Abstract
Objective: There is a paucity and inconsistency of data regarding the natural history of patients affected by idiopathic dilated cardiomyopathy (IDCM) and atrial fibrillation (AF). We examined the prognostic implications of AF in a subset of patients with IDCM.
Methods: We analyzed the data of 539 patients with IDCM enrolled in the Heart Muscle Disease Registry of Trieste.
Results: At baseline, 52 (9.6%) of 539 patients had AF. There was no difference in survival of patients with either AF or sinus rhythm at enrollment (P=.28). During long-term follow-up (90±58 months), AF was detected on ECG/ECG-Holter monitoring in 28 (5.7%) of 487 patients in sinus rhythm at baseline. Predictors of new onset of AF at multivariate analysis were a more dilated left atrium (OR 1.35, 95% CI 1.06–1.72; P=.01) and a lower left ventricle ejection fraction (for 10% decrease, OR 2.41, 95% CI 1.24–4.69, P=.016). Patients developing AF had higher mortality/heart transplantation rate compared to patients who maintained sinus rhythm during follow-up (P<.001). At multivariate analysis, new onset AF (HR 3.67, 95% CI 2.07–6.5; P<.001) in the first three years after diagnosis, but not baseline AF, was found to be independently associated with a worse outcome.
Conclusions: Atrial fibrillation is relatively frequent in patients with IDCM. The early development of AF during follow-up, but not its presence at baseline, is associated with poor survival.
Keywords: Atrial fibrillation, Dilated cardiomyopathy, Heart failure, Prognosis
Atrial fibrillation (AF) and heart failure are very closely linked.1 Atrial fibrillation is the most common sustained arrhythmia in patients with heart failure.2 Patients with heart failure have a five to ten-fold greater probability of developing this arrhythmia than those without heart failure.3–5
To what extent the presence and development of this arrhythmia retains a prognostic role in patients with heart failure remains debatable.6–13 To date, few studies have evaluated the predictive value of new onset AF in heart failure populations; the results of which have been contradictory.7,9–11,14–17
Analyses specifically exploring the role of AF in the subset of patients affected by idiopathic dilated cardiomyopathy (IDCM) are even more scarce and report highly conflicting data.18–20 Most of these studies lack information about the natural history of AF in patients who are optimally managed with angiotensin-converting enzyme (ACE) inhibitor and beta-blocker therapy. Thus, we sought to examine the prevalence, incidence, risk factors for AF development, and long-term prognostic implications of this arrhythmia in a large, single center, well-characterized population of young patients with IDCM on optimal therapy.
Methods
Subjects
Data were obtained from the Heart Muscle Disease Registry of Trieste, Italy, a single-center database. This Registry was developed by the Department of Cardiology in Trieste, an established Italian referral center for screening, diagnosis, and treatment of IDCM. All patients signed informed consent prior to enlistment in the Registry. This study conformed to the principles of the Declaration of Helsinki and was approved by the ethics committee of the University Hospital “Ospedali Riuniti” Trieste, Italy.
From January 1988 to April 2006, 539 consecutive patients with IDCM (with/without heart failure symptoms) were prospectively enlisted in the Registry. The diagnosis of IDCM was made according to World Health Organization criteria.21 Accordingly, patients with left ventricular (LV) systolic dysfunction (left ventricular ejection fraction [LVEF] <50%) in the absence of any other known cardiac disease were included in the Registry. Patients were excluded in the presence of LV dysfunction secondary to one of the following: hypertension (>160/100 mmHg), significant coronary artery disease, history of alcohol abuse (>100g alcohol/day), tachycardia-induced cardiomyopathy, cor pulmonale, diseases of the pericardium, or congenital heart diseases.
All patients underwent complete evaluation at their first visit in the Cardiology Department of Trieste, including accurate clinical history, physical examination, blood sampling for laboratory tests, 12-lead electrocardiogram (ECG), standard chest radiograph, 24-hour Holter monitoring, echocardiogram, exercise stress test, and coronary angiogram. Until 1996, patients routinely underwent endomyocardial biopsy to exclude active myocarditis (according to the “Dallas criteria”22). More recently, myocardial biopsy was performed only in patients with recent onset of heart failure and/or clinical history suggesting active myocarditis. All patients were initially stabilized on treatment with diuretics and ACE inhibitors (maximum tolerated doses). Beta blockers were then added (initially metoprolol, later carvedilol, and more recently bisoprolol). Patients had serial follow-up evaluation at the heart failure outpatient clinic of the Cardiology Department of Trieste at 6, 12, and 24 months, and subsequently every two years, or more frequently if clinically indicated.
From 1991, treatment with oral anticoagulants was recommended to patients with permanent or recurrent episodes of paroxysmal/persistent AF. Starting in 1998 implantable cardioverter defibrillators (ICDs) were implanted as primary prevention in all high-risk patients with IDCM (defined as severe systolic dysfunction [LVEF <35%] and New York Heart Association [NYHA] class II or III, despite the optimal medical therapy), in accordance with the results of the trials of ICD implantation in secondary prevention23–25 and on the empiric data of our clinical experience with patients with IDCM.
Definitions and End-Points
Atrial fibrillation was diagnosed using standard electrocardiographic criteria: irregular undulation of the baseline, generally associated with an irregular ventricular response. All patients with AF at baseline ECG or ECG-Holter monitoring were included in the baseline AF group. Patients in stable sinus rhythm at study enrollment who subsequently developed AF during follow-up (documented on ECG/ECG-Holter monitoring performed on programmed follow-up visits or with symptoms suggesting arrhythmias) were considered to have new onset AF. Patients with spontaneous restoration of sinus rhythm were considered as patients with paroxysmal AF. Patients with restoration of sinus rhythm obtained by pharmacological/electrical cardioversion were considered to have persistent AF. Patients with AF who underwent one or more unsuccessful attempts of pharmacological/electrical cardioversion to restore sinus rhythm were considered as patients with permanent AF.
The onset of heart failure was defined by the onset of heart failure symptoms. Patients who were asymptomatic at the first evaluation in our clinic, without a history of heart failure symptoms and with LV systolic dysfunction at echocardiogram, were considered as patients affected by IDCM in NYHA class I. We considered patients with a previous episode of acute heart failure or receiving diuretics at index evaluation in our center as patients with a history of heart failure. Body mass index (BMI) was estimated using the standard formula (body weight in kilograms divided by squared height in meters).
We analyzed the impact of AF present at baseline or developed during follow-up on all cause mortality/urgent heart transplantation. For the purpose of our study, urgent heart transplantation was considered in status I, indicated in patients with refractory heart failure needing inotropic treatment and/or mechanical support of circulation.
Statistical Analysis
Continuous variables are reported as mean ± standard deviation. Nominal data are reported as counts and percentages. Continuous variables were compared across the groups by analysis of variance (ANOVA). Chi-square test with Yates’ correction for continuity when necessary was used for the comparison of binary variables. A two-tailed P<.05 was considered statistically significant for all test results. To assess the relationship between AF at enrollment and the long-term outcome, event-free survival curves were plotted by using the Kaplan-Meier method and the Log-Rank test for differences in survival was applied.
Factors associated with new onset AF were evaluated with logistic multivariable regression analysis, with a backward stepwise selection procedure. Cox proportional hazards multivariable model was used to evaluate the relationship between clinical and instrumental data of all patients and long-term outcome. The following covariates (selected with backward stepwise selection procedure) were included in the model: age, gender, history of diabetes mellitus, BMI, NYHA class, heart failure duration, glomerular filtration rate, creatinine, hemoglobin, baseline AF, left bundle branch block, LVEF, indexed left atrial area, indexed LV end diastolic volume, moderate to severe mitral regurgitation, restrictive filling pattern, and treatment medications such as ACE inhibitors and beta blockers. The same model was fitted for patients in sinus rhythm at enrollment, substituting baseline AF with new onset AF. Proportional hazard assumption for this variable was checked by plotting corresponding Schoenfeld residuals against fitted time and with the Grambsh and Therneau test.26 New onset AF considered as a time-dependent covariate was then adjusted for other significant variables (also considered as time dependent) in the Cox regression model. The effect of new onset AF on outcome was also investigated by a landmark analysis. According to this method, the follow-up time was divided into several periods of one year starting from the time of the first “new AF.” Patient vital status/heart transplantation was assessed at the beginning of each period. This approach provides a general trend of the adjusted association between the independent variable (new AF) and dependent variable (death/urgent heart transplantation) over time.
All analyses were performed using software SPSS Statistical Package for Windows, Release 10.0 and R statistical package version 2.5.0.
Results
Patient Population
Table 1▶ summarizes the baseline characteristics of patients in the entire study cohort. At enrollment 52 patients (9.6%) in our study population had AF (1 patient had persistent AF; the remaining 51 had permanent AF). Patients with AF were more likely to be males, slightly older, had more dilated left atrium and less dilated left ventricle. Among patients with or without AF there were no differences in terms of comorbidities, treatment with ACE inhibitors (89% vs. 91%, P=.33), beta blockers (79% vs. 82%, P=.2), aldosterone antagonists (25% vs. 16.4%, P=.1), calcium channel blockers (0% vs. 1.6%, P=.3), and ICD implantation in primary prevention (3.8% vs. 9.4%, P=.2). Patients with AF at baseline were more frequently receiving digoxin (88.5% vs. 68%, P=.002). Most patients with baseline AF (39 [75%] of 52 patients) were treated with oral anticoagulants. The remaining 13 patients (25%) were not receiving oral anticoagulants because they were enrolled before 1990 (5 patients; 38%), were non-compliant (1 patient; 8%), or because they had a single episode of AF (7 patients; 54%).
Table 1.
Baseline characteristics by the presence of AF.
| All (n=539) | Sinus rhythm (n=487, 90.4%) | Atrial fibrillation (n=52, 9.6%) | P* | |
|---|---|---|---|---|
| Age (years) | 46±13 | 46 ±13 | 50±12 | .03 |
| Male sex (%) | 73 | 72 | 87 | .02 |
| Weight (kg) | 76±14 | 75±14 | 79±14 | .06 |
| Height (cm) | 171±9 | 171±9 | 173±10 | .12 |
| BSA (cm2) | 1.87±0.2 | 1.87±0.2 | 1.93±0.19 | .04 |
| BMI (kg/m2) | 26 ± 4 | 26 ± 4 | 27 ± 4 | .15 |
| Heart Failure duration (months) | 13±25 | 12±24 | 16±29 | .3 |
| Charlson score | 0.4±0.8 | 0.4±0.8 | 0.5±0.8 | .52 |
| History of diabetes (%) | 9 | 9 | 12 | .5 |
| History of mild hypertension (%) | 23 | 23 | 25 | .8 |
| Heart Rate (bpm) | 78±15 | 78±15 | 79±15 | .56 |
| NYHA III–IV(%) | 25 | 24 | 27 | .7 |
| LBBB (%) | 34 | 35 | 20 | .03 |
| Indexed LA diameter (mm/m2) | 21±4 | 21±4 | 22±5 | .08 |
| Indexed LA area (cm2/m2) | 14±4 | 14±4 | 16±4 | <.001 |
| LVEF (%) | 30±9 | 30±9 | 33±9 | .1 |
| LVESDI (mm/m2) | 31±6 | 31±6 | 27±5 | <.001 |
| LVEDDI (mm/m2) | 36±5 | 37±5 | 33±4 | <.001 |
| LVESVI (ml/m2) | 75±35 | 77±35 | 59±25 | <.001 |
| LVEDVI (ml/m2) | 105±38 | 107±39 | 84±28 | .001 |
| MR (grade II–IV) (%) | 39 | 39 | 37 | .7 |
| Restrictive LV filling pattern (%) | 29 | 28 | 36 | .4 |
*P-value: difference between patients in atrial fibrillation and sinus rhythm.
BMI=Body Mass Index; BSA=Body Surface Area; LA=Left Atrium; LBBB=Left Bundle Branch Block; LV=Left Ventricular; LVEF=Left Ventricular Ejection Fraction; LVEDDI=Indexed Left Ventricular End Diastolic Diameter; LVESDI=Indexed Left Ventricular End Systolic Diameter; LVEDVI=Indexed Left Ventricular End Diastolic Volume; LVESVI=Indexed Left Ventricular End Systolic Volume; MR=Mitral Regurgitation; NYHA=New York Heart Association Class.
Development of AF during the Study
Twenty-eight (5.7%) of 487 patients in sinus rhythm at baseline developed new AF in the course of the study. The rate of occurrence of AF was 0.8% per year and was equally distributed during follow-up. Ten patients (36%) with new onset AF, after one or more attempts to convert to sinus rhythm, remained in permanent AF. At baseline, patients who would later develop AF were in a more advanced NYHA class and had more severe LV systolic and diastolic dysfunction (table 2▶) compared to patients who maintained sinus rhythm. Patients with new onset AF and those maintaining sinus rhythm throughout the study course were equally treated with ACE inhibitors (89% vs. 90%, P=.84), beta blockers (79% vs. 83%, P=.55), aldosterone antagonists (28.6% vs. 15.7%, P=.07) and calcium channel blockers (0% vs. 1.7%, P=.5). Patients with new onset AF were more frequently treated with digoxin (85.7% vs. 66.9%, P=.03) and ICD implantation in primary prevention (25% vs. 8.5%, P=.01).
Table 2.
Baseline characteristics of patients in sinus rhythm at enrollment by the development of AF during the follow-up.
| All (n=487) | Sinus rhythm (n=459, 94.3%) | Atrial fibrillation (n=28, 5.7%) | P* | |
|---|---|---|---|---|
| Age (years) | 46 ±13 | 46±13 | 48±15 | .423 |
| Male sex (%) | 72 | 71 | 82 | .205 |
| Weight (kg) | 75±14 | 75±14 | 82±16 | .019 |
| Height (cm) | 171±9 | 171±9 | 175±7 | .024 |
| BSA (cm2) | 1.87±0.2 | 1.86±0.20 | 1.96±0.19 | .011 |
| BMI (kg/m2) | 26 ± 4 | 25.6±3.9 | 26.7±4.7 | .164 |
| Heart Failure duration (months) | 12±24 | 12±24 | 14±27 | .609 |
| Charlson score | 0.4±0.8 | 0.37±0.76 | 0.5±0.84 | .377 |
| History of diabetes (years) | 9 | 9 | 7 | .729 |
| History of mild hypertension (%) | 23 | 24 | 15 | .299 |
| Heart Rate (bpm) | 78±15 | 77±15 | 82±13 | .109 |
| NYHA III–IV (%) | 24 | 23 | 43 | .018 |
| LBBB (%) | 35 | 35 | 39 | .635 |
| Indexed LA diameter (mm/m2) | 21±4 | 21±4 | 22±5 | .138 |
| Indexed LA area (cm2/m2) | 14±4 | 13±4 | 16±5 | .002 |
| LVEF (%) | 30±9 | 30±9 | 26±11 | .029 |
| LVESDI (mm/m2) | 31±6 | 31±6 | 33±6 | .115 |
| LVEDDI (mm/m2) | 37±5 | 37±5 | 37±5 | .658 |
| LVESVI (ml/m2) | 77±35 | 76±35 | 87±46 | .143 |
| LVEDVI (ml/m2) | 107±39 | 107±38 | 113±50 | .400 |
| MR (grade II–IV) (%) | 39 | 38 | 64 | .010 |
| Restrictive LV filling pattern (%) | 28 | 26 | 57 | .002 |
*P-value: difference between patients in atrial fibrillation and sinus rhythm.
BMI=Body Mass Index; BSA=Body Surface Area; LA=Left Atrium; LBBB=Left Bundle Branch Block; LV=Left Ventricular; LVEF=Left Ventricular Ejection Fraction; LVEDDI=Indexed Left Ventricular End Diastolic Diameter; LVESDI=Indexed Left Ventricular End Systolic Diameter; LVEDVI=Indexed Left Ventricular End Diastolic Volume; LVESVI=Indexed Left Ventricular End Systolic Volume; MR=Mitral Regurgitation; NYHA=New York Heart Association Class.
At multivariate logistic regression analysis, the independent predictors for new AF were a more dilated left atrium (for every 2 cm2/m2 increase of the indexed left atrial area [OR 1.35, 95% CI 1.06–1.72, P=.01]), and a lower LVEF (for every 10% decrease, OR 2.41, 95% CI 1.24–4.69, P=.016).
Survival Analysis
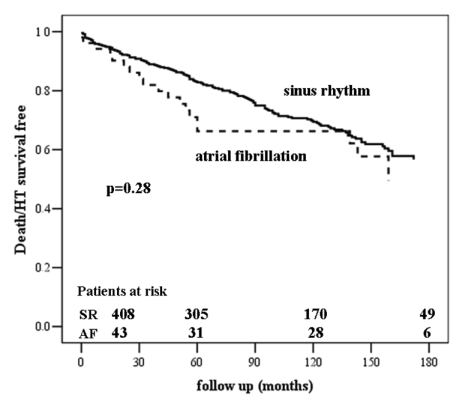
During a mean follow-up of 90±58 months, 156 (28.9%) of 539 patients with IDCM died or underwent urgent heart transplantation in status I; 137 (28%) of 487 patients in sinus rhythm, and 19 (36.5%) of 52 patients with AF (P=.28) (figure 1▶). In a Cox proportional hazards model including all 539 patients, baseline AF was not independently associated with death/urgent heart transplantation (table 3▶).
Figure 1.
All-cause mortality/urgent heart transplantation by baseline atrial fibrillation.
Table 3.
Multivariate predictors for death/urgent heart transplantation for all study population.
| HR | 95% CI | P | |
|---|---|---|---|
| Male sex | 1.73 | 1.13–2.67 | .014 |
| HF duration (for 12 months increase) | 1.10 | 1.06–1.14 | .002 |
| NYHA class (III–IV vs. I–II) | 1.88 | 1.24–2.83 | .002 |
| LBBB | 1.10 | 0.73–1.66 | .6 |
| LVEF (for 10 points % decrease) | 1.22 | 1.004–1.49 | .04 |
| Indexed LVEDV (for 10 ml/m2 increase) | 1.06 | 1.02–1.10 | .02 |
| Beta blockers | 0.46 | 0.31–0.70 | .0002 |
| Baseline AF | 1.49 | 0.83–2.66 | .19 |
AF=Atrial Fibrillation; HF=Heart Failure; LBBB=Left Bundle Branch Block; LVEF=Left Ventricular Ejection Fraction; LVEDV=Left Ventricular End Diastolic Volume; NYHA=New York Heart Association Class.
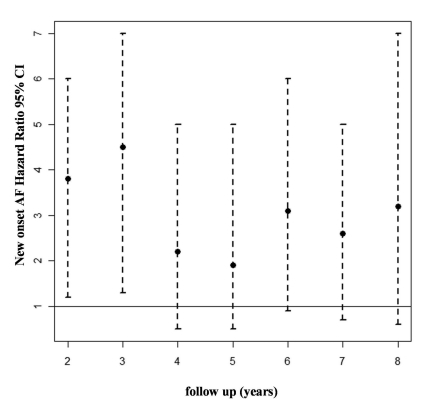
The impact of new onset AF was analyzed in 487 patients without previous documentation or symptoms of AF and in sinus rhythm at enlistment in the Registry. Of 28 patients with new onset AF, 16 patients (57.1%) died or underwent urgent heart transplantation in comparison to 121 patients (26.4%) of 459 patients who maintained sinus rhythm during follow-up (P<.001). The landmark analysis showed that patients developing AF in the first three years after diagnosis had a significantly higher risk of death or urgent heart transplantation (figure 2▶). Moreover, multivariate analysis demonstrated new onset AF (treated as a time dependent variable and adjusted for the other significant time-dependent parameters) was an independent predictor of subsequent all-cause mortality/heart transplantation (HR 3.67, 95% CI 2.07–6.5, P<.001) (table 4▶).
Figure 2.
Landmark analysis examining the time dependent association between the new onset atrial fibrillation and rate of death/urgent heart transplantation.
Table 4.
Multivariate predictors for death or urgent heart transplantation for the 487 patients in sinus rhythm at baseline.
| HR | 95% CI | P | |
|---|---|---|---|
| Male sex | 1.63 | 1.05–2.52 | .03 |
| HF duration (for 12 months increase) | 1.13 | 1.08–1.18 | <.001 |
| NYHA class (III–IV vs. I–II) | 1.6 | 1.1–2.4 | .01 |
| LVEF (for 10 points % decrease) | 5.81 | 5.55–6.09 | .02 |
| Indexed LVEDV (for 10 ml/m2 increase) | 1.06 | 1.02–1.1 | .006 |
| Beta blockers | 0.57 | 0.32–0.88 | .01 |
| New onset AF (time-dependent) | 3.67 | 2.07–6.5 | <.001 |
AF=Atrial Fibrillation; HF=Heart Failure; LVEF=Left Ventricular Ejection Fraction; LVEDV=Left Ventricular End Diastolic Volume; NYHA=New York Heart Association Class.
Discussion
Idiopathic dilated cardiomyopathy is a rare heart muscle disease (incidence 5 to 8 cases per 100,000 population per year27) characterized by left ventricular or biventricular dilatation and impaired myocardial contractility.21 Most patients are first seen between the ages of 20 and 50 years, but the disorder may also affect children and the elderly.28–30 The most common initial manifestation is heart failure, which occurs in 75% to 85% of patients.19,30,31 Some patients affected by IDCM have prevalent rhythm disturbances. Olson et al32 reported that heritable SCN5A defects are associated with susceptibility to early-onset IDCM and AF.
Since previous studies that explored the impact of AF in the setting of IDCM are outdated and contradictory,18–20 we evaluated the impact of AF on outcome in a large, single center, well-characterized population of patients with IDCM on long-term, optimal medical treatment with ACE inhibitors and beta blockers. The prevalence of AF was found to depend on the severity of heart failure. The prevalence of AF at baseline in our patients was relatively low (9.6%). Similarly, in a large study by Dries et al,7 including patients with mild to moderate heart failure, the prevalence of AF was 6%. In clinical trials enrolling patients with more advanced heart failure, AF at baseline ranges between 19% and 50%.6,8,12,33
Patients from our study with either AF or sinus rhythm at enrollment did not differ in duration and severity of heart failure. We did not observe a difference in survival between patients with AF and sinus rhythm at baseline.
There is no consensus regarding the prognostic role of baseline AF in heart failure patients. Two trials have analyzed the prognostic impact of AF in heart failure patients on optimal medical treatment with ACE inhibitors and beta blockers to date. The first one is a sub-analysis of the COMET trial9 that included 3,029 patients treated with beta blockers during a follow-up of 58 months. The authors found that the presence of AF at baseline was associated with more severe symptoms, longer duration of heart failure, and a 28% increased risk of death. However, baseline AF did not independently predict all-cause mortality. More recently, in a CHARM sub-study, Olsson et al10 observed that baseline AF remained an independent predictor of all-cause mortality (HR 1.22, 95% CI 1.04–1.43), even after covariate adjustment in multiple regression analysis.
The difference in our results may be due to the diversity of the study population and the etiology of heart failure. Since, in our population, no difference existed regarding the duration and severity of the heart failure among patients with AF or sinus rhythm, one may speculate that AF present at baseline is not a marker of important hemodynamic impairment, but possibly the expression of a specific genetic mutation that causes early susceptibility to arrhythmias in some patients with IDCM. Consequently, there is no association between AF at enrollment and outcome. Furthermore, the patients enrolled in pharmacologic trials do not fully represent patients from clinical practice due to exclusion criteria such as relevant comorbidities or prevalent diastolic dysfunction.
Clinical Implications of New Onset AF
During a mean follow-up of over seven years, 5.7% of patients in sinus rhythm at baseline and without previous history of AF developed this arrhythmia. Patients with new onset AF were more frequently in advanced NYHA class. The development of AF was associated with a more important left atrial dilatation, and with a more severe LV systolic dysfunction. The sustained atrial overload present in heart failure causing atrial enlargement may facilitate the occurrence and persistence of AF.34 Other mechanisms contributing to AF development are the elevated concentrations of catecholamine and angiotensin II that may promote atrial fibrosis,35,36 and thus induce changes in atrial conduction. Neurohumoral modulation and blockade of adrenergic receptors with ACE inhibitors and beta blockers could explain the efficacy of these drugs in preventing AF in patients with heart failure.37,38 Among our patients the incidence and prevalence of AF was relatively low, and this may be due to the extensive use of ACE inhibitors and beta blockers.
In our study, new onset AF, considered as a time dependent covariate and adjusted for other important covariates, was found to be an independent predictor of poor outcome. Only a few other studies have evaluated the incidence and predictive role of new onset AF. The incidence of new onset AF in the study by Pozzoli et al,14 including patients with heart failure referred for evaluation for heart transplantation, was 9% during a mean follow-up of 19 months. Similar to our study, new onset AF was associated with major cardiac events only in the first 10 months from the onset, but had no influence on patients who lived longer than that period. In the COMET study,9 the incidence of new onset AF was higher (23%) than in our study. The higher incidence might be due to the inclusion of older patients with a lower LVEF. In addition, patients with a history of AF but in sinus rhythm at baseline ECG were included in the non-AF group. However, new onset AF was an independent predictor of subsequent all-cause mortality in the COMET trial.9
The development of AF during follow-up might represent a late, secondary event in patients with long-lasting and more advanced chronic heart failure. On the other hand, its development may itself contribute to the progression of the disease and to a poor outcome. However, patients with IDCM who develop AF late during follow-up represent a subgroup of patients with more advanced disease who need a closer follow-up and further optimization of standard therapy.
The difference we observed in the impact of baseline AF and new onset AF on outcome may be due to the different underlying pathophysiological mechanism of the arrhythmia. The first may represent an early manifestation of IDCM in the context of specific genetic variants. New onset AF characterizes a late stage of the disease with a more important hemodynamic impairment; however, further studies are necessary to elucidate this issue.
Study Limitations
There are some limitations in our study that must be acknowledged. First, we cannot rule out undocumented, asymptomatic, short, self-limited episodes of AF in patients who were in sinus rhythm at follow-up visits. Second, our study is a retrospective study and may be subject to the potential biases of such studies. However, it included a large population of patients with IDCM enrolled in a single-center Registry, with prospective and strict inclusion criteria, and a long-term follow-up. Usually, in Registries there is a particular concern about the change of treatment through different historical periods. In our Registry all patients without contraindications were treated with ACE inhibitors and beta blockers. Third, atrial fibrillation is usually associated with a more advanced stage of heart failure, and its development and specific role on long-term prognosis may be difficult to analyze. In our study, the development of AF through the study period was independently associated with a worse outcome using a time-dependent model and after adjustment for all other clinical and instrumental prognostic variables. In spite of these limitations, we believe that our analysis provides an important and reliable addition to the literature in this field.
Conclusions
The prevalence of AF in our cohort of optimally treated patients with IDCM was close to 10%. The incidence of new onset AF throughout the long-term follow-up was nearly 1/100 patients/year. New onset AF was independently predicted by a more severe left ventricular dysfunction and a more dilated left atrium. The early development of AF during follow-up but not baseline AF was an independent marker of worse outcome in IDCM.
References
- 1.Cha YM, Redfield MM, Shen WK, Gersh BJ. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation 2004;109:2839–2843. [DOI] [PubMed] [Google Scholar]
- 2.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003;91:2D–8D. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort study: the Framingham Heart Study. JAMA 1994;271:840–844. [PubMed] [Google Scholar]
- 4.Onundarson PT, Thorgeirsson G, Jonmundsson E, Sigfusson N, Hardarson T. Chronic atrial fibrillation--epidemiologic features and 14 year follow-up: a case-control study. Eur Heart J 1987;3:521–527. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen OD, Bagger H, Køber L, Torp-Pedersen C. The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. TRACE Study group. TRAndolapril Cardiac Evalution. Eur Heart J 1999;20:748–754. [DOI] [PubMed] [Google Scholar]
- 6.Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation 1991;84:40–48. [DOI] [PubMed] [Google Scholar]
- 7.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 8.Mahoney P, Kimmel S, DeNofrio D, Wahl P, Loh E. Prognostic significance of atrial fibrillation in patients at a tertiary medical center referred for heart transplantation because of severe heart failure. AmJ Cardiol 1999;83:1544–1547. [DOI] [PubMed] [Google Scholar]
- 9.Swedberg K, Olsson LG, Charlesworth A, Cleland J, Hanrath P, Komajda M, Metra M, Torp-Pedersen C, Poole-Wilson P. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta blockers: results from COMET. Eur Heart Jornal 2005;26:1303–1308. [DOI] [PubMed] [Google Scholar]
- 10.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA; CHARM Investigators. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol 2006;47:1997–2004. [DOI] [PubMed] [Google Scholar]
- 11.Crijns HJ, Tjeerdsma G, de Kam PJ, Boomsma F, van Gelder IC, van den Berg MP, van Veldhuisen DJ. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure. Eur Heart J 2000;21:1238–1245. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA, Tillisch JH. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol 1996;28:1458–1163. [DOI] [PubMed] [Google Scholar]
- 13.Shotan A, Garty M, Blondhein DS, Meisel SR, Lewis BS, Shochat M, Grossman E, Porath A, Boyko V, Zimlichman R, Caspi A, Gottlieb S; HFSIS Steering Committee and Investigators. Atrial fibrillation and long-term prognosis in patients hospitalized for heart failure: results from heart failure survey in Israel (HFSIS). Eur Heart J. 2010; 31:309–317. [DOI] [PubMed] [Google Scholar]
- 14.Pozzoli M, Cioffi G, Traversi E, Pinna GD, Cobelli F, Tavazzi L. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J Am Coll Cardiol 1998;32:197–204. [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 16.Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, Masson S, Cerè E, Tognoni G, Cohn JN; Val-HeFT Investigators. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: Results from the Valsartan Heart Failure Trial (Val-HeFT) Am Heart J 2005;149:548–557. [DOI] [PubMed] [Google Scholar]
- 17.Bunch TJ, Day JD, Olshansky B, Stolen KQ, Mullin CM; INTRINSIC RV Study Investigators. Newly detected atrial fibrillation in patients with an implantable cardioverter-defibrillator is a strong risk marker of increased mortality. Heart Rhythm. 2009;6:2–8. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann T, Meinertz T, Kasper W, Geibel A, Zehender M, Hohnloser S, Stienen U, Treese N, Just H. Mode of death in idiopathic dilated cardiomyopathy: a multivariate analysis of prognostic determinants. Am Heart J 1988;116:1455–1463. [DOI] [PubMed] [Google Scholar]
- 19.Diaz RA, Obasohan A, Oakley CM. Prediction of outcome in dilated cardiomyopathy. Br Heart J 1987;58:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Convert G, Delaye J, Beaune J, Biron A, Gonin A. Prognosis of primary non-obstructive cardiomyopathies. [Article in French] Arch Mal Coeur Vaiss 1980;73:227–237. [PubMed] [Google Scholar]
- 21.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation 1996;93: 841–842. [DOI] [PubMed] [Google Scholar]
- 22.Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol 1987;18:619–624. [DOI] [PubMed] [Google Scholar]
- 23.Kuck KH, Cappato R, Siebels J, Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the Cardiac Arrest Study Hamburg (CASH). Circulation 2000;102:748–754. [DOI] [PubMed] [Google Scholar]
- 24.Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, Mitchell LB, Green MS, Klein GJ, O’Brien B. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–1302. [DOI] [PubMed] [Google Scholar]
- 25.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med 1997;337:1576–1583. [DOI] [PubMed] [Google Scholar]
- 26.Grambsh P, Therneau T. Proportional hazard tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526. [Google Scholar]
- 27.Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation 2000;102:IV14–23. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RA, Palacios I. Dilated cardiomyopathies of the adult (first of two parts). N Engl J Med 1982;307:1051–1058. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RA, Palacios I. Dilated cardiomyopathies of the adult (second of two parts). N Engl J Med 1982;307:1119–1126. [DOI] [PubMed] [Google Scholar]
- 30.Komajda M, Jais JP, Reeves F, Goldfarb B, Bouhour JB, Juillieres Y, Lanfranchi J, Peycelon P, Geslin P, Carrie D, Grosgogeat Y. Factors predicting mortality in idiopathic dilated cardiomyopathy. Eur Heart J 1990;11:824–831. [DOI] [PubMed] [Google Scholar]
- 31.Sugrue DD, Rodeheffer RJ, Codd MB, Ballard DJ, Fuster V, Gersh BJ. The clinical course of idiopathic dilated cardiomyopathy: a population-based study. Ann Intern Med 1992;117:117–123. [DOI] [PubMed] [Google Scholar]
- 32.Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ, Horton SC, Rodeheffer RJ, Anderson JL. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA 2005;293:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Effect of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987, 316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 34.Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation 2002; 105:2672–2678. [DOI] [PubMed] [Google Scholar]
- 35.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 1999;100:87–95. [DOI] [PubMed] [Google Scholar]
- 36.Cha YM, Dzeja PP, Shen WK, Jahangir A, Hart CY, Terzic A, Redfield MM. Failing atrial myocardium: energetic deficits accompany structural remodelling and electrical instability. Am J Physiol Heart Circ Physiol 2003;284:H1313–1320. [DOI] [PubMed] [Google Scholar]
- 37.Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, Connolly SJ. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol 2005;45:1832–1839. [DOI] [PubMed] [Google Scholar]
- 38.Nasr IA, Bouzamondo A, Hulot JS, Dubourg O, Le Heuzey JY, Lechat P. Prevention of atrial fibrillation onset by beta-blocker treatment in heart failure: a meta-analysis. Eur Heart J 2007;28:457–462. [DOI] [PubMed] [Google Scholar]