Abstract
Adenoviral infections are typically acute, self-limiting, and not associated with death. However, we present the genomic and bioinformatics analysis of a novel recombinant human adenovirus (HAdV-D56) isolated in France that caused a rare neonatal fatality, and keratoconjunctivitis in three health care workers who cared for the neonate. Whole genome alignments revealed the expected diversity in the penton base, hexon, E3, and fiber coding regions, and provided evidence for extensive recombination. Bootscan analysis confirmed recombination between HAdV-D9, HAdV-D26, HAdV-D15, and HAdV-D29 in the penton base and hexon proteins, centered around hypervariable loops within the putative proteins. Protein structure analysis of the fiber coding region revealed similarity with HAdV-D8, HAdV-D9, and HAdV-D53, possibly accounting for the ocular tropism of the virus. Based on these data, this virus appears to be a new HAdV-D type (HAdV-D56), underscoring the importance of recombination events in human adenovirus evolution and the emergence of new adenovirus pathogens.
Keywords: Adenovirus, viral genomics, viral recombination, viral evolution, viral bioinformatics
Introduction
Human adenoviruses (HAdV) were first isolated in 1953 as respiratory pathogens (Hilleman and Werner, 1954; Rowe et al., 1953), and cause an array of human diseases including respiratory, gastrointestinal, renal, urinary tract, ocular surface infections, opportunistic infections in immune deficient individuals, and possibly obesity (Arnold et al., 2010; Chu and Pavan-Langston, 1979; Dhurandhar et al., 1992; Dingle and Langmuir, 1968; Kojaoghlanian, Flomenberg, and Horwitz, 2003; Wood, 1988). HAdVs cause infections in infancy and childhood, but are rarely diagnosed in neonates. Moreover, while adenoviruses cause significant morbidity, fatal infections are less common. Since the first adenovirus was characterized, a total of 55 types have been formally recognized and classified into 7 species (A–G) on the basis of serology, whole genome sequencing, and/or phylogenomics (Jones et al., 2007; Kaneko et al., 2009; Walsh et al., 2009; Walsh et al., 2010).
Recently a HAdV was associated with fatal pneumonia in a 10-day-old neonate in France (Henquell et al., 2009). Ten days after death, three health care providers developed keratoconjunctivitis. Hemagglutination inhibition for viral isolates from the infant and health care providers was similar and revealed a HAdV species D type 9 (HAdV-D9) fiber protein. (Henquell et al., 2009) However, hexon sequence was consistent with a HAdV-D15, D29 origin – HAdV-D15 and D29 are known to be nearly identical in the hexon region. Notably, another HAdV was previously reported as a putative recombinant HAdV-D15/H9 (“H” for hemagglutinin identity) (Adrian et al., 1985; Hierholzer and Rodriguez, 1981; Notzel et al., 1985; Wigand, Keller, and Werling, 1982) and is known to cause acute follicular conjunctivitis as well as infections of the respiratory tract, but the complete genome sequence of the virus previously described as HAdV-D15/H9, was never determined.
Since serology and hexon sequencing reveal only a small fraction of the complete viral genome, and did not provide a clear picture of the origins of the virus newly isolated in France, the entire genome of this apparently novel HAdV was sequenced. Complete genome analysis confirmed the previously reported fiber and hexon protein coding region origins (Henquell et al., 2009). Further, 70% of the virus appears highly similar to HAdV-D9. The hexon gene was identical to HAdV-D15 and D29, indicating a recombination event. Based on the presence of recombination resulting in a virus with parts from at least two other viruses, and a change in biological behavior and tropism – neither HAdV-D9, HAdV-D15, HAdV-D26, or HAdV-D29 have been associated with keratoconjunctivitis – it is proposed that this virus should be classified as a new type, HAdV-D56. The apparent emergence of this virus underscores the importance of homologous recombination within HAdV-D as a fundamental mechanism for the emergence of new human pathogens. (Kaneko et al., 2009; Robinson et al., 2009a; Walsh et al., 2009; Walsh et al., 2010).
Results
Nucleotide sequence analysis of HAdV-D56
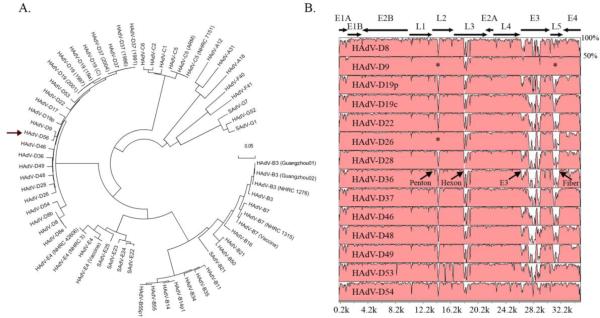
The complete genome of an adenovirus isolate from the neonate as well as an isolate from one care provider who developed keratoconjunctivitis was sequenced. Computational analysis confirmed the genomes to be 100% identical. Whole genome phylogenetic analysis established that the virus belongs to HAdV-D and further identified a close sequence relationship with HAdV-D9 (Fig. 1A). The HAdV-D56 genome contains 4 early, 2 intermediate, and 5 late transcription units, including 36 open reading frames (ORFs), similar to those previously identified in other sequenced HAdV-Ds. The genome of HAdV-D56 was determined to be 35,066 base pairs in length, with a GC content of 57%, also consistent with other HAdV-D (57–59%) (Shenk, 1996).
Fig. 1.
Whole genome analysis of HAdV-D56. (A) Bootstrap- confirmed neighbor-joining tree designed from MEGA 4.0.2 demonstrates relationships between HAdV-D56 and all other completely sequenced adenovirus genomes. (arrow: HAdV-D56). Bootstrap values for the species nodes were 100. The bootstrap value for the HAdV-D56 and HAdV-D9 node was also 100. (B) Global pairwise sequence comparison of HAdV-D56 with fourteen other completely sequenced HAdV-D types. Percent sequence conservation is reflected in the height of each data point along the y-axis. The penton base, hexon, E3, and fiber protein coding regions are divergent within species D, except for the penton base and fiber regions of HAdV-D9 and HAdV-D26 (*).
An on-line sequence alignment program, mVISTA Limited Area Global Alignment of Nucleotides (LAGAN), was used for global pair-wise sequence alignment (Brudno et al., 2003; Frazer et al., 2004; Mayor et al., 2000; Robinson et al., 2009a; Robinson et al., 2008; Robinson et al., 2009b) of the whole genome of HAdV-D56 to the 14 completely sequenced HAdV-D types available in GenBank. Distinct differences in the penton base, hexon, E3, and fiber genes were found, with a higher degree of conservation in other regions (Fig. 1B). Interestingly, HAdV-D56 revealed sequence conservation in the penton base compared to HAdV-D9 and HAdV-D26, which are themselves nearly identical in this region, and with HAdV-D9 in the fiber protein coding region (Fig. 1B)
Bootscan analysis shows recombination in the HAdV-D56 penton base and hexon genes
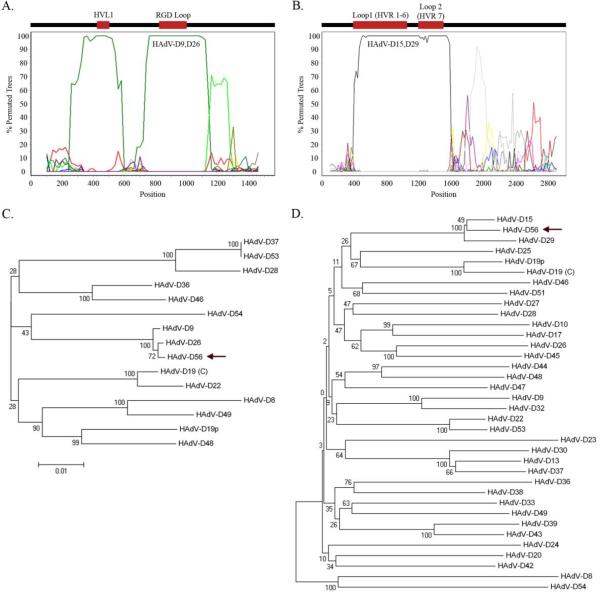
Based on the similar sequence seen in the penton base, Simplot 3.5.1 was used to identify possible recombination sites (Lole et al., 1999). Bootscan analysis identified two possible recombination loci in the penton base coding region between HAdV-D56, HAdV-D9, and HAdV-D26 at nucleotides 200–600 (Variable Loop 1 region) and 650–1150 (RGD loop) (Fig. 2A). Bootstrap-confirmed neighbor-joining phylogenetic trees of HAdV-D penton base genes using Molecular Evolutionary Genetics Analysis (MEGA) 4.0.2 were constructed to impute viral evolution (Tamura et al., 2007). Phylogenetic analysis of the entire penton base gene confirmed a close relationship of HAdV-D56 with HAdV-D9 and HAdV-D26 (Fig. 2C).
Fig. 2.
Bootscan recombination and phylogenetic analysis of HAdV-D56 penton base and hexon genes. (A) Bootscan analysis comparing the HAdV-D56 penton base gene with completely sequenced HAdV types. (B) Bootscan analysis comparing the HAdV-D56 hexon gene with other HAdV-D types. (C) Phylogenetic analysis of the penton base gene from completely sequenced HAdV-D types (arrow: HAdV-D56). (D) Phylogenetic analysis of the hexon gene from all available HAdV-D types (arrow: HAdV-D56). Bootstrap values below 80 are indicative of low confidence.
Global pairwise alignment of HAdV-D56 to other HAdV-D types also identified areas of sequence divergence in the hexon (principal viral capsid protein) (Fig. 1B). The bootscan analysis was extended to all HAdV hexon genes in GenBank and this identified recombination between HAdV-D56, HAdV-D15, and HAdV-D29 (Fig. 2B). Upon closer examination, it was revealed that loop 1, consisting of hypervariable regions 1–6, was 100% identical to both HAdV-D15 and HAdV-D29 (previously shown to be identical in this region (Madisch et al., 2005)), while loop 2, consisting of hypervariable region 7, was 100% identical to HAdV-D29, which in this loop is distinct from HAdV-D15. The conserved region of the hexon gene, located just downstream of loop 2, was 98% identical to HAdV-D15; amino acid sequence for this region was 100% identical to HAdV-D15 as well as other HAdV-D types. Phylogenetic analysis confirmed the similarity identified in Bootscan analysis (Fig. 2D).
HAdV-D56 fiber analysis
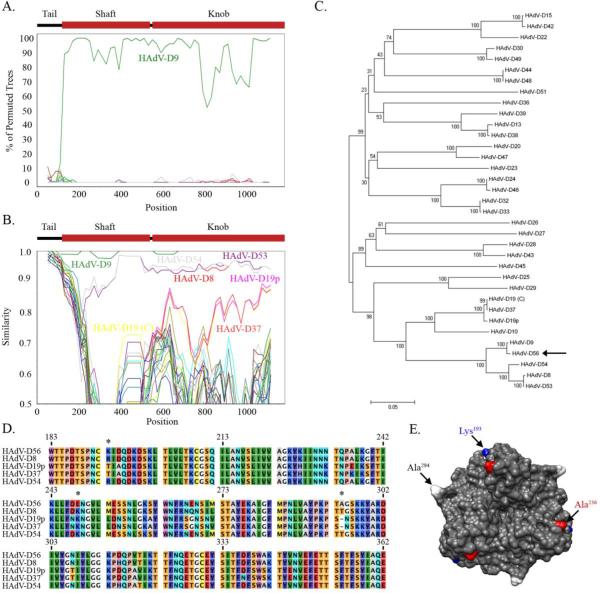
Next, the entire fiber protein coding region (primary host cell binding ligand) was analyzed. Previous serological data as well as mVISTA LAGAN analysis revealed sequence conservation between HAdV-D56 and HAdV-D9. Blast analysis revealed HAdV-D56 fiber nucleotide sequence and its amino acid sequence were each 99% identical to HAdV-D9, with the imputed amino acid sequence differing in only one amino acid (Ser2 to Ala2) (data not shown). Interestingly, the fiber knob, which acts as the primary ligand for binding to the target cell, differs only in one base, and its imputed amino acid sequence is 100% identical to that of HAdV-D9. This amino acid sequence was also 100% identical to the previously sequenced HAdV-D15/H9 fiber knob (Darr et al., 2009). A comparison of the Bootscan and Simplot analyses of the HAdV-D56 fiber gene (Figs. 3A and B, respectively) suggested high levels of identity with the fiber genes of multiple HAdV-D genomes. In addition to HAdV-D9, regions of the HAdV-D8, HAdV-D53, and HAdV-D54 fiber genes were highly similar (~90%) to HAdV-D56. In the fiber knob coding region, HAdV-D19p, HAdV-D19 (C), and HAdV-D37 also showed high similarity to the same region in HAdV-D56. Phylogenetic analysis of the fiber protein coding region confirmed the Simplot results and revealed a close relationship between HAdV-D56 and HAdV-D9, and also with HAdV-D8, HAdV-D53, and HAdV-D54 (Fig. 3C). Also, the fiber gene of HAdV-D56 appears by phylogenetic analysis to be closely related to HAdV-D19 (C) and HAdV-D37. Interestingly HAdV-D8, HAdV-D19 (C), HAdV-D37, HAdV-D53, and HAdV-D54 are all known to cause epidemic keratoconjunctivitis (EKC) (Desmyter et al., 1974; Engelmann et al., 2006; Jawetz et al., 1955; Kaneko et al., 2009; Mitsui and Jawetz, 1957; Robinson et al., 2009b; Walsh et al., 2009)
Fig. 3.
Genomic and structural analysis of the HAdV-D56 fiber. (A) Bootscan analysis comparing the HAdV-D56 fiber gene with each HAdV-D type. (B) Simplot analysis comparing the HAdV-D56 fiber gene with each HAdV-D type. (C) Phylogenetic analysis of sequenced HAdV-D fiber genes (arrow: HAdV-D56). (D) Multi-sequence alignment of the fiber knob designed from MEGA 4.0.2 software. HAdV-D56 unique residues compared to other EKC viruses designated by *. HAdV-D19(C) and HAdV-D53 were left out of the analysis based on 100% identity to HAdV-D37 and HAdV-D8, respectively. (E) HAdV-D56 protein trimer model based on homology modeling from the crystal structure of HAdV-D19p. Unique residues Lys193 and Ala294 are highlighted in blue and white, respectively. Ala236, which may play a key role in host receptor binding, is highlighted in red.
To analyze possible amino acid similarities between viruses associated with EKC and HAdV-D56, a multi-sequence amino acid alignment was performed. Analysis revealed HAdV-D56 to have three unique residues when compared to EKC-causing HAdVs (Fig. 3D). A model of the HAdV-D56 fiber knob was constructed using HAdV-D19p (Burmeister et al., 2004) (PDB: 1UXB) as a template. Residues Lys193 and Ala294 are unique to HAdV-D56 compared to the EKC-associated HAdVs (Fig. 3E). It is unclear how these mutations might affect binding to the host cellular receptor. Glu248 is also disparate, but is located on the posterior side of the fiber knob (data not shown). Previously, Lys240 in HAdV-D37 was implicated as the main determinant for binding to conjunctival cells when compared to the nonpathogenic HAdV-D19p (Huang et al., 1999). HAdV-D56 shares a conserved residue (Ala236) with HAdV-D8 and HAdV-D54 at this position while Glu240 appears to be unique to HAdV-19p (Figs. 3C and D), confirming the prior analysis by Huang and coworkers (Huang et al., 1999). The two key residues Tyr309 and Lys342, identified in binding of HAdV-D37 to sialic acid, are also conserved in HAdV-D56 (Burmeister et al., 2004).
Discussion
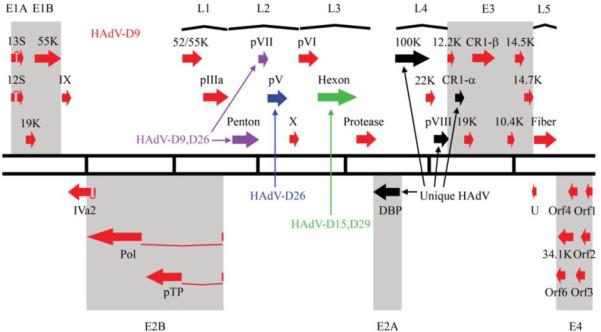
In summary, the 35,066 base pair genome of a novel human adenovirus causing death of a 10-day-old neonate and subsequent keratoconjunctivitis in three health care providers, was determined and computationally analyzed. HAdV-D56 showed 70% genome similarity with HAdV-D9, and bioinformatics analysis reveals possible prior recombination events with HAdV-D15, HAdV-D29, HAdV-D9 and HAdV-D26 as well as a genomic sequence that is unique to HAdV-D56 or a recombinant from a currently un-sequenced and unknown HAdV (Fig. 4). HAdV-D56 fulfills the criteria for a new type within species D, as it is recombinant between several previously characterized viruses, contains previously unknown nucleotide sequence, and shows a change in tropism from its most closely related virus, HAdV-D9, not previously associated with either fatality or keratoconjunctivitis (Javier et al., 1991; Javier, Raska, and Shenk, 1992; Shenk, 1996; Tabrizi et al., 2007; Weiss et al., 1997; Weiss, McArthur, and Javier, 1996). These criteria for a new adenovirus type were used previously to identify HAdV-G52, HAdV-D53, HAdV-D54 and HAdV-B55 (Jones et al., 2007; Kaneko et al., 2009; Walsh et al., 2009; Walsh et al., 2010).
Fig. 4.
Transcriptional map of the recombinant HAdV-D56 genome. Genes with high sequence identity to HAdV-D9; HAdV-D9,D26; HAdV-D26; and HAdV-D15,D29 are indentified in red, purple, blue, and green, respectively, where HAdV-D9,D26 refers to genomic sequence for which HAdV-D9 and HAdV-D26 are nearly identical, and HAdVD15,D29 refers to sequence where HAdV-D15 and HAdV-D29 are nearly identical. Genes with unique sequence are identified in black.
Previously an emergent pathogen referred to as HAdV-D15/H9 was described as a recombinant adenovirus (Hierholzer and Rodriguez, 1981; Notzel et al., 1985), based on its serological properties. Serotyping by viral neutralization revealed a HAdV-D15 hexon epitope, while hemagglutination was consistent with a HAdV-D9 fiber. HAdV-D15/H9 reportedly caused acute respiratory tract infections as well as follicular conjunctivitis (Hierholzer and Rodriguez, 1981), similar to HAdV-D56 (Henquell et al., 2009). It is possible that HAdV-D15/H9 and HAdV-D56 are the same virus, but corneal infection (EKC) by HAdV-D56 suggests an expanded tropism. Genomic analysis of the earlier isolate would be necessary to determine if HAdV-D15/H9 and HAdV-D56 are otherwise related.
In neonates, adenoviral infections (including keratoconjunctivitis) are rare, but when they occur are often fatal (Abbondanzo et al., 1989; Abzug and Levin, 1991; Bhat et al., 1984; Elnifro et al., 2005; Kim et al., 1997). In adults, adenovirus keratoconjunctivitis occurs commonly and is caused predominantly by HAdV-D8, HAdV-D19, and HAdV-D37 (Ford, Nelson, and Warren, 1987). Recently two new adenovirus types causing EKC, HAdV-D53 and HAdV-D54, were identified in Germany and Japan, respectively (Engelmann et al., 2006; Kaneko et al., 2009; Walsh et al., 2009). Although the HAdV-D56 genome appears to be a recombinant between viruses not typically associated with keratoconjunctivitis, examination of the fiber knob binding site to the host cell receptor revealed conservation with HAdV-D8 and HAdV-D54, both of which cause EKC. Also, it is known that HAdV-D8 and HAdV-D9 show cross-reactivity serologically via hemagglutination (Wigand, Keller, and Werling, 1982), suggesting that HAdV-D9 might be an occult cause of EKC, because of the misidentification as HAdV-D8. While the cellular receptor for specific HAdV-Ds remains controversial, analysis of HAdV-D56 fiber knob suggested it may bind to a common corneal epithelial receptor, either CD46 or sialic acid. A possible low affinity interaction with the coxsackie and adenovirus receptor (CAR), based on sequence identity to HAdV-D9 is also feasible (Arnberg, Pring-Akerblom, and Wadell, 2002; Kirby et al., 2001; Roelvink, Kovesdi, and Wickham, 1996; Wu et al., 2004). Interaction between the EKC-associated viruses and their cellular receptor is also believed to be charge dependent (Arnberg et al., 2002). The theoretical isoelectric point (pI) for HAdV-D56 fiber knob is 7.8, lower than the EKC viruses (pI ~ 9.0), but higher than what was previously reported for HAdV-D9 (Arnberg, Mei, and Wadell, 1997).
The mechanism of acquisition of HAdV-D56 by the affected neonate is unknown. Neonatal infection may occur by horizontal or vertical transmission. HAdV-D8, HAdV-D19, and HAdV-D37, the principal causes of EKC, are commonly isolated from the genitourinary tract (de Jong et al., 1981; Phillips, Harnett, and Gollow, 1982; Swenson et al., 1995), suggesting vertical transmission. HAdV-C1, HAdV-C2, HAdV-D9, HAdV-D10, HAdV-A18, HAdV-D22, HAdV-D26, and HAdV-D32 have also been reported as genitourinary pathogens (Harnett, Phillips, and Gollow, 1984), but are not associated with keratoconjunctivitis. Coinfection of cells within the genitourinary tract could facilitate recombination within HAdV-D, leading to new types. This genomic and computational analysis of HAdV-D56 provide new insights into the genetic determinants of tropism and the molecular evolution of adenoviruses.
Materials and Methods
Cells, virus stock, DNA purification
HAdV-D56 was originally cultured from both a pulmonary biopsy of a 10-day old neonate and from a conjunctival swab from a care provider (Henquell et al., 2009). HAdV-D56 was kindly provided by Henquell and coworkers (Henquell et al., 2009). Virus stocks were grown in A-549 cells (ATCC, CCL-185). Virus was purified by CsCl gradient, dialyzed, and stored at −80°C. DNA extraction was accomplished by the addition of proteinase K, phenol:chloroform extraction, and finally ethanol precipitation.
Sequencing and annotation
Purified DNA was sequenced on a Roche 454 DNA sequencer (Branford, CT), by Eurofins MWG Operon (Huntsville, Alabama). Quality control included sequence annotation and comparison with HAdV genome landmarks. The genomes from both virus isolates from the neonate and health care worker were found be 100% identical (data not shown). Annotation was performed using a custom annotation engine (Dyer and coworkers, unpublished), with confirmation from the NCBI open reading frame (ORF) finder (http://www.ncbi.nlm.nih.gov/projects/gorf/). Artemis, a genome viewer (http://www.sanger.ac.uk/resources/software/artemis/), was used to record and manually review the data (Carver et al., 2008; Rutherford et al., 2000). Open reading frames were compared against available databases in GenBank for confirmation and protein similarity. Splice sites were predicted using the GenScan web server at MIT (http://genes.mit.edu/GENSCAN.html), and confirmed by comparisons to previously annotated genomes.
Sequence analysis
An on-line sequence alignment program, mVISTA Limited Area Global Alignment of Nucleotides (LAGAN, http://genome.lbl.gov/vista/lagan/submit.shtml), was used for global pair-wise sequence alignment (Brudno et al., 2003; Frazer et al., 2004; Mayor et al., 2000; Robinson et al., 2009a; Robinson et al., 2008; Robinson et al., 2009b). Sequences were aligned using the ClustalW (Larkin et al., 2007) option within the software Molecular Evolutionary Genetics Analysis (MEGA) 4.0.2 (http://www.megasoftware.net/index.html) (Tamura et al., 2007). Phylogenetic analysis was preformed using bootstrap-confirmed neighbor-joining trees (500 replicates) also designed with MEGA 4.0.2. DNA sequence recombination analysis was performed with Simplot 3.5.1 software (http://sray.med.som.jhmi.edu/SCRoftware/simplot/), including the options of Bootscan and Simplot analysis, to identify possible recombination sites (Lole et al., 1999). The default settings were used and HAdV-D56 was used as the reference sequence for each analysis.
Protein analysis and modeling
Multi-sequence amino acid alignment was performed using CLC sequence viewer 6 (http://www.clcbio.com/index.php?id=28). A model of the HAdV-D56 fiber knob was constructed using SWISS-MODEL (http://swissmodel.expasy.org/) for homology modeling (Arnold et al., 2006; Kiefer et al., 2009). The crystal structure of HAdV-D19p (Burmeister et al., 2004) (PDV: 1UXB) was used as a template for the model. The identity between HAdV-D19p fiber knob and HAdV-D56 fiber knob was 85%. UCSF Chimera software (http://www.cgl.ucsf.edu/chimera) (Pettersen et al., 2004) was used visualization and analysis. The theoretical isoelectric point (pI) was calculated using pI/Mw tool within the ExPASy Proteomics Server (http://ca.expasy.org/tools/pi_tool.html) (Bjellqvist et al., 1994; Gasteiger et al., 2003)
Nucleotide sequence accession numbers
The HAdV-D56 genome and annotation were deposited in GenBank prior to manuscript submission; accession no. HM770721. The following HAdV genomes (GenBank accession numbers) were used: HAdV-C1 (AF534906), HAdV-C2 (AC_000007), HAdV-B3 (AY599834), HAdV-B3 (Guangzhou01) (DQ099432), HAdV-B3 (Guangzhou02) (DQ105654), HAdV-B3 (NHRC 1276) (AY599836), HAdV-E4 (AY594253), HAdV-E4 (Vaccine) (AY594254), HAdV-E4 (NHRC 3) (AY599837), HAdV-E4 (NHRC 42606) (AY599835), HAdV-C5 (AC_000008), HAdV-C5 (ARM) (AY339865), HAdV-C5 (NHRC 7151) (AY601635), HAdV-C6 (FJ349096), HAdV-B7 (AY594255), HAdV-B7 (vaccine) (AY594256), HAdV-B7 (NHRC 1315) (AY601634), HAdV-D8 (AB448767), HAdV-D8b (AB448768), HAdV-D8e (AB448769), HAdV-D9 (AJ854486), HAdV-B11 (AY163756), HAdV-A12 (AC_000005), HAdV-B14 (AY803294), HAdV-B14p1 (FJ822614), HAdV-D15 (DQ149617), HAdV-B16 (AY601636), HAdV-D17 (AC_000006), HAdV-A18 (GU191019), HAdV-D19p (AB448771), HAdV-D19 (C) (EF121005), HAdV-D19 (19a) (AB448772), HAdV-D19 (1997) (AB448773), HAdV-D19 (2001) (AB448774), HAdV-B21 (AY601633), HAdV-D22 (FJ404771), HAdV-D26 (EF153474), HAdV-D28 (FJ824826), HAdV-D29 (DQ149627) HAdV-A31 (AM749299), HAdV-B34 (AY737797), HAdV-B35 (AY128640), HAdV-D36 (GQ384080), HAdV-D37 (DQ900900), HAdV-D37 (1991) (AB448776), HAdV-D37 (1996) (AB448777), HAdV-D37 (2004) (AB448778), HAdV-F40 (NC_001454), HAdV-F41 (DQ315364), HAdV-D46 (AY875648), HAdV-D48 (EF153473), HAdV-D49 (DQ393829), HAdV-B50 (AY737798), HAdV-G52 (DQ923122), HAdV-D53 (FJ169625), HAdV-D54 (AB448770), HAdV-B55 (FJ643676), HAdV-B55p1 (FJ597732), SAdV-G1 (NC_006879), SAdV-G7 (DQ792570), SAdV-B21 (AC_000010), SAdV-E22 (AY530876), SAdV-E23 (AY530877), SAdV-E24 (AY530878), SAdV-E25 (AC_000011).
Acknowledgments
Supported by NIH grants EY013124-S1 and P30EY014104, and an unrestricted grant to the Department of Ophthalmology, Harvard Medical School from Research to Prevent Blindness, Inc. The views expressed in this material are those of the authors, and do not reflect the official policy or position of the U.S. Government, the Department of Defense, or the Department of the Air Force. The funding sponsors played no role in any aspect of the study. The authors wish to thank Sarah Torres for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbondanzo SL, English CK, Kagan E, McPherson RA. Fatal adenovirus pneumonia in a newborn identified by electron microscopy and in situ hybridization. Arch Pathol Lab Med. 1989;113(12):1349–53. [PubMed] [Google Scholar]
- Abzug MJ, Levin MJ. Neonatal adenovirus infection: four patients and review of the literature. Pediatrics. 1991;87(6):890–6. [PubMed] [Google Scholar]
- Adrian T, Bastian B, Benoist W, Hierholzer JC, Wigand R. Characterization of adenovirus 15/H9 intermediate strains. Intervirology. 1985;23(1):15–22. doi: 10.1159/000149562. [DOI] [PubMed] [Google Scholar]
- Arnberg N, Kidd AH, Edlund K, Nilsson J, Pring-Akerblom P, Wadell G. Adenovirus type 37 binds to cell surface sialic acid through a charge-dependent interaction. Virology. 2002;302(1):33–43. doi: 10.1006/viro.2002.1503. [DOI] [PubMed] [Google Scholar]
- Arnberg N, Mei Y, Wadell G. Fiber genes of adenoviruses with tropism for the eye and the genital tract. Virology. 1997;227(1):239–44. doi: 10.1006/viro.1996.8269. [DOI] [PubMed] [Google Scholar]
- Arnberg N, Pring-Akerblom P, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J Virol. 2002;76(17):8834–41. doi: 10.1128/JVI.76.17.8834-8841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J, Janoska M, Kajon AE, Metzgar D, Hudson NR, Torres S, Harrach B, Seto D, Chodosh J, Jones MS. Genomic characterization of human adenovirus 36, a putative obesity agent. Virus Res. 2010;149(2):152–61. doi: 10.1016/j.virusres.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Bhat AM, Meny RG, Aranas EA, Yehia F. Fatal adenoviral (type 7) respiratory disease in neonates. Clin Pediatr (Phila) 1984;23(7):409–11. doi: 10.1177/000992288402300710. [DOI] [PubMed] [Google Scholar]
- Bjellqvist B, Basse B, Olsen E, Celis JE. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis. 1994;15(3–4):529–39. doi: 10.1002/elps.1150150171. [DOI] [PubMed] [Google Scholar]
- Brudno M, Do CB, Cooper GM, Kim MF, Davydov E, Green ED, Sidow A, Batzoglou S. LAGAN and Multi-LAGAN: efficient tools for large-scale multiple alignment of genomic DNA. Genome Res. 2003;13(4):721–31. doi: 10.1101/gr.926603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister WP, Guilligay D, Cusack S, Wadell G, Arnberg N. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J Virol. 2004;78(14):7727–36. doi: 10.1128/JVI.78.14.7727-7736.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver T, Berriman M, Tivey A, Patel C, Bohme U, Barrell BG, Parkhill J, Rajandream MA. Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics. 2008;24(23):2672–6. doi: 10.1093/bioinformatics/btn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W, Pavan-Langston D. Ocular surface manifestations of the major viruses. Int Ophthalmol Clin. 1979;19(2):135–67. [PubMed] [Google Scholar]
- Darr S, Madisch I, Hofmayer S, Rehren F, Heim A. Phylogeny and primary structure analysis of fiber shafts of all human adenovirus types for rational design of adenoviral gene-therapy vectors. J Gen Virol. 2009;90(Pt 12):2849–54. doi: 10.1099/vir.0.014514-0. [DOI] [PubMed] [Google Scholar]
- de Jong JC, Wigand R, Wadell G, Keller D, Muzerie CJ, Wermenbol AG, Schaap GJ. Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J Med Virol. 1981;7(2):105–18. doi: 10.1002/jmv.1890070204. [DOI] [PubMed] [Google Scholar]
- Desmyter J, De Jong JC, Slaterus KW, Verlaeckt H. Letter: Keratoconjunctivitis caused by Adenovirus Type 19. Br Med J. 1974;4(5941):406. doi: 10.1136/bmj.4.5941.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhurandhar NV, Kulkarni P, Ajinkya SM, Sherikar A. Effect of adenovirus infection on adiposity in chicken. Vet Microbiol. 1992;31(2–3):101–7. doi: 10.1016/0378-1135(92)90068-5. [DOI] [PubMed] [Google Scholar]
- Dingle JH, Langmuir AD. Epidemiology of acute, respiratory disease in military recruits. Am Rev Respir Dis. 1968;97(6)(Suppl):1–65. doi: 10.1164/arrd.1968.97.1.1. [DOI] [PubMed] [Google Scholar]
- Elnifro EM, Cooper RJ, Dady I, Hany S, Mughal ZM, Klapper PE. Three nonfatal cases of neonatal adenovirus infection. J Clin Microbiol. 2005;43(11):5814–5. doi: 10.1128/JCM.43.11.5814-5815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann I, Madisch I, Pommer H, Heim A. An outbreak of epidemic keratoconjunctivitis caused by a new intermediate adenovirus 22/H8 identified by molecular typing. Clin Infect Dis. 2006;43(7):e64–6. doi: 10.1086/507533. [DOI] [PubMed] [Google Scholar]
- Ford E, Nelson KE, Warren D. Epidemiology of epidemic keratoconjunctivitis. Epidemiol Rev. 1987;9:244–61. doi: 10.1093/oxfordjournals.epirev.a036304. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32(Web Server issue):W273–9. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31(13):3784–8. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett GB, Phillips PA, Gollow MM. Association of genital adenovirus infection with urethritis in men. Med J Aust. 1984;141(6):337–8. doi: 10.5694/j.1326-5377.1984.tb132799.x. [DOI] [PubMed] [Google Scholar]
- Henquell C, Boeuf B, Mirand A, Bacher C, Traore O, Dechelotte P, Labbe A, Bailly JL, Peigue-Lafeuille H. Fatal adenovirus infection in a neonate and transmission to health-care workers. J Clin Virol. 2009;45(4):345–8. doi: 10.1016/j.jcv.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Hierholzer JC, Rodriguez FH., Jr. Antigenically intermediate human adenovirus strain associated with conjunctivitis. J Clin Microbiol. 1981;13(2):395–7. doi: 10.1128/jcm.13.2.395-397.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleman MR, Werner JH. Recovery of new agent from patients with acute respiratory illness. Proc Soc Exp Biol Med. 1954;85(1):183–8. doi: 10.3181/00379727-85-20825. [DOI] [PubMed] [Google Scholar]
- Huang S, Reddy V, Dasgupta N, Nemerow GR. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J Virol. 1999;73(4):2798–802. doi: 10.1128/jvi.73.4.2798-2802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier R, Raska K, Jr., Macdonald GJ, Shenk T. Human adenovirus type 9-induced rat mammary tumors. J Virol. 1991;65(6):3192–202. doi: 10.1128/jvi.65.6.3192-3202.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier R, Raska K, Jr., Shenk T. Requirement for the adenovirus type 9 E4 region in production of mammary tumors. Science. 1992;257(5074):1267–71. doi: 10.1126/science.1519063. [DOI] [PubMed] [Google Scholar]
- Jawetz E, Kimura S, Nicholas AN, Thygeson P, Hanna L. new type of APC virus from epidemic keratoconjunctivitis. Science. 1955;122(3181):1190–1. doi: 10.1126/science.122.3181.1190-a. [DOI] [PubMed] [Google Scholar]
- Jones MS, 2nd, Harrach B, Ganac RD, Gozum MM, Dela Cruz WP, Riedel B, Pan C, Delwart EL, Schnurr DP. New adenovirus species found in a patient presenting with gastroenteritis. J Virol. 2007;81(11):5978–84. doi: 10.1128/JVI.02650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Iida T, Ishiko H, Ohguchi T, Ariga T, Tagawa Y, Aoki K, Ohno S, Suzutani T. Analysis of the complete genome sequence of epidemic keratoconjunctivitis-related human adenovirus type 8, 19, 37 and a novel serotype. J Gen Virol. 2009;90(Pt 6):1471–6. doi: 10.1099/vir.0.009225-0. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37(Database issue):D387–92. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Han HS, Park SH, Chun YK, Lee HJ, Chi JG. Neonatal adenoviral pneumonia--report of three autopsy cases. J Korean Med Sci. 1997;12(2):146–50. doi: 10.3346/jkms.1997.12.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby I, Lord R, Davison E, Wickham TJ, Roelvink PW, Kovesdi I, Sutton BJ, Santis G. Adenovirus type 9 fiber knob binds to the coxsackie B virus-adenovirus receptor (CAR) with lower affinity than fiber knobs of other CAR-binding adenovirus serotypes. J Virol. 2001;75(15):7210–4. doi: 10.1128/JVI.75.15.7210-7214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojaoghlanian T, Flomenberg P, Horwitz MS. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003;13(3):155–71. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73(1):152–60. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisch I, Harste G, Pommer H, Heim A. Phylogenetic analysis of the main neutralization and hemagglutination determinants of all human adenovirus prototypes as a basis for molecular classification and taxonomy. J Virol. 2005;79(24):15265–76. doi: 10.1128/JVI.79.24.15265-15276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor C, Brudno M, Schwartz JR, Poliakov A, Rubin EM, Frazer KA, Pachter LS, Dubchak I. VISTA : visualizing global DNA sequence alignments of arbitrary length. Bioinformatics. 2000;16(11):1046–7. doi: 10.1093/bioinformatics/16.11.1046. [DOI] [PubMed] [Google Scholar]
- Mitsui Y, Jawetz E. Isolation of adenovirus type 8 (APC type 8) from a case of epidemic keratoconjunctivitis in Japan. Am J Ophthalmol. 1957;43(4 Part 2):91–3. doi: 10.1016/0002-9394(57)91485-x. [DOI] [PubMed] [Google Scholar]
- Notzel R, Adrian T, Wigand R, Wadell G. Characterization of hemagglutinin variant strains of adenovirus 15 and 9. Brief report. Arch Virol. 1985;85(1–2):171–4. doi: 10.1007/BF01317018. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Phillips PA, Harnett GB, Gollow MM. Adenovirus type 19 and a closely related new serotype in genital infection. Br J Vener Dis. 1982;58(2):131–2. doi: 10.1136/sti.58.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Rajaiya J, Walsh MP, Seto D, Dyer DW, Jones MS, Chodosh J. Computational analysis of human adenovirus type 22 provides evidence for recombination among species D human adenoviruses in the penton base gene. J Virol. 2009a;83(17):8980–5. [Google Scholar]
- Robinson CM, Shariati F, Gillaspy AF, Dyer DW, Chodosh J. Genomic and bioinformatics analysis of human adenovirus type 37: new insights into corneal tropism. BMC Genomics. 2008;9:213. doi: 10.1186/1471-2164-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Shariati F, Zaitshik J, Gillaspy AF, Dyer DW, Chodosh J. Human adenovirus type 19: Genomic and bioinformatics analysis of a keratoconjunctivitis isolate. Virus Res. 2009b;139(1):122–126. doi: 10.1016/j.virusres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelvink PW, Kovesdi I, Wickham TJ. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70(11):7614–21. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953;84(3):570–3. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16(10):944–5. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- Shenk T. Adenoviridae: The Viruses and Their Replication. In: Fields DMKBN, Howle PM, et al., editors. Fields Virology. Third Edition Lippincott - Raven Publishers; Philadelphia: 1996. pp. 2111–2148. [Google Scholar]
- Swenson PD, Lowens MS, Celum CL, Hierholzer JC. Adenovirus types 2, 8, and 37 associated with genital infections in patients attending a sexually transmitted disease clinic. J Clin Microbiol. 1995;33(10):2728–31. doi: 10.1128/jcm.33.10.2728-2731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SN, Ling AE, Bradshaw CS, Fairley CK, Garland SM. Human adenoviruses types associated with non-gonococcal urethritis. Sex Health. 2007;4(1):41–4. doi: 10.1071/sh06054. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Walsh MP, Chintakuntlawar A, Robinson CM, Madisch I, Harrach B, Hudson NR, Schnurr D, Heim A, Chodosh J, Seto D, Jones MS. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS ONE. 2009;4(6):e5635. doi: 10.1371/journal.pone.0005635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MP, Seto J, Jones MS, Chodosh J, Xu W, Seto D. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J Clin Microbiol. 2010;48(3):991–3. doi: 10.1128/JCM.01694-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RS, Gold MO, Vogel H, Javier RT. Mutant adenovirus type 9 E4 ORF1 genes define three protein regions required for transformation of CREF cells. J Virol. 1997;71(6):4385–94. doi: 10.1128/jvi.71.6.4385-4394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RS, McArthur MJ, Javier RT. Human adenovirus type 9 E4 open reading frame 1 encodes a cytoplasmic transforming protein capable of increasing the oncogenicity of CREF cells. J Virol. 1996;70(2):862–72. doi: 10.1128/jvi.70.2.862-872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigand R, Keller D, Werling I. Immunological relationship among human adenoviruses of subgenus D. Arch Virol. 1982;72(3):199–209. doi: 10.1007/BF01348965. [DOI] [PubMed] [Google Scholar]
- Wood DJ. Adenovirus gastroenteritis. Br Med J (Clin Res Ed) 1988;296(6617):229–30. doi: 10.1136/bmj.296.6617.229-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E, Trauger SA, Pache L, Mullen TM, von Seggern DJ, Siuzdak G, Nemerow GR. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J Virol. 2004;78(8):3897–905. doi: 10.1128/JVI.78.8.3897-3905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]