Abstract
Ablation therapy is one of the best curative treatment options for malignant liver tumors, and can be an alternative to resection. Radiofrequency ablation (RFA) of primary and secondary liver cancers can be performed safely using percutaneous, laparoscopic, or open surgical techniques, and RFA has markedly changed the treatment strategy for small hepatocellular carcinoma (HCC). Percutaneous RFA can achieve the same overall and disease-free survival as surgical resection for patients with small HCC. The use of a laparoscopic or open approach allows repeated placements of RFA electrodes at multiple sites to ablate larger tumors. RFA combined with transcatheter arterial chemoembolization will make the treatment of larger tumors a clinically viable treatment alternative. However, an accurate evaluation of treatment response is very important to secure successful RFA therapy. Since a sufficient safety margin (at least 0.5 cm) can prevent local tumor recurrences, an accurate evaluation of treatment response is very important to secure successful RFA therapy. To minimize complications of RFA, clinicians should be familiar with the imaging features of each type of complication. Appropriate management of complications is essential for successful RFA treatment.
Keywords: Hepatocellular carcinoma, Radiofrequency ablation, Transcatheter arterial chemoembolization
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common solid cancers worldwide, with an estimated annual incidence of at least one million new patients[1-4]. Furthermore, the liver is second only to lymph nodes as a common site of metastasis from other solid cancers[5-8]. Surgery is the only curative option for HCC; however, the majority of primary liver cancers are not suitable for curative resection at the time of diagnosis. Difficulties in surgical resection may be related to size, site, and number of tumors, vascular and extrahepatic involvement as well as the general condition and liver function of the patient[9-12]. There is, therefore, a need to develop a simple and effective technique for the treatment of unresectable tumors within the liver. In recent years, local ablative techniques [percutaneous ethanol injection (PEI), microwave coagulation therapy (MCT) and radiofrequency ablation (RFA)] have emerged in clinical practice to expand the pool of patients considered for liver-directed therapies[13-16].
Localized application of thermal energy induces tumor cell destruction. When tumor cells are heated above 45-50°C, intracellular proteins are denatured and cell membranes are destroyed through the dissolution and melting of lipid bilayers[17]. RFA is a localized thermal treatment technique designed to produce tumor destruction by heating tumor tissue to temperatures that exceed 60°C[17]. The alternating current of radiofrequency waves passing down from an uninsulated electrode tip into the surrounding tissues generates changes in the direction of ions and creates ionic agitation and frictional heating. This tissue heating then drives extracellular and intracellular water out of the tissue, resulting in tissue destruction by coagulative necrosis[18,19]. Currently, RFA has gained popularity based on the ease of use, safety, reasonable cost and applicability to minimally invasive techniques. This paper reviews the current status of RFA for HCC.
EQUIPMENT
RFA electrodes and generators
Three types of RF electrodes are currently available commercially: two brands of retractable needle electrodes (model 70 and model 90 Starburst XL needles, RITA Medical Systems, Mountain View, CA, USA; LeVeen needle electrode, Boston Scientific, Boston, MA, USA) and an internally cooled electrode (Cool-Tip RF electrode; Radionics, Burlington, MA, USA)[15].
The needle electrodes of RITA consist of a 14-gauge insulated outer needle that houses nine retractable curved electrodes of various lengths. When the electrodes are extended, the device assumes the approximate configuration of a Christmas tree. Nine of the electrodes are hollow and contain thermocouples in their tips in order to measure the temperature of adjacent tissue. The alternating electric current generator comes in a 250-W model at 460 kHz (Model 1500X RF Generator, RITA Medical Systems). The ablation algorithm is based on temperature at the tips of the electrodes. After the ablation cycle is completed, a temperature reading from the extended electrodes in excess of 50°C at 1 min is considered to indicate satisfactory ablation.
Another RFA device (LeVeen Needle Electrode; Radiotherapeutics) has retractable curved electrodes and an insulated 17-gauge outer needle that houses 10 solid retractable curved electrodes that, when deployed, assume the configuration of an umbrella. The electrodes are manufactured in different lengths (2- to 4.0-cm umbrella diameter). The alternating electric current generator is 200 W operated at 480 kHz (RF 3000; Boston Scientific). The ablation algorithm is based on tissue impedance, and ablation is considered successful if the device impedes out.
The third RFA device (Cool-Tip radiofrequency electrode; Radionics) has an insulated hollow 17-gauge needle with an exposed needle tip of variable length (2- or 3-cm). The tip of the needle contains a thermocouple to record the temperature of adjacent tissue. The shaft of the needle has two internal channels to allow the needle to be perfused with chilled water. In an attempt to further increase the size of the ablation area, the manufacturer placed three of the cooled needles in a parallel triangular cluster with a common hub. The generator has a peak power output of 200 W and is operated at 480 kHz (CC-1; Radionics). The ablation algorithm is based on tissue impedance, and ablation is considered successful if the device impedes out. As a result, successful ablations usually increase the temperature of the ablated tissue to above 60°C.
Selection criteria of patients with HCC
In patients with HCC, exclusion criteria should include evidence of extrahepatic metastases and/or lobar and local portal venous thrombosis or uncontrolled liver disease decompensation, patients with clotting impairment, renal failure, or Child-Pugh class C cirrhosis. In the EASL Consensus Conference criteria[20], all patients that had tumor nodules with a maximum diameter of 3 cm and not more than three in number with contraindications for surgery are included.
Assessment of technical effectiveness
The technical effectiveness of ablation is commonly assessed by findings on contrast-enhanced computed tomography (CT) or magnetic resonance imaging. A tumor was considered to have been successfully ablated when there were no longer any enhanced regions within the entire tumor during the arterial phase and at least a 0.5 cm margin of apparently normal hepatic tissue surrounding the tumor during the portal phase (Figure 1)[21-23]. This safety margin for RFA therapy is necessary from the perspective of partial volume effect. Failure to establish a sufficient ablative safety margin was shown to be an independently significant risk factor for local tumor progression on multivariate analysis[24]. Part of the tumor was diagnosed as remaining viable when images of the ablated area showed nodular peripheral enhancement[25].
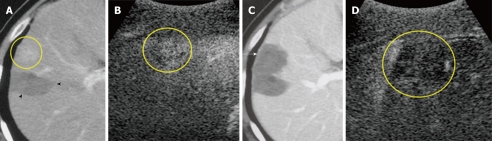
Figure 1.
A 61-year-old man with 1.5-cm recurrent hepatocellular carcinoma after ablation therapy in segment 5 of the liver. A: Early-phase dynamic computed tomography (CT) scan shows recurrent tumor (circle). Non-enhanced area (arrowheads) was previously treated by radiofrequency ablation (RFA); B: Contrast harmonic ultrasound (US) using Levovist shows enhancement of viable focus of a hepatocellular carcinoma (HCC) nodule (circle); C: Portal-phase dynamic CT scan, which was obtained 3 d after RFA shows that the tumor was not enhanced, indicating complete necrosis of the lesion (arrow); D: Contrast harmonic US, which was obtained 3 d after ablation shows non-enhanced area (circle).
CLINICAL OUTCOMES
Percutaneous approach
A randomized control trial (RCT) has shown that RFA achieved survival rates similar to those achieved following resection[26] (Table 1). Chen et al[26] conducted a RCT on 180 patients with a solitary HCC ≤ 5 cm indicated to receive either percutaneous RFA or surgical resection. This study showed percutaneous RFA achieved the same overall and disease-free survival rates as surgical resection for patients with small solitary HCC. The 1- and 4-year overall survival rates after percutaneous RFA and surgery were 95.8%, 67.9% and 93.3%, 64.0%, respectively. The corresponding disease-free survival rates were 85.9%, 46.4% and 86.6%, 51.6%, respectively. However, in cases of primary liver cancer in which local curative therapy was achieved by securing a safety margin, the 4-year survival rate was relatively high, at 66%-82% (results in Japan)[27,28]. Percutaneous RFA has an advantage over liver resection in providing a better short-term postoperative result because local ablative therapy is a less invasive procedure. Although promising, these data need to be confirmed in larger RCTs before local ablative therapy can replace partial hepatectomy in the treatment of good surgical candidates.
Table 1.
Studies comparing radiofrequency ablation vs hepatic resection for hepatocellular carcinoma
| Author, yr | Study type | RFA/resection | RFA/resection (mean tumor size, cm) | RFA vs resection (%) (overall survival) | P |
| Chen, 2006 | RCT | 90/90 | -/- | 65.9 vs 64.0 (4-yr) | NS |
| Takayama, 2009 | Retrospective | 1315/1235 | 1.6/1.8 | 95 vs 94 (2-yr) | 0.28 |
| Ueno, 2009 | Retrospective | 123/110 | 2.0/2.7 | 63 vs 80 (5-yr) | 0.06 |
| Hiraoka, 2008 | Retrospective | 105/59 | -/- | 59.3 vs 59.4 (5-yr) | NS |
| Abu-Hilal, 2008 | Retrospective | 34/34 | 3.0/3.8 | 57 vs 56 (5-yr) | 0.3 |
| Gnglielmi, 2008 | Retrospective | 23/33 | -/- | 45 vs 55 (5-yr) | 0.7 |
| Wakai, 2006 | Retrospective | 64/85 | -/- | 30 vs 53 (10-yr) | 0.012 |
| Ogihara, 2005 | Retrospective | 40/47 | 4.6/7.4 | 39 vs 31 (5-yr) | 0.79 |
| Montorsi, 2005 | Prospective | 58/40 | -/- | 30 vs 53 (4-yr) | 0.018 |
| Vivarelli, 2004 | Retrospective | 79/79 | -/- | 33 vs 65 (3-yr) | 0.002 |
RFA: Radiofrequency ablation; RCT: Randomized control trial; NS: Not significant.
RFA has also been investigated for treating patients with large or multifocal tumors. However, the size and number of tumors are important factors determining the local recurrence rate after RFA[29]. Apart from the larger tumor volume, large liver cancers more frequently have irregular borders and satellite lesions. Therefore, precise tailoring of the size and shape of the thermal lesion is important in RFA for large liver cancers. A number of precisely calculated overlapping coagulation zones are necessary to treat large liver cancers. To increase the size of the coagulation zone in RFA, investigators tried using vascular occlusion during RFA[30,31]. Temporary interruption of hepatic blood flow using vascular occlusion techniques (e.g. balloon catheter occlusion of the hepatic artery, transcatheter arterial embolization (TAE), or transcatheter arterial chemoembolization (TACE) has been shown to increase the efficacy of interstitial thermotherapy due to a significant increase in lesion volume. Vascular occlusion causes a reduction of heat dispersion, thus increasing the range of therapeutic thermal coagulation. Peng et al[32] reported a series of 120 patients with HCC, and the 1-, 2-, 3-, and 5-year overall survival rates for the TACE-RFA and RFA groups were 93%, 83%, 75%, 50%, and 89%, 76%, 64%, 42%, respectively (P = 0.045).
Ultrasound (US)-guided procedures are necessary but have limited use when the tumor is located under the diaphragm. However, saline solution injection into the pleural cavity can separate the lung and liver on B-mode US, i.e. artificial pleural effusion acts as an acoustic media. There are reports on the feasibility and safety of RFA with artificially induced pleural effusion for HCC located in the right subphrenic region[33-36]. In a series of 24 patients with HCC located in the hepatic dome, 200-1100 mL of 5% glucose solution was infused intrathoracically to separate the lung and liver, thus, complete tumor necrosis in a single session was achieved in 96.4% of patients[36].
Multiple RFA sessions for locally progressive HCCs were previously required because it is frequently difficult to distinguish viable tumors from necrotic tissue on B-mode US[37]. However, contrast-enhanced harmonic US imaging is able to evaluate small hypervascular HCCs even when B-mode US cannot adequately characterize the tumors[38-43]. In particular, contrast harmonic US has been improved by the development of second-generation contrast agents such as sulfur hexafluoride microbubbles (SonoVue; Bracco SpA, Milan, Italy), perflutren lipid microbubbles (Definity; Bristol- Myers Squibb, North Billerica, MA, USA), perflutren protein microbubbles (Optison; GE Healthcare, Buckinghamshire, UK), and perfluorocarbon microbubbles (Sonazoid; Daiichi-Sankyo, Tokyo, Japan). These microbubbles provide stable nonlinear oscillation in a low power acoustic field due to the hard shell of these bubbles, producing great detail in the harmonic signals in real-time[44-49]. It has been reported that contrast harmonic sonography-guided RFA is an efficient approach for guiding further ablation of hepatic malignancies that are not clearly demarcated by B-mode US (Figure 2)[50-54].
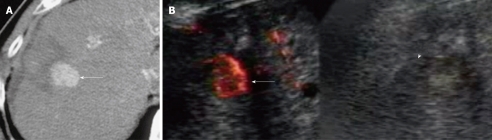
Figure 2.
A 71-year-old man with 2.0 cm local tumor progression of hepatocellular carcinoma after radiofrequency ablation therapy in segment 8 of the liver. A: Early-phase dynamic computed tomography (CT) scan shows outgrowth pattern of locally progressive hepatocellular carcinoma (HCC) (arrow). The lesion borders an unenhanced area, which was previously treated; B: Left: Contrast harmonic Doppler ultrasound (US) using Levovist shows enhancement of local tumor progression of HCC (arrow). Therefore, an enhanced lesion can be identified as a target for the insertion of a single RF electrode; Right: B-mode US shows a HCC nodule demonstrated as a low echoic lesion with an unclear border (arrowhead).
Laparoscopic/open surgical approach
The use of a laparoscopic or open approach allows repeated placements of RFA electrodes at multiple sites to ablate larger tumors. The laparoscopic approach appears to be the safest and most effective method for small tumors on the liver surface, and offers the advantages of laparoscopic US, which provides better resolution of the number and location of liver tumors[55,56]. Moreover, a hand-assisted technique can be applied safely and effectively to laparoscopic liver surgery[57-59]. An intraoperative US probe is inserted into the peritoneal cavity together with the surgeon’s hand through a hand-access device. An RF electrode can be subcostally or intercostally advanced into a liver tumor under direct guidance by intraoperative US. Therefore, a hand-assisted laparoscopic US-guided method has advantages for both laparoscopic and open surgical approaches. The postoperative recovery of patients was shorter compared with that after an open surgical approach. Ishiko et al[57] reported that the surgical procedures consisted of 5 RFA to tumors in the caudate lobe with hand-assisted laparoscopic surgery (HALS), and a postoperative CT scan demonstrated sufficient ablation in all patients and there was no surgical mortality. The HALS approach has several advantages; it facilitates and expedites the procedure, reduces the stress factor on the surgeon, greatly improves exposure, and facilitates immediate and efficient control of bleeding vessels with the internal hand. The hand-access device, which essentially consists of a cuff with a spiral inflatable valve, enables withdrawal and reinsertion of the hand without loss of pneumoperitoneum during the procedure. However, the local treatment failure rate of the laparoscopic approach was higher in patients with HCC nodules situated deep within the liver and measuring 4 cm or more in diameter[60]. Great difficulty can be encountered during treatment of lesions located close to the gallbladder or in contact with the diaphragm.
Although more invasive, open RFA can be performed more easily and the puncture course of the RF needle can be more widely selected than that during the laparoscopic approach. It has been reported that patients undergoing radical open RFA demonstrated few ablation site recurrences even though the nodules measured more than 4 cm in diameter and/or there were more than three nodules[61,62]. Open RFA can be indicated for patients who are considered suitable for open surgery with large, numerous, or deeply located tumors that cannot be accurately accessed by a laparoscopic approach. Furthermore, when patients have synchronous liver metastases, open surgical RFA can be performed in conjunction with resection of the primary cancer.
Local controllability (local tumor progression)
The local recurrence rate after RFA for HCC ranged from 1.7% to 41%[63-70] (Table 2). As reported by Kudo[28], in a series of 141 HCC patients who underwent curative RFA therapy, local tumor progression was observed in 9 cases (local tumor progression rate, 6.3%), whereas the cumulative local tumor progression rate, calculated by the Kaplan-Meier method, was 12% at 4 years. The rate may have depended on the size of nodules treated and the skill of the surgeons. There has not been any definitive report of local recurrence of nodules measuring 2-cm or smaller, and we ourselves have not encountered any case showing such recurrence, suggesting that recurrence in such cases is exceptional. The risk of local tumor progression increases with size, but the local tumor progression rate differs markedly depending on whether or not a circumferential 5-mm safety margin is secured. In a meta-analysis of RFA vs PEI in HCC, the survival rate showed a significant benefit for RFA over PEI at 1, 2, 3 and 4 years[71]. The survival advantage increased over time with Relative Risk values of: 1.28 (95% CI: 1.12-1.45) and 1.24 (95% CI: 1.05-1.48) for RFA vs PEI at 3- and 4-years, respectively. Likewise, RFA achieved significantly lower rates of local recurrence (RR: 0.37, 95% CI: 0.23-0.59)[71].
Table 2.
Studies comparing local tumor progression rates of radiofrequency ablation for hepatocellular carcinoma
| Author | Yr | n | Tumor size (mean, cm) | Follow-up period (mean, mo) | Local tumor progression rate (%) |
| Rossi et al[63] | 1996 | 41 | 2.3 | 22.6 | 5.0 |
| Buscarini et al[64] | 2001 | 60 | - | 26.8 | 14 |
| Choi et al[65] | 2004 | 53 | 2.1 | 23 | 21 |
| Lu et al[66] | 2005 | 87 | 2.5 | 12.7 | 5.8 |
| Shiina et al[67] | 2005 | 118 | - | 34.8 | 1.7 |
| Solmi et al[68] | 2006 | 63 | 2.8 | 32.3 | 41 |
| Hänsler et al[69] | 2007 | 21 | 4.2 | - | 21 |
| Waki et al[70] | 2010 | 88 | - | 36 | 4.8 |
Complications
Complications reported following percutaneous RFA of malignant liver tumors in 2320 patients treated at 41 different hospitals in Italy indicate that the mortality rate was 0.3% with an overall complication rate of 7.1%[72,73]. The authors described major complications (2.4% incidence) including death, hemorrhage, RFA needle-track seeding, RFA lesion abscess, perforation of gastrointestinal viscus, liver failure, biloma, biliary stricture, portal vein thrombosis, and hemothorax or pneumothorax requiring drainage, and minor complications (4.7% incidence) including pain, fever, and asymptomatic pleural effusion. Another recent review indicated that complication rates for percutaneous, laparoscopic, and open RFA of hepatic tumors in 3670 patients were 7.2%, 9.5%, and 9.9%, respectively[74]. Complications directly related to the liver included bleeding (1.6%), intrahepatic abscess (1.1%), biliary or hepatic vascular injury (1.7%), and liver failure (0.8%). Complications that arose in less than 1% of hepatic tumor RFA patients included pulmonary problems (pneumothorax, hydrothorax, pleural effusion), grounding pad skin burn, myoglobinemia or myoglobinuria, renal failure, coagulopathy, tumor seeding of the needle track, excessive hormone release from treated neuroendocrine tumors, cardiac problems (myocardial infarction, arrhythmia), and injury to the diaphragm or adjacent viscera. Although Llovet et al[75] reported that dissemination along the puncture route was observed in 12.5% of their patients, only a few such cases have been reported in Japan, and dissemination may not occur at such a high frequency. This complication was almost absent in many reports from Japan[28]. Overall, the frequency of major complications of percutaneous RFA was 0.6%-8.9%, which was higher than that of PEI, but generally lower than that of MCT[28].
Some investigators have suggested that tumor location is closely related to the risk of major complications. Central tumors close to the hepatic hilum were reported to be unsuitable for percutaneous RFA because of the risk of injuring adjacent bile ducts[15]. It was also suggested that RFA for nodules adjacent to large vessels might often result in incomplete necrosis because of a heat sink effect. In addition, peripheral tumors adjacent to extrahepatic organs were also suggested to be unsuitable because of the risk of heat injuries, such as intestinal perforation and pleural effusion[72,76]. Thus, there may be difficulty with RFA of nodules in such high-risk locations, possibly resulting in complications or preventing adequate treatment. However, Teratani et al[77] reported that there was no difference in early complication rates according to tumor location. The effort to achieve thorough ablation increased the total number of electrode insertions, and this may have led to an increase in complications.
To minimize complications of RFA for malignant liver tumors, knowledge of risk factors and prevention methods is required. In addition, because early and accurate diagnosis is necessary for the proper management of complications, not only radiologists but also hepatologists and surgeons should be familiar with the imaging features of each type of complication. Appropriate management of complications is essential for successful treatment with RFA.
CONCLUSION
RFA can be performed safely using percutaneous, laparoscopic, or open surgical techniques, and has markedly changed the treatment strategy for small HCC. RFA combined with TACE will likely make the treatment of larger tumors a clinically viable treatment alternative. Moreover, an accurate evaluation of treatment response is very important to secure successful RFA therapy. A sufficient safety margin can prevent local tumor recurrences. However, surgery is still the recommended treatment modality for patients with both primary hepatic malignancies. For inoperable lesions, RFA will likely play a significant role with a potential curative intent. Currently, the important clinical issue is that follow-up studies need to be performed for the early detection and treatment of recurrence, either locally or at different sites after RFA.
Footnotes
Peer reviewers: Yicheng Ni, MD, PhD, Professor, Biomedical Imaging, Interventional Therapy and Contrast Media Research, Department of Radiology, University Hospitals, K.U. Leuven, Herestraat 49, B-3000, Leuven, Belgium; Sergio Sartori, MD, Department of Internal Medicine, Section of Interventional Ultrasound, St. Anna Hospital, I-44100 Ferrara, Italy
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
- 1.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, Tanaka E. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17–S26. doi: 10.1053/j.gastro.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Di Bisceglie AM. Epidemiology and clinical presentation of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S169–S171. doi: 10.1016/s1051-0443(07)61783-7. [DOI] [PubMed] [Google Scholar]
- 4.McCaughan GW, Koorey DJ, Strasser SI. Hepatocellular carcinoma: current approaches to diagnosis and management. Intern Med J. 2002;32:394–400. doi: 10.1046/j.1445-5994.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- 5.Zavadsky KE, Lee YT. Liver metastases from colorectal carcinoma: incidence, resectability, and survival results. Am Surg. 1994;60:929–933. [PubMed] [Google Scholar]
- 6.Liu LX, Zhang WH, Jiang HC. Current treatment for liver metastases from colorectal cancer. World J Gastroenterol. 2003;9:193–200. doi: 10.3748/wjg.v9.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsim NC, Frampton AE, Habib NA, Jiao LR. Surgical treatment for liver cancer. World J Gastroenterol. 2010;16:927–933. doi: 10.3748/wjg.v16.i8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalski CW, Erkan M, Hüser N, Müller MW, Hartel M, Friess H, Kleeff J. Resection of primary pancreatic cancer and liver metastasis: a systematic review. Dig Surg. 2008;25:473–480. doi: 10.1159/000184739. [DOI] [PubMed] [Google Scholar]
- 9.Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284–1290. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- 10.Rust C, Gores GJ. Locoregional management of hepatocellular carcinoma. Surgical and ablation therapies. Clin Liver Dis. 2001;5:161–173. doi: 10.1016/s1089-3261(05)70159-8. [DOI] [PubMed] [Google Scholar]
- 11.Lee WS, Yun SH, Chun HK, Lee WY, Kim SJ, Choi SH, Heo JS, Joh JW, Choi D, Kim SH, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol. 2008;42:945–949. doi: 10.1097/MCG.0b013e318064e752. [DOI] [PubMed] [Google Scholar]
- 12.Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg. 2008;25:445–460. doi: 10.1159/000184736. [DOI] [PubMed] [Google Scholar]
- 13.Bartolozzi C, Lencioni R. Ethanol injection for the treatment of hepatic tumours. Eur Radiol. 1996;6:682–696. doi: 10.1007/BF00187673. [DOI] [PubMed] [Google Scholar]
- 14.Okada S. Local ablation therapy for hepatocellular carcinoma. Semin Liver Dis. 1999;19:323–328. doi: 10.1055/s-2007-1007121. [DOI] [PubMed] [Google Scholar]
- 15.McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176:3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 16.Shiina S, Teratani T, Obi S, Hamamura K, Koike Y, Omata M. Nonsurgical treatment of hepatocellular carcinoma: from percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology. 2002;62 Suppl 1:64–68. doi: 10.1159/000048278. [DOI] [PubMed] [Google Scholar]
- 17.McGahan JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3:291–297. doi: 10.1016/s1051-0443(92)72028-4. [DOI] [PubMed] [Google Scholar]
- 18.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267–270. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol. 1996;3:212–218. doi: 10.1016/s1076-6332(96)80443-0. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 21.Ni Y, Mulier S, Miao Y, Michel L, Marchal G. A review of the general aspects of radiofrequency ablation. Abdom Imaging. 2005;30:381–400. doi: 10.1007/s00261-004-0253-9. [DOI] [PubMed] [Google Scholar]
- 22.Ni Y, Chen F, Mulier S, Sun X, Yu J, Landuyt W, Marchal G, Verbruggen A. Magnetic resonance imaging after radiofrequency ablation in a rodent model of liver tumor: tissue characterization using a novel necrosis-avid contrast agent. Eur Radiol. 2006;16:1031–1040. doi: 10.1007/s00330-005-0094-0. [DOI] [PubMed] [Google Scholar]
- 23.Mori K, Fukuda K, Asaoka H, Ueda T, Kunimatsu A, Okamoto Y, Nasu K, Fukunaga K, Morishita Y, Minami M. Radiofrequency ablation of the liver: determination of ablative margin at MR imaging with impaired clearance of ferucarbotran--feasibility study. Radiology. 2009;251:557–565. doi: 10.1148/radiol.2512081161. [DOI] [PubMed] [Google Scholar]
- 24.Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59:432–441. doi: 10.1016/j.ejrad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, Lee JH, Lim JH, Choo IW. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447–454. doi: 10.1148/radiol.2212010446. [DOI] [PubMed] [Google Scholar]
- 26.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78 Suppl 1:113–124. doi: 10.1159/000315239. [DOI] [PubMed] [Google Scholar]
- 28.Kudo M. Local ablation therapy for hepatocellular carcinoma: current status and future perspectives. J Gastroenterol. 2004;39:205–214. doi: 10.1007/s00535-003-1280-y. [DOI] [PubMed] [Google Scholar]
- 29.Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237–257. [PubMed] [Google Scholar]
- 30.Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353–2360. doi: 10.1002/cncr.10966. [DOI] [PubMed] [Google Scholar]
- 31.Yamakado K, Nakatsuka A, Akeboshi M, Shiraki K, Nakano T, Takeda K. Combination therapy with radiofrequency ablation and transcatheter chemoembolization for the treatment of hepatocellular carcinoma: Short-term recurrences and survival. Oncol Rep. 2004;11:105–109. [PubMed] [Google Scholar]
- 32.Peng ZW, Chen MS, Liang HH, Gao HJ, Zhang YJ, Li JQ, Zhang YQ, Lau WY. A case-control study comparing percutaneous radiofrequency ablation alone or combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Eur J Surg Oncol. 2010;36:257–263. doi: 10.1016/j.ejso.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Uehara T, Hirooka M, Ishida K, Hiraoka A, Kumagi T, Kisaka Y, Hiasa Y, Onji M. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol. 2007;42:306–311. doi: 10.1007/s00535-006-1949-0. [DOI] [PubMed] [Google Scholar]
- 34.Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004;183:583–588. doi: 10.2214/ajr.183.3.1830583. [DOI] [PubMed] [Google Scholar]
- 35.Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Shiozaki H. Percutaneous radiofrequency ablation guided by contrast-enhanced harmonic sonography with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. AJR Am J Roentgenol. 2004;182:1224–1226. doi: 10.2214/ajr.182.5.1821224. [DOI] [PubMed] [Google Scholar]
- 36.Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Inoue T, Sakaguchi Y, Sakamoto H, Shiozaki H. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol. 2003;38:1066–1070. doi: 10.1007/s00535-003-1197-5. [DOI] [PubMed] [Google Scholar]
- 37.Cioni D, Lencioni R, Rossi S, Garbagnati F, Donati F, Crocetti L, Bartolozzi C. Radiofrequency thermal ablation of hepatocellular carcinoma: using contrast-enhanced harmonic power doppler sonography to assess treatment outcome. AJR Am J Roentgenol. 2001;177:783–788. doi: 10.2214/ajr.177.4.1770783. [DOI] [PubMed] [Google Scholar]
- 38.Kudo M. Imaging diagnosis of hepatocellular carcinoma and premalignant/borderline lesions. Semin Liver Dis. 1999;19:297–309. doi: 10.1055/s-2007-1007119. [DOI] [PubMed] [Google Scholar]
- 39.Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Maekawa K. Hepatocellular carcinoma: depiction of tumor parenchymal flow with intermittent harmonic power Doppler US during the early arterial phase in dual-display mode. Radiology. 2001;220:349–356. doi: 10.1148/radiology.220.2.r01au07349. [DOI] [PubMed] [Google Scholar]
- 40.Ding H, Kudo M, Maekawa K, Suetomi Y, Minami Y, Onda H. Detection of tumor parenchymal blood flow in hepatic tumors: value of second harmonic imaging with a galactose-based contrast agent. Hepatol Res. 2001;21:242–251. doi: 10.1016/s1386-6346(01)00106-1. [DOI] [PubMed] [Google Scholar]
- 41.Kudo M. Contrast harmonic ultrasound is a breakthrough technology in the diagnosis and treatment of hepatocellular carcinoma. J Med Ultrasonics. 2001;28:79–81. [Google Scholar]
- 42.Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Chung H, Kawasaki T, Maekawa K. Evaluation of posttreatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology. 2001;221:721–730. doi: 10.1148/radiol.2213010358. [DOI] [PubMed] [Google Scholar]
- 43.Minami Y, Kudo M, Kawasaki T, Kitano M, Chung H, Maekawa K, Shiozaki H. Transcatheter arterial chemoembolization of hepatocellular carcinoma: usefulness of coded phase-inversion harmonic sonography. AJR Am J Roentgenol. 2003;180:703–708. doi: 10.2214/ajr.180.3.1800703. [DOI] [PubMed] [Google Scholar]
- 44.Meloni MF, Goldberg SN, Livraghi T, Calliada F, Ricci P, Rossi M, Pallavicini D, Campani R. Hepatocellular carcinoma treated with radiofrequency ablation: comparison of pulse inversion contrast-enhanced harmonic sonography, contrast-enhanced power Doppler sonography, and helical CT. AJR Am J Roentgenol. 2001;177:375–380. doi: 10.2214/ajr.177.2.1770375. [DOI] [PubMed] [Google Scholar]
- 45.Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420–430. doi: 10.1148/radiol.2322031401. [DOI] [PubMed] [Google Scholar]
- 46.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898–906. doi: 10.1148/radiol.2443061520. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Tang J, An L, Wang W, Luo Y, Li J, Xu J. Contrast-enhanced ultrasonography for assessment of tumor vascularity in hepatocellular carcinoma. J Ultrasound Med. 2007;26:757–762. doi: 10.7863/jum.2007.26.6.757. [DOI] [PubMed] [Google Scholar]
- 48.Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del Maschio A, Summaria V, Maresca G, Pezzoli C, Llull JB. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol. 2002;41:200–206. doi: 10.1016/s0720-048x(01)00457-0. [DOI] [PubMed] [Google Scholar]
- 49.Kono Y, Lucidarme O, Choi SH, Rose SC, Hassanein TI, Alpert E, Mattrey RF. Contrast-enhanced ultrasound as a predictor of treatment efficacy within 2 weeks after transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:57–65. doi: 10.1016/j.jvir.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 50.Kudo M, Minami Y. Radiofrequency ablation therapy under harmonic imaging guidance for the recurring cancer after local therapy for HCC: a randomized controlled study with RFA under B-mode guidance. Ultrasound Med Biol. 2003;29:S145. [Google Scholar]
- 51.Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Shiozaki H. Treatment of hepatocellular carcinoma with percutaneous radiofrequency ablation: usefulness of contrast harmonic sonography for lesions poorly defined with B-mode sonography. AJR Am J Roentgenol. 2004;183:153–156. doi: 10.2214/ajr.183.1.1830153. [DOI] [PubMed] [Google Scholar]
- 52.Minami Y, Kudo M, Chung H, Kawasaki T, Yagyu Y, Shimono T, Shiozaki H. Contrast harmonic sonography-guided radiofrequency ablation therapy versus B-mode sonography in hepatocellular carcinoma: prospective randomized controlled trial. AJR Am J Roentgenol. 2007;188:489–494. doi: 10.2214/AJR.05.1286. [DOI] [PubMed] [Google Scholar]
- 53.Minami Y, Kudo M. Contrast-enhanced harmonic ultrasound imaging in ablation therapy for primary hepatocellular carcinoma. World J Radiol. 2009;1:86–91. doi: 10.4329/wjr.v1.i1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minami Y, Kudo M, Hatanaka K, Kitai S, Inoue T, Hagiwara S, Chung H, Ueshima K. Radiofrequency ablation guided by contrast harmonic sonography using perfluorocarbon microbubbles (Sonazoid) for hepatic malignancies: an initial experience. Liver Int. 2010;30:759–764. doi: 10.1111/j.1478-3231.2010.02226.x. [DOI] [PubMed] [Google Scholar]
- 55.Santambrogio R, Podda M, Zuin M, Bertolini E, Bruno S, Cornalba GP, Costa M, Montorsi M. Safety and efficacy of laparoscopic radiofrequency ablation of hepatocellular carcinoma in patients with liver cirrhosis. Surg Endosc. 2003;17:1826–1832. doi: 10.1007/s00464-002-8960-1. [DOI] [PubMed] [Google Scholar]
- 56.Okabayashi T, Kobayashi M, Akimori T, Akisawa N, Iwasaki S, Onishi S, Araki K. Usefulness of laparoscopic radiofrequency ablation of hepatocellular carcinoma. Surg Technol Int. 2005;14:177–181. [PubMed] [Google Scholar]
- 57.Ishiko T, Beppu T, Sugiyama S, Masuda T, Takahashi M, Komori H, Takamori H, Hirota M, Kanemitu K, Baba H. Radiofrequency ablation with hand-assisted laparoscopic surgery for the treatment of hepatocellular carcinoma in the caudate lobe. Surg Laparosc Endosc Percutan Tech. 2008;18:272–276. doi: 10.1097/SLE.0b013e31816a24bf. [DOI] [PubMed] [Google Scholar]
- 58.Schumacher G, Eisele R, Spinelli A, Schmidt SC, Jacob D, Pratschke J, Neuhaus P. Indications for hand-assisted laparoscopic radiofrequency ablation for liver tumors. J Laparoendosc Adv Surg Tech A. 2007;17:153–159. doi: 10.1089/lap.2006.0001. [DOI] [PubMed] [Google Scholar]
- 59.Hirooka M, Kisaka Y, Uehara T, Ishida K, Kumagi T, Watanabe Y, Abe M, Matsuura B, Hiasa Y, Onji M. Efficacy of laparoscopic radiofrequency ablation for hepatocellular carcinoma compared to percutaneous radiofrequency ablation with artificial ascites. Dig Endosc. 2009;21:82–86. doi: 10.1111/j.1443-1661.2009.00836.x. [DOI] [PubMed] [Google Scholar]
- 60.Santambrogio R, Opocher E, Montorsi M. Laparoscopic radiofrequency ablation of hepatocellular carcinoma: A critical review from the surgeon's perspective. J Ultrasound. 2008;11:1–7. doi: 10.1016/j.jus.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minami Y, Kawasaki T, Kudo M, Haji S, Shiraishi O, Kawabe T, Yasuda C, Nakai T, Takeyama Y, Shiozaki H. Treatment of large and/or multiple hepatic malignancies: open surgical approaches of radiofrequency ablation. Hepatogastroenterology. 2007;54:2358–2360. [PubMed] [Google Scholar]
- 62.Tanaka S, Shimada M, Shirabe K, Taketomi A, Maehara S, Tsujita E, Ito S, Kitagawa D, Maehara Y. Surgical radiofrequency ablation for treatment of hepatocellular carcinoma: an endoscopic or open approach. Hepatogastroenterology. 2009;56:1169–1173. [PubMed] [Google Scholar]
- 63.Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–768. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 64.Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914–921. doi: 10.1007/s003300000659. [DOI] [PubMed] [Google Scholar]
- 65.Choi D, Lim HK, Kim MJ, Lee SH, Kim SH, Lee WJ, Lim JH, Joh JW, Kim YI. Recurrent hepatocellular carcinoma: percutaneous radiofrequency ablation after hepatectomy. Radiology. 2004;230:135–141. doi: 10.1148/radiol.2301021182. [DOI] [PubMed] [Google Scholar]
- 66.Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, Durazo F, Saab S, Han S, Finn R, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130–1137. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 67.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Solmi L, Nigro G, Roda E. Therapeutic effectiveness of echo-guided percutaneous radiofrequency ablation therapy with a LeVeen needle electrode in hepatocellular carcinoma. World J Gastroenterol. 2006;12:1098–1104. doi: 10.3748/wjg.v12.i7.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hänsler J, Frieser M, Tietz V, Uhlke D, Wissniowski T, Bernatik T, Hothorn T, Hahn EG, Strobel D. Percutaneous radiofrequency ablation of liver tumors using multiple saline-perfused electrodes. J Vasc Interv Radiol. 2007;18:405–410. doi: 10.1016/j.jvir.2006.12.729. [DOI] [PubMed] [Google Scholar]
- 70.Waki K, Aikata H, Katamura Y, Kawaoka T, Takaki S, Hiramatsu A, Takahashi S, Toyota N, Ito K, Chayama K. Percutaneous radiofrequency ablation as first-line treatment for small hepatocellular carcinoma: results and prognostic factors on long-term follow up. J Gastroenterol Hepatol. 2010;25:597–604. doi: 10.1111/j.1440-1746.2009.06125.x. [DOI] [PubMed] [Google Scholar]
- 71.Bouza C, López-Cuadrado T, Alcázar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009;9:31. doi: 10.1186/1471-230X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 73.Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 75.Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 76.Meloni MF, Goldberg SN, Moser V, Piazza G, Livraghi T. Colonic perforation and abscess following radiofrequency ablation treatment of hepatoma. Eur J Ultrasound. 2002;15:73–76. doi: 10.1016/s0929-8266(01)00171-9. [DOI] [PubMed] [Google Scholar]
- 77.Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–1108. doi: 10.1002/hep.21164. [DOI] [PubMed] [Google Scholar]