Abstract
Human neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis have been termed “protein misfolding disorders.” Upregulation of heat shock proteins that target misfolded aggregation-prone proteins has been proposed as a potential therapeutic strategy to counter neurodegenerative disorders. The heat shock protein 70 (HSP70) family is well characterized for its cytoprotective effects against cell death and has been implicated in neuroprotection by overexpression studies. HSP70 family members exhibit sequence and structural conservation. The significance of the multiplicity of HSP70 proteins is unknown. In this study, coimmunoprecipitation was employed to determine if association of HSP70 family members occurs, including Hsp70B′ which is present in the human genome but not in mouse and rat. Heteromeric complexes of Hsp70B′, Hsp70, and Hsc70 were detected in differentiated human SH-SY5Y neuronal cells. Hsp70B′ also formed complexes with Hsp40 suggesting a common co-chaperone for HSP70 family members.
Keywords: SH-SY5Y neuronal cells, Hsp70B′, Hsp70, Hsc70, Heteromeric complexes, Celastrol
Introduction
Manipulation of the cellular stress response involving induction of heat shock proteins (Hsps) in differentiated neurons offers a potential therapeutic strategy to counter conformational changes in neuronal proteins that trigger pathogenic cascades resulting in human neurodegenerative disorders (Selkoe 2004; Brown 2007; Haass and Selkoe 2007; Asea and Brown 2008; Brown 2008). Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis (ALS) have been termed “protein misfolding disorders.” Hsps are protein repair agents that provide a line of defense against misfolded, aggregation-prone proteins. The HSP70 family is well characterized for its cytoprotective effects against cell death and has previously been implicated as having neuroprotective capability in overexpression studies (Jana et al. 2000; Auluck et al. 2002; Karunanithi et al. 2002; Magrane et al. 2004; Patel et al. 2005; Rujano et al. 2007).
HSP70 is a multigene family composed of constitutively expressed members such as Hsc70 (HSPA8) and stress-inducible members such as Hsp70 (HSPA1A) and Hsp70B′ (HSPA6; Tavaria et al. 1996; Daugaard et al. 2007; Kampinga et al. 2009). Interestingly, the human genome includes stress-inducible Hsp70B′ that is not found in the mouse and rat genomes and hence, is not present as a potential beneficial factor in animal models of neurodegenerative diseases to combat misfolded proteins (Parsian et al. 2000; Chow and Brown 2007; Noonan et al. 2007a). Although stress-inducible Hsp70B′ and Hsp70 share 84% sequence homology, differences in the substrate-binding pocket and activation profiles may confer Hsp70B′ with distinct cellular roles (Noonan et al. 2007a; Hageman and Kampinga 2009). Hsp70B′ has previously been examined in human colon cells (Noonan et al. 2007a, b; Noonan et al. 2008a, b). Other than our work (Chow and Brown 2007), Hsp70B′ has not received attention in the field of human neurodegenerative diseases.
The significance of the multiplicity of HSP70 proteins is unknown. We have employed co-immunoprecipitation (Co-IP) to determine if association of HSP70 family members occurs, including Hsp70B′. After stringent tests of antibody specificity, heteromeric complexes of HSP70 family members were detected in differentiated human neuronal cells. We also show that Hsp70B′ forms complexes with Hsp40 suggesting a common cochaperone for HSP70 family members. Formation of heteromeric complexes of HSP70 family members could be a potential mechanism for regulatory control of HSP70 specificity in neuronal cells. Human SH-SY5Y neuronal cells were selected for the present study because they have been widely used as a model system for neurodegenerative diseases such as Alzheimer’s disease (Bandyopadhyay et al. 2006; Bate et al. 2006; Westmark et al. 2006) and Parkinson’s disease (Ho et al. 2006; Inden et al. 2006).
Materials and methods
Cell culture and neuronal cell differentiation
The human SH-SY5Y neuronal cell line (American Type Culture Collection, Manassas, VA) was maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum. Cultures were maintained at 37ºC in a humidified 5% CO2 atmosphere. After plating and allowing for cell adhesion for 24 h, neuronal differentiation was induced by treatment with 10 µM of all-trans-retinoic acid at 37ºC for 72 h under serum-free conditions (Chow and Brown 2007).
Treatment of cells
All experiments were performed on differentiated neuronal cell cultures plated at a cell density of 4 × 104 cells per square centimeter. Heat shock was performed by immersion of culture dishes in a circulating water bath calibrated to the required temperature 43 ± 0.1ºC for 10 or 20 min before returning to incubation at 37ºC until harvest at specific time points. Celastrol was obtained from Gaia Chemical Corporation (Gaylordsville, CT) and dissolved in dimethyl sulfoxide (DMSO) as the vehicle (Chow and Brown 2007).
Western blotting analysis
At specific time points, cells were harvested, and samples containing 30 µg of protein per lane were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) with 4% stacking gel and 12% resolving gel, and transferred to nitrocellulose membranes. After blocking for 1 h with 5% skim milk powder in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4), blots were incubated overnight at 4ºC with primary antibodies. The following primary antibodies were used: Hsp70B′ (SPA-754, Stressgen Biotechnologies, Victoria, BC, Canada); Hsp70 (SPA-810, SPA-812, Stressgen; 3096, BioVision, Mountain View, CA; ab45133, Abcam, Cambridge, MA; 554243, Lab Vision, Fremont, CA); Hsc70 (SPA-815, Stressgen); Hsp40 (SPA-400, Stressgen); yellow fluorescent protein (YFP; ab1218, Abcam); and β-tubulin (MAB3408, Chemicon, Temecula, CA). After washes, blots were incubated with horseradish peroxidase-conjugated secondary antibodies (Sigma, St. Louis, MO). Incubations with primary and secondary antibodies were performed in 1% skim milk powder in PBS. After three washes with PBS (containing 0.1% Tween-20), immunoactivity was detected by enhanced chemiluminescence assay (Amersham, Piscataway, NJ) with exposure to BioFlex MSI Film (Clonex, Mississauga, ON, Canada) and Hyperfilm ECL (Amersham). The purified HSP70 proteins employed for testing of antibody specificity were: recombinant human Hsp70B′ protein (SPP-762, Stressgen), recombinant human Hsp70 protein (NSP-555, Stressgen), and recombinant human Hsc70 protein (H00003312-P01, Abnova, Taiwan). Western blots representative of six experimental repeats are shown.
Coimmunoprecipitation
Differentiated human SH-SY5Y neuronal cells (6 × 106 cells) treated with DMSO or 0.75 μM of celastrol for 24 h were homogenized in 200 μl of lysis buffer (20 mM Tris pH 7.4, 20 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 1 mM DTT) in the presence of a protease inhibitor cocktail (Roche Diagnostics, Mississauga, ON, Canada). Coimmunoprecipitation was performed with magnetic Dynabeads preconjugated to secondary antibodies (Dynal Biotech, Lake Success, NY) bypassing the use of traditional protein A or G cross-linked to agarose beads that yields a high background signal due to nonspecific binding of Hsps to agarose beads. Dynabeads preconjugated with secondary antibodies of either sheep anti-mouse immunoglobulin (IgG), sheep anti-rat IgG, or sheep anti-rabbit IgG were washed in 1% bovine serum albumin (BSA) in PBS before complexing with the indicated primary antibodies by incubation for 2 h at 4ºC with slow rotation. The following primary antibodies were used: Hsp70B′ (SPA-754, Stressgen); Hsp70 (SPA-810, Stressgen); Hsc70 (SPA-815, Stressgen); YFP (ab1218, Abcam). Dynabead–antibody complexes were then washed with 1% BSA in PBS prior to incubation with cell lysates for 6 h at 4ºC with slow rotation. After three washes with PBS, Dynabeads were separated from protein complexes by boiling in Laemmli buffer followed by their removal using a magnetic concentrator. The solubilized protein samples were subjected to Western blotting analysis. Data representative of four experimental repeats are shown.
Generation of YFP–Hsp70 fusion construct and stable cell line
A YFP–Hsp70 construct was generated by in-frame insertion of a wild-type human Hsp70 transgene (Chow et al. 2009) into the plasmid pEYFP-C1 (Clontech). This vector expresses YFP (at the N terminus) and wild-type human Hsp70 as a single fusion transcript. Human SH-SY5Y neuronal cells expressing YFP–Hsp70 fusion proteins were selected based on YFP fluorescence by fluorescence-activated cell sorting (FACS; FACSAria flow cytometer, Becton Dickinson, Mississauga, ON, Canada). Expression of YFP–Hsp70 in differentiated neuronal cells was confirmed by Western blotting. Data representative of three experimental repeats are shown.
Results
Induction of Hsp70B′ and Hsp70 in differentiated human neuronal cells
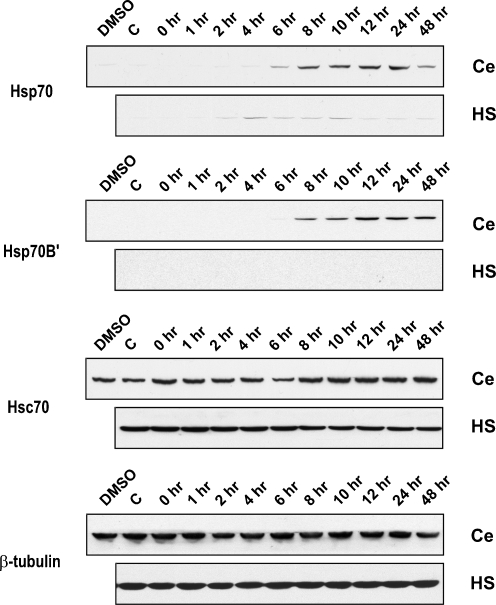
Upregulation of Hsps could alleviate neurodegenerative diseases by modulating protein misfolding in affected neurons (Selkoe 2004; Brown 2007; Haass and Selkoe 2007; Asea and Brown 2008; Brown 2008). This has led to a quest for pharmacological agents that can induce Hsps in differentiated neurons as a potential therapeutic approach for combating neurodegenerative diseases that have been termed “protein misfolding disorders” (Westerheide et al. 2004; Brown 2007; Chow and Brown 2007; Brown 2008). As shown in Fig. 1, conventional heat shock (43ºC for 10 min) results in a weak, transient induction of Hsp70 in differentiated human SH-SY5Y neuronal cells grown in tissue culture. Increasing the severity of the heat shock (43ºC for 20 min) did not alter this (data not shown). These observations are in agreement with previous reports showing that differentiated neurons in both in vivo and in vitro systems are refractory to Hsp induction following conventional heat shock (Manzerra et al. 1993; Foster et al. 1995; Dwyer et al. 1996; Hatayama et al. 1997; Batulan et al. 2003). However, treatment with celastrol triggered a robust and sustained Hsp70 induction at a dosage of 0.75 µM that does not affect neuronal viability (Chow and Brown 2007). Celastrol also induced a robust and sustained induction of Hsp70B′, whereas it was not detected following conventional heat shock (Fig. 1). Celastrol was therefore employed as an inducer of Hsps in subsequent coimmunoprecipitation experiments to determine if HSP70 family members form heteromeric complexes in differentiated human neuronal cells. Constitutively expressed Hsc70 was detected, and the signal showed little change following either celastrol treatment or conventional heat shock (Fig. 1).
Fig. 1.
Expression of HSP70 family proteins in differentiated human neuronal cells following treatment with celastrol. Differentiated human SH-SY5Y neuronal cells were treated with either 0.75 µM of celastrol (Ce), or heat shock (HS) at 43ºC for 10 min, and allowed to recover at 37ºC for time points up to 48 h. Robust and sustained expression of Hsp70 and Hsp70B′ was induced by celastrol, but not by heat shock. Levels of constitutive Hsc70 showed little change following either celastrol or heat shock treatments. β-tubulin was used as a loading control. Antibodies employed for the detection of HSP70 family proteins: Hsp70 (SPA-810), Hsp70B′ (SPA-754), and Hsc70 (SPA-815). DMSO, Cells treated with DMSO, the celastrol vehicle; C, cells receiving no DMSO or celastrol
Specificity of antibodies for HSP70 family proteins
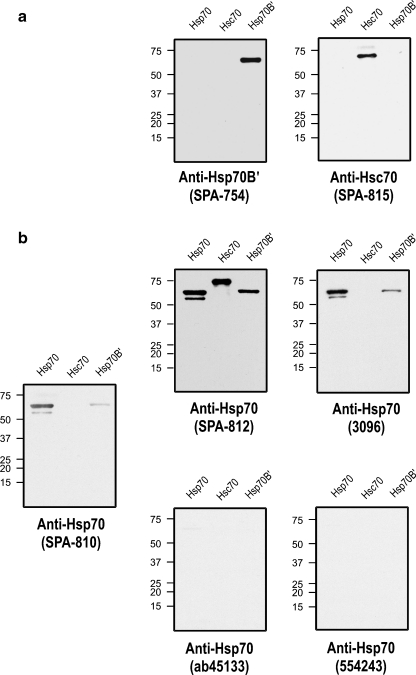
The HSP70 family is highly conserved, and Hsp70B′, Hsp70, and Hsc70 exhibit high sequence and structural homology (Daugaard et al. 2007). As shown in Fig. 2a, the antibodies employed to detect Hsp70B′ and Hsc70 are highly specific and did not cross-react with either Hsp70 and Hsc70 or Hsp70B′ and Hsp70, respectively. Screening of several commercially available Hsp70 antibodies revealed cross-reactivity to Hsp70B′ and Hsc70 (SPA-812, 3096) or no detectable signal (ab45133, 554243; Fig. 2b). The commonly used Hsp70 antibody, SPA-810, did not cross-react to Hsc70, however, cross-reactivity to Hsp70B′ was observed.
Fig. 2.
Specificity of antibodies used to detect HSP70 family proteins. Purified recombinant human proteins (0.5 µg) of Hsp70, Hsc70, and Hsp70B′ were subjected to Western blot analysis. a For the detection of Hsp70B′ and Hsc70, antibodies SPA-754 and SPA-815 were found to be specific. b Screening of Hsp70 antibodies was performed. Commercially available anti-Hsp70 antibodies either cross-reacted with Hsp70B′ (SPA-812, 3096, and the commonly used SPA-810) or failed to detect a signal (ab45133, 554243)
Heteromeric HSP70 complexes in differentiated human neuronal cells
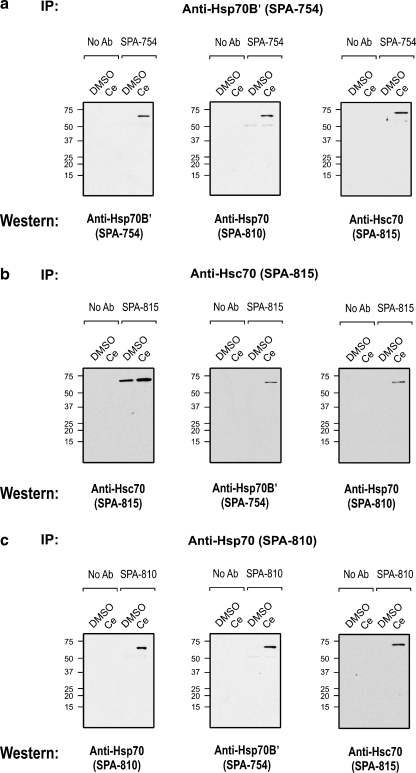
Co-IP was employed to determine if HSP70 family members form complexes in differentiated human neuronal cells treated with the Hsp70/Hsp70B′ inducer celastrol as described in Fig. 1. Co-IP analysis using the Hsp70B′-specific antibody demonstrated complex formation of Hsp70B′ protein with both Hsp70 and Hsc70 in samples derived from celastrol-treated cells that induced Hsp70B′ and Hsp70, but not in control cells treated with DMSO, the celastrol vehicle (Fig. 3a).
Fig. 3.
Heteromeric complexes of HSP70 family proteins Hsp70B′, Hsp70, and Hsc70 in differentiated human neuronal cells. a Co-IP using the Hsp70B′-specific antibody demonstrated complex formation of Hsp70B′ with Hsp70 and Hsc70. b Co-IP of Hsc70 showed association of Hsc70 with Hsp70B′ and Hsp70. c Co-IP of Hsp70 suggested its association with Hsp70B′ and Hsc70. IP, antibody used for Co-IP; Western, antibody used for immunodetection of Co-IP pull-down product
In support of this observation, Co-IP with the Hsc70-specific antibody revealed complex formation of Hsc70 with both Hsp70B′ and Hsp70 in celastrol-treated cells but not in control cells (Fig. 3b). Self-immunoprecipitation with anti-Hsc70 antibody detected the constitutively expressed Hsc70 protein in both celastrol-treated and control cells. Fig. 3c suggests that Hsp70 formed complexes with Hsp70B′ and Hsc70.
Coimmunoprecipitation analysis with YFP–Hsp70 fusion protein
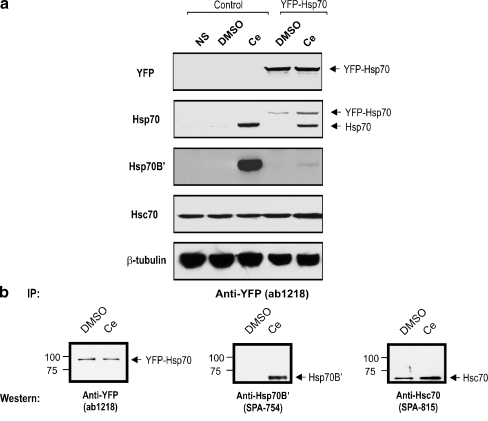
The antibody specificity tests shown in Fig. 2b demonstrated cross-reactivity of the commonly used Hsp70 antibody (SPA-810) to Hsp70B′ that might be amplified by the concentrating effect of co-immunoprecipitation. We therefore generated a stable neuronal cell line expressing a YFP fusion construct of the Hsp70 protein (YFP–Hsp70). The objective was to bypass the specificity issue of the SPA-810 Hsp70 antibody by use of a YFP-specific antibody for Co-IP analysis to pull down YFP–Hsp70. The YFP–Hsp70 fusion protein was detected in the stably transfected neuronal cell line and can be distinguished from celastrol-induced Hsp70 by specific detection with the YFP antibody and by its higher molecular weight (Fig. 4a).
Fig. 4.
Co-immunoprecipitation of YFP–Hsp70 fusion protein with Hsp70B′ and Hsc70. a A human SH-SY5Y neuronal cell line stably expressing YFP–Hsp70 fusion protein was generated to bypass the need for the use of an anti-Hsp70 antibody in the Co-IP assay. Stable expression of YFP–Hsp70 fusion protein was detected with an anti-YFP antibody and by the increased molecular weight of the signal. β-tubulin was used as a loading control. b Co-IP was performed with the YFP-specific antibody. Immunodetection with YFP antibody demonstrated successful pull-down of YFP–Hsp70 fusion protein. Association of Hsp70B′ and Hsc70 with the YFP–Hsp70 fusion protein was detected in the pull-down products
Immunodetection with the YFP antibody demonstrated successful pull-down of the YFP–Hsp70 fusion protein (Fig. 4b). Analysis of YFP–Hsp70 pull-down products showed the association of YFP–Hsp70 with both Hsp70B′ and Hsc70. This indicates that Hsp70 forms complexes with Hsp70B′ and Hsc70 in differentiated human neuronal cells.
Association of Hsp70B′ with Hsp40
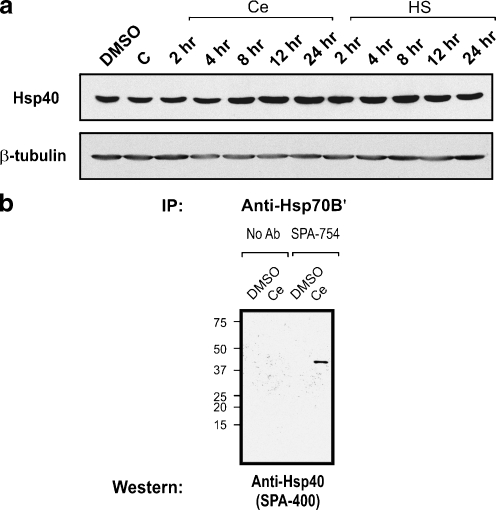
Hsp40 has been shown to interact with HSP70 family proteins as a co-chaperone (Fan et al. 2003; Hennessy et al. 2005). However, it is not known if this is the case for Hsp70B′ which is present in the human but not in the mouse or rat genomes. As shown in Fig. 5a, Hsp40 was detected in differentiated human neuronal cells. Co-IP revealed that Hsp70B′ formed complex with Hsp40 (Fig. 5b).
Fig. 5.
Association of Hsp70B′ with Hsp40. a Constitutive expression of Hsp40 was detected in differentiated human neuronal cells under all conditions examined (DMSO, cells treated with DMSO, the celastrol vehicle; C, cells receiving no DMSO or celastrol; Ce, cells treated with celastrol at 0.75 µM; HS, cells heat shocked at 43ºC for 10 min). β-tubulin was used as a loading control. b Co-IP of Hsp70B′ demonstrated its association with Hsp40
Discussion
The HSP70 family of heat shock (stress) proteins has been well characterized for its roles in protein homeostasis, cytoprotection, and cellular signaling (Arya et al. 2007; Morano 2007; Meimaridou et al. 2009). Interestingly, eukaryotes exhibit multiple genes that encode a family of HSP70 family proteins that are highly conserved in sequence and structure (Daugaard et al. 2007). In human, the HSP70 protein family is comprised of constitutively expressed members (Hsc70) and stress-inducible members (Hsp70), including Hsp70B′ that is not found in the mouse and rat genomes (Daugaard et al. 2007; Kampinga et al. 2009). The significance of this multiplicity of HSP70 proteins is unknown.
HSP70 family members including Hsp70, Hsc70, and the endoplasmic reticulum member BiP have been shown to self-associate (Freiden et al. 1992; Benaroudj et al. 1995; Wei et al. 1995; Gao et al. 1996; Angelidis et al. 1999; Nemoto et al. 2006). In the present report, we demonstrate that Hsp70B′, Hsp70, and Hsc70 form heteromeric complexes in differentiated human neuronal cells. Association of HSP70 family members has been noted in non-neural cells using the commonly used anti-Hsp70 antibody SPA-810 (Brown et al. 1993; Noonan et al. 2008a). Screening of the SPA-810 antibody showed cross-reactivity to Hsp70B′. A YFP–Hsp70 fusion protein was therefore employed to demonstrate that Hsp70B′, Hsp70, and Hsc70 form heteromeric complexes in differentiated human neuronal cells.
HSP70 multiplicity has generally been presumed to serve the purpose of increasing the copy number of HSP70 proteins with similar functions (Angelidis et al. 1999). However, a different picture of HSP70 family diversity and specificity is emerging (Daugaard et al. 2007; Vos et al. 2008). For example, Hsp70 and Hsc70 differ in their ability to interact with lipids, with Hsc70 being more effective in promoting liposome aggregation (Arispe et al. 2002). Hsp70 demonstrates an enhanced ability to associate with immunogenic peptides compared to Hsc70 (Callahan et al. 2002). Colocalization with actin microfilament under physiological growth conditions is observed for Hsp70, but not for Hsc70, in mouse mesoangioblast cells (Turturici et al. 2008). Although little is known about Hsp70B′, recent work in human colon cells has shown that the inhibition of either Hsp70B′ or Hsp70 induction by small interfering RNA renders cells sensitive to stress, suggesting that both proteins play indispensable roles in the protection of cells against stress-induced cell death (Noonan et al. 2007a). These observations support the suggestion that HSP70 multiplicity does not solely serve the purpose of increasing HSP70 copy number but could contribute to functional diversity of HSP70 proteins (Daugaard et al. 2007; Vos et al. 2008).
Structural studies to pinpoint the region involved in self-association have focused on Hsc70 (Angelidis et al. 1999; Fouchaq et al. 1999; Chou et al. 2003; Nemoto et al. 2006; Amor-Mahjoub et al. 2007). The regions essential for self-association of Hsc70 were found to be the conserved hydrophobic residues within the interdomain linker region and the helices at the end of the C terminus (Amor-Mahjoub et al. 2007). Comparing the amino acid sequence of human Hsp70B′, Hsp70, and Hsc70, the highest sequence diversity exists in the last 110 amino acids of the C terminus. Differences in the patterns of self-association of Hsc70 and Hsp70 have been reported, with Hsc70 having a tendency to remain self-associated under both resting and heat shock conditions, while Hsp70 alters its self-association pattern after heat stress (Angelidis et al. 1999). Formation of heteromeric complexes of HSP70 proteins could add an additional level of regulatory control of HSP70 function that is based on the multiplicity of HSP70 proteins. Yeast HSP70 family members utilize multiplicity of HSP70 proteins for task delegation. The yeast Ssa subfamily of HSP70 proteins focuses on broad spectrum protein folding, while the yeast Ssb family interacts with a more specific subset of targets (Boorstein et al. 1994; Craig et al. 1994).
Recently, Hsp110 and Grp170 have been grouped within the HSP70 family and termed “atypical” HSP70 family members (Shaner and Morano 2007). These “atypical” HSP70 members share identical structural domains with typical HSP70 family proteins but exhibit a longer C-terminal loop linking the β-sandwich and the α-helical lid within the substrate binding domain. In support of the suggestion that heteromeric complex formation of HSP70 proteins is a potential mechanism for regulatory control of HSP70 function, it has been shown that “atypical” HSP70 members function as nucleotide exchange factors regulating HSP70 chaperone function (Morano 2007; Shaner and Morano 2007).
Interestingly, HSP70 heteromeric complex formation is not restricted to inducible members. Our present results indicate that a constitutive member of the HSP70 family, Hsc70, forms heteromeric complexes with stress-inducible members such as Hsp70B′ and Hsp70. This suggests that constitutively expressed Hsc70 is a player in the neuronal stress response, rather than solely serving housekeeping functions under non-stress conditions. We have previously reported high levels of constitutively expressed Hsc70 in the mammalian nervous system (Manzerra et al. 1997), and have proposed that neurons may utilize constitutively expressed Hsc70 as a “pre-protection” mechanism for defense against misfolded proteins triggered by stress or associated with neurodegenerative diseases (Chen and Brown 2007a, b). Here, we provide experimental evidence that constitutively expressed Hsc70 associates with stress-inducible Hsp70B′ and Hsp70 following the induction of stress-inducible HSP70 members. This supports our suggestion that constitutive and stress-inducible Hsps play cooperative roles in the neuronal stress response and neuroprotective mechanisms.
The future direction of our work will be to investigate the functional roles of heteromeric complexes of HSP70 family members. Additional techniques, including native gel electrophoresis, will be employed to further our understanding of the interaction of HSP70 family proteins in differentiated human neuronal cells.
Human neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and ALS have been termed “protein misfolding disorders.” Upregulation of Hsps has been proposed as a potential therapeutic strategy to counter conformational changes in neuronal proteins that trigger pathogenic cascades resulting in neurodegenerative disorders (Selkoe 2004; Muchowski and Wacker 2005; Brown 2007; Haass and Selkoe 2007; Asea and Brown 2008; Brown 2008). Hsps are protein repair agents that provide a line of defense against misfolded aggregation-prone proteins. This has led to a search for pharmacological agents that can induce Hsps in differentiated neurons (Westerheide et al. 2004; Brown 2007; Chow and Brown 2007; Brown 2008). We have demonstrated that celastrol induces a set of Hsps in differentiated human neuronal cells that includes Hsp70B′ that is present in human genome but not in mouse and rat (Chow and Brown 2007). Our present results indicate that, in contrast to conventional heat shock treatment, celastrol triggers a robust and sustained induction of Hsp70B′ and Hsp70 in differentiated human neuronal cells.
Acknowledgments
We thank Erin Chang, Abelyn K. Lim, and Nasim M. Zamir for technical assistance. This study was supported by grants to I.R.B. from National Science and Engineering Research Council of Canada.
References
- Amor-Mahjoub M, Gomez-Vrielyunck N, Suppini JP, Fouchaq B, Benaroudj N, Ladjimi M. Analysis of monomeric mutants of HSC70: a possible relationship between oligomerization and functional properties. Protein Pept Lett. 2007;14:761–765. doi: 10.2174/092986607781483624. [DOI] [PubMed] [Google Scholar]
- Angelidis CE, Lazaridis I, Pagoulatos GN. Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur J Biochem. 1999;259:505–512. doi: 10.1046/j.1432-1327.1999.00078.x. [DOI] [PubMed] [Google Scholar]
- Arispe N, Doh M, Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones. 2002;7:330–338. doi: 10.1379/1466-1268(2002)007<0330:LIDTCA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya R, Mallik M, Lakhotia SC. Heat shock genes—integrating cell survival and death. J Biosci. 2007;32:595–610. doi: 10.1007/s12038-007-0059-3. [DOI] [PubMed] [Google Scholar]
- Asea AA, Brown IR, editors. Heat shock proteins and the brain: implications for neurodegenerative diseases and neuroprotection. New York: Springer Science Publishers; 2008. [Google Scholar]
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Ni J, Ruggiero A, Walshe K, Rogers MS, Chattopadhyay N, Glicksman MA, Rogers JT. A high-throughput drug screen targeted to the 5′untranslated region of Alzheimer amyloid precursor protein mRNA. J Biomol Screen. 2006;11:469–480. doi: 10.1177/1087057106287271. [DOI] [PubMed] [Google Scholar]
- Bate C, Kempster S, Last V, Williams A. Interferon-gamma increases neuronal death in response to amyloid-beta1-42. J Neuroinflammation. 2006;3:7. doi: 10.1186/1742-2094-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batulan Z, Shinder GA, Minotti S, He BP, Doroudchi MM, Nalbantoglu J, Strong MJ, Durham HD. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaroudj N, Batelier G, Triniolles F, Ladjimi MM. Self-association of the molecular chaperone HSC70. Biochemistry. 1995;34:15282–15290. doi: 10.1021/bi00046a037. [DOI] [PubMed] [Google Scholar]
- Boorstein WR, Ziegelhoffer T, Craig EA. Molecular evolution of the HSP70 multigene family. J Mol Evol. 1994;38:1–17. doi: 10.1007/BF00175490. [DOI] [PubMed] [Google Scholar]
- Brown CR, Martin RL, Hansen WJ, Beckmann RP, Welch WJ. The constitutive and stress inducible forms of hsp 70 exhibit functional similarities and interact with one another in an ATP-dependent fashion. J Cell Biol. 1993;120:1101–1112. doi: 10.1083/jcb.120.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IR. Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci. 2007;1113:147–158. doi: 10.1196/annals.1391.032. [DOI] [PubMed] [Google Scholar]
- Brown IR. Heat shock proteins at the synapse: implications for functional protection of the nervous system. In: Asea AA, Brown IR, editors. Heat shock proteins and the brain: implications for neurodegenerative diseases and neuroprotection. New York: Springer Science Publishers; 2008. pp. 239–254. [Google Scholar]
- Callahan MK, Chaillot D, Jacquin C, Clark PR, Menoret A. Differential acquisition of antigenic peptides by Hsp70 and Hsc70 under oxidative conditions. J Biol Chem. 2002;277:33604–33609. doi: 10.1074/jbc.M202890200. [DOI] [PubMed] [Google Scholar]
- Chen S, Brown IR. Translocation of constitutively expressed heat shock protein Hsc70 to synapse-enriched areas of the cerebral cortex after hyperthermic stress. J Neurosci Res. 2007;85:402–409. doi: 10.1002/jnr.21124. [DOI] [PubMed] [Google Scholar]
- Chen S, Brown IR. Neuronal expression of constitutive heat shock proteins: implications for neurodegenerative diseases. Cell Stress Chaperones. 2007;12:51–58. doi: 10.1379/CSC-236R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CC, Forouhar F, Yeh YH, Shr HL, Wang C, Hsiao CD. Crystal structure of the C-terminal 10-kDa subdomain of Hsc70. J Biol Chem. 2003;278:30311–30316. doi: 10.1074/jbc.M304563200. [DOI] [PubMed] [Google Scholar]
- Chow AM, Brown IR. Induction of heat shock proteins in differentiated human and rodent neurons by celastrol. Cell Stress Chaperones. 2007;12:237–244. doi: 10.1379/CSC-269.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AM, Steel R, Anderson RL. Hsp72 chaperone function is dispensable for protection against stress-induced apoptosis. Cell Stress Chaperones. 2009;14:253–263. doi: 10.1007/s12192-008-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Baxter BK, Becker J, Halladay J, Ziegelhoffer T. Cytosolic hsp70s of Saccharomyces cerevisiae: roles in protein synthesis, protein translocation, proteolysis, and regulation. In: Morimoto RI, Tissieres A, Georgopolous C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1994. pp. 31–52. [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Liu Y, Miao S, Bradley RJ. Neuronal differentiation in PC12 cells is accompanied by diminished inducibility of Hsp70 and Hsp60 in response to heat and ethanol. Neurochem Res. 1996;21:659–666. doi: 10.1007/BF02527722. [DOI] [PubMed] [Google Scholar]
- Fan CY, Lee S, Cyr DM. Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones. 2003;8:309–316. doi: 10.1379/1466-1268(2003)008<0309:MFROHF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, Rush SJ, Brown IR. Localization of constitutive and hyperthermia-inducible heat shock mRNAs (hsc70 and hsp70) in the rabbit cerebellum and brainstem by non-radioactive in situ hybridization. J Neurosci Res. 1995;41:603–612. doi: 10.1002/jnr.490410506. [DOI] [PubMed] [Google Scholar]
- Fouchaq B, Benaroudj N, Ebel C, Ladjimi MM. Oligomerization of the 17-kDa peptide-binding domain of the molecular chaperone HSC70. Eur J Biochem. 1999;259:379–384. doi: 10.1046/j.1432-1327.1999.00053.x. [DOI] [PubMed] [Google Scholar]
- Freiden PJ, Gaut JR, Hendershot LM. Interconversion of three differentially modified and assembled forms of BiP. EMBO J. 1992;11:63–70. doi: 10.1002/j.1460-2075.1992.tb05028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Eisenberg E, Greene L. Effect of constitutive 70-kDa heat shock protein polymerization on its interaction with protein substrate. J Biol Chem. 1996;271:16792–16797. doi: 10.1074/jbc.271.28.16792. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hageman J, Kampinga HH. Computational analysis of the human HSPH/HSPA/DNAJ family and cloning of a human HSPH/HSPA/DNAJ expression library. Cell Stress Chaperones. 2009;14:1–21. doi: 10.1007/s12192-008-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatayama T, Takahashi H, Yamagishi N. Reduced induction of HSP70 in PC12 cells during neuronal differentiation. J Biochem. 1997;122:904–910. doi: 10.1093/oxfordjournals.jbchem.a021851. [DOI] [PubMed] [Google Scholar]
- Hennessy F, Nicoll WS, Zimmermann R, Cheetham ME, Blatch GL. Not all J domains are created equal: implications for the specificity of Hsp40–Hsp70 interactions. Protein Sci. 2005;14:1697–1709. doi: 10.1110/ps.051406805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PW, Chu AC, Kwok KH, Kung MH, Ramsden DB, Ho SL. Knockdown of uncoupling protein-5 in neuronal SH-SY5Y cells: effects on MPP+-induced mitochondrial membrane depolarization, ATP deficiency, and oxidative cytotoxicity. J Neurosci Res. 2006;84:1358–1366. doi: 10.1002/jnr.21034. [DOI] [PubMed] [Google Scholar]
- Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, Takata K, Yanagisawa D, Nishimura K, Taniguchi T, Kiso Y, Yoshimoto K, Agatsuma T, Koide-Yoshida S, Iguchi-Ariga SM, Shimohama S, Ariga H. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson’s disease rat model. Neurobiol Dis. 2006;24:144–158. doi: 10.1016/j.nbd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Jana NR, Tanaka M, Wang G, Nukina N. Polyglutamine length-dependent interaction of Hsp40 and Hsp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity. Hum Mol Genet. 2000;9:2009–2018. doi: 10.1093/hmg/9.13.2009. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithi S, Barclay JW, Brown IR, Robertson RM, Atwood HL. Enhancement of presynaptic performance in transgenic Drosophila overexpressing heat shock protein Hsp70. Synapse. 2002;44:8–14. doi: 10.1002/syn.10048. [DOI] [PubMed] [Google Scholar]
- Magrane J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzerra P, Rush SJ, Brown IR. Temporal and spatial distribution of heat shock mRNA and protein (hsp70) in the rabbit cerebellum in response to hyperthermia. J Neurosci Res. 1993;36:480–490. doi: 10.1002/jnr.490360414. [DOI] [PubMed] [Google Scholar]
- Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997;170:130–137. doi: 10.1002/(SICI)1097-4652(199702)170:2<130::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Meimaridou E, Gooljar SB, Chapple JP. From hatching to dispatching: the multiple cellular roles of the Hsp70 molecular chaperone machinery. J Mol Endocrinol. 2009;42:1–9. doi: 10.1677/JME-08-0116. [DOI] [PubMed] [Google Scholar]
- Morano KA. New tricks for an old dog: the evolving world of Hsp70. Ann N Y Acad Sci. 2007;1113:1–14. doi: 10.1196/annals.1391.018. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Nemoto TK, Fukuma Y, Itoh H, Takagi T, Ono T. A disulfide bridge mediated by cysteine 574 is formed in the dimer of the 70-kDa heat shock protein. J Biochem. 2006;139:677–687. doi: 10.1093/jb/mvj071. [DOI] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Giardina C, Hightower LE. Hsp70B′ regulation and function. Cell Stress Chaperones. 2007;12:393–402. doi: 10.1379/CSC-278e.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Rasoulpour RJ, Giardina C, Hightower LE. Cell number-dependent regulation of Hsp70B′ expression: evidence of an extracellular regulator. J Cell Physiol. 2007;210:201–211. doi: 10.1002/jcp.20875. [DOI] [PubMed] [Google Scholar]
- Noonan E, Giardina C, Hightower L. Hsp70B′ and Hsp72 form a complex in stressed human colon cells and each contributes to cytoprotection. Exp Cell Res. 2008;314:2468–2476. doi: 10.1016/j.yexcr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Noonan EJ, Fournier G, Hightower LE. Surface expression of Hsp70B′ in response to proteasome inhibition in human colon cells. Cell Stress Chaperones. 2008;13:105–110. doi: 10.1007/s12192-007-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsian AJ, Sheren JE, Tao TY, Goswami PC, Malyapa R, Rheeden R, Watson MS, Hunt CR. The human Hsp70B gene at the HSPA7 locus of chromosome 1 is transcribed but non-functional. Biochim Biophys Acta. 2000;1494:201–205. doi: 10.1016/s0167-4781(00)00203-7. [DOI] [PubMed] [Google Scholar]
- Patel YJ, Payne Smith MD, Belleroche J, Latchman DS. Hsp27 and Hsp70 administered in combination have a potent protective effect against FALS-associated SOD1-mutant-induced cell death in mammalian neuronal cells. Brain Res Mol Brain Res. 2005;134:256–274. doi: 10.1016/j.molbrainres.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Rujano MA, Kampinga HH, Salomons FA. Modulation of polyglutamine inclusion formation by the Hsp70 chaperone machine. Exp Cell Res. 2007;313:3568–3578. doi: 10.1016/j.yexcr.2007.07.034. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–638. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- Shaner L, Morano KA. All in the family: atypical Hsp70 chaperones are conserved modulators of Hsp70 activity. Cell Stress Chaperones. 2007;12:1–8. doi: 10.1379/CSC-245R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:AHSGTT>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturici G, Geraci F, Candela ME, Giudice G, Gonzalez F, Sconzo G. Hsp70 localizes differently from chaperone Hsc70 in mouse mesoangioblasts under physiological growth conditions. J Mol Histol. 2008;39:571–578. doi: 10.1007/s10735-008-9197-7. [DOI] [PubMed] [Google Scholar]
- Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- Wei J, Gaut JR, Hendershot LM. In vitro dissociation of BiP-peptide complexes requires a conformational change in BiP after ATP binding but does not require ATP hydrolysis. J Biol Chem. 1995;270:26677–26682. doi: 10.1074/jbc.270.44.26677. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- Westmark PR, Shin HC, Westmark CJ, Soltaninassab SR, Reinke EK, Malter JS. Decoy mRNAs reduce beta-amyloid precursor protein mRNA in neuronal cells. Neurobiol Aging. 2006;27:787–796. doi: 10.1016/j.neurobiolaging.2006.03.003. [DOI] [PubMed] [Google Scholar]