Abstract
Although immune reactions against heat shock proteins have been implicated in the pathogenesis of atherosclerosis, conflicting associations between Hsp70, anti-Hsp70 antibody and coronary heart disease (CHD) have been reported. This study assessed whether there is a significant association between extracellular human Hsp70, anti-Hsp70 antibody and acute coronary syndrome (ACS) and stable angina (SA), and examined dynamic changes in Hsp70 and anti-Hsp70 antibody levels induced by acute myocardial infarction (AMI). Plasma Hsp70 and anti-Hsp70 antibody levels in 291 patients with ACS (179 AMI, 112 unstable angina), 126 patients with SA and 417 age and sex-matched healthy subjects, and in 40 patients after admission for AMI, and on day 2, 3, and 7 after the onset of AMI were determined using enzyme-linked immunosorbent assays. Hsp70 levels were significantly higher in ACS and SA and anti-Hsp70 antibody levels were only markedly lower in ACS than controls. After adjustment for traditional CHD risk factors, increasing levels of Hsp70 were significantly associated with an increased risk and severity of ACS (P for trend < 0.001), whereas increasing levels of anti-Hsp70 antibody were associated with a decreased risk of ACS (P for trend = 0.0003). High levels of Hsp70 combined with low levels of anti-Hsp70 antibody had a joint effect on the risk of ACS (OR, 5.14, 95% CI, 3.00-8.79; P < 0.0001). In patients with AMI, Hsp70 levels decreased rapidly from days 1-7 after onset, whereas anti-Hsp70 antibody levels increased in patients with AMI. These findings suggest that higher Hsp70 levels or lower anti-Hsp70 antibody levels are independently associated with a higher risk of ACS. Higher Hsp70 levels and lower anti-Hsp70 antibody levels combine to further increase this risk.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-010-0180-3) contains supplementary material, which is available to authorized users.
Keywords: Heat shock protein 70, Antibody, Heart diseases, Acute coronary syndrome
Introduction
Accumulating evidence suggests that an immunological mechanism is involved in the pathogenesis of atherosclerosis, including autoimmune reactions against heat shock (stress) proteins (Hsps; Wick et al. 2004). Hsps are typically regarded as being intracellular proteins. However, under particular conditions, they can be released into the extracellular environment (Hightower and Guidon 1989), in which they can act as autoantigenic agents (Asea 2005). A number of studies have shown that Hsps and their corresponding autoantibodies play a crucial role in the pathogenesis and/or prognosis of certain diseases (Pockley et al. 2002; Pockley et al. 2003; Prohaszka et al. 2001; Wu and Tanguay 2006; Zhu et al. 2001). Several investigations, including some of our own, have identified that human Hsp60 and anti-Hsp60 antibodies are independently associated with the development and severity of coronary heart disease (CHD; Prohaszka et al. 2001; Zhang et al. 2008a; Zhang et al. 2008b; Zhu et al. 2001).
Hsp70 has been studied extensively, and circulating Hsp70 and anti-Hsp70 antibody have been found in the peripheral circulation of normal individuals (Pockley et al. 1998). Elevation of circulating Hsp70 and anti-Hsp70 antibody has also been observed in certain diseases states, such as different types of vascular disease (Chan et al. 1999; Wright et al. 2000), chronic heart failure (Genth-Zotz et al. 2004), after acute myocardial infarction (AMI; Dybdahl et al. 2005), dilated cardiomyopathy (Portig et al. 1997), and electrocardiography abnormality (Yuan et al. 2005), thereby suggesting that Hsp70 and anti-Hsp70 antibody might possibly play a harmful role in the pathogenesis and progression of atherosclerosis or cardiovascular diseases. However, limited epidemiological studies have reported conflicting associations between Hsp70, anti-Hsp70 antibody and CHD. Zhu et al. (2003) found that increased serum levels of Hsp70 are associated with low CHD risk, but that there is no association between anti-Hsp70 IgG antibody seropositivity and the prevalence of CHD (n = 421). Kocsis et al. (Kocsis et al. 2002) also failed to find any significant difference in anti-Hsp70 antibody levels between patients with severe CHD patients and healthy subjects (n = 99 pairs). A small study has documented that patients with stable (n = 40) and unstable angina (n = 91) exhibit lower serum anti-Hsp70 antibody levels compared to controls (n = 18; Herz et al., 2006). In addition, the presence of anti-Hsp70 IgG indicated a better outcome in patients suffering from severe angina undergoing coronary artery bypass grafting (n = 17; Vogt et al. 2004).
These discrepant results might be related to both different patient profiles and different inclusion criteria. Although the pathogenesis in various phenotypes of CHD may differ, no studies have clinically subdivided CHD events into acute and chronic presentations, such as acute coronary syndrome (ACS) and stable angina (SA). Therefore, we investigated if there was association between Hsp70 or anti-Hsp70 antibody levels and the risk or severity of ACS and SA, as well as the possible joint effects of Hsp70 and anti-Hsp70 antibody on ACS and SA in a case-control study. We further explored this relationship by evaluating plasma Hsp70 and anti-Hsp70 antibody levels in a prospective observational study in AMI patients.
Subjects and methods
Study population
The case-control study was composed of 417 CHD patients and 417 age (±1 years) and sex-matched healthy controls. The study design and assessment of demographic data, lifestyle, and medical history of the subjects have been described in detail previously (Zhang et al. 2008a). Briefly, patients from 40 to 79 years old who were hospitalized at three hospitals (Tongji Hospital, Union Hospital, and Wugang Hospital) in Wuhan (Hubei, China) between May 2006 and September 2007 were consecutively recruited for the present study. All patients with CHD having significant clinical symptoms or typical electrocardiogram (ECG) abnormalities, and enzymatic abnormalities underwent coronary angiography, and exhibited stenoses ≥ 50% in at least one major coronary artery. Patients with congenital heart disease and vascular disease were excluded. Overall patients with CHD comprised 291 ACS and 126 SA patients, and ACS patients included 179 AMI and 112 unstable angina (UA). The diagnosis of AMI was based on the following criteria (Alpert et al. 2000; Galvani et al. 2002): (1) chest pain lasting longer than 20 min, (2) development of pathologic Q waves on the ECG or ST segment elevation or depression, and (3) elevation of biochemical markers of myocardial necrosis (preferably troponin). UA was defined as new onset of severe angina, accelerated angina, or angina at rest. SA was considered as no change in frequency, duration, or intensity of symptoms within 6 weeks before admission (Mintz et al. 2003; Ozdemir et al. 2008). Fasting blood samples were collected into the ethylenediaminetetraacetic acid (EDTA)-treated tubes from the patients on the morning following admission. Controls were randomly selected from healthy subjects residing in the same communities as the cases on the basis of medical history, clinical examinations, and electrocardiography. None of these exhibited clinical or diagnostic evidence of CHD, nor had received any intervention therapy for CHD.
The prospective study was conducted in 40 patients with AMI who were consecutively admitted (October 2007 to November 2008) to Wugang and Wuhan Second Hospital within 12 h of the onset of symptoms, and only at daytime. Blood samples were drawn immediately after admission, on the following morning (12-24 h after admission), and on 3 and 7 days after the admission blood draw. The average time from symptom onset to the initial draw was 2.5 ± 2.2 h.
Structured questionnaires were used to collect information on demographic variables, medical history, medications, and lifestyle habits by trained interviewers. The Ethics Committee of Tongji Medical College approved this study and written informed consent was obtained from each subject.
Assay of plasma Hsp70 and anti-Hsp70 antibody levels
Hsp70 and anti-Hsp70 antibody levels were measured in EDTA-plasma using Hsp70 and anti-human Hsp70 antibody (total IgA/G/M) enzyme-linked immunosorbent assay (ELISA) kits (Stressgen Biotechnologies Corp, EKS-715 and EKS-750, Victoria, British Columbia, Canada, now Assay Designs, Ann Arbor, USA) with a sensitivity of 0.09 and 6.79 ng/mL, respectively. The Hsp70 assay only detects inducible Hsp70 and does not detect other Hsp70 family members such as constitutive Hsp70, Grp78, DnaK (Escherichia coli), or Hsp71 (Mycobacterium tuberculosis). The inter-assay and intra-assay coefficients of variation of the assays was <10%.
Other clinical assays
Fasting glucose, total cholesterol, and triglyceride were assayed using standard laboratory procedures in the Department of Clinical Laboratory at the Union Hospital, Tongji Medical College. The markers of myocardial necrosis (creatine phosphokinase MB (CKMB) and cardiac troponin I (cTNI)) were measured by the Department of Clinical Laboratory at Wugang and Wuhan Second Hospitals.
Statistical analyses
Hsp70 and anti-Hsp70 antibody levels exhibited a skewed distribution and were log transformed (log10), or divided into groups based on a priori selected cut points. Continuous variables were analyzed using two-tailed t test and repeated measures ANOVA for two or more group comparisons, whereas categorical data were analyzed using the χ2 test. A logistic regression analysis was used to evaluate associations between Hsp70 or anti-Hsp70 antibody levels and CHD. Spearman correlations were calculated to estimate the interrelationship between continuous variables. All P values presented are two-tailed, and P values below 0.05 were considered to reflect statistically significant differences. Analyses were performed using SPSS 12.0 software (SPSS Inc., Chicago, USA).
Results
Characteristics of the study population
The general characteristics of the case-control study population are presented in Table 1. Fasting glucose was significantly higher in patients with CHD, including ACS and SA. Systolic blood pressure was only significantly higher in SA patients, but not in ACS patients. In contrast, diastolic blood pressure was markedly lower in ACS patients, not in SA patients. Total cholesterol in all patients was significantly lower than in controls. This is probably due to higher use of anti-hypertensive and cholesterol-lowering medications in the patient group, especially in those with ACS. As expected, all patients were more likely to have history of hypertension, diabetes, and family history of CHD than controls. Hsp70 was detectable in 91% (380/417), 91% (264/291), and 92% (116/126) of patients with CHD, ACS, and SA, respectively, and in 94% (392/417) of control subjects, whereas anti-Hsp70 antibody was detectable in all plasma samples. Hsp70 levels in patients with CHD, ACS, and SA (3.54, 3.77, and 2.26 ng/mL, respectively) were statistically higher than in controls (1.76 ng/mL), and Hsp70 levels were significantly higher in ACS compared with SA. On the contrary, anti-Hsp70 antibody levels in patients with CHD or ACS were significantly lower than those in their corresponding controls (260.35 µg/mL vs. 297.93 µg/mL, P < 0.01; 252.03 µg/mL vs. 297.93 µg/mL, P < 0.01, respectively), and anti-Hsp70 antibody levels were significantly lower in ACS in comparison to SA. No differences in anti-Hsp70 antibody levels between SA patients and controls were observed. Hsp70 and anti-Hsp70 antibody levels in patients with AMI and UA were not significantly different.
Table 1.
Characteristics of study population
| Parameters | Control (n = 417) | CHD (n = 417) | ||
|---|---|---|---|---|
| ACS + SA (n = 417) | ACS (n = 291) | SA (n = 126) | ||
| Age, y | 60.6 ± 9.9 | 60.5 ± 9.9 | 60.3 ± 10.2 | 61.2 ± 9.3 |
| Sex, male/female | 315/102 | 315/102 | 226/65 | 89/37 |
| BMI, kg/m2 | 24.4 ± 3.4 | 24.5 ± 3.3 | 24.5 ± 3.39 | 24.5 ± 3.11 |
| Blood pressure, mmHg | ||||
| Systolic | 132 ± 21.4 | 136 ± 21.9* | 135 ± 22.1 | 138 ± 21.2** |
| Diastolic | 83 ± 11.4 | 81 ± 13.6* | 80 ± 13.7* | 82 ± 13.6 |
| Fasting glucose, mmol/L | 5.36 ± 1.92 | 6.16 ± 2.52** | 6.26 ± 2.60** | 5.93 ± 2.31** |
| Total cholesterol, mmol/L | 4.90 ± 1.32 | 4.56 ± 1.02** | 4.57 ± 1.02** | 4.54 ± 1.01** |
| Triglyceride, mmol/L | 1.65 ± 1.37 | 1.81 ± 1.40 | 1.86 ± 1.51 | 1.70 ± 1.11 |
| Smoking,% | 62.6 | 67.2 | 63.2 | 75.4** |
| Alcohol drinking,% | 29.9 | 21.4** | 21.4** | 21.6** |
| History of hypertension,% | 35.7 | 74.6** | 74.6** | 74.6** |
| History of diabetes,% | 6.9 | 29.7** | 29.2** | 30.9** |
| Family history of CHD,% | 2.2 | 11.3** | 11.7** | 10.3** |
| Hsp70, ng/mL | 1.76 (0.73-3.55) | 3.54 (1.20-5.52)** | 3.77 (1.33-6.02)**,**** | 2.26 (1.01-4.73)* |
| Anti-Hsp70 antibody, µg/mL | 297.93 (212.60-430.14) | 260.35 (190.78-391.01)** | 252.03 (179.71-371.17)**,**** | 295.56 (220.12-434.17) |
Variables are mean ± SD, percentage or median (25th-75th quartile)
*P < 0.05 vs. control
**P < 0.01 vs. control
***P < 0.05 vs. SA
****P < 0.01 vs. SA
Of the 40 patients with AMI (34 men, six women; average age 65.5 ± 12.1 years) included in the prospective study, 24 had a history of hypertension, 12 had diabetes mellitus, and 26 had a history of smoking (see the Supplementary Table 1).
Hsp70 and anti-Hsp70 antibody levels and medication use
Median Hsp70 and anti-Hsp70 antibody levels were similar in patients with CHD, ACS and SA who reported using medications (aspirin and/or statins; n = 171, 110, and 61, respectively) and in those who did not use these medications (n = 246, 181, and 65, respectively; P > 0.05). Furthermore, we did not observe any association between Hsp70 or anti-Hsp70 antibody levels and other classical risk factors for CHD, including age, sex, smoking, body mass index (BMI), cholesterol, triglyceride levels, hypertension, and diabetes (data not shown).
Hsp70 and anti-Hsp70 antibody levels and risk of ACS
As shown in Table 2, after adjustment for traditional CHD risk factors by multiple regression analysis, an elevated Hsp70 level (>median, 2.34 ng/mL)) was markedly associated with an increased risk of ACS (OR = 2.41, 95% CI, 1.68-3.45, P = 0.000), whereas higher anti-Hsp70 antibody levels (>median, 275.26 µg/mL) were significantly associated with a lower risk of ACS (OR = 0.49, 95% CI, 0.34-0.70, P = 0.001). No such significant difference was found between Hsp70 and anti-Hsp70 antibody levels and the risk of SA. A quartile analysis revealed that the trend for the presence of ACS increased with increasing Hsp70 levels and decreased with anti-Hsp70 antibody levels (P for trend < 0.0001; P for trend = 0.0003, respectively; Table 3). This elevated or reduced risk persisted after multivariate adjustment for traditional CHD risk factors such as age, sex, smoking, BMI, hypercholesterolemia, hypertension, and diabetes.
Table 2.
Logistic regression analysis of the association between the risk of CHD and Hsp70 and anti-Hsp70 antibody levels
| Variable | ACS + SA | ACS | SA | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age ≥ 60y | 1.56 | 1.09-2.24 | 1.67 | 1.10-2.54 | 1.51 | 0.90-2.54 |
| Male | 0.92 | 0.61-1.38 | 0.91 | 0.57-1.44 | 1.14 | 0.66-1.96 |
| Smoking | 1.52 | 1.02-2.26 | 1.42 | 0.91-2.22 | 1.88 | 1.03-3.42 |
| BMI | 0.96 | 0.91-1.01 | 0.97 | 0.91-1.03 | 0.94 | 0.87-1.02 |
| Hypercholesterolemia | 0.81 | 0.58-1.14 | 0.81 | 0.56-1.19 | 0.86 | 0.53-1.40 |
| Hypertension | 5.34 | 3.78-7.56 | 5.76 | 3.87-8.58 | 5.02 | 3.02-8.35 |
| Diabetes | 5.31 | 3.28-8.61 | 5.21 | 3.10-8.78 | 5.58 | 3.06-10.15 |
| Family history of CHD | 6.97 | 3.10-15.70 | 7.87 | 3.30-18.77 | 6.60 | 2.38-18.27 |
| High Hsp70 (ng/mL) | 2.07 | 1.50-2.86 | 2.41 | 1.68-3.45 | 1.36 | 0.86-2.17 |
| High anti-Hsp70 antibody (µg/mL) | 0.61 | 0.44-0.84 | 0.49 | 0.34-0.70 | 0.89 | 0.56-1.41 |
High Hsp70 Hsp70 level > median; high anti-Hsp70 antibody anti-Hsp70 antibody level > median
Table 3.
ORs for the risk of ACS by quartiles of Hsp70 and anti-Hsp70 antibody levels
| Quartile | P trend | ||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||
| Hsp70 | |||||
| Range, ng/mL | <0.91 | ≥0.91 to <2.36 | ≥0.91 to < 4.39 | ≥4.39 | |
| Control/ACS | 120/56 | 126/51 | 95/83 | 76/101 | |
| Crude (95% CI) | 1.00 | 0.87 (0.55-1.37) | 1.87 (1.21-2.89) | 2.85 (1.84-4.40) | <0.0001 |
| Adjusted ORa (95% CI) | 1.00 | 0.75 (0.44-1.28) | 1.66 (1.00-2.73) | 2.61 (1.57-4.33) | <0.0001 |
| Anti-Hsp70 antibody | |||||
| Range, µg/mL | <200.21 | ≥200.21 to <275.26 | ≥275.26 to < 413.24 | ≥413.24 | |
| Control/ACS | 85/92 | 99/78 | 119/58 | 114/63 | |
| Crude (95% CI) | 1.00 | 0.73 (0.48-1.11) | 0.45 (0.29-0.69) | 0.51 (0.33-0.78) | 0.0003 |
| Adjusted ORa (95% CI) | 1.00 | 0.67 (0.41-1.09) | 0.36 (0.21-0.59) | 0.46 (0.28-0.76) | 0.0003 |
aAdjusted for age, sex, smoking, BMI, hypercholesterolemia, hypertension, diabetes, and family history of CHD
Hsp70 and anti-Hsp70 antibody levels and the severity of ACS
We next examined if there is any relationship between Hsp70 and its corresponding antibody and the severity of ACS. As shown in Table 4, an increased level of Hsp70 was significantly associated with an increased severity of ACS, as assessed by ≥2-diseased vessels (P for trend = 0.001). Adjusted OR of having multi-vessel disease (≥2-diseased vessels) for patients in the highest quartile of Hsp70 levels was 3.51 (95% CI, 1.58-7.79), as compared with the lowest quartile, after controlling for age, sex, smoking, BMI, hypercholesterolemia, hypertension, and diabetes. However, the level of anti-Hsp70 antibody was not significantly associated with severity of ACS.
Table 4.
ORs for the severity of ACS by quartiles of Hsp70 and anti-Hsp70 antibody levels
| Quartile | P trend | ||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||
| Hsp70 | |||||
| Range, ng/mL | <0.91 | ≥0.91 to <2.36 | ≥0.91 to < 4.39 | ≥4.39 | |
| 1-disease vessel/≥2-diseased vessels | 25/31 | 17/34 | 21/62 | 19/82 | |
| Crude (95% CI) | 1.00 | 1.61 (0.74-3.54) | 2.46 (1.19-5.06) | 3.40 (1.64-7.02) | 0.001 |
| Adjusted ORa (95% CI) | 1.00 | 1.36 (0.58-3.18) | 2.35 (1.08-5.13) | 3.51 (1.58-7.79) | 0.001 |
| Anti-Hsp70 antibody | |||||
| Range, μg/mL | <200.21 | ≥200.21 to <275.26 | ≥275.26 to < 413.24 | ≥413.24 | |
| 1 disease vessel/≥2 diseased vessels | 24/68 | 22/56 | 22/36 | 14/49 | |
| Crude (95% CI) | 1.00 | 0.97 (0.49-1.90) | 0.61 (0.30-1.22) | 1.22 (0.57-2.58) | 0.955 |
| Adjusted ORa (95% CI) | 1.00 | 1.20 (0.57-2.52) | 0.61 (0.29-1.28) | 1.19 (0.54-2.63) | 0.972 |
aAdjusted for age, sex, smoking, BMI, hypercholesterolemia, hypertension, diabetes and family history of CHD.
Joint effects of Hsp70 and anti-Hsp70 antibody levels on the risk of ACS
After multivariate adjustment for other potential factors, high Hsp70 levels (≥median, 2.36 ng/mL) and low anti-Hsp70 antibody levels (<median, 275.26 µg/mL) were associated with a more than fivefold greater risk of ACS compared with subjects with low Hsp70 levels and high anti-Hsp70 antibody levels (OR, 5.14, 95% CI, 3.00-8.79; P < 0.0001; Table 5).
Table 5.
ORs for the risk of ACS by combined Hsp70 and anti-Hsp70 antibody levels
| Variable | Control (n) | ACS (n) | Crude OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|---|---|
| Low Hsp70 + high anti-Hsp70 antibody | 136 | 41 | 1.00 | 1.00 |
| Low Hsp70 + low anti-Hsp70 antibody | 110 | 66 | 1.99 (1.25-3.17) | 2.81 (1.62-4.86) |
| High Hsp70 + high anti-Hsp70 antibody | 97 | 80 | 2.74 (1.73-4.32) | 3.27 (1.92-5.56) |
| High Hsp70 + low anti-Hsp70 antibody | 74 | 104 | 4.66 (2.95-7.38) | 5.14 (3.00-8.79) |
P trend < 0.0001
Low Hsp70 Hsp70 level < median (2.36 ng/mL); high Hsp70 Hsp70 level ≥ median (2.36 ng/mL); low anti-Hsp70 antibody anti-Hsp70 antibody level < median (275.26 µg/mL); high anti-Hsp70 antibody anti-Hsp70 antibody level ≥ median (275.26 µg/mL)
aAdjusted for age, sex, smoking, BMI, hypercholesterolemia, hypertension, diabetes, and family history of CHD
Dynamic changes of Hsp70 and anti-Hsp70 antibody levels in patients with AMI
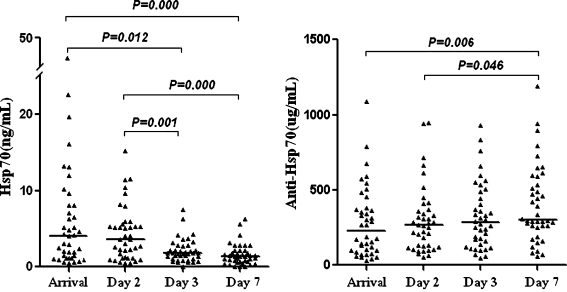
Finally, we examined dynamic changes in Hsp70 and anti-Hsp70 antibody levels induced by AMI. Hsp70 was undetectable in one of the 40 patients with AMI on day 3 and in 3 patients with AMI on day 7. There was a clear tendency for AMI to lead to decreases in Hsp70 levels. Hsp70 levels on the day of arrival and the following day were significantly elevated in comparison to those on day 3 and 7 after the onset of AMI (all P < 0.05). Plasma anti-Hsp70 antibody was detectable in all of the 40 patients with AMI, and levels gradually increased and to reach a relative peak value on day 7. Anti-Hsp70 antibody levels on day 7 were significantly more elevated in comparison to those on the day of arrival and the following day after the onset of AMI (P = 0.023 and P = 0.045, respectively; Fig. 1).
Fig. 1.
Hsp70 and anti-Hsp70 antibody levels at the different time points after AMI (n = 40). Individual and median levels (horizontal bars) are shown. The median (25th and 75th quartiles) levels of Hsp70 were 4.06 (1.23-8.09), 3.59 (1.16-5.54), 1.80 (1.05-2.77) and 1.40 (0.66-2.09) ng/mL, and anti-Hsp70 antibody were 225.81(96.72-376.01), 266.53 (121.40-366.32), 282.84 (143.49-477.99) and 299.65 (254.08-574.44) µg/mL at the four time points respectively. Log10 transformed Hsp70 and anti-Hsp70 antibody levels were compared over the time course using the repeated one-way ANOVA test (LSD)
Correlations of Hsp70 and anti-Hsp70 antibody with markers of myocardial necrosis in patients with AMI
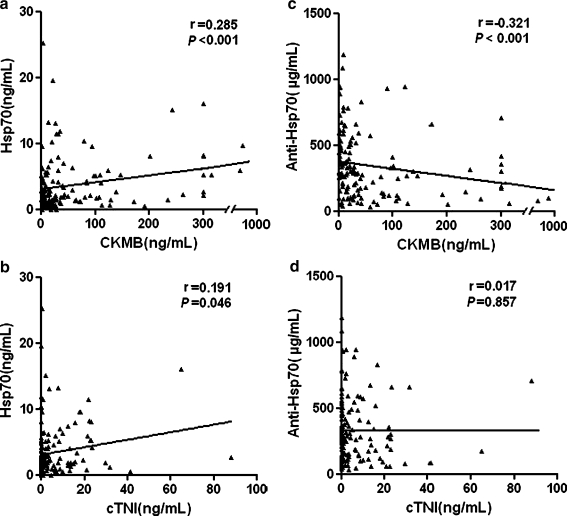
Hsp70 levels were significantly related to the levels of CKMB (r = 0.285, P < 0.001, Fig. 2a), however, a weaker positive correlation of marginal significance was found between Hsp70 and cTnI (r = 0.191, P = 0.046, Fig. 2b) measured at the four time points. In contrast, anti-Hsp70 antibody levels were inversely correlated with CKMB levels (r = −0.321, P < 0.001, Fig. 2c), and no correlation between anti-Hsp70 and cTnI was found (r = 0.017, P = 0.857, Fig. 2d).
Fig. 2.
Correlations between Hsp70 levels and (a) CKMB, (b) cTnI, and anti-Hsp70 antibody levels and (c) CKMB, (d) cTnI. Data are scatter plots with fit lines
Discussion
In the present study, we found that median levels of Hsp70 were markedly higher in patients with CHD including ACS and SA than in the controls, and also that Hsp70 levels were higher in ACS than in SA. There is an increased risk and severity of ACS as Hsp70 levels increase. In contrast, anti-Hsp70 antibody levels were significantly lower in ACS compared to controls and SA, and no differences were found between SA patients and controls. Anti-Hsp70 antibody levels were inversely associated with the risk of ACS. Furthermore, high levels of Hsp70 combined with low levels of anti-Hsp70 antibody was associated with more than a fivefold higher risk of ACS, compared with subjects with low levels of Hsp70 and high levels of anti-Hsp70 antibody.
ACS and SA are two different clinical manifestations of CHD. Our results show that the elevations in Hsp70 levels are more pronounced in ACS than in SA, and that lower anti-Hsp70 antibody levels are present only in ACS, not in SA, when compared to the controls. This interesting finding might be explained by differences in the degree of immunoreaction or severity of myocardial damage because of different degree of myocardial ischemia and nature of the plaque in ACS and SA. Pathological and autopsy studies have reported that rupture or erosion of multiple vulnerable plaques and subsequent formation of thrombus are the most important mechanisms leading to ACS, whereas SA closely correlates with the stability of an atherosclerotic plaque (Hong et al. 2004). Consistent with our results, increased Hsp70 levels have been detected in heart biopsies from patients with unstable, as compared with stable angina (Valen et al. 2000). It is possible that soluble Hsp70 release into the circulation is higher in the sudden fissure or rupture of unstable plaque, and acute events, following AMI for example, rather than in stable plaque. This hypothesis is supported by the findings of Caligiuri et al. (Caligiuri et al. 1998) who demonstrated that markers of immune activation (CD4+ and CD3+/HLA-DR+ cells, IL-2 and IgM) were higher in UA than in SA, and that acute and transient immune system activation was only observed in UA, but not in SA. Consequently, anti-Hsp70 antibody neutralized by increased circulating Hsp70 leads to decreased plasma levels of anti-Hsp70 antibody only in ACS. These findings suggest that clinical stabilization of disease might be associated with a change in Hsp70 and anti-Hsp70 antibody levels, and levels of this protein and its antibody might be more predictive of ACS than SA.
Few studies have examined the association between Hsp70 levels and the risk of CHD. Zhu et al. (Zhu et al. 2003) reported that Hsp70 levels were higher in non-CHD patients than CHD patients. In this study, cases were defined as having ≥ 50% vessel stenosis and controls included patients with chest pain or with noninvasive tests compatible with myocardial ischemia, and blood samples were drawn from all subjects before the time of coronary angiography. In contrast, our controls were selected from healthy subjects and blood samples were obtained from the patients on the following morning after admission. Therefore, differences in population characteristics and timing of blood drawing between our study and Zhu et al. (Zhu et al. 2003) might contribute to the discrepant findings. Furthermore, the assay used to measure Hsp70 and anti-Hsp70 antibody levels were different between our study and that of Zhu and Kocsis et al. (Kocsis et al. 2002; Zhu et al. 2003). We measured plasma Hsp70 and anti-Hsp70 antibody (IgG/A/M) levels using commercial kits, whereas Zhu et al. (Zhu et al. 2003) detected IgG of anti-Hsp70 levels using an in-house ELISA. In contrast to our result, anti-Hsp70 antibody was present in only one third of subjects in their study, this might reflect the fact that the commercial assay measures all antibody isotypes, whereas Zhu’s ‘in-house’ assays measured only IgG antibodies.
The inducible Hsp70 is part of the Hsp70 family which contains a number of highly related protein isoforms ranging in size from 66 to 78 kDa (Tavaria et al. 1996). Coronary endothelial cells are the main site of induction of Hsp70 in the heart and Hsp70 has been found to be highly expressed in cardiovascular tissues and atherosclerotic lesions in humans, in which it has a cytoprotective role (Kleindienst et al. 1993; Wick et al. 1997). In contrast, extracellular Hsp70 has been shown to have protective or deleterious effects, protecting against or modifying the progression of atherosclerosis. Although it is still unclear how and from where Hsp70 is released into the circulation, circulating Hsp70 levels may reflect intracellular Hsp70 concentrations, especially if tissue damage is present. Gombos et al. (Genth-Zotz et al. 2004; Gombos et al., 2008) observed that serum Hsp70 levels were increased in patients with severe heart failure. Such results indicated that myocardial injury and worsening heart function might result in the release of Hsp70. It has been suggested that the release of Hsp70 from the myocardium into the circulation elicits an immediate innate immune response which leads to the production of pro-inflammatory cytokines including tumor necrosis factor-α, interleukin (IL)-1, IL-6, and the expression of adhesion molecules on macrophages and endothelial cells (Asea et al. 2000; Mandal et al. 2004). Simultaneously, Hsp70 activates adaptive immune responses and stimulates the production of anti-Hsp70 antibody. In contrast to innate immunity, adaptive immunity is a more specialized and delayed response which typically takes several days to become protective (Binder et al. 2002; Binder et al. 2005). Thus, anti-Hsp70 antibody has been speculated to contribute to the elimination of autoantigens, which might explain why anti-Hsp70 antibody levels were low in ACS. Such a hypothesis has been supported by Schett et al. (Schett et al. 1999) who found that myocardial ischemic injury leads to a release of Hsp60 and subsequent binding to circulating anti-Hsp65, and lower antibody titers via the rapid clearance of immune complex. Therefore, higher plasma Hsp70 levels might indicate cell necrosis and thus represent a danger signal, whereas higher anti-Hsp70 antibody levels could have a possible protective role in ACS.
To further evaluate the association between Hsp70, anti-Hsp70 antibody, and CHD, we examined the kinetic changes of plasma Hsp70 and anti-Hsp70 antibody levels in AMI over time. The novel findings in this prospective study were that Hsp70 levels peaked after the onset of AMI admission and gradually decreased during the 7 days of recovery, whereas anti-Hsp70 antibody levels progressively increased during the period. Furthermore, Hsp70 levels in patients with AMI were positively correlated with markers of myocardial necrosis, CKMB and cTnI, and anti-Hsp70 antibody levels were negatively correlated with CKMB. CKMB and cTNI are well-known markers of myocardial damage, with high sensitivity and cardio-specificity. Their circulating levels are strongly correlated with the extent of infarct (Laurino et al. 1996; Lindbloom and Stevermer 1999). The positive correlations of Hsp70 with CKMB and cTNI support the concept that increased plasma levels indeed resulted from myocardial necrosis (Dybdahl et al. 2005). Thus, high Hsp70 levels were indicative of injury, and neutralization of Hsp70 by anti-Hsp70 antibody might be a part of down-regulatory mechanism. This is consistent with the observation that there is an association between high Hsp70 or low anti-hsp70 antibody levels and the risk of ACS. Similar results have been found by Dybdahl et al. (Dybdahl et al. 2005) who observed that the release of Hsp70 in the patients with AMI peaked 6 h after their arrival and decreased significantly from the day of arrival to the following morning. These results indicate that a large amount of Hsp70 is rapidly released into the circulation from infracted heart tissue several hours following heart ischemia and that it is cleared from the circulation after AMI. It is possible that the stress response is only transient or short term, not chronic and persistent state. Tanaka et al. (Tanaka et al. 1998) reported that expression of Hsp70 appeared within 3 h after ischemic stress, that it persisted for up to 72 h, and that it was not detected at 168 h in rabbit hearts. Based on this finding, we suggest that the sources of circulating Hsp70 after AMI may result from the damaged or necrosis of cardiomyocytes and severe endothelial dysfunction in the early phase of AMI (Bennett and Boyle 1998; Mayr and Xu 2001; Toga et al. 2007).
Several studies have found that autoantibodies against human Hsp70 are present in normal individuals (Pockley et al. 1998; Rea et al. 2001). When soluble Hsp70 originates from acute myocardial cell injury and is released into the circulation it may bind preexisting anti-Hsp70 antibody, thereby leading to immune complex formation and a reduction in measurable levels of free anti-Hsp70 antibody. However, antigen-antibody complexes are relatively unstable and can be easily dissociated into free antigen and free antibody, the dynamic equilibrium of which partly depending on the relative proportions of antigen and antibody (Knutson et al. 1979; van Es et al. 1979). The biological half life of Hsp70 has been reported to be about 18 h (Gerner et al. 2002), thus Hsp70 can be cleared rapidly and promote the dissociation of immune complexes, which could lead to the increase of free anti-Hsp70 antibody. Our data are consistent with Hoppichler’s et al. (Hoppichler et al. 1996) finding that anti-Hsp65 antibody titers show a significant drop after the occurrence of MI, suggesting that the decrease of anti-Hsp65 antibody after AMI might result from the removal of the antigen-antibody complexes from the circulation. The negative correlation of anti-Hsp70 antibody with CKMB strengthened this argument. This result suggests the co-existence of an increased autoimmune response and cardiac injury in patients suffering an AMI. The dynamic change of Hsp70 and anti-Hsp70 antibody during acute and recovery phase in AMI could be an indirect reflection of myocardial ischemic injury and endothelial dysfunction in the immediate post-AMI period and the gradual return to homeostasis thereafter. It may indicate that the qualitative nature of the immune response is a time-dependent phenomenon. These results suggest that circulating Hsp70 and anti-Hsp70 antibody may be an early marker for detrimental effects at the acute stages of AMI and may serve as an indicator to evaluate disease states or pathological processes of AMI.
Some potential limitations to the present study must be considered. First, the case-control design cannot establish the causal relationships between Hsp70, anti-Hsp70 antibody and ACS. It is possible that the observed relationship is due to changes in Hsp70 and anti-Hsp70 antibody that are secondary to events that are associated with ACS. Second, our controls did not undergo angiography. Although the possibility of having CHD in healthy population-based samples in a Chinese population is very low, this cannot be completely excluded (Wu and Tanguay 2006). Third, we did not systematically investigate inflammatory markers, such as hsCRP, an important predictor of CHD risk in most epidemiologic studies (Burke et al. 2002; Liuzzo et al. 1994). However, Park et al. (Park et al. 2006) have reported there was no significant correlation between Hsp70 levels and CRP levels. Finally, the sample size in the prospective study is relatively small. Nevertheless, to the best of our knowledge, our case-control study is the largest thus far and cases and controls were well matched by age and sex, and for Hsp70 and anti-Hsp70 antibody evaluation, we used commercially available, easy to perform, and standardized assays.
In summary, our study shows that Hsp70 levels are significantly higher in ACS and SA, whereas anti-Hsp70 antibody levels are significantly lower in ACS than controls. Higher Hsp70 and lower anti-Hsp70 antibody levels are independently associated with a relatively higher risk of ACS, and higher Hsp70 levels are related to an increased severity of ACS. Furthermore, high levels of Hsp70 combined with low levels of anti-Hsp70 antibody have a joint effect on the risk of ACS. Hsp70 levels were significantly increased, but anti-Hsp70 antibody levels were markedly decreased after the onset of AMI. The relationship between circulating Hsp70 and anti-Hsp70 antibody in CHD is undoubtedly complex and further studies are required to confirm these findings in prospective cohorts and elucidate molecular mechanisms of these associations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 36 kb)
Acknowledgments
We are particularly grateful to all CHD patients and volunteers for participating in the present study and to the medical personnel of Tongji Hospital, Union Hospital, Wugang Hospital, Wuhan Second Hospital, and Dr Tangchun Wu’s lab in Wuhan City, Hubei Province, China for their kind assistance in collecting the data and samples. We also thank A. Graham Pockley (University of Sheffield, Sheffield, UK) for his critical review of this manuscript. This work was supported by research funds from the National Natural Scientific Foundation of China (NNSFC 30430590 and 30525031 to Tangchun Wu) and a China-Canada collaborative research grant of the Canadian Institutes of Health Research (CIHR, CCI85673, Robert M Tanguay) and NNSFC (30711120579, Tangchun Wu).
Abbreviations
- Hsps
heat shock proteins
- CHD
coronary heart disease
- ACS
acute coronary syndrome
- SA
stable angina
- AMI
acute myocardial infarction
- UA
unstable angina
- CKMB
creatine phosphokinase MB
- cTNI
cardiac troponin I
- BMI
body mass index
References
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/S0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Boyle JJ. Apoptosis of vascular smooth muscle cells in atherosclerosis. Atherosclerosis. 1998;138:3–9. doi: 10.1016/S0021-9150(98)00013-6. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Shaw PX, Chang MK, Boullier A, Hartvigsen K, Horkko S, Miller YI, Woelkers DA, Corr M, Witztum JL. The role of natural antibodies in atherogenesis. J Lipid Res. 2005;46:1353–1363. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, Pestaner J, Smialek J, Virmani R. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–2023. doi: 10.1161/01.CIR.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- Caligiuri G, Liuzzo G, Biasucci LM, Maseri A. Immune system activation follows inflammation in unstable angina: pathogenetic implications. J Am Coll Cardiol. 1998;32:1295–1304. doi: 10.1016/S0735-1097(98)00410-0. [DOI] [PubMed] [Google Scholar]
- Chan YC, Shukla N, Abdus-Samee M, Berwanger CS, Stanford J, Singh M, Mansfield AO, Stansby G. Anti-heat-shock protein 70 kDa antibodies in vascular patients. Eur J Vasc Endovasc Surg. 1999;18:381–385. doi: 10.1053/ejvs.1999.0885. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvani M, Panteghini M, Ottani F, Cappelletti P, Chiarella F, Chiariello M, Crea F, Dolci A, Golino P, Greco C, Nicolosi GL, Plebani M, Tubaro M, Zaninotto M. The new definition of myocardial infarction: analysis of the ESC/ACC Consensus Document and reflections on its applicability to the Italian Health System. Ital Heart J. 2002;3:543–557. [PubMed] [Google Scholar]
- Genth-Zotz S, Bolger AP, Kalra PR, Haehling S, Doehner W, Coats AJ, Volk HD, Anker SD. Heat shock protein 70 in patients with chronic heart failure: relation to disease severity and survival. Int J Cardiol. 2004;96:397–401. doi: 10.1016/j.ijcard.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Gerner C, Vejda S, Gelbmann D, Bayer E, Gotzmann J, Schulte-Hermann R, Mikulits W. Concomitant determination of absolute values of cellular protein amounts, synthesis rates, and turnover rates by quantitative proteome profiling. Mol Cell Proteomics. 2002;1:528–537. doi: 10.1074/mcp.M200026-MCP200. [DOI] [PubMed] [Google Scholar]
- Gombos T, Förhécz Z, Pozsonyi Z, Jánoskuti L, Prohászka Z. Interaction of serum 70-kDa heat shock protein levels and HspA1B (+1267) gene polymorphism with disease severity in patients with chronic heart failure. Cell Stress Chaperones. 2008;13:199–206. doi: 10.1007/s12192-007-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz I, Rosso R, Roth A, Keren G, George J. Serum levels of anti heat shock protein 70 antibodies in patients with stable and unstable angina pectoris. Acute Card Care. 2006;8:46–50. doi: 10.1080/14628840600606950. [DOI] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Hong MK, Mintz GS, Lee CW, Kim YH, Lee SW, Song JM, Han KH, Kang DH, Song JK, Kim JJ, Park SW, Park SJ. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three-vessel intravascular ultrasound study in 235 patients. Circulation. 2004;110:928–933. doi: 10.1161/01.CIR.0000139858.69915.2E. [DOI] [PubMed] [Google Scholar]
- Hoppichler F, Lechleitner M, Traweger C, Schett G, Dzien A, Sturm W, Xu Q. Changes of serum antibodies to heat-shock protein 65 in coronary heart disease and acute myocardial infarction. Atherosclerosis. 1996;126:333–338. doi: 10.1016/0021-9150(96)05931-X. [DOI] [PubMed] [Google Scholar]
- Kleindienst R, Xu Q, Willeit J, Waldenberger FR, Weimann S, Wick G. Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am J Pathol. 1993;142:1927–1937. [PMC free article] [PubMed] [Google Scholar]
- Knutson DW, Es LA, Kayser BS, Glassock RJ. Soluble oligovalent antigen–antibody complexes. II. The effect of various selective forces upon relative stability of isolated complexes. Immunology. 1979;37:495–503. [PMC free article] [PubMed] [Google Scholar]
- Kocsis J, Veres A, Vatay A, Duba J, Karadi I, Fust G, Prohaszka Z. Antibodies against the human heat shock protein hsp70 in patients with severe coronary artery disease. Immunol Invest. 2002;31:219–231. doi: 10.1081/IMM-120016242. [DOI] [PubMed] [Google Scholar]
- Laurino JP, Bender EW, Kessimian N, Chang J, Pelletier T, Usategui M. Comparative sensitivities and specificities of the mass measurements of CK-MB2, CK-MB, and myoglobin for diagnosing acute myocardial infarction. Clin Chem. 1996;42:1454–1459. [PubMed] [Google Scholar]
- Lindbloom EJ, Stevermer JJ. Cardiac troponin I as a marker for AMI. Am Heart J. 1999;138:798–800. doi: 10.1016/S0002-8703(99)70199-7. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- Mandal K, Jahangiri M, Xu Q. Autoimmunity to heat shock proteins in atherosclerosis. Autoimmun Rev. 2004;3:31–37. doi: 10.1016/S1568-9972(03)00088-0. [DOI] [PubMed] [Google Scholar]
- Mayr M, Xu Q. Smooth muscle cell apoptosis in arteriosclerosis. Exp Gerontol. 2001;36:969–987. doi: 10.1016/S0531-5565(01)00090-0. [DOI] [PubMed] [Google Scholar]
- Mintz GS, Maehara A, Bui AB, Weissman NJ. Multiple versus single coronary plaque ruptures detected by intravascular ultrasound in stable and unstable angina pectoris and in acute myocardial infarction. Am J Cardiol. 2003;91:1333–1335. doi: 10.1016/S0002-9149(03)00323-0. [DOI] [PubMed] [Google Scholar]
- Ozdemir O, Gundogdu F, Karakelleoglu S, Sevimli S, Pirim I, Acikel M, Arslan S, Serdar S. Comparison of serum levels of inflammatory markers and allelic variant of interleukin-6 in patients with acute coronary syndrome and stable angina pectoris. Coron Artery Dis. 2008;19:15–19. doi: 10.1097/MCA.0b013e3282f27bf7. [DOI] [PubMed] [Google Scholar]
- Park HK, Park EC, Bae SW, Park MY, Kim SW, Yoo HS, Tudev M, Ko YH, Choi YH, Kim S, Kim DI, Kim YW, Lee BB, Yoon JB, Park JE. Expression of heat shock protein 27 in human atherosclerotic plaques and increased plasma level of heat shock protein 27 in patients with acute coronary syndrome. Circulation. 2006;114:886–893. doi: 10.1161/CIRCULATIONAHA.105.541219. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20:1815–1820. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42:235–238. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- Portig I, Pankuweit S, Maisch B. Antibodies against stress proteins in sera of patients with dilated cardiomyopathy. J Mol Cell Cardiol. 1997;29:2245–2251. doi: 10.1006/jmcc.1997.0463. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Duba J, Horvath L, Csaszar A, Karadi I, Szebeni A, Singh M, Fekete B, Romics L, Fust G. Comparative study on antibodies to human and bacterial 60 kDa heat shock proteins in a large cohort of patients with coronary heart disease and healthy subjects. Eur J Clin Invest. 2001;31:285–292. doi: 10.1046/j.1365-2362.2001.00819.x. [DOI] [PubMed] [Google Scholar]
- Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001;36:341–352. doi: 10.1016/S0531-5565(00)00215-1. [DOI] [PubMed] [Google Scholar]
- Schett G, Metzler B, Kleindienst R, Amberger A, Recheis H, Xu Q, Wick G. Myocardial injury leads to a release of heat shock protein (hsp) 60 and a suppression of the anti-hsp65 immune response. Cardiovasc Res. 1999;42:685–695. doi: 10.1016/S0008-6363(99)00012-7. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fujiwara H, Yamasaki K, Yokota R, Doyama K, Inada T, Ohtani S, Fujiwara T, Sasayama S. Expression of heat shock protein after ischemic preconditioning in rabbit hearts. Jpn Circ J. 1998;62:512–516. doi: 10.1253/jcj.62.512. [DOI] [PubMed] [Google Scholar]
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:AHSGTT>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga W, Tanonaka K, Takeo S. Changes in Hsp60 level of the failing heart following acute myocardial infarction and the effect of long-term treatment with trandolapril. Biol Pharm Bull. 2007;30:105–110. doi: 10.1248/bpb.30.105. [DOI] [PubMed] [Google Scholar]
- Valen G, Hansson GK, Dumitrescu A, Vaage J. Unstable angina activates myocardial heat shock protein 72, endothelial nitric oxide synthase, and transcription factors NFkappaB and AP-1. Cardiovasc Res. 2000;47:49–56. doi: 10.1016/S0008-6363(00)00071-7. [DOI] [PubMed] [Google Scholar]
- Es LA, Knutson DW, Kayser BS, Glassock RJ. Soluble oligovalent antigen-antibody complexes. I. The effect of antigen valence and combining ratio on the composition of fluorescein-carrier anti-fluorescein complexes. Immunology. 1979;37:485–493. [PMC free article] [PubMed] [Google Scholar]
- Vogt S, Portig I, Kusch B, Pankuweit S, Sirat AS, Troitzsch D, Maisch B, Moosdorf R. Detection of anti-hsp70 immunoglobulin G antibodies indicates better outcome in coronary artery bypass grafting patients suffering from severe preoperative angina. Ann Thorac Surg. 2004;78:883–889. doi: 10.1016/j.athoracsur.2004.03.082. [DOI] [PubMed] [Google Scholar]
- Wick G, Romen M, Amberger A, Metzler B, Mayr M, Falkensammer G, Xu Q. Atherosclerosis, autoimmunity, and vascular-associated lymphoid tissue. Faseb J. 1997;11:1199–1207. doi: 10.1096/fasebj.11.13.9367355. [DOI] [PubMed] [Google Scholar]
- Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15:18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- Wu T, Tanguay RM. Antibodies against heat shock proteins in environmental stresses and diseases: friend or foe? Cell Stress Chaperones. 2006;11:1–12. doi: 10.1379/CSC-155R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yang M, Yao H, Zheng J, Yang Q, Chen S, Wei Q, Tanguay RM, Wu T. Plasma antibodies to heat shock protein 60 and heat shock protein 70 are associated with increased risk of electrocardiograph abnormalities in automobile workers exposed to noise. Cell Stress Chaperones. 2005;10:126–135. doi: 10.1379/CSC-95R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, He MA, Cheng L, Zhou L, Zeng H, Wang J, Wang F, Chen Y, Hu FB, Wu T. Joint effects of antibody to heat shock protein 60, hypertension, and diabetes on risk of coronary heart disease in chinese. Clin Chem. 2008;54:1046–1052. doi: 10.1373/clinchem.2007.101451. [DOI] [PubMed] [Google Scholar]
- Zhang X, He M, Cheng L, Chen Y, Zhou L, Zeng H, Pockley AG, Hu FB, Wu T. Elevated heat shock protein 60 levels are associated with higher risk of coronary heart disease in Chinese. Circulation. 2008;118:2687–2693. doi: 10.1161/CIRCULATIONAHA.108.781856. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Rott D, Csako G, Wu H, Halcox J, Epstein SE. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation. 2001;103:1071–1075. doi: 10.1161/01.cir.103.8.1071. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:1055–1059. doi: 10.1161/01.ATV.0000074899.60898.FD. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(DOC 36 kb)