inhibition of the Na+/K+-ATPase pump with 10 μM ouabain abolished the oscillatory activity (N = 6). B, Bar graphs showing that inhibition of the Na+/K+-ATPase pump by 0.1 to 10 μM ouabain resulted in concentration-dependent inhibition of oscillatory activity. Maximal force development during oscillatory activity was measured, and the magnitude of contraction was corrected by initial contraction with PE, which was considered to be 100% contraction. PE = phenylephrine; ACh = acetylcholine. Data are reported as means ± SEM. *P < 0.05 vs control (one-way ANOVA and Bonferroni’s post-test).
Other approaches that modify/inhibit Na+/K+-ATPase activity were also tested, such as changes in the temperature or in the extracellular K+ concentration. When the temperature of the myograph was set to 20°C and vessels were equilibrated for 20 min in PSS, the addition of 5 μM PE did not induce oscillatory activity (data not shown). Similarly, if the temperature was maintained at 37°C, but the composition of the PSS was changed to a K+-free solution, no oscillatory activity was observed upon stimulation with PE (data not shown). Force development during initial PE contraction was slightly increased in the presence of 10 μM ouabain (7.2 ± 0.3 mN) and significantly decreased (4.4 ±0.5 mN) when temperature was decreased to 20°C, compared to control conditions (6.5 ± 0.3 mN).
Taken together, these observations suggest that the Na+/K+-ATPase pump plays a major role in the oscillatory activity in murine small mesenteric arteries.
Vasomotion in small mesenteric vessels from ouabain-treated mice
In view of the central function of the Na+/K+-ATPase pump in mouse small mesenteric artery vasomotion, we hypothesized that chronic blockade of the Na+/K+ pump with ouabain would abolish the oscillatory activity.
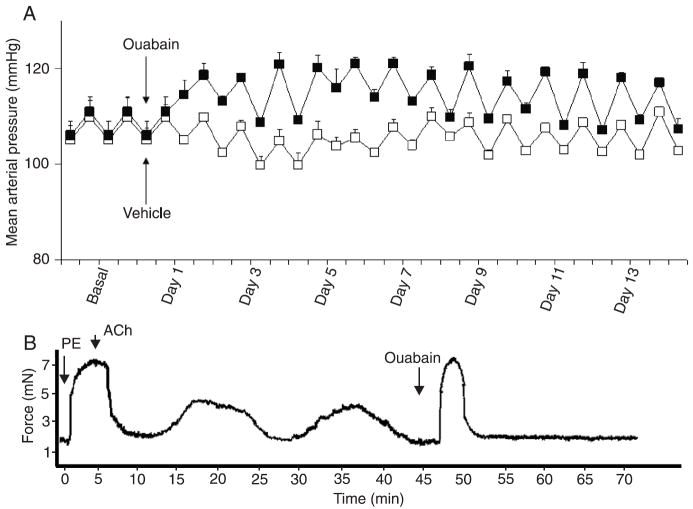
Chronic administration of ouabain to C57BL/6 mice increased MAP in comparison to vehicle-infused mice. During the baseline period (before implantation of mini-pumps), MAP was similar for the vehicle (105 ± 4 mmHg) and ouabain (106 ± 3 mmHg) groups. Ouabain infusion induced a significant increase in MAP. From days 1 to 14, MAP average was 105 ± 1.7 in vehicle-treated vs 117 ± 2 mmHg in ouabain-treated mice (Figure 6A).
Figure 6.
Mean arterial pressure and vasomotion in small mesenteric arteries from ouabain-treated mice. A, Mean arterial pressure of animals treated (black squares) or not (white squares) with ouabain (300 μg·kg-1·day-1) for 14 days. B, Representative tracing showing that oscillatory activity occurs in murine mesenteric vessels after chronic-infusion of ouabain. Vessels were contracted with 3 μM PE and endothelium was tested with 10 μM ACh. The addition of 10 μM ouabain to the muscle bath induced a significant transitory contraction and abolished vasomotion (N = 9). Contraction data are reported in millinewtons (mN). PE = phenylephrine; ACh = acetylcholine.
Ex vivo vasomotion was not abolished in vessels from ouabain-treated animals, as shown in Figure 6. Ex vivo oscillatory activity in small mesenteric arteries from ouabain-treated animals was observed upon PE-induced contractile response (Figure 6B), but not at basal tension levels, similar to those in vessels from vehicle-treated animals. Introduction of 10 μM ouabain into the bath induced a significant transient contractile response (much greater than that observed in arteries from control animals) and completely abolished ex vivo vasomotion. The magnitude and frequency of oscillatory contraction in arteries from mice infused with ouabain were similar to those in vessels from vehicle-treated animals.
Expression of Na+/K+-ATPase pump
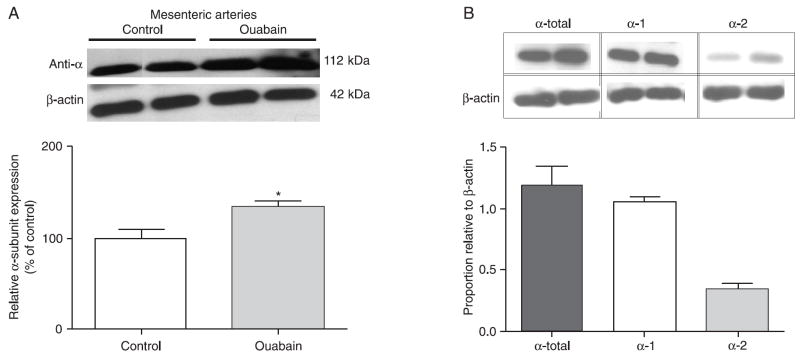
Since vasomotion was not affected by chronic infusion of ouabain, but was indeed abolished by the addition of ouabain to the muscle bath, which in this condition induced a greater transient contraction, we speculated about the possibility of up-regulation of the Na+/K+-ATPase pump in small mesenteric arteries from ouabain-treated mice. The Western blot assays showed that expression of total Na+/K+-ATPase α-subunit (the antibody recognizes all α-subunits) is increased in mesenteric arteries (with endothelium) from ouabain-infused mice (Figure 7A). Similar results were observed in aorta and heart from these animals (data not shown).
Figure 7.
Western blots of the Na+/K+-ATPase alpha subunit. A) Up-regulation of Na+/K+-ATPase in small mesenteric arteries from ouabain-infused and control mice (pool of 2 animals, N = 3). *P < 0.05 vs control (unpaired t-test with Welch’s correction). B) Expression of the α-1 and α-2 subunits in small mesenteric arteries from control mice. *P < 0.05 vs control (one-way ANOVA and Bonferroni’s post-test). Densitometric analyses were performed on samples from control and ouabain-infused mice. Data were normalized by β-actin protein expression and are reported as means ± SEM.
Since the α-subunit of Na+/K+-ATPase has four isoforms, each with a tissue-specific distribution, and that the α-1 and α-2 isoforms display differential sensitivity to ouabain (whereas α-2 is sensitive to low concentrations of ouabain, the α-1 isoform is inhibited by higher ouabain concentrations) (19,20), we determined the expression of α-1 and α-2 subunits in resistance mesenteric arteries from untreated mice. Mouse resistance mesenteric arteries display expression of both α-1 and α-2 isoforms (Figure 7B).
Discussion
The present study shows that murine small mesenteric vessels display endothelium-dependent oscillatory contractions, which do not occur at basal tonus and are only observed upon contractile stimuli. Na+/K+-ATPase activity, driven by the Na+/K+-ATPase α-1 and α-2 isoforms, but not K+ channels, NO or COX-derived products, plays a major role in the phenomenon. Oscillatory activity is not abolished in vessels from ouabain-treated mice possibly due to the reversible binding of ouabain to Na+/K+-ATPase or because ouabain infusion was able to up-regulate Na+/K+-ATPase expression.
The role of the endothelium in vasomotion differs between vascular beds and species. In some regions, a functional endothelium is essential for vasomotion (21,22), whereas in others, vasomotion is observed after endothelium removal (13,23). In mouse small mesenteric arteries, this is an endothelium-dependent phenomenon. Specifically in the mesenteric bed, the endothelium seems to play an essential role in vasomotion, as previously reported (11).
Whereas different mechanisms are able to generate vasomotion, the Na+/K+-ATPase pump plays a key role in the oscillatory activity of murine small mesenteric vessels, as evidenced by the inhibition of the oscillatory phenomenon upon 1) blockade of Na+/K+-ATPase with ouabain, and 2) decreased Na+/K+-ATPase activity achieved by either a low temperature or K+-free solution. Na+/K+-ATPase seems to play an important role in the local vasomotion of the mesenteric vascular bed. In rats, the oscillations are modulated by variations in potassium conductance and Na+/K+-ATPase directly participates in the generation of rhythmic contractions in these vessels (24).
Na+/K+-ATPase is a membrane protein that transports Na+ and K+ across the plasma membrane against their concentration gradients (19,25). The enzyme is involved in several cellular functions, such as transmembrane potential, regulation of intracellular ionic concentrations and blood pressure regulation (19,26). An increase in Na+/K+-ATPase activity leads to hyperpolarization and relaxation of smooth muscle, whereas its inhibition by cardiac glycosides induces the opposite effects (25,27). It is possible that the oscillatory contraction in small mesenteric vessels reflects a contribution of Na+/K+-ATPase to the membrane potential: the relaxation phase matches membrane hyperpolarization driven by the enzyme whereas the contraction phase is coincident to the enzyme being inactive.
The electrogenic nature of the Na+/K+ pump leads to oscillations in membrane potential, which could spread to neighboring cells. This suggestion is supported by the observation of several investigators that ouabain-mediated inhibition of Na+/K+-ATPase blocks vasomotion (23,24,28,29). However, how the Na+ pumps of individual cells are entrained in this model to effect synchronized vasomotion in larger areas has not been discussed.
Chronic blockade of Na+/K+-ATPase with ouabain was induced to determine if this pharmacological maneuver would abolish or alter the oscillatory activity. The present study shows that ex vivo vasomotion is preserved in ouabain-treated animals, possibly due to increased Na+/K+-ATPase expression, demonstrated here both by molecular (Western blots) and pharmacological (contraction induced by ouabain incubation) approaches. Interestingly, vasomotion is increased in pathophysiological conditions such as hypertension, both in human and experimental models (30-32), but was not altered in ouabain-treated mice despite the increased arterial pressure. The possibility that plasma levels of ouabain in our treatment were too low and, therefore, ineffective to block Na+/K+-ATPase, can be ruled out by our observation that ouabain infusion induced up-regulation of Na+/K+-ATPase expression and blood pressure. In agreement, Dostanic et al. (33) showed that mice treated with ouabain (300 μg·kg-1·day-1) displayed serum ouabain-like reactivity significantly higher than the corresponding saline control groups and that these levels were sufficient to induce hypertension.
Although one may question the physiological relevance of vasomotion that is not changed in a pathological situation, others may argue that the phenomenon per se has such a relevant function that the organism compensates. In this case, by up-regulating the enzyme, Na+/K+-ATPase-associated vasomotion is maintained when ouabain is present. We have eliminated the possibility that oscillations are the result of injury in these vessels by testing vessel responsiveness to a series of agents.
The Na+/K+-ATPase α-1 isoform of rodents has unusually low affinity for ouabain (EC50 >50 µM) whereas, in mammals, the α-2 isoform has high ouabain affinity (EC50 >50 nM) (19,34). Our data show that both α-1 and α-2 isoforms of the Na+/K+-ATPase are expressed in mouse small mesenteric arteries. In addition, micromolar concentrations of ouabain in the muscle bath (concentration range of 1 to 10 µM) were able to completely abolish vasomotion. Although the α-1 isoform was expressed relatively more than the other isoforms, oscillatory activity was inhibited by concentrations of ouabain that inhibit the α-2 isoform, but not the “ouabain-insensitive” α-1 isoform (35). Our results, suggesting that the α-2 isoform subunit contributes to oscillatory activity, are consistent with reports demonstrating that the Na+/K+-ATPase α-2 isoform mediates ouabain-induced hypertension in mice as well as changes in vascular contractility following chronic administration of ouabain (33,35-37).
The results of the present study show that Na+/K+-ATPase plays a major role in oscillatory activity in mouse resistance arteries. In addition, mesenteric resistance arteries express both the α-1 and α-2 isoforms of Na+/K+-ATPase and both isoforms may contribute to oscillatory activity. Since oscillatory activity occurs upon agonist stimulation, it will be interesting to determine whether protein kinase C-induced phosphorylation of Na+/K+-ATPase is the mechanism driving the pump activity. In addition, because it is an endothelium-dependent event and Na+/K+-ATPase exhibits a wide tissue distribution, the initial event driving Na+/K+-ATPase activation will be addressed in the future.
Acknowledgments
The authors wish to thank Dr. Deborah L. Carr and Dr. Thomas A. Presley, Texas Tech University Health Sciences Center, for their generous donation of antibodies.
Research supported by grants from the National Institutes of Health (HL71138 and HL74167) and FAPESP (#2006/01773-0).
References
- 1.Aalkjaer C, Nilsson H. Vasomotion: cellular background for the oscillator and for the synchronization of smooth muscle cells. Br J Pharmacol. 2005;144:605–616. doi: 10.1038/sj.bjp.0706084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv. 2003;3:79–89. 51. doi: 10.1124/mi.3.2.79. [DOI] [PubMed] [Google Scholar]
- 3.Gratton RJ, Gandley RE, McCarthy JF, Michaluk WK, Slinker BK, McLaughlin MK. Contribution of vasomotion to vascular resistance: a comparison of arteries from virgin and pregnant rats. J Appl Physiol. 1998;85:2255–2260. doi: 10.1152/jappl.1998.85.6.2255. [DOI] [PubMed] [Google Scholar]
- 4.Meyer C, de Vries G, Davidge ST, Mayes DC. Reassessing the mathematical modeling of the contribution of vasomotion to vascular resistance. J Appl Physiol. 2002;92:888–889. doi: 10.1152/jappl.2002.92.2.888. [DOI] [PubMed] [Google Scholar]
- 5.Rucker M, Strobel O, Vollmar B, Roesken F, Menger MD. Vasomotion in critically perfused muscle protects adjacent tissues from capillary perfusion failure. Am J Physiol Heart Circ Physiol. 2000;279:H550–H558. doi: 10.1152/ajpheart.2000.279.2.H550. [DOI] [PubMed] [Google Scholar]
- 6.Iida N. Effects of vasomotion and venous pressure elevation on capillary-tissue fluid exchange across hetero-porous membrane. Biorheology. 1990;27:205–224. doi: 10.3233/bir-1990-27206. [DOI] [PubMed] [Google Scholar]
- 7.Lamb FS, Webb RC. Regenerative electrical activity and arterial contraction in hypertensive rats. Hypertension. 1989;13:70–76. doi: 10.1161/01.hyp.13.1.70. [DOI] [PubMed] [Google Scholar]
- 8.Myers JH, Lamb FS, Webb RC. Norepinephrine-induced phasic activity in tail arteries from genetically hypertensive rats. Am J Physiol. 1985;248:H419–H423. doi: 10.1152/ajpheart.1985.248.3.H419. [DOI] [PubMed] [Google Scholar]
- 9.Watts SW, Traub O, Webb RC. Effects of ramipril on contractile oscillations in arteries from genetically hypertensive rats. Clin Exp Hypertens. 1994;16:881–898. doi: 10.3109/10641969409078032. [DOI] [PubMed] [Google Scholar]
- 10.Struijker Boudier HA. Arteriolar and capillary remodelling in hypertension. Drugs. 1999;59(Spec No):37–40. [PubMed] [Google Scholar]
- 11.Gustafsson H, Nilsson H. Rhythmic contractions of isolated small arteries from rat: role of calcium. Acta Physiol Scand. 1993;149:283–291. doi: 10.1111/j.1748-1716.1993.tb09623.x. [DOI] [PubMed] [Google Scholar]
- 12.Griffith TM. Temporal chaos in the microcirculation. Cardiovasc Res. 1996;31:342–358. [PubMed] [Google Scholar]
- 13.Rocha ML, Bendhack LM. Spontaneous oscillatory contractions in aortas of rats with arterial pressure lability caused by sinoaortic denervation. Clin Exp Pharmacol Physiol. 2007;34:708–713. doi: 10.1111/j.1440-1681.2007.04612.x. [DOI] [PubMed] [Google Scholar]
- 14.Hilgers RH, Webb RC. Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H2275–H2284. doi: 10.1152/ajpheart.00949.2006. [DOI] [PubMed] [Google Scholar]
- 15.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinton JM, Langton PD. Inhibition of EDHF by two new combinations of K+-channel inhibitors in rat isolated mesenteric arteries. Br J Pharmacol. 2003;138:1031–1035. doi: 10.1038/sj.bjp.0705171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard TJ, Parvatiyar M, Bullard DP, Lynch RM, Lorenz JN, Paul RJ. Transgenic mice expressing Na+-K+-ATPase in smooth muscle decreases blood pressure. Am J Physiol Heart Circ Physiol. 2007;293:H1172–H1182. doi: 10.1152/ajpheart.00279.2007. [DOI] [PubMed] [Google Scholar]
- 21.Jackson WF, Mulsch A, Busse R. Rhythmic smooth muscle activity in hamster aortas is mediated by continuous release of NO from the endothelium. Am J Physiol. 1991;260:H248–H253. doi: 10.1152/ajpheart.1991.260.1.H248. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson H. Vasomotion and underlying mechanisms in small arteries. An in vitro study of rat blood vessels. Acta Physiol Scand Suppl. 1993;614:1–44. [PubMed] [Google Scholar]
- 23.Griffith TM, Edwards DH. Ca2+ sequestration as a determinant of chaos and mixed-mode dynamics in agonist-induced vasomotion. Am J Physiol. 1997;272:H1696–H1709. doi: 10.1152/ajpheart.1997.272.4.H1696. [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson H, Nilsson H. Rhythmic contractions in isolated small arteries of rat: role of K+ channels and the Na+,K(+)-pump. Acta Physiol Scand. 1994;150:161–170. doi: 10.1111/j.1748-1716.1994.tb09673.x. [DOI] [PubMed] [Google Scholar]
- 25.Fleming WW. The electrogenic Na+, K+-pump in smooth muscle: physiologic and pharmacologic significance. Annu Rev Pharmacol Toxicol. 1980;20:129–149. doi: 10.1146/annurev.pa.20.040180.001021. [DOI] [PubMed] [Google Scholar]
- 26.Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci U S A. 2005;102:15845–15850. doi: 10.1073/pnas.0507358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Vizcaino F, Cogolludo A, Tamargo J. Modulation of arterial Na+-K+-ATPase-induced [Ca2+]i reduction and relaxation by norepinephrine, ET-1, and PMA. Am J Physiol. 1999;276:H651–H657. doi: 10.1152/ajpheart.1999.276.2.H651. [DOI] [PubMed] [Google Scholar]
- 28.Osol G, Halpern W. Spontaneous vasomotion in pressurized cerebral arteries from genetically hypertensive rats. Am J Physiol. 1988;254:H28–H33. doi: 10.1152/ajpheart.1988.254.1.H28. [DOI] [PubMed] [Google Scholar]
- 29.Sabouni MH, Mustafa SJ. Effects of adenosine analogs and ouabain on rhythmicity in human coronary artery. Eur J Pharmacol. 1989;168:271–276. doi: 10.1016/0014-2999(89)90787-5. [DOI] [PubMed] [Google Scholar]
- 30.Lamb FS, Myers JH, Hamlin MN, Webb RC. Oscillatory contractions in tail arteries from genetically hypertensive rats. Hypertension. 1985;7:I-25–I-30. doi: 10.1161/01.hyp.7.3_pt_2.i25. [DOI] [PubMed] [Google Scholar]
- 31.Tostes RC, Storm DS, Chi DH, Webb RC. Intracellular calcium stores and oscillatory contractions in arteries from genetically hypertensive rats. Hypertens Res. 1996;19:103–111. doi: 10.1291/hypres.19.103. [DOI] [PubMed] [Google Scholar]
- 32.Webb RC, Schreur KD, Papadopoulos SM. Oscillatory contractions in vertebral arteries from hypertensive subjects. Clin Physiol. 1992;12:69–77. doi: 10.1111/j.1475-097x.1992.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 33.Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB. The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H477–H485. doi: 10.1152/ajpheart.00083.2004. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien WJ, Lingrel JB, Wallick ET. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch Biochem Biophys. 1994;310:32–39. doi: 10.1006/abbi.1994.1136. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, et al. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol. 2005;569:243–256. doi: 10.1113/jphysiol.2005.091801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossoni LV, Salaices M, Marin J, Vassallo DV, Alonso MJ. Alterations in phenylephrine-induced contractions and the vascular expression of Na+,K+-ATPase in ouabain-induced hypertension. Br J Pharmacol. 2002;135:771–781. doi: 10.1038/sj.bjp.0704501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shelly DA, He S, Moseley A, Weber C, Stegemeyer M, Lynch RM, et al. Na(+)pumpalpha2-isoform to contractility in vascular smooth muscle: evidence from gene-targeted neonatal mice. Am J Physiol Cell Physiol. 2004;286:C813–C820. doi: 10.1152/ajpcell.00389.2003. [DOI] [PubMed] [Google Scholar]