Abstract
AIM: To assess prospectively parameters of computed tomography perfusion (CT p) for evaluation of vascularity of liver metastases from neuroendocrine tumors.
METHODS: This study was approved by the hospital’s institutional review board. All 18 patients provided informed consent. There were 30 liver metastases from neuroendocrine tumors. Patients were divided into three groups depending on the appearance of the liver metastases at the arterial phase of morphological CT (hyperdense, hypodense and necrotic). Sequential acquisition of the liver was performed before and for 2 min after intravenous injection of 0.5 mg/kg contrast medium, at 4 mL/s. Data were analyzed using deconvolution analysis to calculate blood flow (BF), blood volume (BV), mean transit time (MTT), hepatic arterial perfusion index (HAPI) and a bi-compartmental analysis was performed to obtain vascular permeability-surface area product (PS). Post-treatment analysis was performed by a radiologist and regions of interest were plotted on the metastases, normal liver, aorta and portal vein.
RESULTS: At the arterial phase of the morphological CT scan, the aspects of liver metastases were hyperdense (n = 21), hypodense (n = 7), and necrotic (n = 2). In cases of necrotic metastases, none of the CT p parameters were changed. Compared to normal liver, a significant difference in all CT p parameters was found in cases of hyperdense metastases, and only for HAPI and MTT in cases of hypodense metastases. No significant difference was found for MTT and HAPI between hypo- and hyperdense metastases. A significant decrease of PS, BV and BF was demonstrated in cases of patients with hypodense lesions PS (23 ± 11.6 mL/100 g per minute) compared to patients with hyperdense lesions; PS (13.5 ± 10.4 mL/100 g per minute), BF (93.7 ± 75.4 vs 196.0 ± 115.6 mL/100 g per minute) and BV (9.7 ± 5.9 vs 24.5 ± 10.9 mL/100 g).
CONCLUSION: CT p provides additional information compared to the morphological appearance of liver metastases.
Keywords: Computed tomography perfusion scanning, Tumor angiogenesis, Hepatic metastases, Endocrine tumors
INTRODUCTION
Neuroendocrine tumors are rare[1], and are usually described as having a slow rate of progression. The presence of hepatic metastases from neuroendocrine tumors is frequent (25%-90%) and has obvious implications for quality of life (in the presence of debilitating functional syndromes), which affects the overall prognosis[2]. The 5-year survival rate depends on the presence of hepatic metastases and represents an independent prognostic factor, such as tumor cell differentiation and complete resection of the primary tumor[3]. Accordingly, the presence of hepatic metastases influences the strategies available for treatment.
Neuroendocrine tumors are characterized by a dense and specialized capillary network[4]. This specificity has been commonly used for diagnosis on conventional and multiphase helical computed tomography (CT) scanning with contrast enhancement. Currently, various patterns of enhancement have been described on contrast-enhanced CT scanning, which suggest that the angiogenesis process is variable among liver metastases of neuroendocrine tumors[3]. CT perfusion (CT p) is a technology that allows quantitative assessment of various parameters, such as tumor blood flow (BF), blood volume (BV), mean transit time (MTT), and permeability-surface area product (PS). CT p is currently used for brain, lung, and head and neck tumors.
The purpose of our study was to describe, prospectively, parameters of CT p in evaluating the vascularity of tumors in different aspects of liver metastases from neuroendocrine tumors.
MATERIALS AND METHODS
Selection
This prospective single center study included patients with pathologically proven liver metastases from neuroendocrine tumors, during the period between February 2007 and January 2008 at the Department of Gastrointestinal Imaging of the University Hospital Edouard Herriot, and for whom liver CT scanning was performed. Patients without adequate renal function (creatinine clearance < 50 mL/min), allergy, liver metastases not detected by non-contrast enhancement CT, non-cooperative patients, and patients with dyspnea were excluded from the study.
Patient population/study cohort
The study cohort included 10 men and eight women (age range: 38-81 years; mean age: 55 years).
The site of the main tumor was either gastrointestinal (n = 8), pancreas (n = 6), kidney (n = 2), lung (n = 1) or indeterminate (n = 1). All patients had a well-differentiated neuroendocrine tumor; digestive tumors were all classified as group 2 according to WHO 2000 criteria (well-differentiated endocrine carcinoma)[5], lung tumor as atypical carcinoid tumor, and other tumors as differentiated neuroendocrine tumors by a referent pathologist. Seventeen out of 18 patients benefited initially from surgical excision of the primary tumor and were in the course of additional treatment. Our institutional review board approved the study, and all patients gave their informed consent.
The cohort was divided into three different groups depending on the aspect of liver metastases at the arterial phase of the CT scan in comparison with background liver parenchyma; two of the groups being patients with hypodense liver metastases (n = 4) and hyperdense liver metastases (n = 12). If the lesion has a cystic element, the third group classified patients as having necrotic liver metastases (n = 2).
CT p technique
CT p of the liver was obtained to estimate the following parameters in metastases of neuroendocrine tumors and the background liver: BF, BV, MTT, hepatic arterial perfusion index (HAPI), and capillary PS[6,7]. CT p of the liver was performed with a 32-section multi-row CT scanner (Light Speed; GE Medical Systems, Milwaukee, WI, USA). A non-contrast CT scan of the liver was obtained to localize the tumor for further investigation by dynamic scanning. A 4-cm region of interest (ROI) was selected and dynamic scanning of this area was performed at a static table position 5 s after initiation of intravenous injection. The scanning was made at breath-holding after deep inspiration. A total of 0.5 mg/kg non-ionic iodinated contrast medium (Xenetix; 350 mg/mL iodine; Guerbet, France) was injected at a rate of 4 mL/s through an 18-gauge intravenous cannula. The following CT parameters were used to acquire dynamic data: 1-s gantry rotation time, 80 kVp, 100 mAs, acquisition with 8 sections per gantry rotation and 5-mm reconstructed section thickness. Scanning was initiated after a 5-s delay from the start of the injection, and images were acquired for a total duration of 120 s: 40 acquisitions with 1 rotation/s during 40 s, 10 acquisitions with 1 rotation/2 s during 20 s, and 12 acquisitions with 1 rotation/4 s during 60 s, to limit the radiation dose.
Data analysis
Data were processed at a workstation (Advantage Windows 4.0; GE Medical Systems) with CT p software (GE Perfusion 3.0) by an experienced radiologist in the field of abdominal imaging. This software is based on the mathematical models of Johnson and Wilson. Functional maps were obtained by displaying images at an appropriate window and filter, placing an ROI in the aorta and portal vein manually to provide reference arterial and portal vein curves. Functional maps of BF, BV, MTT, PS and HAPI were generated and were displayed in a range of colors. ROIs for tumors (range: 28-1333.3 mm2) and background liver parenchyma (range: 41-1118.5 mm2) were hand drawn.
Statistical analysis
The value of BF, BV, HAPI, PS and MTT for each ROI of liver metastases and background parenchyma were recorded. Data were compiled in an Excel 2003 database to calculate the mean, SD and variance of each parameter. One-way ANOVA was used to compare the difference in CT p parameters between hepatic metastases from neuroendocrine tumors and the background liver. In addition, ANOVA was used to compare CT p parameters between the three subgroups. Statistical analysis using Student’s t test was performed to calculate the P value for each comparison. P ≤ 0.05 was considered to indicate a statistically significant difference.
RESULTS
CT p parameters of liver metastases of neuroendocrine tumors vs normal liver parenchyma
In the cohort of 18 patients, the CT scan aspect of liver metastases of neuroendocrine tumors in the arterial phase (scanning after 30 s from the injection of the contrast material) was hyperdense (n = 21), hypodense (n = 7), and necrotic (n = 2). The average size of liver metastases was 35 ± 23.5 mm (range: 11-89 mm). A CT p scan could not be performed on two patients due to technical problems (a delay starting the acquisition). These two patients were subsequently excluded from the study. Comparison of CT p parameters between hepatic metastases from neuroendocrine tumors and the background liver was performed.
Overall, liver metastases from neuroendocrine tumors demonstrated higher CT p parameters (BF, BV, PS) than the background liver (Table 1). There was a significant difference in perfusion parameters between liver metastases and the background liver (P < 0.05). In cases of necrotic metastases, none of the CT p parameters were changed.
Table 1.
Computed tomography perfusion parameters of metastases from neuroendocrine tumors and background liver
| CT p parameters | Liver metastases from neuroendocrine tumors | Background liver | P value |
| BF (mL/100 g per minute) | 170.5 ± 114.9 | 80.6 ± 38.3 | < 0.001 |
| BV (mL/100 g) | 20.8 ± 11.8 | 13.9 ± 6.7 | < 0.01 |
| PS (mL/100 g per minute) | 20.9 ± 11.8 | 15.9 ± 6.6 | < 0.05 |
| MTT (s) | 11.4 ± 3.6 | 15.2 ± 3.3 | < 0.001 |
| HAPI | 0.66 ± 0.21 | 0.21 ± 0.17 | < 0.001 |
CT p: Computed tomography perfusion; BF: Blood flow; BV: Blood volume; MTT: Mean transit time; HAPI: Hepatic arterial perfusion index; PS: Permeability-surface area product.
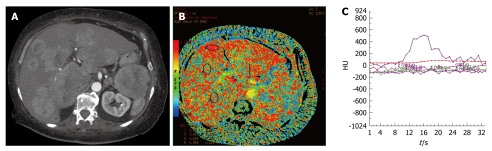
MTT was equal to 11.4 s (SD: 3.6) for the metastases group and 15.2 s (SD: 3.3) for the parenchyma group (P < 0.001). There is an increased arterial perfusion in hepatic metastases of neuroendocrine tumors with HAPI equal to 0.66 ± 0.21 for metastases and 0.21 ± 0.17 for the background liver (P < 0.001).
Comparison of CT p parameters between patients with hyperdense and hypodense liver metastases in the arterial phase of the CT scan
Mean HAPI was similar in both hyperdense (0.67 ± 0.14) and hypodense ( 0.64 ± 0.35) liver metastases. The mean BF and BV parameters were lower in patients with hypodense liver metastases compared to those with hyperdense liver metastases (BF: 93.7 ± 75.4 mL/100 g per minute vs 196.0 ± 115.6 mL/100 g per minute; BV: 9.7 ± 5.9 mL/100 g vs 24.5 ± 10.9 mL/100 g). MTT was not significantly different when comparing hypodense and hyperdense liver metastases (11.7 ± 4.7 s vs 10.7 ± 2.9 s). Permeability was 13.5 ± 10.4 mL/100 g per minute (hypodense liver lesions) vs 23 ± 11.6 mL/100 g per minute (hyperdense liver metastases).
Necrotic liver metastases from neuroendocrine tumors
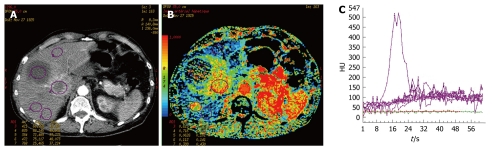
Two patients of our cohort presented with necrotic liver metastases from neuroendocrine tumors. Their curves of contrast enhancement had a null slope. On the parametric maps, HAPI was equal to 0.36 ± 0.03, BF was 20.2 ± 0.2 mL/100 g per minute, BV was 2.2 ± 0.4 mL/100 g, MTT was 14.2 ± 1.4 s, and PS was 1.7 ± 1.1 mL/100 g per minute.
DISCUSSION
The results of our study were significant. Comparison between metastases and normal liver parenchyma demonstrated a significant increase in BV, BF and PS, and a lower MTT within the lesions (P < 0.05) compared to the normal liver.
Quantification of various parameters of vascularization of liver metastasis showed a significant increase in tumor perfusion compared to that of normal liver parenchyma. These results seem similar to those found previously[8,9]. Sahani et al[8] have analyzed the parameters of perfusion in cases of hepatocellular carcinoma (HCC) and have found significant differences between measures performed within the lesion compared with the non-tumoral liver parenchyma. Although HCC and liver metastatic lesions from neuroendocrine tumors are considered to be hypervascular on dynamic imaging, a difference in their CT p parameters seems apparent, which could help in the differential diagnosis of the hepatic lesions. Fabiano et al[10] and Wang et al[11] also have found differences between the values of the CT p parameters of benign and malignant liver lesions. Fournier et al[12] have previously identified differences in CT p values between regenerative and dysplastic carcinomatous nodules, which allowed early detection of HCC. Tsushima et al[13] have performed a comparative study to evaluate CT p of HCC and liver metastases of colorectal origin. All the lesions were hypervascular compared to normal liver parenchyma, but HCC presented with arterial perfusion that was superior to that of colorectal metastases (0.94 ± 0.26 mL/min per milliliter vs 0.67 ± 0.33 mL/min per milliliter, P = 0.03).
BV and BF of normal liver parenchyma in our study were different to those found in the study of Sahani et al[8] (BF: 57 ± 38.3 mL/100 g per minute vs 14.9 ± 2.8 mL/100 g per minute; BV: 13.9 ± 6.8 mL/100 g vs 2.6 ± 0.9 mL/100 g). These differences were explained by the existence of underlying cirrhosis in patient with HCC. Fibrosis of the liver parenchyma involved an increased resistance vascular network, thus revealing a fall in BF and BV. Guan et al[14] have identified in an animal study, modification of the CT p parameters in the liver between different stages of hepatitis, fibrosis and cirrhosis. Van Beers et al[15] also have shown in a human study, decreased BF, and increased HAPI and MTT (P < 0.05) in cases of cirrhosis compared to a normal population with a strong correlation between these modifications and the severity of cirrhosis (Child-Pugh class A, B and C). The increased HAPI should be explained by the development of secondary hyper-arteriolization and the presence of portal hypertension. In our patients with liver metastases from neuroendocrine tumors, five presented with hypodense lesions (31.25%) and 11 with hyperdense lesions (68.75%), which was similar to other findings in the literature[3]. Comparison of metastatic disease according to its hypodense or hyperdense aspect demonstrated decreased tumor perfusion for the hypodense lesion (BF: P < 0.05; BV: P < 0.01). A correlation between the hypo- or hyperdense aspect of neuroendocrine liver metastases, their microvascular index of density, and their degree of tumor differentiation has already been demonstrated[3]. Therefore, the CT p parameters of tumor vascularization could represent a prognostic factor and should be confirmed by the realization of a prospective study that correlates CT p and histological data with a survival curve. Values of HAPI, which have not been systematically calculated in published studies, could be altered by the presence of portal hypertension or portal vein thrombosis. In our study, the HAPI of the normal parenchyma measured 0.21 on average, which corresponds to the arterial part of hepatic vascularization[16]. Neuroendocrine liver metastases mainly develop with an arterial network (HAPI: 0.66)[17-19]. Thus, our study showed that all neuroendocrine liver metastases were hypervascular and arterial (Figure 1). Furthermore, HAPI seemed to be similar for hypodense and hyperdense liver lesions (0.67 and 0.64). Therefore, hypodense liver metastases from neuroendocrine tumors also have preferential arterial vascularization identical to that of hyperdense metastases.
Figure 1.
Functional maps of hepatic arterial perfusion index for a hyperdense metastasis from a neuroendocrine tumor (A-C).
Even if neuroendocrine liver metastases are hypodense by double-phase CT scanning, these lesions have the same type of arterial network but have lower CT p parameters; probably because of architectural differences from the network of neo-microvascularization due to different neoangiogenesis.
In cases of neuroendocrine metastases with necrosis, perfusion parameters could not be measured, due to the lack of vascularization in the necrotic area (Figure 2). Nevertheless, CT p has some limitations. The volume of exploration is limited by the width of the detectors, which is generally 4 cm. The selection of the metastatic area and the portal vein section for comparison are operator dependent, thus, this technical constraint could limit lesion detection.
Figure 2.
Example of results for a necrotic liver metastasis from an neuroendocrine tumor (A-C).
Standardization of CT p values allowed minimization of variations due to cardiac output and circulating BV. This standardization is not available at present for commercial software. It was recently proposed by Miles et al[20] and the principle is similar to that used for positron emission tomography, with the standardized perfusion value (SPV), such that SPV = tissue perfusion/total body perfusion. This requires knowledge of the dose of contrast agent injected and the body weight of the patient. Recently, Goh et al[21] have compared the CT p parameters of colorectal tumors in 44 examinations performed with two different commercial software packages, and have identified a lack of reproducibility for the different values. Commercially available softwares do not use the same mathematical models for calculation, and are not thus interchangeable. However, it does seem possible to apply a factor of correction according to the software used.
Therefore, CT p seems to be promising for exploring diffuse hepatic disease such as fibrosis and cirrhosis[14,15]. Furthermore, CT p seems to be attractive for the diagnosis and follow-up of focal liver lesions. CT p has demonstrated increased arterial flow within macro-metastases in numerous studies[22].
In 2001, a small animal study demonstrated that the presence of micro-metastases of the liver decreased the portal flow by up to 34%, and increased MTT up to 25%, which was explained by increased resistance in the sinusoids[23].
In 2005, Kan et al[7] performed a small animal study that demonstrated significant modification of BV, BF and PS of intrahepatic lesions at days 1 and 14 of daily antiangiogenic treatment, but without correlation with the microvascular index of density. One of the hypotheses could be modification of the number of circulating microvessels with the appearance of non-functional vessels and not the total number of microvessels. In 2006, Meijerink et al[24] measured the BF of primary lesions (melanoma, fibrosarcoma, mesothelioma, osteosarcoma, colorectal carcinoma) and of hepatic, pleural, mediastinal and pelvic secondary tumors before and after treatment, by association of a vascular endothelial growth factor receptor inhibitor and an epidermal growth factor receptor inhibitor in a phase I clinical trial. The arterial BF was decreased in 12 out of 13 patients. CT p should be one of the tools that are indispensable for evaluation of antiangiogenic treatment, as well early detection of patients who respond well to such treatment.
However, the approach of using CT p imaging of liver metastases from neuroendocrine tumors highlighted some disadvantages. First of all, we did not estimate the reproducibility of the technique, which has already been demonstrated in the literature[6-8]. Second, we did not study the reproducibility of the intra- and inter-operator values because a single radiologist did the post-treatment analysis of the CT p. Strong agreement of the intra- and inter-observer values has already been demonstrated for the quantification of CT p in colorectal tumors[9]. Finally, only a relatively small number of patients were included in our study because of limited time of inclusion and the low incidence of this pathology.
Furthermore, our study demonstrated the arterial predominance of the neo-microcirculation of the neuroendocrine liver metastases, and the advantage of chemoembolization could be extended to all the hyper- and hypodense lesions. Recent studies have demonstrated the advantages of CT p for detection of rectal tumors in patients who respond well to chemotherapy and radiotherapy, by comparing BF and MTT (P < 0.05). CT p could be used in the evaluation of antiangiogenic treatment of liver metastases[25,26].
Neuroendocrine tumors constitute a homogeneous group of tumors that are disseminated within the body, which are characterized by their histological and biological properties. Liver metastases of neuroendocrine tumors are frequent, which is one of the main factors for poor prognosis. CT p is useful for characterization of liver metastases of neuroendocrine tumors, especially those that are hypervascular.
As in any neoplastic lesions, neoangiogenesis plays an essential role in tumor growth and the response to various therapeutic options. Quantification of tumor angiogenesis without an invasive procedure is essential in oncology for the characterization and staging of the lesions[27].
CT p is a feasible technique for quantification of tumor vascularity and angiogenesis in liver metastases of neuroendocrine tumors. CT p could help in the future to identify subgroups of patients who could benefit from antiangiogenic therapy and help clinicians to choose the most appropriate treatment. Another challenge is to establish the accuracy of CT p to evaluate prospectively therapeutic efficacy.
COMMENTS
Background
Neuroendocrine tumors are characterized by their histological and biological properties. Liver metastases of neuroendocrine tumors are frequent, and are one of the main factors for poor prognosis. Computed tomography perfusion (CT p) is useful for characterization of liver metastases of neuroendocrine tumors, especially those that are hypervascular, which constitute a homogeneous group of tumors that are disseminated throughout the body.
Innovations and breakthroughs
CT p could help in the future to identify subgroups of patients who could benefit from antiangiogenic therapies and help clinicians to choose the most appropriate treatment to administer. Another challenge is to evaluate the accuracy of CT p to evaluate prospectively treatment efficiency.
Applications
As with any neoplastic lesions, neoangiogenesis plays an essential role in tumoral growth and in the response to various therapeutic options. Quantification of tumoral angiogenesis without an invasive procedure is essential in oncology for the characterization and staging of the lesions. CT p is a feasible technique for quantification of tumor vascularity and angiogenesis in liver metastases of neuroendocrine tumors.
Terminology
Neuroendocrine tumors are rare and slow growing. They are characterized by a dense and specialized capillary network. The presence of hepatic metastases from neuroendocrine tumors is frequent (25%-90%). CT p is a technique that allows quantitative assessment of various parameters, such as tumor blood flow, blood volume, mean transit time, and permeability-surface area product.
Peer review
This manuscript may be helpful to recognize the tumour vascularity of liver metastases from endocrine tumor and would be a reference for radiological practice.
Footnotes
Peer reviewers: Xiao-Ming Zhang, MD, Professor, Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Wenhua Road 63, Nanchong 637000, Sichuan Province, China; Kenneth Coenegrachts, MD, PhD, Department of Radiology, AZ St.-Jan AV, Ruddershove 10, B-8000 Bruges, Belgium
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Ruszniewski P, O'Toole D. Ablative therapies for liver metastases of gastroenteropancreatic endocrine tumors. Neuroendocrinology. 2004;80 Suppl 1:74–78. doi: 10.1159/000080746. [DOI] [PubMed] [Google Scholar]
- 2.Dromain C, de Baere T, Baudin E, Galline J, Ducreux M, Boige V, Duvillard P, Laplanche A, Caillet H, Lasser P, et al. MR imaging of hepatic metastases caused by neuroendocrine tumors: comparing four techniques. AJR Am J Roentgenol. 2003;180:121–128. doi: 10.2214/ajr.180.1.1800121. [DOI] [PubMed] [Google Scholar]
- 3.Rodallec M, Vilgrain V, Couvelard A, Rufat P, O'Toole D, Barrau V, Sauvanet A, Ruszniewski P, Menu Y. Endocrine pancreatic tumours and helical CT: contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology. 2006;6:77–85. doi: 10.1159/000090026. [DOI] [PubMed] [Google Scholar]
- 4.Scarsbrook AF, Ganeshan A, Statham J, Thakker RV, Weaver A, Talbot D, Boardman P, Bradley KM, Gleeson FV, Phillips RR. Anatomic and functional imaging of metastatic carcinoid tumors. Radiographics. 2007;27:455–477. doi: 10.1148/rg.272065058. [DOI] [PubMed] [Google Scholar]
- 5.Solcia E, Klöppel G, Sobin L. In: WHO international histological classification of tumours: histological typing of endocrine tumours. 2nd ed. Berlin: Springer; 2000. [Google Scholar]
- 6.Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement? Br J Radiol. 2003;76:220–231. doi: 10.1259/bjr/13564625. [DOI] [PubMed] [Google Scholar]
- 7.Kan Z, Phongkitkarun S, Kobayashi S, Tang Y, Ellis LM, Lee TY, Charnsangavej C. Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model. Radiology. 2005;237:151–158. doi: 10.1148/radiol.2363041293. [DOI] [PubMed] [Google Scholar]
- 8.Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007;243:736–743. doi: 10.1148/radiol.2433052020. [DOI] [PubMed] [Google Scholar]
- 9.Goh V, Halligan S, Hugill JA, Bassett P, Bartram CI. Quantitative assessment of colorectal cancer perfusion using MDCT: inter- and intraobserver agreement. AJR Am J Roentgenol. 2005;185:225–231. doi: 10.2214/ajr.185.1.01850225. [DOI] [PubMed] [Google Scholar]
- 10.Fabiano S, Squillaci E, Carlani M, Fusco N, Stefanini M, Simonetti G. CT Perfusion Studies of Liver Lesions with a 64-detector Row Scanner: Initial Clinical Results. RSNA. 2005;SSQ08-07:508. [Google Scholar]
- 11.Wang S, Zhou C, Zhao X. Perfusion studies of hepatic tumors: use of multi-detector row helical CT and liver perfusion software. In: Radiological Society of North America scientific assembly and annual meeting program 2004., editor. Oak Brook: Radiological Society of North America; 2004. p. 238. [Google Scholar]
- 12.Fournier LS, Cuenod CA, de Bazelaire C, Siauve N, Rosty C, Tran PL, Frija G, Clement O. Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol. 2004;14:2125–2133. doi: 10.1007/s00330-004-2339-8. [DOI] [PubMed] [Google Scholar]
- 13.Tsushima Y, Funabasama S, Aoki J, Sanada S, Endo K. Quantitative perfusion map of malignant liver tumors, created from dynamic computed tomography data. Acad Radiol. 2004;11:215–223. doi: 10.1016/s1076-6332(03)00578-6. [DOI] [PubMed] [Google Scholar]
- 14.Guan S, Zhao WD, Zhou KR, Peng WJ, Mao J, Tang F. CT perfusion at early stage of hepatic diffuse disease. World J Gastroenterol. 2005;11:3465–3467. doi: 10.3748/wjg.v11.i22.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol. 2001;176:667–673. doi: 10.2214/ajr.176.3.1760667. [DOI] [PubMed] [Google Scholar]
- 16.Bézy-Wendling J, Kretowski M, Rolland Y. Hepatic tumor enhancement in computed tomography: combined models of liver perfusion and dynamic imaging. Comput Biol Med. 2003;33:77–89. doi: 10.1016/s0010-4825(02)00056-2. [DOI] [PubMed] [Google Scholar]
- 17.Miles KA, Leggett DA, Kelley BB, Hayball MP, Sinnatamby R, Bunce I. In vivo assessment of neovascularization of liver metastases using perfusion CT. Br J Radiol. 1998;71:276–281. doi: 10.1259/bjr.71.843.9616236. [DOI] [PubMed] [Google Scholar]
- 18.Miles KA. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: a review. Eur J Radiol. 1999;30:198–205. doi: 10.1016/s0720-048x(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 19.Dugdale PE, Miles KA. Hepatic metastases: the value of quantitative assessment of contrast enhancement on computed tomography. Eur J Radiol. 1999;30:206–213. doi: 10.1016/s0720-048x(99)00013-3. [DOI] [PubMed] [Google Scholar]
- 20.Miles KA, Griffiths MR, Fuentes MA. Standardized perfusion value: universal CT contrast enhancement scale that correlates with FDG PET in lung nodules. Radiology. 2001;220:548–553. doi: 10.1148/radiology.220.2.r01au26548. [DOI] [PubMed] [Google Scholar]
- 21.Goh V, Halligan S, Bartram CI. Quantitative tumor perfusion assessment with multidetector CT: are measurements from two commercial software packages interchangeable? Radiology. 2007;242:777–782. doi: 10.1148/radiol.2423060279. [DOI] [PubMed] [Google Scholar]
- 22.Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661–673. doi: 10.1148/radiol.2343031362. [DOI] [PubMed] [Google Scholar]
- 23.Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, Poulet B, Frouin F, Clément O, Frija G. Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology. 2001;218:556–561. doi: 10.1148/radiology.218.2.r01fe10556. [DOI] [PubMed] [Google Scholar]
- 24.Meijerink MR, van Cruijsen H, Hoekman K, Kater M, van Schaik C, van Waesberghe JH, Giaccone G, Manoliu RA. The use of perfusion CT for the evaluation of therapy combining AZD2171 with gefitinib in cancer patients. Eur Radiol. 2007;17:1700–1713. doi: 10.1007/s00330-006-0425-9. [DOI] [PubMed] [Google Scholar]
- 25.Sahani DV, Kalva SP, Hamberg LM, Hahn PF, Willett CG, Saini S, Mueller PR, Lee TY. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology. 2005;234:785–792. doi: 10.1148/radiol.2343040286. [DOI] [PubMed] [Google Scholar]
- 26.Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology. 2007;244:486–493. doi: 10.1148/radiol.2442061189. [DOI] [PubMed] [Google Scholar]
- 27.Pauls S, Gabelmann A, Heinz W, Fröhlich E, Juchems MS, Brambs HJ, Schmidt SA. Liver perfusion with dynamic multidetector-row computed tomography as an objective method to evaluate the efficacy of chemotherapy in patients with colorectal cancer. Clin Imaging. 2009;33:289–294. doi: 10.1016/j.clinimag.2008.10.030. [DOI] [PubMed] [Google Scholar]