Abstract
Atherosclerotic cardiovascular disease is a major health problem around the world. Obesity is a primary risk factor for atherosclerosis and is associated with increased morbidity and mortality of cardiovascular diseases. However, the precise molecular pathways underlying this close association remain poorly understood. Adipokines are cytokines, chemokines and hormones secreted by adipose tissue that couple the regulation of lipid accumulation, inflammation, and atherogenesis, and therefore serve to link obesity with cardiovascular disorders. Obesity-related disorders including metabolic syndrome, diabetes, atherosclerosis, hypertension, and coronary artery disease are associated with dysregulated adipokine(s) expression. Recent studies demonstrate the proinflammatory effects as well as atherogenic properties of adipokines. Adipokines also participate in the regulation of endothelial function, which is an early event in atherosclerosis. By contrast, adiponectin, an adipocyte-derived hormone, exerts anti-inflammatory, anti-atherogenic and vascular protective effects. Furthermore, there is an interactive association among adipokines, by which adipokines reciprocally regulate each other’s expression. Understanding this interplay may reveal plausible mechanisms for treating atherosclerosis and coronary heart disease by modulating adipokine(s) expression. In this review, we discuss insights into the role and the therapeutic potential of adipokines as mediators of atherosclerosis.
Keywords: Obesity, Inflammation, Adipokines, Endothelial function, Atherosclerosis
INTRODUCTION
Obesity is becoming pandemic[1]. Obesity and overweight conditions pose a major risk for a number of comorbidities including atherosclerotic diseases. The increasing prevalence of obesity, and recognition of the role of abdominal adiposity, has again focused attention on the relationship of obesity to atherosclerosis and coronary heart diseases[2].
Although the association of obesity with atherosclerotic diseases has been widely reported[3], mechanisms that describe how excess fat causes impairment of vascular function and atherosclerosis formation have not yet been fully elucidated. Recent advances in obesity research strongly indicate that adipose tissue is an active endocrine organ that secretes multiple bioactive factors categorized as adipokines[4,5]. The adipokines include a large number of cytokines [e.g. tumor necrosis factor (TNF)-α and interleukin (IL)-6], chemokines [e.g. IL-8 and monocyte chemoattractant protein (MCP)-1] and hormones (e.g. leptin, resistin and adiponectin)[6-8]. This review focuses on the contribution of excess adipose tissue to the major underlying cause of cardiovascular death, atherosclerosis and the evidence regarding potential mechanisms by which excess adipose tissue and the dysregulated adipokine expression profile could adversely affect the vessel wall.
OBESITY AND ATHEROSCLEROSIS
Atherosclerosis is characterized by the progressive deposition of fatty substances, cholesterol, etc. (called plaques) in the intima and contributes to many cardiovascular diseases. Plaques can rupture and lead to formation of a blood clot, which can cause fatal complications, such as heart attack or stroke. The classical perception of atherosclerosis merely as a cholesterol storage disease has been replaced by the notion that inflammatory processes regulate all stages of athrosclerosis[9]. Obesity is an independent risk factor for the development of coronary artery atherosclerosis[3]. The fact that the prevalence of obesity is increasing among youth as well as adults, highlights the necessity for closely examining the association of obesity with atherosclerosis and the prevention of obesity[2]. Abdominal adiposity in particular is a major factor associated with accelerated progression of atherosclerosis, which emphasizes the need to develop strategies to avoid abdominal obesity and prevent atherosclerotic diseases[10].
There are a number of mechanisms by which obesity can adversely affect the vasculature and thereby increase cardiovascular mortality. Obesity has various consequences known to accelerate atherosclerosis, including hypertension, diabetes, and dyslipidemia[11]. Adipokines released by adipose tissue can target distant organs, such as liver, skeletal muscle and hypothalamus. These adipokines impact the atherogenic environment of the vessel wall through metabolic changes, such as hyperlipidemia, hyperglycemia and insulin resistance. Moreover, systemic inflammation attributed to proinflammatory adipokines produced by inflamed adipose tissue serve as an important factor contributing to the adverse effects of adiposity on the vasculature[12]. Visceral adipose tissue, with its favored access to the portal circulation, could be particularly important in this pathway. In addition to the systemic effects, perivascular adipose tissue exhibiting a proinflammatory phenotype may exert paracrine effects and promote inflammatory cell infiltration into the vascular wall, thereby exerting local effects[13]. In addition to proinflammatory effects and atherogenic properties, the most abundant adipocyte protein, adiponectin, has potent anti-inflammatory as well as anti-atherogenic effects[14]. Those adipose-derived factors influence gene expression and cell function in endothelial cells, arterial smooth muscle cells, and monocytes/macrophages, which represent the major cell types of the artery wall and are major components for defending vessel wall homeostasis.
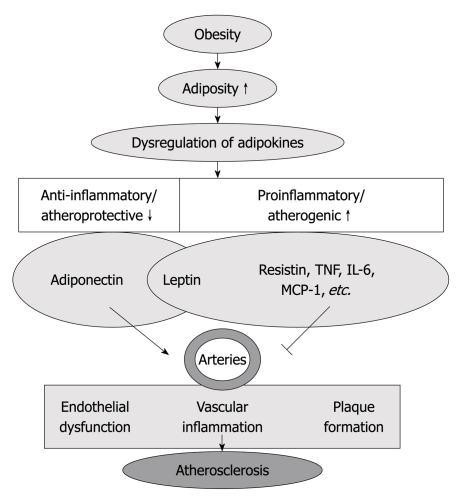
Therefore, adipokines may provide a link between adipose tissue lipid accumulation and atherosclerotic plaque formation, as well as the crosstalk between perivascular adipose and blood vessels in the regulation of atherosclerotic disease[13] (Figure 1).
Figure 1.
Increased adiposity (obesity) is associated with dysregulated adipokine production, which is characterized by decrease in anti-inflammatory/atheroprotective adipokines (adiponectin) and increase in proinflammatory/atherogenic adipokines [resistin, tumor necrosis factor (TNF)-α, interleukin (IL)-6, macrophage chemoattractant protein (MCP)-1, etc.]. Those adipokines participate in the regulation of endothelial function, vascular inflammation and plaque formation, which contribute to the inception and progression of atherosclerosis.
THE PROINFLAMMATORY CAPACITY OF ADIPOSE TISSUE
Obesity is associated with a chronic low-grade inflammatory condition in adipose tissue[15]. During obesity, adipose tissue is infiltrated by macrophages and displays secretion of proinflammatory cytokines[16,17]. Very recently, T cells have also been detected in adipose tissue with increased infiltration in obesity[18-20], suggesting the potential role of adaptive immunity in obesity-related inflammation. Meanwhile, chemokines expressed in obese adipose tissue likely mediate the recruitment of these cells[21], supporting the view of the existence of feedback regulation in perpetuating inflammatory status. Although it remains unclear which inflammatory cell types play predominant roles in the regulation of adipose inflammation, it is widely accepted that adipose inflammation, especially visceral adipose inflammation, increases vascular risk of disease due to secretion of adipokines by cellular constituents of the adipose tissue[22]. Atherosclerotic mice exhibit increased inflammation in periadventitial and visceral adipose tissue[23]. The transplanted fat depots revealed chronically increased macrophage infiltration with characteristics identical to those observed in fat harvested from obese animals. Interestingly, by transplanting epididymal fat depots into atherosclerosis-prone apolipoprotein E knockout (ApoE KO) mice, plasma levels of leptin, resistin, and MCP-1 were increased. Mice transplanted with visceral fat developed significantly more atherosclerosis compared with sham-operated animals. The above results further support the idea that adipose inflammation contributes to atherosclerosis formation[24].
The cellular source of adipokines released by adipose are examined in several studies. Leptin and adiponectin are predominantly produced by adipocytes in adipose and are released to the blood as hormones[25]. Over 90% of the adipokines released by adipose tissue, except for adiponectin and leptin, could be attributed to nonfat cells[25]. The location of adipose depots also affects the production and release of adipokines. Visceral adipose tissue released greater amounts of vascular endothelial growth factor, IL-6, and plasminogen activator inhibitor 1 compared with abdominal subcutaneous adipose tissue[25].
Therefore, the proinflammatory properties may serve as the molecular basis for linking obesity with cardiovascular disorders. Knowledge of how alterations in the endocrine function of adipose tissue occur may help to identify mechanisms underlying the high cardiovascular risk associated with obesity[26].
ROLE OF ADIPOKINES IN ENDOTHELIAL DYSFUNCTION DURING ATHEROSCLEROSIS
Endothelial dysfunction is a key early event in the development of atherosclerosis[27,28], which can be detected before structural changes to the vessel wall are apparent using angiography or ultrasound. Atherosclerotic endothelial dysfunction, particularly in the early disease stages, is primarily due to dysregulation of endothelial nitric oxide synthase (eNOS) enzymatic activity and inactivation of nitric oxide (NO) through oxidative stress[29]. Reduced bioavailability of NO is involved in the initiation, progression and complications of atherosclerosis[30,31]. NO opposes the effects of endothelium-derived vasoconstrictors and inhibits oxidation of low-density lipoprotein (LDL)[30]. Thus, reduced NO bioavailability leads to endothelial dysfunction[30].
Recent studies by our and other groups suggest adipokines play a role in regulating atherosclerotic endothelial function by mediating NO production and oxidative stress. Adiponectin induces eNOS activation and NO production in endothelial cells[32]. Adiponectin reduces reactive oxygen species (ROS) production as well as improving endothelial function in aortas of ApoE KO mice[33]. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. The protective role of globular adiponectin in inhibiting leukocyte-endothelium adhesion was abolished by the blockade of eNOS with N(omega)-nitro-L-arginine methyl ester[34]. TNF-α (TNF-α plays an important role in both atherogenesis and vascular dysfunction. Inhibition of TNF-α reduces atherosclerosis[35] and improves endothelial function in ApoE KO mice (unpublished data). Aortic ROS formation and nuclear factor-κB (NF-κB) expression were higher in aortas of ApoE KO mice compared with control mice[33]. Genetic deletion of TNF-α reduced aortic superoxide production and improved NO availability[36]. Thus, vascular inflammation and oxidative stress may contribute to TNF-α-induced endothelial dysfunction.
Thus, maintaining endothelial health is important in preventing atherosclerotic diseases. The role of adipokines in atherosclerotic endothelial dysfunction warrants further attention.
ROLE OF ADIPOKINES IN ATHEROGENESIS
Some adipokines are atherogenic while others have atheroprotective effects.
Adiponectin exerts atheroprotective effects. In in vitro studies, adiponectin inhibited TNF-α-induced increase in endothelial expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selection[37]. Adiponectin also suppresses vascular smooth muscle cell proliferation[38], as well as macrophage to foam cell transformation[39]. Adiponectin reduces lipid accumulation in macrophage foam cells[40] and prevents atherosclerosis by increasing cholesterol efflux from macrophages[41]. In vivo studies further demonstrated that apolipoprotein E/adiponectin double-deficient mice had increased plasma IP-10 levels, accelerated T-lymphocyte accumulation in atheromata, and augmented atherogenesis compared with ApoE single-deficient mice. Indeed, adiponectin inhibits the production of CXC Receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis[42]. Adiponectin prevents adventitial fibroblasts from proliferating, transforming to myofibroblasts, and migrating to the intima, thus worsening atherosclerosis[43]. Macrophage adiponectin transgenic mice exhibited reduced macrophage foam cell formation in the arterial wall when these transgenic mice were crossed with a LDL receptor knockout (ldlr-/-) mouse model and were fed a high-fat diet[44]. A previous study suggested that an adenovirus-mediated increase in plasma adiponectin significantly suppressed the progression of atherosclerotic lesions in aortic sinus by 30% in ApoE KO mice[45]. Globular adiponectin also showed effects on amelioration of atherosclerosis, which was associated with decreased expression of class A scavenger receptor and TNF[46]. Thus, adiponectin modulates multiple pathways to impact the development of atherosclerosis.
There is some discrepancy regarding the role of leptin in atherogenesis. One study suggested that leptin treatment of ApoE KO mice did not affect lesion size and surface area occupied by atherosclerotic lesions, but did increase lesion calcification and the expression of the osteoblast-specific markers, osteocalcin and osteopontin. Thus, leptin may increase cardiovascular risk by promoting osteogenic differentiation and vascular calcification[47]. Another study showed that recombinant leptin treatment resulted in an increase in atherosclerosis (lesion surface coverage) and a shortened time to occlusive thrombosis after vascular injury, which promotes atherosclerosis and thrombosis in ApoE KO mice[48]. Leptin deficient hyperlipidemic mice (ob/ob; ApoE KO mice) developed significantly less atherosclerosis than ApoE KO mice, when fed an atherogenic diet for 16 wk from 8 wk of age. Histological analysis revealed that most of the atherosclerotic lesions in leptin deficient ApoE KO mice remained as fatty streaks, while those in ApoE KO mice were mainly fibrous plaques[49]. Leptin-deficiency (ob/ob) in LDL receptor deficient mice induces an unexpected 2.2- to 6-fold reduction in atherosclerotic lesion development[50]. The above findings support the notion that leptin accelerates atherosclerosis. However, one previous study reported that leptin deficiency in atherosclerosis-susceptible LDL receptor knockout or ApoE knockout background resulted in the development of larger atherosclerotic lesions[51], suggesting a protective role for leptin in atherosclerosis. These results identify a critical role for the leptin/leptin receptor pathway in the modulation of atherogenesis, and further studies are needed before drawing conclusions. Resistin is a cardiovascular and atherosclerotic risk factor[52]. Resistin is a 12.5 kDa protein originally found to be secreted by mouse adipocytes[53]. Whereas in rodents the adipocyte is the major source of resistin, in humans resistin is mainly expressed in macrophages[54]. Resistin protein is present in both murine and human atherosclerotic lesions. ApoE KO mice had significantly higher resistin mRNA and protein levels in their aortas, and elevated serum resistin levels. Incubation of murine aortic endothelial cells with recombinant resistin increased MCP-1 and soluble VCAM-1 protein levels in the conditioned medium, suggesting a possible mechanism where resistin contributes to atherosclerotic diseas[55].
TNF-α also plays an important role in atherosclerosis[56]. TNF-α has been detected in atherosclerotic lesions throughout all stages of human atherosclerosis[57,58], and was found to be associated with atherosclerosis in mouse models[35,59]. Mice deficient in both ApoE and TNF-α showed less advanced atherosclerosis than ApoE KO mice[60]. mRNA levels of pro-atherosclerotic factors, i.e. IL-1β, interferon-γ, ICAM-1, VCAM-1, MCP-1, GM-CSF and NF-κB (p65) were significantly downregulated in ApoE and TNF-α double-knockout mice[60]. Lectin-like oxidized LDL receptor-1 (LOX-1) is an important mediator of atherogenesis. TNF-α-induced increase in LOX-1 expression was demonstrated in various cell types, including endothelial cells, macrophages, vascular smooth muscle cells, etc[61-63].
IL-6 contributes to both atherosclerotic plaque development and plaque destabilization via a variety of mechanisms[64], which involve the release of other pro-inflammatory cytokines and prothrombotic mediators, oxidation of lipoproteins by phospholipases, stimulation of acute phase protein secretion, and the activation of matrix metalloproteinases[64].
MCP-1 plays a key role in monocyte/macrophage infiltration to the sub-endothelial space of the blood vessel wall, which is a crucial initial step in atherosclerosis[65]. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cell proliferation[66], suggesting it has atheroprotective potential.
In summary, adiponectin is anti-atherogenic, but resistin, TNF-α, IL-6 and MCP-1, etc. exert atherogenic effects through profound mechanisms. The role of leptin in atherogenesis remains to be controversial. The role of various adipokines in the pathogenesis of atherosclerosis and the therapeutic potential of modulating adipokine expression warrants further investigation (Figure 1).
ADIPOKINES INTERPLAY
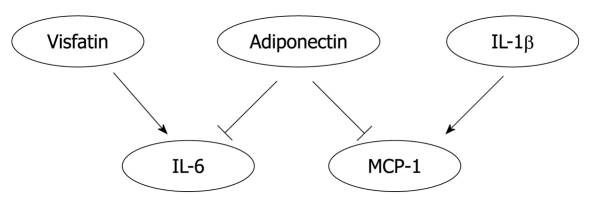
Reciprocal regulation may occur among adipokines. Transgenic mice that specifically express the gene coding for human adiponectin in mouse macrophages exhibit enhanced whole-body glucose tolerance and insulin sensitivity with reduced MCP-1 and TNF-α levels both in macrophages and serum[44]. Visfatin (pre-B cell colony-enhancing factor) has recently been identified as a new adipocytokine affecting insulin resistance[67]. Visfatin treatment increased the level of circulating IL-6 without affecting that of TNF[68]. In addition to systemic effects, adipokines showed interactive regulation in various cell types. Both full length and globular adiponectin attenuated IL-6 and MCP-1 production from inflamed adipocytes[69]. IL-1β promotes the expression of MCP-1 in human aortic smooth muscle cells via the NF-κB signaling pathway[70]. Thus, the interplay among adipokines may be an important contributor in the pathogenesis of atherogenesis (Figure 2).
Figure 2.
An heuristic diagram positing the interplay of adipokines. Visfatin treatment increased the level of interleukin (IL)-6 in circulation. Adiponectin reduced the production of IL-6 and macrophage chemoattractant protein (MCP)-1 by inflamed adipocytes. IL-1β increased the expression of MCP-1 in aortic smooth muscle cells. Thus, the interplay among adipokines may significantly affect the pathogenesis of atherogenesis.
FUTURE PERSPECTIVES
A strong correlation is observed between obesity and atherosclerosis. However, the existence and nature of a communication/linkage between adipose tissue-derived factors and pathogenesis of atherosclerosis warrants further investigation. Future studies may better elucidate the mechanisms of this communication by delving into these aspects: (1) Direct and specific evidence is needed to determine if/how adipose communicates with vasculature through adipose proinflammatory cells and/or various adipose-derived cytokines, hormones and lipid signals; (2) The role of adipose, as an endocrine organ, results in altered levels of systemic regulators of chronic inflammation and energy metabolism. Therefore, the metabolic parameters associated with adipose dysfunction may indirectly affect the vascular tissues. Thus, studies are needed to examine the local pathogenic effects of perivascular fat vs. the systemic effects of obesity-induced metabolic changes in the pathophysiology of atherosclerosis; (3) Investigate using animal models relevant to atherosclerosis in human beings; and (4) If obesity is rescued, can that rescue atherosclerosis?
Of particular importance is the need to identify novel adipokines that play crucial roles in atherogenesis or exert atheroprotective effects and to improve the translation of the exciting scientific results to patients.
CONCLUSION
Obesity, especially visceral obesity, is associated with dyslipidemia, impaired glucose metabolism, and hypertension, all of which exacerbate atherosclerosis. One plausible mechanism involves adipokines, produced by adipose tissue in obesity that can directly impact the atherogenic environment of the vessel wall by regulating gene expression and function in endothelial, arterial smooth muscle, and macrophage cells, etc. Thus, this review explores the connection between adipose and atherosclerosis with particular emphasis on the role of adipokines as mediators in the development of atherosclerosis. We suggest that the proinflammatory capacity of adipose tissue provides new insights in the pathophysiology of atherosclerosis.
Acknowledgments
We appreciate the expert assistance Dr. Marvin K Harris provided.
Footnotes
Supported by Grants from American Heart Association SDG, No. 110350047A; NIH Grants, No. RO1-HL077566 and No. RO1-HL085119 to Zhang C; and American Heart Association Pre-doctoral Fellowship, No. 10PRE4300043 to Zhang H
Peer reviewers: Khalid Rahman, BSc (Hons), PhD, Professor of Physiological Biochemistry/Academic Recruitment Co-ordinator, School of Pharmacy and Biomolecular Sciences, Max Perutz Building, Byrom Street, Liverpool, L3 3AF, United Kingdom; Ehud Goldhammer, Professor, Head Cardiac Rehabilitation Center, Department of Cardiology, Bnai Zion Medical Center, 35 Golomb Str., Haifa 31048, Israel
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
References
- 1.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 2.McGill HC Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 3.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 4.MacDougald OA, Burant CF. The rapidly expanding family of adipokines. Cell Metab. 2007;6:159–161. doi: 10.1016/j.cmet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 7.Eringa EC, Bakker W, Smulders YM, Serné EH, Yudkin JS, Stehouwer CD. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation. 2007;14:389–402. doi: 10.1080/10739680701303584. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Zhang C. Regulation of Microvascular Function by Adipose Tissue in Obesity and Type 2 Diabetes: Evidence of an Adipose-Vascular Loop. Am J Biomed Sci. 2009;1:133–142. doi: 10.5099/aj090200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 10.Lakka TA, Lakka HM, Salonen R, Kaplan GA, Salonen JT. Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis. 2001;154:497–504. doi: 10.1016/s0021-9150(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 11.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 12.Ikeoka D, Mader JK, Pieber TR. Adipose tissue, inflammation and cardiovascular disease. Rev Assoc Med Bras. 2010;56:116–121. doi: 10.1590/s0104-42302010000100026. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacifico L, Di Renzo L, Anania C, Osborn JF, Ippoliti F, Schiavo E, Chiesa C. Increased T-helper interferon-gamma-secreting cells in obese children. Eur J Endocrinol. 2006;154:691–697. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- 19.O'Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, Jobe BA, Roberts CT Jr, Slifka MK, Marks DL. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 22.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 23.Lohmann C, Schäfer N, von Lukowicz T, Sokrates Stein MA, Borén J, Rütti S, Wahli W, Donath MY, Lüscher TF, Matter CM. Atherosclerotic mice exhibit systemic inflammation in periadventitial and visceral adipose tissue, liver, and pancreatic islets. Atherosclerosis. 2009;207:360–367. doi: 10.1016/j.atherosclerosis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 26.Fortuño A, Rodríguez A, Gómez-Ambrosi J, Frühbeck G, Díez J. Adipose tissue as an endocrine organ: role of leptin and adiponectin in the pathogenesis of cardiovascular diseases. J Physiol Biochem. 2003;59:51–60. doi: 10.1007/BF03179868. [DOI] [PubMed] [Google Scholar]
- 27.Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Rönnemaa T, Raitakari OT. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004;110:2918–2923. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 28.Williams IL, Wheatcroft SB, Shah AM, Kearney MT. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord. 2002;26:754–764. doi: 10.1038/sj.ijo.0801995. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4:53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 31.Schächinger V, Zeiher AM. Atherosclerosis-associated endothelial dysfunction. Z Kardiol. 2000;89 Suppl 9:IX/70–74. doi: 10.1007/s003920070033. [DOI] [PubMed] [Google Scholar]
- 32.Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Zhang H, McAfee S, Zhang C. The reciprocal relationship between adiponectin and LOX-1 in the regulation of endothelial dysfunction in ApoE knockout mice. Am J Physiol Heart Circ Physiol. 2010;299:H605–H612. doi: 10.1152/ajpheart.01096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 38.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 39.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 40.Tian L, Luo N, Klein RL, Chung BH, Garvey WT, Fu Y. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis. 2009;202:152–161. doi: 10.1016/j.atherosclerosis.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsubakio-Yamamoto K, Matsuura F, Koseki M, Oku H, Sandoval JC, Inagaki M, Nakatani K, Nakaoka H, Kawase R, Yuasa-Kawase M, et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2008;375:390–394. doi: 10.1016/j.bbrc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–225. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 43.Cai XJ, Chen L, Li L, Feng M, Li X, Zhang K, Rong YY, Hu XB, Zhang MX, Zhang Y, et al. Adiponectin inhibits lipopolysaccharide-induced adventitial fibroblast migration and transition to myofibroblasts via AdipoR1-AMPK-iNOS pathway. Mol Endocrinol. 2010;24:218–228. doi: 10.1210/me.2009-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo N, Liu J, Chung BH, Yang Q, Klein RL, Garvey WT, Fu Y. Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes. 2010;59:791–799. doi: 10.2337/db09-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 47.Zeadin M, Butcher M, Werstuck G, Khan M, Yee CK, Shaughnessy SG. Effect of leptin on vascular calcification in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:2069–2075. doi: 10.1161/ATVBAHA.109.195255. [DOI] [PubMed] [Google Scholar]
- 48.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:e119–e122. doi: 10.1161/01.ATV.0000173306.47722.ec. [DOI] [PubMed] [Google Scholar]
- 49.Chiba T, Shinozaki S, Nakazawa T, Kawakami A, Ai M, Kaneko E, Kitagawa M, Kondo K, Chait A, Shimokado K. Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis. 2008;196:68–75. doi: 10.1016/j.atherosclerosis.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 50.Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clément K, Holvoet P, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2691–2698. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 51.Hasty AH, Shimano H, Osuga J, Namatame I, Takahashi A, Yahagi N, Perrey S, Iizuka Y, Tamura Y, Amemiya-Kudo M, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem. 2001;276:37402–37408. doi: 10.1074/jbc.M010176200. [DOI] [PubMed] [Google Scholar]
- 52.Cohen G, Hörl WH. Resistin as a cardiovascular and atherosclerotic risk factor and uremic toxin. Semin Dial. 2009;22:373–377. doi: 10.1111/j.1525-139X.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 53.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 54.Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 55.Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Peppel K, Sivashanmugam P, Orman ES, Brian L, Exum ST, Freedman NJ. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1087–1094. doi: 10.1161/ATVBAHA.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, Forrester JS. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990;65:297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- 58.Rus HG, Niculescu F, Vlaicu R. Tumor necrosis factor-alpha in human arterial wall with atherosclerosis. Atherosclerosis. 1991;89:247–254. doi: 10.1016/0021-9150(91)90066-c. [DOI] [PubMed] [Google Scholar]
- 59.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180:11–17. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Xiao N, Yin M, Zhang L, Qu X, Du H, Sun X, Mao L, Ren G, Zhang C, Geng Y, et al. Tumor necrosis factor-alpha deficiency retards early fatty-streak lesion by influencing the expression of inflammatory factors in apoE-null mice. Mol Genet Metab. 2009;96:239–244. doi: 10.1016/j.ymgme.2008.11.166. [DOI] [PubMed] [Google Scholar]
- 61.Chiba Y, Ogita T, Ando K, Fujita T. PPARgamma ligands inhibit TNF-alpha-induced LOX-1 expression in cultured endothelial cells. Biochem Biophys Res Commun. 2001;286:541–546. doi: 10.1006/bbrc.2001.5361. [DOI] [PubMed] [Google Scholar]
- 62.Liang M, Zhang P, Fu J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Lett. 2007;258:31–37. doi: 10.1016/j.canlet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Shibata Y, Kume N, Arai H, Hayashida K, Inui-Hayashida A, Minami M, Mukai E, Toyohara M, Harauma A, Murayama T, et al. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis. 2007;193:20–27. doi: 10.1016/j.atherosclerosis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 64.Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009;102:215–222. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
- 65.Ohman MK, Eitzman DT. Targeting MCP-1 to reduce vascular complications of obesity. Recent Pat Cardiovasc Drug Discov. 2009;4:164–176. doi: 10.2174/157489009789152230. [DOI] [PubMed] [Google Scholar]
- 66.Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, de Vries CJ, Egashira K, van Bockel JH, Quax PH. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2063–2069. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- 67.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 68.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 69.Zoico E, Garbin U, Olioso D, Mazzali G, Fratta Pasini AM, Di Francesco V, Sepe A, Cominacini L, Zamboni M. The effects of adiponectin on interleukin-6 and MCP-1 secretion in lipopolysaccharide-treated 3T3-L1 adipocytes: role of the NF-kappaB pathway. Int J Mol Med. 2009;24:847–851. doi: 10.3892/ijmm_00000302. [DOI] [PubMed] [Google Scholar]
- 70.Lim JH, Um HJ, Park JW, Lee IK, Kwon TK. Interleukin-1beta promotes the expression of monocyte chemoattractant protein-1 in human aorta smooth muscle cells via multiple signaling pathways. Exp Mol Med. 2009;41:757–764. doi: 10.3858/emm.2009.41.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]