Abstract
Objective To compare the effectiveness of elective single embryo transfer versus double embryo transfer on the outcomes of live birth, multiple live birth, miscarriage, preterm birth, term singleton birth, and low birth weight after fresh embryo transfer, and on the outcomes of cumulative live birth and multiple live birth after fresh and frozen embryo transfers.
Design One stage meta-analysis of individual patient data.
Data sources A systematic review of English and non-English articles from Medline, Embase, and the Cochrane Central Register of Controlled Trials (up to 2008). Additional studies were identified by contact with clinical experts and searches of bibliographies of all relevant primary articles. Search terms included embryo transfer, randomised controlled trial, controlled clinical trial, single embryo transfer, and double embryo transfer.
Review methods Comparisons of the clinical effectiveness of cleavage stage (day 2 or 3) elective single versus double embryo transfer after fresh or frozen in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatments were included. Trials were included if the intervention differed only in terms of the intended number of embryos to be transferred. Trials that involved only blastocyst (day five) transfers were excluded.
Results Individual patient data were received for every patient recruited to all eight eligible trials (n=1367). A total of 683 and 684 women randomised to the single and double embryo transfer arms, respectively, were included in the analysis. Baseline characteristics in the two groups were comparable. The overall live birth rate in a fresh IVF cycle was lower after single (181/683, 27%) than double embryo transfer (285/683, 42%) (adjusted odds ratio 0.50, 95% confidence interval 0.39 to 0.63), as was the multiple birth rate (3/181 (2%) v 84/285 (29%)) (0.04, 0.01 to 0.12). An additional frozen single embryo transfer, however, resulted in a cumulative live birth rate not significantly lower than the rate after one fresh double embryo transfer (132/350 (38%) v 149/353 (42%) (0.85, 0.62 to 1.15), with a minimal cumulative risk of multiple birth (1/132 (1%) v 47/149 (32%)). The odds of a term singleton birth (that is, over 37 weeks) after elective single embryo transfer was almost five times higher than the odds after double embryo transfer (4.93, 2.98 to 8.18).
Conclusions Elective single embryo transfer results in a higher chance of delivering a term singleton live birth compared with double embryo transfer. Although this strategy yields a lower pregnancy rate than a double embryo transfer in a fresh IVF cycle, this difference is almost completely overcome by an additional frozen single embryo transfer cycle. The multiple pregnancy rate after elective single embryo transfer is comparable with that observed in spontaneous pregnancies.
Introduction
Increasing success after in vitro fertilisation (IVF) and intracytoplasmic sperm injection has been accompanied by concerns about rising rates of multiple pregnancies.1 Multiple pregnancy is associated with increased maternal and perinatal morbidity and mortality, as well as increased costs to the health service.1 2 3 These concerns have prompted a change in practice that has led to a reduction in triple embryo transfers and an increase in double embryo transfers. In 2006, however, twins accounted for nearly 20% of all live births in Europe resulting from IVF.4
Considerations of safety in the context of expanding use of assisted reproduction has encouraged many clinics in northern Europe to adopt a policy of elective single embryo transfer as an effective means of minimising the rate of twin pregnancy associated with IVF.2 5 The Human Embryology and Fertilisation Authority (HFEA), which regulates IVF in the United Kingdom, now advocates the use of elective single embryo transfer as an effective way of reducing twin births associated with IVF6 and recommends a national target twin rate of under 10%.7 A systematic review of randomised trials with aggregated data on elective single versus double embryo transfer concluded that, though single transfer reduced the odds of multiple pregnancies, it halved the odds of live birth per fresh cycle.8 Subsequent transfer of a single frozen thawed embryo resulted in comparable cumulative live birth outcomes to those after double embryo transfer. Since that review was performed, however, results from other relevant unpublished trials have become available (M Davies, University of Adelaide, personal communication).9 10 Also, meta-analysis of published aggregated data cannot provide information on which subgroups of women might be most likely to benefit from elective single embryo transfer or present data on important outcomes such as the birth of a healthy singleton baby at term, which have not been reported in existing trials. This is important, as characteristics of the patients and embryos are key prognostic indices that can influence chances of a live birth, as well as multiple pregnancies.11
Meta-analysis of individual patient data has several advantages over a traditional review of published data,12 including the ability to carry out data checks, standardise outcome measures, and perform appropriate subgroup analyses.13 We carried out a systematic review of randomised controlled trials to compare the effectiveness of cleavage stage (day 2 or 3) elective single versus double embryo transfer on the outcomes of live birth and multiple live birth after a fresh embryo transfer and on the outcomes of cumulative live birth and multiple live birth after fresh and frozen embryo transfers. Our use of individual patient data allowed us to address some of the acknowledged shortcomings of existing meta-analyses of aggregated data, including assessment of study quality, intention to treat analysis of all randomised women, and subgroup analysis based on important prognosticators such as age, duration of infertility, and embryo quality. In addition, we used the combined dataset of randomised women to investigate key outcomes such as term singleton live birth, miscarriage, and preterm birth not previously reported by the relevant trials.
Methods
The systematic review was based on a protocol designed with widely recommended methods to allow us to comply with reporting guidelines.13 14 15 16 We undertook a “one stage” meta-analysis of individual patient data from randomised trials of cleavage stage (day 2 or 3) single versus double embryo transfer.13 17 18
Literature search and study selection
We searched Medline and Embase databases up to 2008 using relevant terms and word variants for the interventions and study design. The detailed search strategy is in appendix 1 on bmj.com. We also hand searched bibliographies of all relevant primary articles and reviews to identify any articles missed by the electronic searches. Several trial registers were searched to identify ongoing trials and experts were contacted to identify further studies. No language restriction was applied.
Studies were selected in a two step process. We first scrutinised the citations identified by the electronic searches and then obtained full manuscripts of all the citations that met or were thought likely to meet the predetermined inclusion criteria based on criteria for patients at entry, type of intervention, and study design.
Types of studies
Only randomised controlled trials that compared the clinical effectiveness of cleavage stage elective single with double embryo transfer were deemed eligible for inclusion. Quasi-randomised trials were excluded, as were trials with other co-interventions. Ongoing trials and crossover trials were considered for inclusion—the latter only if data from before the crossover occurred were available.
Types of participants
Women undergoing IVF or intracytoplasmic sperm injection, or both, with their own oocytes were considered eligible for inclusion.
Type of intervention
We included comparisons of the clinical effectiveness of elective single versus double cleavage stage embryo transfer after fresh or frozen IVF or intracytoplasmic sperm injection treatments. Frozen treatment cycles were included if the intention was to follow the randomised allocation of electively transferring one or two embryos. Trials that involved only blastocyst (day five) transfers were excluded because of their higher success rates,19 which could lead to biased results.
Trials were included if the intervention differed only in terms of the intended number of embryos to be transferred—that is, similar protocols were used for controlled ovarian stimulation, embryo culture, and the replacement of embryos.
Types of outcome measures
The primary outcomes were live births and multiple live births after the initial fresh embryo transfer, cumulative live births, and cumulative multiple live births (after fresh and frozen embryo transfers accruing from a single oocyte retrieval).
Secondary outcomes included miscarriage rate and perinatal events such as rates of preterm delivery, term singleton delivery, and delivery of at least one low birthweight baby (<2500 g). The denominator for miscarriage rate was defined by using all pregnancies, including biochemical pregnancy.
Data collection
We sought individual patient data from all identified eligible trials. Every attempt was made to contact the corresponding authors of published and registered trials via post, email, or telephone to access data. Authors were supplied with a data extraction sheet and asked to supply anonymised data for every patient in the trial in a password protected or encrypted database. Received data were merged into a specifically constructed master database. The data were subjected to standard range and internal consistency checks. In addition, the results from the review’s analysis were cross checked against the published reports of the trials. Authors were contacted for clarification where discrepancies existed and asked to supply missing data when possible.
Data items
From each trial we extracted information on the characteristics of the couples (body mass index (BMI), woman’s age, duration, type and cause of infertility, and type of treatment); the characteristics of the cycles (randomised treatment (elective single embryo transfer or double embryo transfer), number of embryos available for transfer, day of transfer, and number of blastomeres); the type of outcome (live birth, multiple live birth, cumulative live birth, cumulative multiple live birth, miscarriage, preterm delivery, term singleton birth, and low birth weight).
Risk of bias in individual studies
To ascertain validity, we assessed all selected trials for their methodological quality on the basis of information reported in the original published papers, trial protocols (for non-published trials), and responses to specific queries to the authors. Study quality was scrutinised by checking the adequacy of randomisation, comparability of groups at baseline (examining baseline characteristics for any substantive differences), the use of blinding (where appropriate), the use of intention to treat analysis, the completeness of follow-up, reliability with a priori sample size estimation, and generalisability with a description of the sample recruited. The adequacy of randomisation was assessed by exploring methods used for sequence generation, treatment allocation, and allocation concealment. When these details were unclear in the initial publication, we contacted authors to provide further clarification.
Statistical analysis
Receipt of data from each trial was followed by checks to ensure the accuracy of the data, integrity of randomisation, and the extent of follow-up. Standard range and internal consistency checks were used to assess accuracy. Final analyses were based on all patients randomised with the intention to treat principle, and the data were checked to confirm that no patients were excluded after trial entry and that key prognostic variables were similar in the randomised groups. Queries were resolved in discussion with the relevant trialists, who received results based on re-analysis of their original trial data.
Trial heterogeneity
We carried out formal checks for statistical heterogeneity between the trials by summarising randomised treatment effects for each trial using odds ratios obtained from the individual patient data. The I2 statistic was applied to these summary data to describe the percentage of variation across trials caused by heterogeneity, as opposed to sampling error. A value greater than 50% was considered to show substantial heterogeneity.20 To assess the risk of bias across trials, we used funnel plots and formally assessed their symmetry using the Horbold-Egger test.
Meta-analysis of individual patient data
On the condition that no trial heterogeneity was present, we carried out a “one stage” meta-analysis of individual patient data. A logistic regression model was fitted describing the effect of elective single versus double embryo transfer on live birth, adjusted for trial. Trial was included as a fixed effect dummy variable that allowed the log odds to vary across the trials. This model was further adjusted separately for duration (years) and primary cause of infertility (male factor, female factor, unexplained, mixed/other), type of infertility (primary or secondary), type of treatment (IVF or intracytoplasmic sperm injection), woman’s age and BMI, number of embryos available for transfer, day of transfer, and quality of embryos transferred. Embryo quality was defined by the number of blastomeres observed, which was the only consistent objective measure used across all the included trials. Grade A was used to describe all embryos that contained four cells on day two after fertilisation or more than six cells on day three after fertilisation. All other embryos were graded as grade B. When two embryos were transferred, the higher grade was included in the model. This classification was informed by data on the association between the number of blastomeres and successful implantation rate.21 22
We included any of the above covariates with P<0.3 in a backward stepwise logistic regression modelling procedure to determine multiple covariates that, together, had a significant effect on the outcome, while holding the trial and elective single versus double embryo transfer effects fixed. A similar process was performed for the other primary and secondary outcomes. The odds ratios and their 95% confidence intervals of all outcomes were calculated from the models based on the intention to treat principle. In the presence of substantial heterogeneity between trials or over-fitting because of small numbers in each trial, we performed sensitivity analyses by excluding the offending trial(s).
Subgroup analyses
We fitted further logistic regression models to investigate the effect of single versus double embryo transfer on live births and multiple live births between different subgroups of women. The subgroups were woman’s age (<33 and ≥33), duration of infertility (<3 and ≥3 years), and embryo quality (grade A and B). Subgroups were coded as binary factors and included in the model along with trial and single versus double embryo transfer. To investigate whether the effect of single versus double embryo transfer on live birth differed among the subgroups, we added an interaction term to the model of single versus double embryo transfer by subgroup factor.
In addition, for singleton live births only, we compared birth weight between single versus double embryo transfer using a linear regression model adjusted for trial. Because of the small percentage of missing data, all analyses were undertaken with complete data only. We used Review Manager (RevMan) v5.0 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen) and SAS v9.1 (SAS Institute, Cary, USA) for statistical analyses.
Results
Study selection
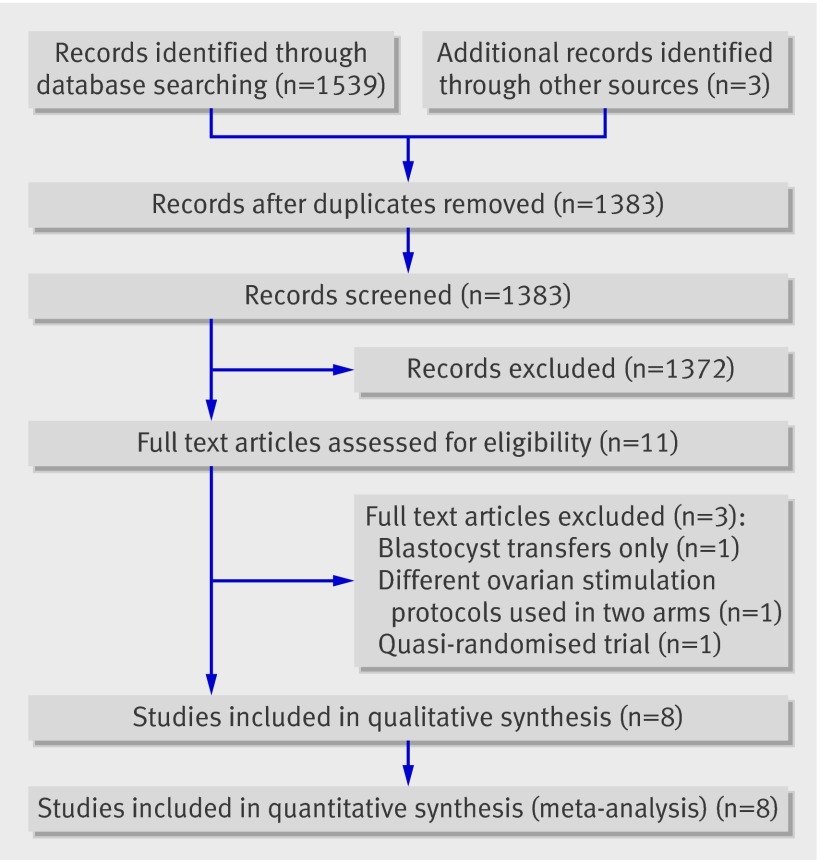
Electronic searches identified 1539 citations, from which we assessed 11 full text articles for eligibility. Three of these trials were excluded for different reasons23 24 25 (table 1). Eight trials were considered to be suitable for inclusion in the review (fig 1). Three were unpublished trials.9 10 One was a pilot trial, which was published only as part of a PhD dissertation.9 The other two were externally funded national trials, in Australia (National Health and Medical Research Council Grant no: 158006) (M Davies, University of Adelaide, personal communication) and the UK.10 The Australian trial, known as the “Australian study of single embryo transfer (ASSET),” was stopped because its implementation immediately and substantially altered patients’ decision making, which more than tripled the rates of elective single embryo transfer during the study period and reduced participation rates (M Davies, University of Adelaide, personal communication). The UK trial, known as the “efficacy and cost effectiveness of selective single embryo transfer (ECOSSE) study” was stopped because of poor recruitment.
Table 1.
Description of identified trials examining effectiveness of single embryo transfer (SET) versus double embryo transfer (DET)
| Authors | Details | Interventions | Clinical outcomes |
|---|---|---|---|
| Included published trials | |||
| Gerris et al, 199929 | First IVF/ICSI cycle. Female age <34. At least one top quality embryo should be available | SET fresh v DET fresh | Clinical ongoing pregnancy (at least one gestational sac with heartbeat), multiple pregnancy, biochemical pregnancy (positive test), implantation, clinical miscarriage (loss of at least one amniotic sac identified at sonography), clinical ectopic pregnancy (required surgical intervention) |
| Lukassen et al, 200527 | First IVF/ICSI cycle. Female age <35. FSH <10I U/L. At least one good quality embryo should be available | SET fresh + SET fresh v DET fresh | Cumulative live birth, singleton live birth, multiple live birth, clinical ongoing pregnancy (at least one gestational sac with heartbeat), abortion, ectopic pregnancy, preterm birth (<37 weeks’ gestation), low birth weight (<2500 g), perinatal death rates and live birth rate after only one treatment cycle |
| Martikainen et al, 200130 | Fresh IVF/ICSI treatment in four centres. No more than one previous failed treatment. Age was not taken into account and first two cycles eligible in two centres. In other two centres, age <36 in first cycle only. At least four good quality embryos should be available | SET fresh v DET fresh | Live birth, singleton live birth, twin live birth, clinical pregnancy (confirmed by ultrasonography), implantation, miscarriage, ectopic pregnancy, preterm birth (<37 weeks’ gestation), low birth weight (<2500 g) rates |
| Thurin et al, 200426 | First or second IVF cycle. At least two good quality embryos available for transfer or freezing. Female age <36 | SET fresh + SET frozen v DET fresh | Cumulative live birth, biochemical pregnancy (positive test), implantation (number of gestational sacs/number of embryos transferred), multiple live birth, spontaneous abortion, ectopic pregnancy |
| Van Montfoort et al, 200628 | First IVF cycle. Participants had to have normal fertilisation of at least two oocytes (two pro-nuclear stage embryos) irrespective of woman’s age or embryo quality | SET fresh v DET fresh | Biochemical pregnancy (positive test), clinical pregnancy (at least one gestational sac with heartbeat at 12 weeks’ gestation), twin pregnancy, abortion |
| Included unpublished data | |||
| Thurin. Elective single embryo transfer [dissertation]. Gothenburg University, 20059 | Female age ≥36 years. First or second IVF/ICSI cycle. At least two good quality embryos available | SET fresh + SET frozen v DET fresh | Cumulative live birth, biochemical pregnancy (positive test), implantation (number of gestational sacs/number of embryos transferred), multiple live birth, spontaneous abortion, ectopic pregnancy |
| Davies M. Australian study of single embryo transfer (ASSET) | Female age <35 if no previous ART pregnancy, <40 if previous ART pregnancy. At least four good quality embryos or at least three if previous ART pregnancy successful | SET fresh versus DET fresh | Cumulative live birth, twin live birth, clinical ongoing pregnancy (fetal heartbeat), complications during pregnancy, delivery and neonatal period, perinatal mortality and morbidity, use of neonatal intensive care |
| Bhattacharya S. Efficacy and cost effectiveness of selective single embryo transfer (ECOSSE)10 | Female age ≤37. First or second IVF/ICSI cycle. At least four good quality embryos at time of embryo transfer | SET fresh + multiple SET frozen v DET fresh + multiple DET frozen | Cumulative live birth, twin live birth, clinical pregnancy (at least one gestational sac with heartbeat), biochemical pregnancy (positive test), miscarriage, ectopic pregnancy preterm delivery, low birth weight, congenital abnormality |
| Excluded trials | |||
| Gardner et al, 200424 | Involved only blastocyst (day 5) transfers | SET fresh v DET fresh | Clinical ongoing pregnancy (at least one gestational sac with heartbeat), twin pregnancy, implantation (number of gestational sacs with heartbeat/number of embryos transferred) |
| Heijnen et al, 200723 | Different ovarian stimulation protocols used in two arms | Mild ovarian stimulation (GnRH) antagonist co-treatment) + SET fresh + SET/DET frozen as per patient’s preference v standard ovarian stimulation (GnRH agonist long protocol) + DET fresh + SET/DET frozen as per patient’s preference. More than one fresh cycle | Cumulative term (≥37 weeks’ gestation) live birth, term singleton live birth, late preterm (32-37 weeks’ gestation) live birth, early preterm (< 32 weeks’ gestation) live birth, clinical pregnancy (positive heartbeat) rates within 1 year of randomisation. Multiple pregnancy. Time to pregnancy leading live birth |
| Moustafa et al, 200825 | Quasi-randomised trial with alternate days used as method of randomisation | SET fresh + multiple SET frozen v DET fresh + multiple DET frozen | Live birth, clinical pregnancy (positive heartbeat), multiple pregnancy. Gestational age at miscarriage, gestational age at birth, birth weight |
IVF=in vitro fertilisation; ISCI=intracytoplasmic sperm injection; FSH=follicle stimulating hormone; SET=single embryo transfer; DET=double embryo transfer; GnRH=gonadotrophin releasing hormone; ART=assisted reproductive technology.
Fig 1 Flow diagram of study selection
Study characteristics
Table 1 describes the characteristics of the eight trials. All compared one fresh cycle of elective single embryo transfer with one fresh double embryo transfer. Additionally, two trials also compared fresh elective single embryo transfer followed by one frozen single embryo transfer cycle with one fresh double embryo transfer,9 26 while one trial compared two fresh elective single embryo transfer cycles versus one fresh double embryo transfer.27
As entry criteria were slightly different among trials, we could not exclude an element of clinical heterogeneity. With the exception of two trials,9 28 all the trials included women with “good prognoses”—that is, younger women in their first or second IVF treatment cycles with good embryo quality. The age criteria were different in the eight included studies. The upper age limit was 34, 35, and 36, respectively, in Gerris et al,29 Lukassen et al,27 and Thurin et al.26 In a multicentre Finnish trial, one of the centres did not take the age of the woman into account, but the other centres included only women aged under 36 in their first treatment cycle.30 There was no upper age limit for entry into van Montfoort et al28 or Thurin,9 while the upper age limits for Davies and Bhattacharya were 35 and 37, respectively.10 All trials included women with good quality embryos, apart from one that included women with at least two normally fertilised oocytes.28 Criteria for embryo quality differed in the individual trials but generally involved visual morphological assessment, in addition to the number of blastomeres.
Ovarian stimulation protocols and oocyte retrieval, embryo culture, and embryo transfer techniques varied slightly among the different trials. Though nearly all trials involved only cleavage stage transfers, one published and one unpublished study included a few blastocyst (day five) transfers,9 26 which we excluded from the analysis because of small numbers.
Individual patient data were received for every patient recruited to all eight eligible trials (n=1367). The received data had varying levels of completeness (see appendix 2 on bmj.com). With the exception of BMI, which was missing for nearly 17%, all required variables were over 95% complete.
Risk of bias within studies
The methodological quality of the included studies varied (table 2). Three of the published trials26 27 30 and all three unpublished trials (M Davies, University of Adelaide, personal communication)9 10 explicitly mentioned computer generated randomisation. One trial used a non-transparent box containing sealed opaque envelopes,28 and one trial did not mention details of the method of randomisation used.29 The first author of the latter trial, however, later confirmed that randomisation was performed at the time of embryo transfer by laboratory staff using a computer generated randomisation list (J Gerris, personal communication).
Table 2.
Quality assessment of studies on single embryo transfer versus double embryo transfer included in meta-analysis
| Target population described adequately | Sample size calculation | Adequate randomisation | Blinding | Intention to treat analysis performed | Two arms comparable at baseline | Follow-up >80% | |
|---|---|---|---|---|---|---|---|
| Published trials (details retrieved from published paper and response from authors) | |||||||
| Gerris et al, 199929 | Yes | No | Not stated in paper. Later confirmed as yes by author | Not stated in paper. Later confirmed as yes by author | Not stated in paper. Later confirmed as yes by author | Yes | Yes |
| Lukassen et al, 200527 | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Martikainen et al, 200130 | Yes | No | Yes | Not stated in paper. Later confirmed as yes by author | Not stated in paper. Later confirmed as yes by author | Yes | Yes |
| Thurin et al, 200426 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Van Montfoort et al, 200628 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Unpublished trials (details retrieved from trial protocols and response from authors) | |||||||
| Thurin A, 20059 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Davies M, 2003 | Yes | Yes | Yes | No | Results not previously analysed | Yes | Yes |
| Bhattacharya S, 200610 | Yes | Yes | Yes | Yes | Results not previously analysed | Yes | Yes |
Concealment of allocation was adequate and explicitly described in Thurin et al,26 Lukassen et al,27 and van Montfoort et al28 but not in Martikainen et al.30 Gerris et al stated only that they used external concealment and gave no further details.29 All the more recent unpublished trials used acceptable methods of concealment of allocation.9 10
Four studies performed intention to treat analysis.9 26 27 28 Although not explicitly mentioned in the original publication, the first authors of two trials29 30 later confirmed that they had performed intention to treat analysis.
Only one of the published studies was double blinded—that is, neither the patient nor the physician knew whether one embryo or two embryos had been transferred.26 Two unpublished trials used double blinding at the time of embryo transfer.9 10 Davies et al enforced double blinding at the time of embryo transfer, but the participants were unblinded when they received photographs of the transferred embryo(s) (M Davies, University of Adelaide, personal communication). In one study only the patients were blinded, but they were told immediately after the transfer whether they received single or double embryo transfer.28 Other trials were not blinded. Thurin et al,26 Lukassen et al,27 and van Montfoort et al28 reported an a priori power calculation; Gerris et al29 and Martikainen et al30 did not.
Results of individual studies
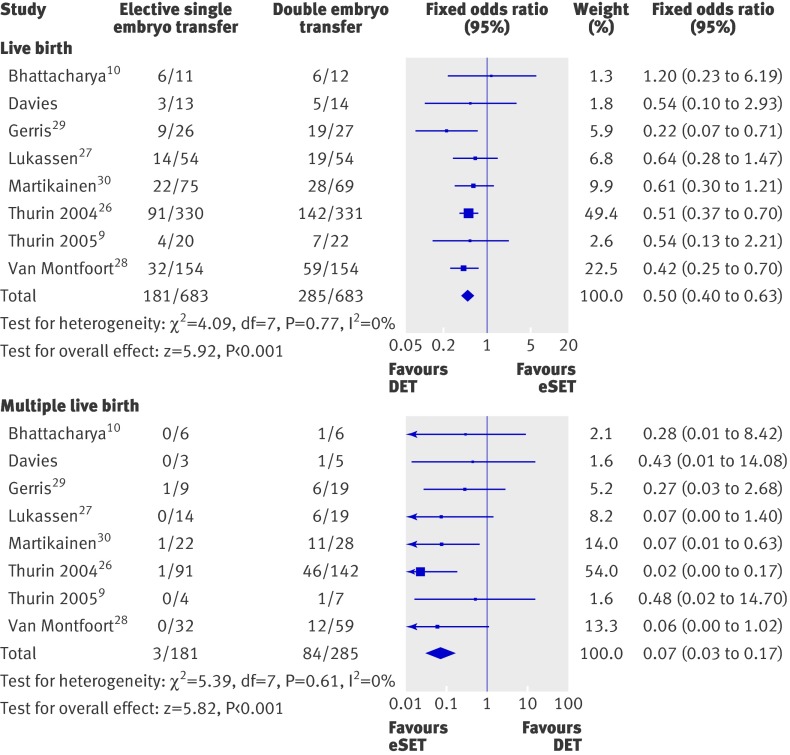
Appendix 3 on bmj.com shows characteristics of the women by trial. Before conducting the analysis of individual patient data, we carried out an aggregated meta-analysis of the individual trials to check for heterogeneity (fig 2). The pooled odds ratio for elective single embryo transfer versus double embryo transfer was 0.50 (95% confidence interval 0.40 to 0.63) for live births and 0.07 (0.03 to 0.17) for multiple live births. The I2 statistic was 0% for both outcomes, suggesting no significant heterogeneity between trials and therefore justified the analysis of individual patient data. The Horbold-Egger test result was not significant for both outcomes, suggesting no significant publication bias.
Fig 2 Odds ratios of elective single embryo transfer v double embryo transfer for separate trials and pooled odds ratios for live births and multiple live births
Synthesis of results
The two randomised groups of women were comparable in terms of baseline characteristics (table 3). In the women, the mean age was 31.3 (SD 3.5) in the single embryo transfer group and 31.4 (SD 3.4) in the double embryo transfer group, and the median BMI was 23.3 (interquartile range 21.3-26.1) and 23.3 (21.1-26.3), respectively. The most common cause of infertility was male factor infertility (41% and 40%, respectively).
Table 3.
Characteristics of couples at randomisation by randomised group: elective single embryo transfer (eSET) or double embryo transfer (DET). Figures are numbers (percentages) unless stated otherwise
| eSET (n=683) | DET (n=684) | |
|---|---|---|
| Mean (SD) age of woman (years) | 31.3 (3.5) | 31.4 (3.4) |
| Missing | 3 (0.4) | 2 (0.3) |
| Median (IQR) BMI of woman | 23.3 (21.3-26.1) | 23.3 (21.1-26.3) |
| Missing | 115 (17) | 110 (16) |
| Type of infertility: | ||
| Primary | 507 (74) | 489 (72) |
| Secondary | 161 (24) | 182 (27) |
| Missing | 15 (2) | 13 (2) |
| Median (IQR) duration of infertility (years) | 3.0 (2.1-4.4) | 3.0 (2.0-4.0) |
| Missing | 11 (1.6) | 10 (1.5) |
| Primary cause of infertility: | ||
| Male | 280 (41) | 276 (40) |
| Female | 170 (25) | 177 (26) |
| Unexplained | 109 (16) | 141 (21) |
| Other/mixed | 122 (18) | 88 (13) |
| Missing | 2 (0.3) | 2 (0.3) |
SD=standard deviation; IQR=interquartile range.
Table 4 summarises details of treatment in the fresh cycle. In both groups the median number of embryos available was five, and most embryo transfers were performed on day two (79% in both groups). The allocated treatment was received by 677/683 (99%) patients in the elective single embryo transfer arm and 676/684 (99%) patients in the double embryo transfer arm. Only one (0.1%) woman was lost to follow-up (in the double embryo transfer group). Thus 683 women in the elective single embryo transfer arm and 683 in the double embryo transfer arm were included in the analysis of primary outcomes.
Table 4.
Characteristics of treatment received by randomised group: elective single embryo transfer (eSET) or double embryo transfer (DET). Figures are numbers (percentages) unless stated otherwise
| eSET (n=683) | DET (n=684) | |
|---|---|---|
| Type of treatment: | ||
| IVF | 365 (53) | 388 (57) |
| Intracytoplasmic sperm injection | 318 (47) | 293 (43) |
| Both | 0 | 2 (0.3) |
| Missing | 0 | 1 (0.1) |
| Median (IQR) No of embryos available for transfer in first fresh cycle | 5 (3-8) | 5 (3-7) |
| Missing | 0 | 2 (0.3) |
| Embryos transferred in first fresh cycle: | ||
| 0 | 2 (0.3) | 1 (0.1) |
| 1 | 677 (99) | 7 (1) |
| 2 | 4 (1) | 676 (99) |
| Missing | 0 | 0 |
| Day of fresh embryo transfer: | ||
| Day 2 | 540 (79) | 539 (79) |
| Day 3 | 130 (19) | 131 (19) |
| Day 5 | 10 (1.5) | 9 (1.3) |
| No transfer | 2 (0.3) | 1 (0.1) |
| Missing | 1 (0.1) | 4 (0.6) |
| Median (IQR) No of blastomeres* | 4 (4-5) | 4 (4-6) |
| Missing | 30 (4) | 32 (5) |
| Grade of embryos transferred*† | ||
| Grade A | 571 (84) | 597 (87) |
| Grade B | 79 (12) | 51 (7) |
| Missing | 31 (5) | 35 (5) |
IQR=interquartile range.
*For DET, embryo with largest number of cells chosen.
†Grade A=four cells transferred on day 2 or more than six cells transferred on day 3; grade B=all others.
Meta-analysis of clinical outcomes with individual patient data
Live birth
After one fresh cycle, the live birth rate in the elective single embryo transfer group (181/683, 27%) was lower than in the double embryo transfer group (285/683, 42%). The odds of a live birth in women randomised to single embryo transfer were half that for women who received a double embryo transfer (table 5). When we did a backward stepwise selection process, the adjusted odds ratio remained similar (adjusted odds ratio 0.50, 95% confidence interval 0.39 to 0.63). A few women 72/1366 (5%) were excluded from this adjusted analysis because of missing values for the covariates. The covariates that were included in this adjusted model were the grade of the embryos transferred, type of treatment, and the primary cause of infertility. The odds of live birth associated with transfer of grade A embryos were significantly higher than for grade B embryos (1.99, 1.26 to 3.13; P=0.003) (table 5). The chance of a live birth was 66% greater after IVF than after intracytoplasmic sperm injection (1.66, 1.15 to 2.38; P=0.006). Compared with male infertility, female infertility was associated with a reduction in the odds of a live birth (0.58, 0.38 to 0.88; P=0.02).
Table 5.
Predictors of live birth after elective single embryo transfer (eSET) and double embryo transfer (DET). Figures are numbers (percentages) unless stated otherwise
| Baseline characteristics | Live birth* | Odds ratio (95% CI) | |||
|---|---|---|---|---|---|
| Yes | No | Crude† | Adjusted‡ | ||
| Randomised treatment: | |||||
| DET | 285/466 (61) | 398/900 (44) | 1 | 1 | |
| eSET v DET | 181/466 (39) | 502/900 (56) | 0.50 (0.40 to 0.63)§ | 0.50 (0.39 to 0.63)§ | |
| Mean (SD) age of woman (years) | 31.0 (3.4) | 31.5 (3.5) | 0.97 (0.94 to 1.01) | — | |
| Median (IQR) BMI of woman | 23.5 (21.2-26.0) | 23.2 (21.2-26.4) | 0.99 (0.96 to 1.02) | — | |
| Median (IQR) duration of infertility (years) | 3.0 (2.0-4.0) | 3.1 (2.1-4.4) | 0.96 (0.90 to 1.02) | — | |
| Type of infertility: | |||||
| Primary | 333/454 (73) | 663/884 (75) | 1 | — | |
| Secondary v primary | 121/454 (27) | 221/884 (25) | 1.03 (0.79 to 1.35) | — | |
| Primary cause of infertility: | |||||
| Male | 187/465 (40) | 368/897 (41) | 1 | 1 | |
| Female v male | 108/465 (23) | 239/897 (27) | 0.86 (0.64 to 1.16) | 0.58 (0.38 to 0.88)¶ | |
| Unexplained v male | 96/465 (21) | 154/897 (17) | 1.17 (0.85 to 1.61) | 0.79 (0.51 to 1.22) | |
| Other/mixed v male | 74/465 (16) | 136/897 (15) | 0.99 (0.69 to 1.41) | 0.77 (0.51 to 1.15) | |
| Type of treatment: | |||||
| ICSI | 190/465 (41) | 420/898 (47) | 1 | 1 | |
| IVF v ICSI | 275/465 (59) | 478/898 (53) | 1.25 (0.99 to 1.59)** | 1.66 (1.15 to 2.38)†† | |
| Day of embryo transfer: | |||||
| Day 2 | 366/465 (79) | 712/893 (80) | 1 | — | |
| Day 3 v day 2 | 95/465 (20) | 166/893 (19) | 1.10 (0.70 to 1.74) | — | |
| Day 5 v day 2 | 4/465 (1) | 15/893 (2) | 0.51 (0.17 to 1.58) | — | |
| Median (IQR) No of embryos available for transfer | 5.0 (4.0-8.0) | 5.0 (3.0-7.0) | 1.03 (1.00 to 1.07) | — | |
| Quality of embryos transferred | |||||
| Grade B | 27/450 (6) | 103/848 (12) | 1 | 1 | |
| Grade A v grade B | 423/450 (94) | 745/848 (88) | 1.93 (1.23 to 3.04)†† | 1.99 (1.26 to 3.13)†† | |
SD=standard deviation; IQR=interquartile range; BMI=body mass index; ISCI=intracytoplasmic sperm injection.
*Means (SD) provided for normally distributed data; medians (IQR) presented for skewed data. Denominator differs because of missing values for some characteristics.
†Estimated from separate logistic models adjusted for eSET v DET and trial.
‡One logistic model resulting from stepwise procedure. Adjusted for eSET v DET, trial, embryo grade, type of treatment, primary cause of infertility.
§P≤0.001.
¶0.01<P≤0.05.
**0.05<P≤0.1.
††0.001<P≤0.01.
Multiple live births
Fewer multiple births occurred after one elective single embryo transfer cycle (3/181, 2%) than after a double embryo transfer cycle (84/285, 29%) with an odds ratio of 0.04 (0.01 to 0.12; P<0.001). No other covariates had a significant effect on multiple live births.
Cumulative live births
Only two trials had an additional frozen single embryo transfer after the initial fresh elective single embryo transfer (both Thurin trials). This resulted in a cumulative live birth rate similar to that after one fresh double embryo transfer (132/350 (38%) v 149/353 (42%); 0.85, 0.62 to 1.15), with a minimal cumulative risk of multiple live birth (1/132 (1%) v 47/149 (32%); 0.02, 0.002 to 0.12).
Miscarriage
The rate of miscarriage in the elective single embryo transfer group (60/245, 24%) was higher than in the double embryo transfer group (63/355, 18%), with an odds ratio of 1.52 (1.01 to 2.28). There was, however, evidence of significant heterogeneity between the trials. When we excluded the two trials that caused the heterogeneity (Davies, 2003; Thurin, 2005) the odds ratio remained similar (1.51, 0.99 to 2.33; P=0.06).
Subgroup analyses
Age group
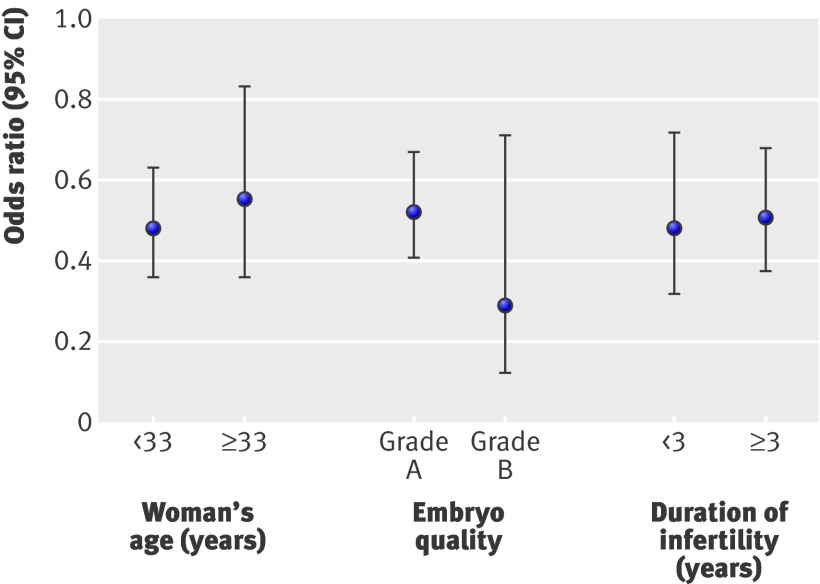
We excluded five (0.4%) women from analysis because of missing data on age. Younger women (<33) had a higher odds of a live birth than older women (≥33) (336/915 (37%) v 130/446 (29%); 1.37, 1.05 to 1.77; P=0.02). As before, the odds of a live birth in women randomised to elective single embryo transfer were half those for women who received a double embryo transfer (0.50, 0.40 to 0.63; P<0.001). The effect of elective single embryo transfer versus double embryo transfer on live birth did not differ between younger (131/461 (28%) v 205/454 (45%)) and older (50/219 (23%) v 80/227 (35%); P=0.60) women. Figure 3 shows the odds ratios for both groups by age .
Fig 3 Odds ratios of live birth for elective single embryo transfer versus double embryo transfer by different subgroups
There was no difference between the odds of multiple live birth for younger versus older women (64/336 (19%) v 23/130 (18%); 1.06, 0.58 to 1.91; P=0.86). As before, the odds of a multiple live birth in women randomised to elective single embryo transfer were significantly smaller than for women who received double embryo transfer (0.04, 0.01 to 0.12; P<0.001). The interaction between elective single versus double embryo transfer and age group could not be fitted in the model as no multiple births occurred in women aged over 32 who received elective single embryo transfer. The rate of multiple live births for elective single versus double embryo transfer, however, were similar for younger women (3/131 (2%) v 61/205 (30%)) and older women (0/50 v 23/80 (29%)).
For embryo grade and duration of infertility subgroup analyses, the odds ratios for elective single versus double embryo transfer were similar to those reported for age group.
Embryo grade
As embryo grade was missing for 68 (5%) women, they could not be included in the analysis. Women who received grade A embryos had higher odds of a live birth than women with grade B embryos (423/1168 (36%) v 27/130 (21%); 1.93, 1.23 to 3.04; P=0.004). The effect of elective single versus double embryo transfer on live birth did not differ between grade A (164/571 (29%) v 259/597 (43%)) and grade B (10/79 (13%) v 17/51 (33%)) embryos (P=0.21) (fig 3).
There was no difference between the odds of multiple live birth for grade A embryos versus grade B embryos (4/27 (15%) v 81/423 (19%); 1.59, 0.49 to 5.17; P=0.44). We could not fit the interaction between elective single versus double embryo transfer and embryo grade in the model because no multiples occurred in women randomised to elective single embryo transfer who had grade B embryos transferred. The rate of multiple live births for elective single versus double embryo transfer, however, were similar for grade A (3/164 (2%) v 78/259 (30%)) and grade B (0/10 v 4/17 (24%)) embryos.
Duration of infertility
Data on duration of infertility was missing for 32 women (2%), who we excluded from this subgroup analysis. Couples with three or more years of infertility had similar odds of a live birth to couples with less than three years infertility (153/460 (33%) v 298/873 (34%); 1.02, 0.79 to 1.32; P=0.87). The effect of elective single versus double embryo transfer on live birth did not differ between shorter (60/234 (26%) v 93/226 (41%)) and longer (115/432 (27%) v 183/441 (42%), P=0.14) duration of infertility (fig 3).
Couples with three or more years of infertility had the same chance of multiple births as couples who experienced less than three years’ infertility (28/153 (18%) v 56/298 (19%); 0.97 (0.55 to 1.72), P=0.91). The effect of elective single versus double embryo transfer on multiple live birth did not differ between shorter term (1/60 (2%) v 27/93 (29%)) and longer term (2/115 (2%) v 54/183 (30%)) duration of infertility (p=0.95). For couples with under three year’s infertility, the odds ratio for multiple live birth rates for eSET versus DET was 0.04 (0.005 to 0.29). In couples who were infertile for three years or more, the odds ratio was similar (0.04, 0.01 to 0.17).
Perinatal outcomes
Table 6 shows perinatal outcomes after elective single versus double embryo transfer. One woman had missing values for all perinatal outcomes and was excluded from this analysis.
Table 6.
Perinatal outcomes after fresh elective single embryo transfer (eSET) versus fresh double embryo transfer (DET) in women with live birth. Figures are numbers (percentages) unless stated otherwise
| eSET (n=181) | DET (n=284) | Odds ratio (95% CI) | ||
|---|---|---|---|---|
| Crude* | Adjusted | |||
| Delivery of at least one low birthweight baby (<2500 g) | 14 (8%) | 69 (24%) | 0.26 (0.14 to 0.48) | 0.36 (0.15 to 0.87)† |
| Term singleton delivery | 158 (87%) | 169 (60%) | 4.93 (2.98 to 8.18) | As crude‡ |
| Preterm delivery (weeks): | ||||
| 24-37 | 23 (13%) | 85 (30%) | 0.33 (0.20 to 0.55)§ | As crude‡ |
| 24-34 | 6 (3%) | 29 (10%) | 0.29 (0.12 to 0.72) | 0.30 (0.12 to 0.75)¶ |
| 24-32 | 1 (<1%) | 16 (6%) | 0.09 (0.01 to 0.70) | 0.08 (0.01 to 0.65)** |
*Estimated from logistic model adjusted for eSET v DET and trial.
†Adjusted for gestational age. Bhattacharya trial excluded as no low birthweight babies.10
‡No significant covariates, so adjusted as for crude odds ratio.
§Bhattacharya trial excluded as no preterm births.10
¶Adjusted for female age. Bhattacharya,10 Gerris et al,29 and Thurin (2005)9 excluded as no preterm births ≤34 weeks.
**Adjusted for female BMI and duration of infertility. Davies, Bhattacharya,10 Gerris et al,29 and Thurin (2005)9 excluded as no preterm births ≤32 weeks.
Birth weight
The odds of delivering at least one low birthweight (<2500 g) baby after elective single embryo transfer were a quarter of those associated with double embryo transfer (table 6). After adjustment for gestational age, this odds ratio rose, only slightly, to a third. For singletons born after elective single or double embryo transfer, the mean birth weights were 3373 g (SD 591 g) and 3275 g (SD 773 g), respectively, and there was no significant difference between them (P=0.18).
Term singleton birth
The odds of a term singleton birth (that is, over 37 weeks) after elective single embryo transfer were almost five times higher than those after double embryo transfer (4.93, 2.98 to 8.18) (table 6).
Preterm birth
Elective single embryo transfer was associated with a significantly reduced risk of preterm birth at ≤37 completed weeks, with an odds ratio of 0.33 (0.20 to 0.55). The odds of preterm births at ≤34 completed weeks for elective single versus double embryo transfer were similar to that for preterm births at ≤37 completed weeks (table 6). Increasing maternal age significantly reduced the odds of a preterm birth at ≤34 completed weeks (adjusted odds ratio 0.86, 0.77 to 0.97).
For very preterm births (≤32 weeks) covariates affecting the odds of this outcome included double embryo transfer, higher female BMI, and longer duration of infertility. For each unit increase in BMI, the odds of a preterm birth at ≤32 weeks increased by 16% (1.16, 1.04 to 1.30; P=0.01), and for each yearly increase in duration of infertility the odds of a preterm birth increased by 52% (1.52, 1.15 to 2.03; P=0.004).
Discussion
Main findings
Term singleton live birth rates after a single fresh cycle of elective single embryo transfer are higher than those associated with double embryo transfer. Compared with double embryo transfer, elective single embryo transfer has a significantly lower risk of preterm birth and delivery of a low birthweight baby. As previously reported in systematic reviews based on aggregated data, elective single embryo transfer in a fresh IVF cycle yields a lower live birth rate than double embryo transfer,8 but this difference is almost completely overcome by an additional fresh or frozen single embryo transfer cycle. The multiple live birth rate after elective single embryo transfer is much lower than that associated with double embryo transfer and comparable with that observed in spontaneous pregnancies.
Strengths and limitations of the review
We carried out an extensive literature search, with no language restrictions, minimising the risk of missing information. Access to data at the level of the patient enabled us to uncover previously unreported data, improve the assessment of study quality, perform rigorous quality checks, standardise outcome measures, and perform intention to treat analysis. We complied with existing guidelines for the reporting of systematic reviews and meta-analyses and were able to provide data on previously unreported perinatal outcomes.15 16
We obtained individual patient data from all eligible trials of cleavage stage elective single versus double embryo transfer in IVF; the overall quality of received data was good and there were few missing values. The quality of the included studies was variable, with some of the earlier papers failing to report key details such as mechanism of randomisation, intention to treat analysis, and a priori power calculation. We subsequently obtained information about these factors by directly communicating with the authors who were part of the collaborative group. These responses suggest that the source of error lay in reporting rather than faulty design. Information regarding the quality of the three unpublished studies was obtained from trial protocols and personal communication with the trialists. These trials had limited sample size (n=93 in total) compared with the published trials, and two of the three were stopped because of poor recruitment. Their inclusion is important, however, as they alleviate systematic bias.31 Detailed information on trial quality allowed us to be strict in our inclusion criteria of potential trials.
Variations in entry criteria and clinical protocols (for ovarian stimulation and embryo culture) among the trials mean that we cannot exclude an element of clinical heterogeneity within this review. Nearly all trials focused on women with “good prognoses” so our findings are not generalisable beyond this group. As most of the trials reported outcomes after a single fresh IVF cycle, cumulative outcomes of fresh followed by frozen single embryo transfer were based on only two Swedish studies.9 26
Meta-analysis of individual patient data
While this type of meta-analysis is more demanding in terms of resources, time, and cooperation than meta-analysis of aggregated patient data,32 there are many benefits. These include the ability to perform consistent data checks and cleaning, having a complete database to analyse all the relevant variables from all relevant trials, the ability to analyse effects at patient level, and collaboration with all the trialists.18 An alternative method to the one stage meta-analysis of individual patient data done in this study could have been the two stage procedure, where each study is analysed separately and the summary estimates combined in an aggregated meta-analysis. We chose the one stage approach because all individual patient data were available from all eight trials, and this allowed for the adjustment of potential confounding factors and the exploration of treatment effects and subgroup interactions.
Interpretation
This study confirms earlier results from aggregated systematic reviews that in a fresh IVF treatment cycle, elective single embryo transfer is associated with a reduced chance of live birth compared with double embryo transfer but that the additional transfer of another frozen single embryo in a successive cycle results in a comparable live birth rate and virtually eliminates the risk of twins.8 33 In the relatively homogeneous population of women who were recruited to these trials (that is, younger women with good quality embryos) elective single embryo transfer in a fresh cycle resulted in lower rates of live birth than double embryo transfer, irrespective of the age of the woman, duration of infertility, and embryo quality. The effect of elective single versus double embryo transfer was similar between the subgroups. However, the numbers of women who belonged to the older age group and received grade B quality embryos were smaller than their opposing subgroups, and this could explain the lack of a significant difference.
Elective single embryo transfer significantly reduced the risk of preterm birth and low birth weight, primarily because of fewer twins. The most appropriate method of reporting outcomes in IVF remains debatable, but many consider term singleton live birth to be a useful measure of describing positive consequences of IVF—that is, offspring who are likely to be healthy at birth.34 We found that the chances of delivering a healthy newborn (term singleton birth) after elective single embryo transfer were higher than those after double embryo transfer. On the other hand, double embryo transfer (because of the higher risks of twins), increased female BMI, and prolonged duration of infertility were associated with very preterm births (≤32 weeks).
Despite the reduced odds of a live birth, the absolute proportion of women who had a baby after a fresh IVF/intracytoplasmic sperm injection cycle with elective single embryo transfer was an acceptable 27%—probably reflecting the entry criteria, which encouraged the inclusion of a population with a favourable prognostic profile. Again, because of the homogeneous nature of the sample, we were unable to identify subgroups of women who were more likely to benefit from elective single embryo transfer and compare them with older women or women with poor quality embryos. The two trials that did recruit older women included too few aged over 36 to make such a comparison possible.9 28 Since the publication of these trials, however, observational data from Scandinavia has confirmed that a policy of elective single embryo transfer has not compromised live birth rates, even for women aged over 36.1 35
We focused on embryo transfers performed at the cleavage stage (two to three days after fertilisation), and the results should be interpreted in light of this. Since the trials included in this meta-analysis were undertaken, many clinics have increased their use of blastocyst transfers (day five after fertilisation). There is still debate on which strategy leads to the best perinatal and neonatal outcomes. A recent study has suggested an increased risk of preterm birth and congenital malformations with blastocyst transfer,36 while early pregnancy loss has been shown to be higher after embryo transfer at the cleavage stage. 37
Implications for practice
Our review should be useful in informing decision making regarding the number of embryos to transfer in IVF. Live birth rates after elective single embryo transfer are high in women with a profile similar to that in the trials included in this systematic review, and a further increase (by means of double embryo transfer) is associated with high rates of preterm birth and low birthweight babies caused by twin pregnancies. Given the opportunity for replacing a single frozen thawed embryo in a subsequent cycle, elective single embryo transfer should thus be the default position.38 It must be emphasised that as nearly all couples recruited to the trials included in this analysis had a good prognostic profile, the results of our meta-analysis are valid only for this group. The entry criteria in these trials ensured that most participants were young, and we were unable to establish the impact of unfavourable prognosticators such as increased female age. When a strategy of elective single embryo transfer is applied in couples with poorer prognosis (for example, in older women), we cannot rule out the possibility that the cumulative live birth rate could shift in favour of double embryo transfer.
Implications for policy
Recruitment data from six of the eight trials included in this study showed that a high total proportion of women (out of those eligible) refused to participate (4270/5788, 74%). These figures were not available for the trials of Davies et al and Thurin (2005). While many centres would consider it unethical to ask couples for their reasons for declining randomisation, one can speculate that in many instances the main reason could be a preference for double embryo transfer.39 Though many clinicians consider multiple pregnancy, which is associated with increased maternal and perinatal morbidity and mortality,1 2 to be the most detrimental effect of assisted reproduction, many infertile couples are not aware of these risks40 and show a clear preference for twins.41 42 43 Many couples who know about the complications associated with twins believe that their personal risk of any adverse outcome is low, while others cannot imagine the effect such potential events would have on their lives.42 43
The safety considerations of assisted reproduction are paramount in the minds of policy makers and clinicians. The HFEA’s concerns about the multiple birth rate with IVF in the UK are echoed by the Society of Obstetricians and Gynaecologists of Canada and the Canadian Fertility and Andrology Society, who have highlighted the need to avoid iatrogenic twins.7 44 The latter also recommended that emphasising healthy singleton live birth as the measure of success “may be beneficial in promoting a reduction in the number of embryos transferred.”44
Our results show that, despite a small reduction of live birth rates in a fresh IVF cycle, elective single embryo transfer does lower the risk of preterm birth compared with double embryo transfer. After an additional fresh or frozen cycle, there is no significant difference in live birth rate between elective single and double embryo transfer and the risk of a multiple birth in the elective single embryo transfer group is still much lower than in the double embryo transfer group. This strategy involves an acceptance of the need to undergo multiple cycles, involving fresh followed by frozen embryo transfers, to achieve similar rates of live birth. The associated inconvenience and stress associated with this should, of course, be taken into account. As some of these additional cycles are frozen embryo replacement cycles, not requiring ovarian stimulation and oocyte retrieval (that is, surgery to harvest the eggs), however, many would consider that this is a relatively small price to pay compared with the long term consequences of preterm birth.45 Furthermore, a study by Thurin Kjellberg et al showed that one fresh cycle elective single embryo transfer followed by one frozen single embryo transfer was more cost effective than one fresh double embryo transfer from both a healthcare and societal perspective.3
Implications for research
Further trials of elective single versus double embryo transfer should focus on women with different prognostic profiles, such as older women. In addition, existing national databases should be examined to identify which women and embryos could benefit from a policy of single embryo transfer. Although costly and time consuming compared with aggregated data meta-analysis, meta-analysis of individual patient data allows the most definitive synthesis of available data and should be promoted in research in reproductive health.
What is already known on this topic
Rising rates of multiple pregnancies with in vitro fertilisation, which are associated with increased maternal and perinatal morbidity and mortality, are a concern
Elective single embryo transfer results in fewer multiple pregnancies than double embryo transfer but lowers the chance of live birth
Evidence of the effect of single versus double embryo transfer in different subgroups of women and on secondary outcomes, such as miscarriage, preterm delivery, and low birth weight, has been lacking
What this study adds
This meta-analysis of individual patient data from clinical trials in infertility and assisted reproduction shows that elective single embryo transfer results in increased rates of term singleton live birth and reduces the risk of preterm birth and low birth weight
We thank all authors of the original published trials. Gerris trial: Jan Gerris, Diane de Neubourg, Kathelijne Mangelschots, Eric Van Royen, Muriel Van de Meerssche, and Marion Valkenburg; Lukassen trial: H G M Lukassen, D D Braat, Alex M M Wetzels, Gerhard A Zielhuis, Eddy M M Adang, Eduard Scheenjes, and Jan A M Kremer; Martikainen trial: Hannu Martikainen, Aila Tiitinen, Candido Tomás, Juha Tapanainen, Mauri Orava, Leena Tuomivaara, Sirpa Vilska, Christel Hydén-Granskog, Outi Hovatta, and the Finnish ET Study Group; Thurin trial: Ann Thurin, Jon Hausken, Torbjörn Hillensjö, Barbara Jablonowska, Anja Pinborg, Annika Strandell, and Christina Bergh; Van Montfoort trial: Aafke PA van Montfoort, Audrey A A Fiddelers, J Marij Janssen, Josien G Derhaag, Carmen D Dirksen, Gerard A J Dunselman, Jolande A Land, Joep P M Geraedts, Johannes L H Evers, and John CM Dumoulin.
We also thank J Wang (ASSET trial) and S Keay, R Gazvani, Y Sajjad, H Lyall, M Jamieson, V Sandison, and P Braude (ECOSSE trial), Maureen Porter for her significant contribution to the ECOSSE trial, and all participants of the original trials that provided the data for this systematic review.
Contributors: SB and BWM conceived the idea for the review. KH with SB and BWM developed the protocol with input from all the authors. KH and SB carried out literature searches and retrieved the identified papers. BWM acted as the third reviewer if consensus could not be reached between KH and SB. KH developed the master database and performed the initial statistical analysis. DJMcL developed the master database, performed the statistical analysis, and wrote the initial draft of the manuscript and all subsequent drafts with input from all coauthors. DJMcL is guarantor. All authors had full access to all of the data and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This review was funded by the Wellcome Trust (Research Leave award for SB). The study funder did not participate in study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. All researchers were independent from the funder.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work apart from the funding from the Wellcome Trust (mentioned above); MJD received a research grant from the National Health and Medical Research Council, DdeN is secretary of the VWRG (Reproductive Medicine subgroup of the Flemish Gynaecological society) and received travel and accommodation sponsored by Schering-Plough for the 6th world congress on ovulation induction; with the exception of DJM, KH, BWM, and AMvanP, all authors were involved in the conduct of the primary randomised trials included in this systematic review.
Ethical approval: Advice regarding ethical approval to conduct this meta-analysis was sought from the local ethics committee of the centre (Aberdeen) where IPD analysis of anonymised data was performed. The North of Scotland Research Ethics Service deemed that a formal ethics application was unnecessary. Where necessary, local ethics approval to share anonymised trial data was obtained by participating trial groups.
Data sharing: No additional data available.
Cite this as: BMJ 2010;341:c6945
Web Extra. Extra material supplied by the author
Appendix 1: Search strategy used for identification of studies
Appendix 2: Amount of missing data by randomised group
Appendix 3: Characteristics of couples at randomisation by trial
References
- 1.Bergh C. Single embryo transfer: a mini review. Hum Reprod 2005;20:323-7. [DOI] [PubMed] [Google Scholar]
- 2.Gerris JM. Single embryo transfer and IVF/ICSI outcome: a balanced appraisal. Hum Reprod Update 2005;11:105-21. [DOI] [PubMed] [Google Scholar]
- 3.Thurin Kjellberg A, Carlsson P, Bergh C. Randomized single versus double embryo transfer: obstetric and paediatric outcome and a cost-effectiveness analysis. Hum Reprod 2006;21:210-6. [DOI] [PubMed] [Google Scholar]
- 4.De Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, et al, and The European IVF-monitoring (EIM) Consortium, for the European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2006: results generated from European registers by ESHRE. Hum Reprod 2010;25:1851-62. [DOI] [PubMed] [Google Scholar]
- 5.Vilska S, Tiitinen A, Hyden-Granskog C, Hovatta O. Elective transfer of one embryo results in an acceptable pregnancy rate and eliminates the risk of multiple birth. Hum Reprod 1999;14:2392-5. [DOI] [PubMed] [Google Scholar]
- 6.El-Toukhy T, Khalaf Y, Braude P. IVF results: optimize not maximize. Am J Obstet Gynecol 2006;194:322-31. [DOI] [PubMed] [Google Scholar]
- 7.Human Fertility and Embryology Authority. Multiple births and single embryo transfer review. 2009. www.hfea.gov.uk/530.html.
- 8.Pandian Z, Bhattacharya S, Ozturk O, Serour G, Templeton A. Number of embryos for transfer following in-vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev 2009;2:CD003416. [DOI] [PubMed] [Google Scholar]
- 9.Thurin A. Elective single embryo transfer. [Dissertation.] Gothenburg University, 2005.
- 10.Bhattacharya S. Efficacy and cost-effectiveness of selective single embryo transfer. 2006. www.controlled-trials.com/isrctn/pf/86466058.
- 11.Roberts SA, Fitzgerald CT, Brison DR. Modelling the impact of single embryo transfer in a national health service IVF programme. Hum Reprod 2009;24:122-31. [DOI] [PubMed] [Google Scholar]
- 12.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 1993;341:418-22. [DOI] [PubMed] [Google Scholar]
- 13.Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof 2002;25:76-97. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.0.2. Cochrane Collaboration, 2009.
- 15.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 1999;354:1896-900. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmonds MC, Higgins JPT, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials 2005;2:209-17. [DOI] [PubMed] [Google Scholar]
- 18.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. John Wiley, 2009.
- 19.Blake D, Farquhar C, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev 2007;4:CD002118. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holte J, Berglund L, Milton K, Garello C, Gennarelli G, Revelli A, et al. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod 2007;22:548-57. [DOI] [PubMed] [Google Scholar]
- 22.Van Royen E, Mangelschots K, de Neubourg D, Laureys I, Ryckaert G, Gerris J. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod 2001;16:326-32.11157828 [Google Scholar]
- 23.Heijnen EMEW, Eijkemans MJC, De Klerk C, Polinder S, Beckers NGM, Klinkert ER, et al. A mild treatment strategy for in-vitro fertilisation: a randomised non-inferiority trial. Lancet 2007;369:743-9. [DOI] [PubMed] [Google Scholar]
- 24.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril 2004;81:551-5. [DOI] [PubMed] [Google Scholar]
- 25.Moustafa MK, Sheded SA, El Aziz Mousta MA. Elective single embryo transfer versus double embryo transfer in assisted reproduction. Reprod Biomed Online 2008;17:82-7. [DOI] [PubMed] [Google Scholar]
- 26.Thurin A, Hausken J, Hillensjö T, Jablonowska B, Pinborg A, Strandell A, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med 2004;351:2392-402. [DOI] [PubMed] [Google Scholar]
- 27.Lukassen HG, Braat DD, Wetzels AM, Zielhuis GA, Adang EMM, Scheenjes E, et al. Two cycles with single embryo transfer versus one cycle with double embryo transfer: a randomized controlled trial. Hum Reprod 2005;20:702-8. [DOI] [PubMed] [Google Scholar]
- 28.Van Montfoort AP, Fiddelers AA, Janssen JM, Derhaag JG, Dirksen CD, Dunselman GAJ, et al. In unselected patients, elective single embryo transfer prevents all multiples, but results in significantly lower pregnancy rates compared with double embryo transfer: a randomized controlled trial. Hum Reprod 2006;21:338-43. [DOI] [PubMed] [Google Scholar]
- 29.Gerris J, de Neubourg D, Mangelschots K, Van Royen E, Van de Meerssche M, Valkenburg M. Prevention of twin pregnancy after in-vitro fertilization or intracytoplasmic sperm injection based on strict embryo criteria: a prospective randomized clinical trial. Hum Reprod 1999;14:2581-7. [DOI] [PubMed] [Google Scholar]
- 30.Martikainen H, Tiitinen A, Tomás C, Tapanainen J, Orava M, Tuomivaara L, et al. One versus two embryo transfer after IVF and ICSI: a randomized study. Hum Reprod 2001;16:1900-3. [DOI] [PubMed] [Google Scholar]
- 31.Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev 2009;1:MR000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyman GH, Kuderer NM. The strengths and limitations of meta-analyses based on aggregate data. BMC Med Res Methodol 2005;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelbaya TA, Tsoumpou I, Nardo LG. The likelihood of live birth and multiple birth after single versus double embryo transfer at the cleavage stage: a systematic review and meta-analysis. Fertil Steril 2010;94:936-45. [DOI] [PubMed] [Google Scholar]
- 34.Min JK, Breheny SA, MacLachlan V, Healy DL. What is the most relevant standard of success in assisted reproduction? The singleton, term gestation, live birth rate per cycle initiated: the BESST endpoint for assisted reproduction. Hum Reprod 2004;19:3-7. [DOI] [PubMed] [Google Scholar]
- 35.Karlström PO, Bergh C. Reducing the number of embryos transferred in Sweden—impact on delivery and multiple birth rates. Hum Reprod 2007;22:2202-7. [DOI] [PubMed] [Google Scholar]
- 36.Källén B, Finnstrom O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Blastocyst versus cleavage stage transfer in in vitro fertilization: differences in neonatal outcome? Fertil Steril 2010;94:1680-3. [DOI] [PubMed] [Google Scholar]
- 37.Papanikolaou EG, Camus M, Fatemi HM, Tournaye H, Verheyen G, van Steirteghem A, et al. Early pregnancy loss is significantly higher after day 3 single embryo transfer than after day 5 single blastocyst transfer in GnRH antagonist stimulated IVF cycles. Reprod Biomed Online 2006;12:60-5. [DOI] [PubMed] [Google Scholar]
- 38.Veleva Z, Karinen P, Tomas C, Tapanainen JS, Martikainen H. Elective single embryo transfer with cryopreservation improves the outcome and diminishes the costs of IVF/ICSI. Hum Reprod 2009;24:1632-9. [DOI] [PubMed] [Google Scholar]
- 39.Porter M, Bhattacharya S. Investigation of staff and patients’ opinions of a proposed trial of elective single embryo transfer. Hum Reprod 2005;20:2523-30. [DOI] [PubMed] [Google Scholar]
- 40.Ryan GL, Zhang SH, Dokras A, Syrop CH, van Voorhis BJ. The desire of infertile patients for multiple births. Fertil Steril 2004;81:500-4. [DOI] [PubMed] [Google Scholar]
- 41.Maheshwari A, Griffiths S, Bhattacharya S. Global variations in the uptake of single embryo transfer. Hum Reprod Update 2010. July 15, epub ahead of print. [DOI] [PubMed]
- 42.Murray S, Shetty A, Rattray A, Taylor V, Bhattacharya S. A randomized comparison of alternative methods of information provision on the acceptability of elective single embryo transfer. Hum Reprod 2004;19:911-6. [DOI] [PubMed] [Google Scholar]
- 43.Ryan GL, Sparks AET, Sipe CS, Syrop CH, Dokras A, van Voorhis BJ. A mandatory single blastocyst transfer policy with educational campaign in a United States IVF program reduces multiple gestation rates without sacrificing pregnancy rates. Fertil Steril 2007;88:354-60. [DOI] [PubMed] [Google Scholar]
- 44.Min JK, Claman P, Hughes E. Guidelines for the number of embryos to transfer following in vitro fertilization. Int J Gynecol Obstet 2008;102:203-16. [DOI] [PubMed] [Google Scholar]
- 45.Templeton A. Avoiding multiple pregnancies in ART. Replace as many embryos as you like—one at a time. Hum Reprod 2000;15:1662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Search strategy used for identification of studies
Appendix 2: Amount of missing data by randomised group
Appendix 3: Characteristics of couples at randomisation by trial