Abstract
We have previously shown that exposure to febrile-range temperature (FRT, 39.5°C) reduces LPS-induced TNF-α transcription in mouse macrophages through at least two mechanisms: (1) by directly recruiting heat shock factor-1 (HSF-1) to a heat shock response element present in the TNF-α 5′-UTR and (2) by markedly reducing LPS-induced recruitment of NFκB-p65 to the κB enhancer (at −510) in the TNF-α gene. In the present study, we used EMSA and chromatin immunoprecipitation assays to further analyze the complex effects of FRT on the recruitment of transcription factors and co-activators on the TNF-α gene in LPS-stimulated RAW 264.7 mouse macrophages. Our results showed that in FRT-exposed RAW cells, HSF-1 was recruited only to the 5′-UTR site, and no additional interaction was evident in the TNF-α gene up to 1,300 nt upstream of the transcription start site. Similarly, FRT exposure selectively reduced LPS-induced NFκB-p65 recruitment to the κB enhancer site at −510 without affecting the other three κB enhancer sites present in the TNF-α 5′-flanking sequence. Finally, we found that FRT exposure abrogated LPS-stimulated recruitment of Sp1 to the proximal TNF-α promoter without any change in associated histone H3 acetylation around the TNF-α promoter and despite a marked increase in the total intra-nuclear Sp1 DNA binding activity. In conclusion, our studies further emphasize the complex and redundant control of TNF-α transcription and identify additional potential mechanisms through which FRT exposure may reduce TNF-α expression by selectively modifying gene-specific recruitment of transcription factors to the proximal TNF-α promoter.
Keywords: Fever, Hyperthermia, TNF-α, Repression, Sp1
Introduction
Exposure to temperatures in the febrile and clinical heatstroke range exerts profound immunomodulatory effects (Hasday and Singh 2000). We have previously demonstrated that exposure to febrile-range temperature (FRT, 39.5°C) reduces LPS-stimulated TNF-α expression in the RAW 264.7 macrophage cell line, murine peritoneal macrophages, liver slices, Kupffer cells, human monocytes and monocyte-derived macrophages, and the human THP-1 promonocytic leukemia cell line (Ensor et al. 1994, 1995; Fairchild et al. 2000; Jiang et al. 1999; Singh et al. 2000, 2002, 2004) Our subsequent studies utilizing the LPS-stimulated RAW 264.7 mouse macrophage model demonstrated three independent mechanisms through which exposure to FRT modifies LPS-induced TNF-α gene expression: (1) by activating the stress-activated transcription factor, heat shock factor-1 (HSF-1), and directly recruiting it to a heat shock response element (HSE)-like sequence present in the 5′-untranslated region (UTR) of the TNF-α gene (Singh et al. 2000, 2002, 2004); (2) by selectively reducing LPS-induced NFκB-p65 recruitment to the κB enhancer site located 510 nt upstream of the TNF-α transcription start site (Cooper et al. 2009); and (3) by destabilizing TNF-α mRNA (Ensor et al. 1995).
Regulation of TNF-α expression is rigorous and redundant. At the transcriptional level, LPS-induced TNF-α expression is regulated by NFκB, which interacts with multiple sites in the TNF-α promoter (Kuprash et al. 1999), and assembly of the core enhanceosome complex containing Sp1, ATF-2, and Ets proteins on the proximal TNF-α promoter (Barthel et al. 2003; Tsai et al. 2000). The murine TNF-α 5′-flanking sequence contains four κB enhancer sites located 210, 510, 655, and 850 nt upstream of the transcription start site. The proximal 210 nucleotide region of the TNF-α promoter is highly conserved across species, including between mouse and human (Kuprash et al. 1999), and is sufficient to confer inducibility to many extracellular stimuli, including LPS (Kuprash et al. 1999). LPS-induced TNF-α expression in mouse J774 macrophages is temporally associated with recruitment of Egr-1, ATF-2, c-jun, Ets-1, Elk-1, and Sp1 to the proximal TNF-α promoter and requires the additional recruitment of the co-activators CBP and p300 (Tsai et al. 2000). Analysis of helical phasing relationships between transcription factor binding sites further suggests that the CRE-κ3-Ets sites, which bind ATF-2, c-jun, and Ets proteins, comprise the composite core element while Sp1 must associate with its proximal Ets site for activation of the promoter (Barthel et al. 2003). Of relevance to this study, it is not known how exposure to FRT modifies LPS-induced assembly of the enhanceosome complex on the proximal TNF-α promoter.
The objective of this study was to determine how exposure to FRT modifies the recruitment of transcription factors and LPS-induced enhanceosome complex formation on the TNF-α proximal promoter. Using the RAW 264.7 mouse macrophages and EMSA and chromatin immunoprecipitation (ChIP) assays, we showed that exposure to FRT abrogated LPS-induced recruitment of Sp1 to the proximal TNF-α promoter despite increasing intranuclear Sp1 DNA binding activity and without any associated changes in histone H3 acetylation.
Materials and methods
Cell culture
The RAW 264.7 cell line, obtained from the American Type Cell Collection (Manassas, VA), was maintained in RPMI 1640 supplemented with 50 U/ml penicillin, 50 µg/ml streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM HEPES buffer (Invitrogen, Carlsbad, CA), pH 7.3, and 10% defined fetal bovine serum (Hyclone, Logan, UT) as described previously (Singh et al. 2000, 2002). Cells were routinely tested for Mycoplasma infection using a commercial assay system (MycoTest, Invitrogen), and new cultures were established monthly from frozen stocks. Cell viability was determined by trypan blue dye exclusion.
For FRT exposures, RAW 264.7 cells were pre-warmed at 39.5°C in automatic CO2 incubators certified to have <0.2°C temperature variation (Forma; Marietta, OH) and calibrated for each experiment using an electronic thermometer (FLUKE Instruments model 5211, Everett, WA). Following pre-incubation, cells were stimulated with either LPS (Escherichia coli 0111B4; Sigma Aldrich; St. Louis, MO) and maintained at 39.5°C (FRT). Normothermic controls were maintained at 37°C prior to and during LPS stimulation.
Electrophoretic mobility shift assay
Nuclear extracts were prepared according to the method of Schreiber et al. (1989) as described earlier (Cooper et al. 2009; Singh et al. 2000, 2002), and total protein concentration was measured using the Bradford method (Biorad; Mountainview, CA). Double-stranded oligonucleotides containing the consensus Sp1 binding sequence (#E3232, Promega) were radiolabeled with [32γP]ATP using T4 polynucleotide kinase (Promega) according to the manufacturer’s protocol. Twenty-microliter EMSA reactions containing 5 µg nuclear extract, 0.035 pmol radiolabeled oligonucleotide, 1 µg poly(dI/dC), 10 mM Tris–HCl, pH 7.8, 10% glycerol, 60 mM NaCl, 1 mM EDTA, and 1 mM dithiothreitol were incubated at room temperature for 30 min. For competition gel shift assays, 4-, 12-, 40-, 120-fold excess unlabeled oligonucleotide for the two potential Sp1 binding sites in the TNF-α promoter was incubated with nuclear extracts at room temperature prior to the addition of the probe. The following pairs of complimentary oligonucleotides were used to generate double-stranded DNA competitor probes for murine Sp1 TNF-α sites: Sp1-1 (−70/−44) Forward 5′-GAG CTT TTC CCC GCC CTC TTC CCC AAG; Reverse 5′-CTT GGG GAA GAG GGC GGG GAA AAG CTC; Sp1-2 (−184/−158) Forward 5′-CCC TCT GCC CCC GCG ATG GAG AAG AAA; and Reverse 5′-TTT CTT CTC CAT CGC GGG GGC AGA GGG; Sp1-1 mut (−70/−44) Forward 5′-GAG CTT TTC CCA AAC CTC TTC CCC AAG; and Reverse 5′-CTT GGG GAA GAG GTT TGG GAA AAG CTC. For supershift, anti-Sp1 antibody (Santa Cruz, sc-14027X) was incubated with nuclear extracts for 30 min prior to addition of the probe. The DNA–protein complexes were electrophoretically resolved on 4% nondenaturing polyacrylamide gels. The dried gels were analyzed by phosphorimaging (PhosphorImager, Molecular Dynamics) and subsequently exposed to X-ray film.
ChIP assays
ChIP assays were performed using a kit from Millipore (Billerica, MA) as described previously (Cooper et al. 2009; Singh et al. 2002, 2008). ChIP-validated antibodies were purchased from the following vendors: anti-HSF-1 (sc-9144X), anti-Sp1 (sc-14027X), anti-Elk-1 (sc-355X), anti-ATF2 (sc-6233X), and anti-NFκB p65 (sc-372X) from Santa Cruz and rabbit anti-acetyl-histone-H3 antibody from Millipore (# 06-599). In brief, RAW 264.7 cells were incubated with or without 100 ng/ml LPS at either 37°C or 39.5°C, cross-linked using 1% formaldehyde for 10 min, washed with PBS, and collected by centrifugation. The cell pellets were resuspended in SDS lysis buffer and sonicated for five 10-s bursts using a Branson Sonifier 450. Sonicated cell lysates were diluted 10-fold using ChIP dilution buffer and precleared for 1 h at 4°C using 80 µl of a 50% salmon sperm DNA–saturated protein A agarose beads (ss-protein A). Cross-linked chromatin was immunoprecipitated with 4 µg of primary antibody or control antibody at 4°C overnight and immune complexes collected with ss-protein A. The immune complexes were washed and eluted, and cross-linked protein-DNA was reverted by incubating at 65°C for 4 h. DNA was extracted and used as template for real-time PCR, which were performed as described previously (Cooper et al. 2009; Singh et al. 2008). Duplicate 25 µl real-time PCRs were performed in 96-well plates using a SYBR-Green reaction mix (#170-8880, BioRad) and a BioRad iCycler IQ Optical Module according to the supplier’s protocol with the primers listed (Table 1). ChIP qRT-PCR data were analyzed using a template from SA Biosciences as described earlier (Cooper et al. 2009).
Table 1.
Primer sequences and amplicon position along the human HSPA1A and TNF-α gene
| Gene | Amplicon distance from start site | Primer sequences |
|---|---|---|
| HSPA1A | −250/−51 | F, AGCACCAGCACTTCCCCACA |
| R, CCGCTGGGCCAATCAGCGAG | ||
| TNF-α | −1,300/−1,175 | F, AGAGAGATGGCGAGAGAATTAGATG |
| R, TGGGTTTCACAGATGGTGCA | ||
| TNF-α | −1,194/−1,063 | F, TGCACCATCTGTGAAACCCA |
| R, TGGACCAACTGAGGCCTCTG | ||
| TNF-α | −1,082/−890 | F, CAGAGGCCTCAGTTGGTCCA |
| R, ACCCTCCAGTGGAGTCACTTCTC | ||
| TNF-α | −912/−763 | F, GAGAAGTGACTCCACTGGAGGGT |
| R, ACTGCGGTACATCAACTCAGACAT | ||
| TNF-α | −786/−662 | F, ATGTCTGAGTTGATGTACCGCAGT |
| R, GAATTCACGGACCTCACAAGC | ||
| TNF-α | −685/−543 | F, AAGGCTTGTGAGGTCCGTGA |
| R, AAGTGGCTGAAGGCAGAGCA | ||
| TNF-α | −586/−468 | F, ATGCACACTTCCCAACTCTAAG |
| R, CTTCTGAAAGCTGGGTGCATAAG | ||
| TNF-α | −490/−356 | F, TTATGCACCCAGCTTTCAGAAG |
| R, TCCTCCAGAGCTAATCATTGTCTG | ||
| TNF-α | −364/−182 | F, TCTGGAGGACAGAGAAGAAATG |
| R, GGTTTGGAAAGTTGGGGACAC | ||
| TNFa | −208/−89 | F, TTCCTTGATGCCTGGGTGTC |
| R, CCACCAGGATTCTGGCAATG | ||
| TNF-α | −89/+32 | F, TTTTCCGAGGGTTGAATGAGA |
| R, TCCTGGCTAGTCCCTTGCTGT | ||
| TNF-α | +4/+152 | F, AGCGAGGACAGCAAGGGA |
| R, TCTTTTCTGGAGGGAGTGTGG |
Primers are listed 5′ to 3′
F forward, R reverse
Statistics
Data are displayed as mean ± SE. Differences between two groups were analyzed by unpaired Student t test, and among multiple groups was analyzed by applying a Tukey–Kramer Honestly Significant Difference test to a one-way ANOVA, and differences with p < 0.05 were considered significant.
Results
Association of HSF-1 with TNF-α 5′-flanking sequence is limited to the 5′-UTR
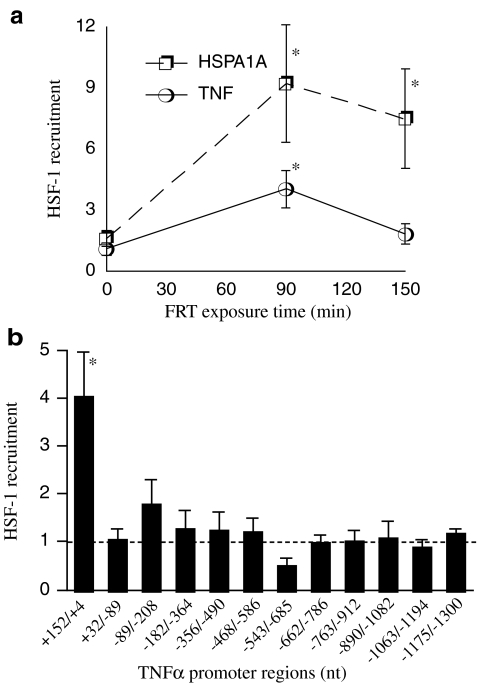
We previously showed that exposure to FRT (39.5°C) causes HSF-1 to bind to the HSE-like element in the 5′-UTR of the TNF-α promoter in the RAW 264.7 mouse macrophage line and that this HSE-like sequence is required for repression of the minimal TNF-α promoter (Singh et al. 2002). We used ChIP assays to confirm that exposure to FRT was sufficient to activate recruitment of HSF-1 to a prototypical HSF-1-activated gene promoter, HSPA1A, and to the TNF-α 5′-UTR region (Fig. 1a). Incubating RAW 264.7 cells at 39.5°C for 90 min stimulated a 9-fold increase in binding of HSF-1 to the HSE-containing region of HSPA1A that persisted during an additional 1 h of warming. Recruitment of HSF-1 to the TNF-α 5′-UTR region increased 4-fold after 90 min incubation at 39.5°C, but unlike HSF-1 recruitment to HSPA1A, HSF-1 recruitment to TNF-α returned to baseline over the next hour despite continued exposure to FRT (Fig. 1a). Treatment with 100 ng/ml LPS, which was used in all subsequent experiments to stimulate TNF-α expression, neither stimulated HSF-1 recruitment to the TNF-α 5′-UTR in Raw 264.7 cells incubated at 37°C nor modified HSF-1 recruitment to TNF-α 5′-UTR in cells exposed to 39.5°C (data not shown). To determine whether HSF-1 was recruited to additional sites in the full-length TNF-α promoter, we measured HSF-1 recruitment to partially overlapping ∼150 nt sequences spanning −1,300 to +152 nt of the TNF-α 5′-flanking sequence. RAW 264.7 cells were exposed to 39.5°C for 90 min, and the cross-linked chromatin was immunoprecipitated using an anti-HSF-1 rat monoclonal antibody (SantaCruz) and the precipitated DNA was quantified by real-time PCR as we described earlier (Cooper et al. 2009) (Fig. 1b). This analysis confirmed HSF-1 recruitment to the 5′-UTR region previously identified in our laboratory (Singh et al. 2002) but did not reveal any additional regions in the TNF-α 5′flanking sequence that exhibited enhanced HSF-1 recruitment following exposure to FRT.
Fig. 1.
FRT recruits HSF-1 only to the 5′-UTR in the TNF-α promoter. a RAW 264.7 macrophages were warmed at 39.5°C for 90 or 150 min, and cross-linked chromatin was analyzed for recruitment of HSF-1 to the HSE-containing region of the HSPA1A promoter and the TNF-α 5′-UTR by ChIP assay and expressed as enrichment compared with nonspecific IgG control. b Cross-linked chromatin from RAW 264.7 cells warmed to 39.5°C for 90 min was analyzed for recruitment of HSF-1 to overlapping ∼150 bp amplicons comprising the 1.3 kb TNF-α 5′ flanking sequence (see Table 1 for sequences) and expressed as enrichment compared with nonspecific IgG control. Data presented as mean ± SE of five independent experiments. *p < 0.05 compared with untreated controls
FRT selectively reduces NFκB recruitment to the −510 nt high-affinity NFκB binding site
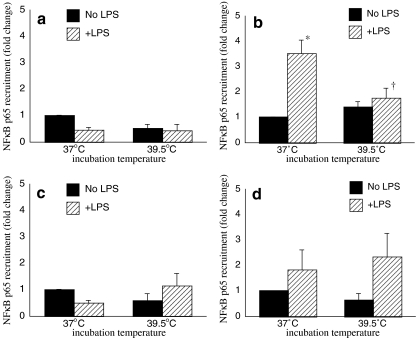
We previously showed that exposure to FRT selectively abrogated LPS-induced NFκB-p65 recruitment to the κB enhancer site centered around −510 nt in the TNF-α promoter without changing total intranuclear levels of NFκB DNA binding activity (Cooper et al. 2009; Singh et al. 2002). We used ChIP assays to extend this analysis by measuring the effect of FRT on NFκB recruitment to the other three NFκB binding sequences in the TNF-α 5′-flanking sequence (Fig. 2). RAW 264.7 cells were stimulated with LPS for 30 min at 37°C or pre-warmed at 39.5°C for 30 min to allow for equilibration of cells to the higher temperature then stimulated with LPS for 30 min at 39.5°C. We confirmed our previous finding that LPS stimulates recruitment of NFκB-p65 to the κB enhancer site centered at −510 nt of the TNF-α promoter, and this was blocked by co-exposure to FRT (Fig. 2b). In contrast, we could not detect any effects of LPS or FRT exposure on NFκB-p65 recruitment to the TNF-α promoter sequence containing the −210, −655, or −850 nt NFκB binding sequence (Fig. 2a, c, d).
Fig. 2.
FRT only affects recruitment of NFκB-p65 to the −586/−468 κB enhancer site in the TNF-α promoter. RAW 264.7 macrophages were prewarmed to 37°C or 39.5°C for 30 min, stimulated with 100 ng/ml LPS and incubation was continued at the same temperature for an additional 30 min. Cross-linked chromatin was immunoprecipitated with anti-p65 antibody and analyzed for recruitment of NFκB-p65 to the κB binding sites by qRT-PCR using primer pairs spanning the regions −364/−182 (a), −586/−468 (b), −685/−543 (c), and −912/−763 (d) and expressed as fold-change vs. unstimulated 37°C cells. Data presented as mean ± SE of four independent experiments. *p < 0.05 compared with untreated controls. †p < 0.05 vs. 37°C cells at the same time point
FRT blocks recruitment of Sp1 to the TNF-α minimal promoter
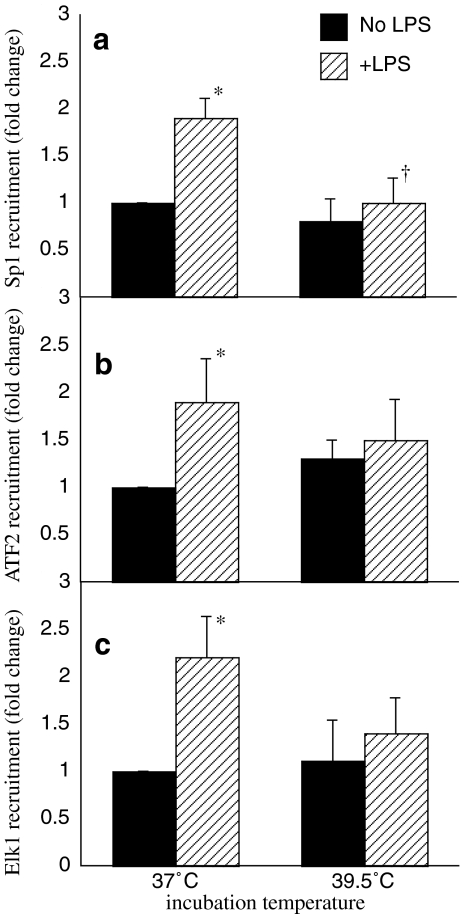
Previous studies analyzing the helical phasing relationships between transcription factor binding sites on the TNF-α promoter suggests the CRE-k3-Ets sites that bind ATF-2 and Ets proteins comprise the composite core elements along with Sp1 (Barthel et al. 2003). To explore the possibility that FRT changes LPS-induced TNF-α expression by affecting recruitment of these core transcriptional regulators to the TNF-α core promoter, we used ChIP to analyze the effect of FRT exposure on recruitment of ATF-2, Elk-1, and Sp1 to the proximal TNF-α promoter sequence bounded by nucleotides −208 to +32. RAW 264.7 cells were stimulated with LPS for 1 h at 37°C or pre-warmed at 39.5°C for 30 min and stimulated with LPS for 1 h at 39.5°C (Fig. 3). In 37°C RAW 264.7 cells, treatment with LPS increased recruitment of ATF-2, Elk-1, and Sp1 to the proximal TNF-α promoter by 86%, 122%, and 90%, respectively (Fig. 3). Pre-exposure to FRT for 30 min and co-exposure to FRT completely abrogated LPS-induced Sp1 recruitment (Fig. 3a) but did not significantly alter ATF-2 or Elk-1 recruitment (Fig. 3b, c).
Fig. 3.
FRT abrogates recruitment of Sp1 to TNF-α minimal promoter. RAW 264.7 macrophages were prewarmed to 37°C or 39.5°C for 30 min, stimulated with 100 ng/ml LPS and incubated at the same temperature for an additional 60 min. Cross-linked chromatin was analyzed for recruitment of Sp1 (a), ATF2 (b), and Elk-1 (c) to the TNF-α minimal promoter (−208/+32) by ChIP assay and expressed as fold-change vs. unstimulated 37°C cells. Data presented as mean ± SE of five independent experiments. *p < 0.05 compared with untreated controls. †p < 0.05 vs. 37°C cells at the same time point
FRT modifies activation of the core transcription elements regulating TNF-α transcription
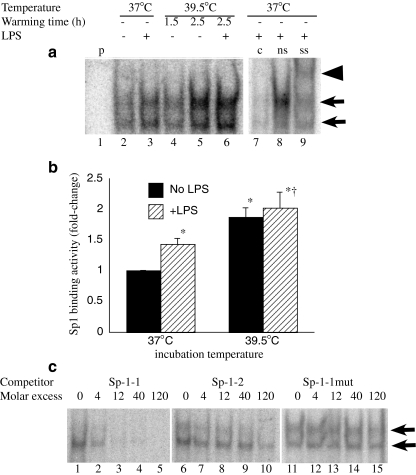
To determine whether the decrease in Sp1 recruitment to the TNF-α promoter in FRT-exposed cells was caused by a reduction in levels of activated Sp1, we analyzed the effect of FRT exposure on intranuclear Sp1 binding activity using EMSA (Fig. 4a, b). Rather than reducing Sp1 DNA binding activity, exposing RAW 264.7 cells to FRT for 2.5 h stimulated a 2-fold increase in Sp1 binding activity (Fig. 4a, b). The DNA binding activity was shown to be specific by competition with 40-fold excess of unlabeled Sp1 binding oligonucleotide (Fig. 4a, lane 7) and confirmed to be Sp1 by supershifting with anti-Sp1 antibody (Fig. 4a, lane 9). There are two potential Sp1 binding sequences in the proximal TNF-α promoter, centered on −49 and −168 nt. The proximity of the two sites precluded using ChIP to distinguish between of Sp1 to the two sites. However, using EMSA with the consensus Sp1 probe, we found that only the more proximal (−49 nt) TNF-α Sp1 binding sequence competed with consensus probe for Sp1 binding (Fig. 4c).
Fig. 4.
FRT enhances Sp1 activation. RAW 264.7 macrophages were prewarmed to 37°C or 39.5°C for 90 min, stimulated with 100 ng/ml LPS and incubated at the same temperature for an additional 1 h or warmed at 39.5°C for 90 or 150 min without LPS. Nuclear extracts were analyzed by EMSA for binding to a consensus Sp1 probe. A representative autoradiograph (a) and mean ± SE of EMSA band density determined by phosphorimaging from three separate experiments (b) are shown. The Sp1 binding complex appears as a doublet band indicated by arrows in a. Lane 1 is probe alone (p without cell extract). Binding specificity was confirmed by competition with unlabeled probe (c) (compare lane 7 with nonspecific (ns) oligonucleotide competition in lane 8) and Sp1 was confirmed by supershifting (ss) with an anti-Sp1 antibody (arrowhead, lane 9). c RAW 264.7 macrophages were stimulated with 100 ng/ml LPS and incubated at 37°C for 1 h and the capacity of the sequences comprising the proximal Sp1 (Sp1-1, −70/−44 nt) and distal Sp1 (Sp1-2, −184/−158 nt) as competitors was analyzed. Lanes 1–5 (cold Sp1-1 oligonucleotides), lanes 6–10 (cold Sp1-2 oligonucleotides), and lanes 11–15 (mutant Sp1-1 oligonucleotide)
LPS-induced histone H3 acetylation on the TNF-α promoter is independent of FRT
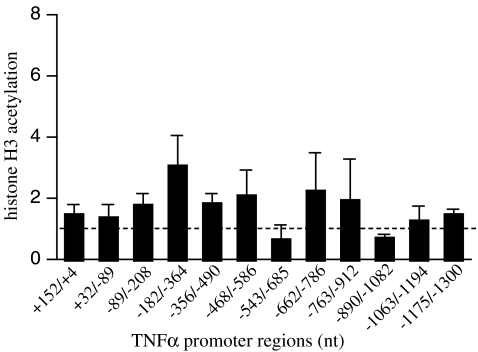
To determine whether the effects of FRT on the recruitment of transcription factors to the TNF-α promoter may be caused by temperature-dependent alterations in chromatin modification, we analyzed histone H3 acetylation on the TNF-α 5′-flanking sequence. Using an anti-acetyl histone H3 antibody (Millipore) and ChIP assay, we measured histone H3 acetylaton on partially overlapping ∼150 nt sequences spanning −1,300 to +152 nt of the TNF-α 5′-flanking sequence using the same strategy as we used for HSF-1 (Fig. 5). Exposing RAW 264.7 cells to 39.5°C for 90 min did not seem to alter histone H3 acetylation on any region of the TNF-α 5′-flanking sequence.
Fig. 5.
Exposure to FRT fails to alter histone H3 acetylation of the TNF-α promoter. RAW 264.7 macrophages were warmed to 39.5°C for 90 min and analyzed for acetylation of histone-H3 by ChIP on overlapping ∼150 bp regions of the TNF-α proximal promoter. Histone acetylation for each promoter region at 39.5°C was expressed as fold-change compared with 37°C cells (indicated by dashed line). Data presented as mean ± SE of five independent experiments
Discussion
In earlier studies, we demonstrated that FRT exposure reduces TNF-α transcription in human and mouse macrophages (Ensor et al. 1994; Fairchild et al. 2000; Singh et al. 2000, 2002, 2004), that HSF-1 is activated at FRT, that HSF-1 represses LPS-induced TNF-α transcription by binding to an HSE in the murine TNF-α 5′-flanking sequence (Singh et al. 2000, 2002), and that HSF-1 is required for the repression of LPS-induced TNF-α expression caused by exposure to FRT in vivo (Cooper et al. 2009) and in vitro (Singh et al. 2002). We also found a second independent mechanism through which FRT reduces TNF-α transcription by blocking LPS-induced recruitment of NFκB to the TNF-α-510 NFκB binding sequence (Cooper et al. 2009). The present study confirms and extends these findings by showing that: (1) FRT exposure selectively recruits HSF-1 to only the 5′-UTR site and not to additional sites in the 1.3 kb 5′-flanking TNF-α sequence; (2) FRT exposure selectively reduces recruitment of NFκB-p65 to only one of the four documented κB enhancer sites present in murine TNF-α promoter (Kuprash et al. 1999); (3) FRT exposure reduces recruitment of Sp1 to the proximal TNF-α promoter despite an increase in total intranuclear Sp1 DNA binding activity, and (4) FRT exposure does not appear to block Sp1 recruitment by modifying histone H3 acetylation on the proximal TNF-α promoter.
We previously showed that HSF-1 binds to the HSE sequence located between +49 and +59 nt in the murine TNF-α 5′-UTR and reduces activity of a TNF-α reporter plasmid (Singh et al. 2002). In that study, we found that HSF-1 overexpression reduced activity of TNF-α reporter plasmids driven by long (−1,080 to +138 nt), intermediate (−244 to +138 nt), and short (−84 to +138 nt) TNF-α promoter fragments, but mutational inactivation of the +49/+59 HSE eliminated HSF-1-mediated repression only in the shortest plasmid. These results suggested that the promoter region spanning −1,080 and −84 nt might contain additional HSF-1 binding sequences (Singh et al. 2002). Moreover, EMSA analysis utilizing recombinant HSF-1 and overlapping ∼200 nt fragments of TNF-α 5′-flanking sequences as probes suggested possible HSF-1 binding sequences between −326 and −39 nt and between −1,080 and −845 nt (Singh et al. 2002). However, in the present study, ChIP analysis of FRT-exposed RAW 264.7 cells with 150 nt resolution showed no additional HSF-1 recruitment to the 5′-flanking region of the TNF-α promoter (Fig. 1c). Considering that ChIP assays analyze DNA–protein interactions in vivo and on the endogenous gene rather than in cell-free protein binding reactions to naked DNA that is measured by EMSA, the results of the present study are more likely to accurately reflect HSF-1 binding to the endogenous TNF-α promoter.
We previously showed that exposure to FRT selectively abrogated LPS-induced NFκB-p65 recruitment to the high-affinity κB enhancer site centered around −510 nt in the murine TNF-α promoter (Cooper et al. 2009), but it was not evident whether FRT also modified NFκB binding to the other three documented κB enhancer sites present in the murine TNF-α promoter (Kuprash et al. 1999). Using p65-ChIP assays, we extended our previous findings by showing that FRT only modified LPS-induced but not constitutive NFκB-p65 recruitment to the −510 nt NFκB site but did not modify NFκB-p65 recruitment to any of the other three sites. That we were able to detect LPS-inducible NFκB-p65 recruitment to the −510 site, and not the −210, −655, or −850 nt κB sites, appears to contradict an earlier study by Kuprash et al. (1999) showing that all four κB enhancer sites contribute to LPS-inducibility in the murine TNF-α promoter. However, Kuprash and colleagues measured activation of reporter plasmids rather than the physical association of NFκB with the endogenous gene promoter as measured by ChIP in our study.
That FRT also represses expression of human TNF-α (Ensor et al. 1994; Fairchild et al. 2000) even though the human gene apparently lacks the two elements that participate in FRT-induced repression of murine TNF-α, the −510 nt κB enhancer, and the 5′-UTR HSE suggests additional mechanisms must contribute to FRT-induced repression of TNF-α. Since the proximal 210 nt region of the mouse and human TNF-α promoter is highly conserved and sufficient to confer LPS inducibility (Tsai et al. 2000) and FRT repressibility (Singh et al. 2002), it was a likely candidate for further study. We found neither HSF-1 recruitment nor FRT-repressible NFκB recruitment in this region, leading us to analyze the effects of FRT on recruitment of the other core transcriptional regulators that assemble on this region. Based on the findings of Tsai et al. (2000) that Elk-1, ATF2, and Sp1 comprise the core transcriptional enhanceosome that assemble on the proximal TNF-α promoter sequence, we analyzed the effects of FRT on activation of these three transcriptional activators and their recruitment to the proximal TNF-α promoter region. ChIP analysis revealed that co-exposure to FRT abrogated LPS-induced Sp1 recruitment to the proximal TNF-α promoter despite an increase in total intranuclear Sp1 DNA binding activity, suggesting that FRT exerted effects on accessibility of this region of the TNF-α promoter. The trend toward reduced LPS-induced recruitment of ATF-2 and Elk-1 to the proximal TNF-α promoter in FRT-exposed cells is consistent with this conclusion. HSF-1 and exposure to hyperthermia have been shown to influence chromatin remodeling. Inouye et al. showed that the presence of HSF-1 was associated with increased histone H3 acetylation on the IL-6 promoter and with an increase in NFκB recruitment (Inouye et al. 2007). However, we found neither HSF-1 recruitment nor an effect of FRT on acetylation of histone-H3 on the proximal promoter sequence, suggesting that core enhanceosome assembly is modified through alternative mechanisms in FRT-exposed cells. Although H3 acetylation is the predominant histone modification on the proximal TNF-α promoter and is required for transcriptional activation, it is not sufficient for chromatin remodeling. Lee et al. showed that histone H3 and H4 on the proximal TNF-α promoter is constitutively acetylated in human monocytes and does not increase further after stimulation with LPS despite an increase in nucleosome accessibility and activation of TNF-α transcription (Lee et al. 2003). That TNF-α is inducibly transcribed in J774 mouse macrophages in the absence of the ATP-dependent histone remodeling complexes, BRG-1 and Brm (Ramirez-Carrozzi et al. 2006), further suggests that the TNF-α promoter is packaged in a relatively accessible or “poised” chromatin structure in resting mature macrophages. It is important to recognize that while these studies indicate a limited role for chromatin remodeling in inducible TNF-α transcription in euthermic macrophages and we have shown that FRT does not reduce histone H3 acetylation on the TNF-α promoter, we have not excluded other FRT-induced chromatin modification that might contribute to reduce transcription factor recruitment in FRT-exposed macrophages. Alternatively, exposure to FRT may modify interactions among Sp1, Elk-1, and ATF2, which Barthel et al. (2003) showed are required for optimal enhanceosome assembly. For example, interference with recruitment of the co-activator CBP/p300, which binds to all three of these transcription factors (Barthel et al. 2003) to the enhanceosome complex, might reduce its stability and reduce the Sp1 that is associated with the proximal TNF-α promoter region. HSF-1, which we have shown is activated at FRT, has been shown to bind to other transcriptional regulators, such as NF-IL6, STAT-1, TATA-binding protein, and TFIID and B with differing effects on transcription (Stephanou et al. 1999; Xie et al. 2002; Yuan and Gurley 2000). Xie et al. (2002) showed that binding between HSF-1 and c/EBPß/NF-IL6 results in reciprocal inhibition with reduced activation of human c-fms and HSPA1B genes. In contrast, STAT1 and HSF-1 act synergistically to activate HSP-70 and HSP-90 genes (Stephanou et al. 1999). Although we previously showed that HSF-1 was required for maximal repression of TNF-α expression in FRT-exposed macrophages (Singh et al. 2002), we have not determined whether it contributes to the altered enhanceosome formation in FRT-exposed cells.
In summary, we have found that exposure to temperatures that are within the range achieved in fever and heat-related illnesses blocks LPS-induced recruitment of Sp1 to the TNF-α proximal promoter. Taken with our previous studies, these results demonstrate that exposure to FRT represses TNF-α expression through multiple mechanisms.
Acknowledgements
Supported by NIH grants GM069431 (ISS) and GM066855, HL69057 and HL085256 (JDH), AI18797 (SNV), and by VA Merit Review grants to JDH and ISS.
References
- Barthel R, Tsytsykova AV, Barczak AK, Tsai EY, Dascher CC, Brenner MB, Goldfeld AE. Regulation of tumor necrosis factor alpha gene expression by mycobacteria involves the assembly of a unique enhanceosome dependent on the coactivator proteins CBP/p300. Mol Cell Biol. 2003;23:526–533. doi: 10.1128/MCB.23.2.526-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZA, Ghosh A, Gupta A, Maity T, Benjamin IJ, Vogel S, Hasday JD, Singh IS. Febrile range temperature modifies cytokine gene expression in LPS-stimulated macrophages by differentially modifying NF-kb recruitment to cytokine promoters. Am J Physiol Cell Physiol. 2009;298(1):C171–C181. doi: 10.1152/ajpcell.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensor JE, Wiener SM, McCrea KA, Viscardi RM, Crawford EK, Hasday JD. Differential effects of hyperthermia on macrophage interleukin-6 and tumor necrosis factor-a expression. Am J Physiol Cell Physiol. 1994;266:C967–C974. doi: 10.1152/ajpcell.1994.266.4.C967. [DOI] [PubMed] [Google Scholar]
- Ensor JE, Crawford EK, Hasday JD. Warming macrophages to febrile range destabilizes tumor necrosis factor-a mRNA without inducing heat shock. Am J Physiol. 1995;269:C1140–C1146. doi: 10.1152/ajpcell.1995.269.5.C1140. [DOI] [PubMed] [Google Scholar]
- Fairchild KD, Viscardi RM, Hester L, Singh IS, Hasday JD. Effects of hypothermia and hyperthermia on cytokine production by cultured human mononuclear phagocytes from adults and newborns. J Interferon Cytokine Res. 2000;20:1049–1055. doi: 10.1089/107999000750053708. [DOI] [PubMed] [Google Scholar]
- Hasday JD, Singh IS. Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress & Chaperones. 2000;5:471–480. doi: 10.1379/1466-1268(2000)005<0471:FATHSR>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S, Fujimoto M, Nakamura T, Takaki E, Hayashida N, Hai T, Nakai A. Heat shock transcription factor 1 opens chromatin structure of interleukin-6 promoter to facilitate binding of an activator or a repressor. J Biol Chem. 2007;282:33210–33217. doi: 10.1074/jbc.M704471200. [DOI] [PubMed] [Google Scholar]
- Jiang Q, DeTolla L, Kalvakolanu I, Roojien N, Singh IS, Fitzgerald B, Cross AS, Hasday JD. Febrile range temperature modifies early systemic TNFa expression in mice challenged with bacterial endotoxin. Infect Immun. 1999;67:1539–1546. doi: 10.1128/iai.67.4.1539-1546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuprash DV, Udalova IA, Turetskaya RL, Kwiatkowski D, Rice NR, Nedospasov SA. Similarities and differences between human and murine TNF promoters in their response to lipopolysaccharide. J Immunol. 1999;162:4045–4052. [PubMed] [Google Scholar]
- Lee JY, Kim NA, Sanford A, Sullivan KE. Histone acetylation and chromatin conformation are regulated separately at the TNF-alpha promoter in monocytes and macrophages. J Leukoc Biol. 2003;73:862–871. doi: 10.1189/jlb.1202618. [DOI] [PubMed] [Google Scholar]
- Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, Smale ST. Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding protein with mini extracts prepared from a small number of cells. Nucl Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IS, Calderwood S, Kalvakolanu I, Viscardi R, Hasday JD. Inhibition of tumor necrosis factor-a transcription in macrophages exposed to febrile range temperature: a possible role for heat shock factor-1. J Biol Chem. 2000;275:9841–9848. doi: 10.1074/jbc.275.13.9841. [DOI] [PubMed] [Google Scholar]
- Singh I, He J-R, Calderwood S, Hasday J. A high affinity HSF-1 binding site in the 5′-untranslated region of the murine tumor necrosis factor-a gene is a transcriptional repressor. J Biol Chem. 2002;277:4981–4988. doi: 10.1074/jbc.M108154200. [DOI] [PubMed] [Google Scholar]
- Singh I, He J-R, Hester L, Fenton M, Hasday J. Bacterial endotoxin modifies heat shock factor-1 activity in RAW 264.7 cells: implications for TNF_ regulation during exposure to febrile range temperatures. J Endotoxin Res. 2004;10:175–184. doi: 10.1179/096805104225004851. [DOI] [PubMed] [Google Scholar]
- Singh IS, Gupta A, Nagarsekar A, Cooper Z, Manka C, Hester L, Benjamin IJ, He J-E, Hasday JD. Heat shock co-activates interleukin-8 transcription. Am J Respir Cell Molec Biol. 2008;39(2):235–242. doi: 10.1165/rcmb.2007-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou A, Isenberg DA, Nakajima K, Latchman DS. Signal transducer and activator of transcription-1 and heat shock factor-1 interact and activate the transcription of the Hsp-70 and Hsp- 90beta gene promoters. J Biol Chem. 1999;274:1723–1728. doi: 10.1074/jbc.274.3.1723. [DOI] [PubMed] [Google Scholar]
- Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH, Fenton MJ, Gordon DC, Dunn IF, Goldfeld AE. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol Cell Biol. 2000;20:6084–6094. doi: 10.1128/MCB.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Chen C, Stevenson MA, Hume DA, Auron PE, Calderwood SK. NF-IL6 and HSF1 have mutually antagonistic effects on transcription in monocytic cells. Biochem Biophys Res Commun. 2002;291:1071–1080. doi: 10.1006/bbrc.2002.6562. [DOI] [PubMed] [Google Scholar]
- Yuan CX, Gurley WB. Potential targets for HSF1 within the preinitiation complex. Cell Stress Chaperones. 2000;5:229–242. doi: 10.1379/1466-1268(2000)005<0229:PTFHWT>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]