Abstract
Recent research on the heat shock proteins (Hsps) in chronic inflammatory diseases indicates that Hsps may have disease-suppressive activities. Our aim was to characterize immune response directed to bacterial (DnaJ) and human Hsp40s in patients with rheumatoid arthritis (RA). We found elevated levels of anti-DnaJ, anti-Hdj2, and anti-Hdj3 (but not ant-Hdj1) serum antibodies in the RA patients (P ≤ 0.001) compared to healthy controls. In peripheral blood mononuclear cells (PBMCs) culture, all tested Hsp40 proteins significantly inhibited the divisions of CD4+ and CD8+ T cells of the RA patients but not those of the controls. Both DnaJ and Hdj2 stimulated secretion of the main anti-inflammatory cytokine IL-10 by PBMCs of the RA patients (P < 0.05), and of IL-6 by PBMCs of the RA (P < 0.001) and control (P < 0.01) groups. DnaJ reduced TNFα secretion (P < 0.05) by both groups of PBMCs. Our results show for the first time that the RA patients have an increased humoral response to human Hsp40 proteins Hdj2 and Hdj3. This is also the first description of immunomodulatory effect of human Hsp40s on T cells and cytokine secretion in RA, suggesting that Hsp40s act as natural anti-inflammatory agents in RA.
Keywords: Heat shock proteins, Rheumatoid arthritis, Hsp40, DnaJ, E. coli
Introduction
Rheumatoid arthritis (RA) is the most common form of chronic inflammatory arthritis in humans (reviewed in Lee and Weinblatt 2001). The pathogenesis of RA is unknown but it is thought that interplay between genetic factors, sex hormones, and possibly an infectious agent or another immune-activating agent initiates an autoimmune pathogenic mechanism resulting in inflammation and cartilage/bone destruction. A strong evidence exists that the components of the immune system, especially autoreactive T and B cells, are involved in the inflammation and joint destruction (reviewed in Fournier 2005).
CD4+ T cells are the dominant population of lymphocytes which play an essential role in immune response. They can be divided into four main functional subtypes, Th1, Th2, Th17, and regulatory T cells—Treg characterized by phenotype CD4+CD25+high (reviewed in Skapenko et al. 2005; Bryl et al. 2009; Shahrara et al. 2008). In RA, chronic inflammation is characterized by a dominant Th1 activation manifested by secretion of pro-inflammatory cytokines such as IL-2, TNFα, or IFNγ (reviewed in Skapenko et al. 2005). The Th2 subset produces cytokines, including IL-4, IL-5, IL-6, and IL-10, which inhibit Th1 cell function and provide potent help for B-cell activation (reviewed in Skapenko et al. 2005; Lafaille 1998; Diehl and Rincón 2002; Taylor and Feldmann 2004). Many studies suggest that induction of Treg cells is one of the host’s natural mechanisms for controlling inflammatory responses (Ulmansky et al. 2002; de Kleer et al. 2004; Cobelens et al 2002; Wendling et al. 2000). Some reports have also indicated a role for autoreactive CD8+ T cells in RA inflammation, via secretion of high amounts of pro-inflammatory cytokines such as TNFα and IFNγ (reviewed in Skapenko et al. 2005).
Recently, a number of studies have investigated a role of the heat shock proteins (Hsps) in T-cell regulation in chronic inflammatory diseases and their outcome indicates that T-cell responses to Hsps have disease-suppressive activities through production of anti-inflammatory cytokines (van Eden et al. 2005; Zlacka et al. 2006; Wieten et al. 2007; van Eden et al. 2007).
Heat shock proteins are a family of evolutionarily conserved proteins, which play an important role in cell physiology under normal and stress conditions. At times of cellular stress, including infection and chronic inflammation, the expression of Hsps is markedly elevated (reviewed in van Eden et al. 2005). Hsps are also a group of major bacterial antigens (Albani et al. 1992). The Hsps are classified into protein families based on their molecular weight, the major families being the Hsp70, Hsp60, Hsp90, Hsp100, Hsp40, and small Hsps. Their structure conserved from bacteria to man and high immunogenicity made them attractive targets for investigation in the area of autoimmunity, with the Hsp60 and Hsp70 being most extensively studied, especially since the discovery that T cells isolated from rats with adjuvant-induced arthritis were responding to mycobacterial Hsp60 (van Eden et al. 2005; Wieten et al. 2007; van Eden et al. 2007). To the contrary, the research on Hsp40 involvement in autoimmune diseases, including RA, has been less extensive, in spite of the fact that Hsp40 is probably the largest Hsp family in humans, with at least 50 members (reviewed in Kampinga et al. 2009).
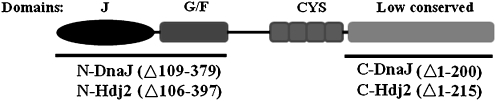
Escherichia coli DnaJ is one of the immunogenic bacterial Hsp proteins and the model representative of the Hsp40 family. In the full-length E. coli DnaJ protein, there are four domains formed by 375 amino acid sequence. The amino-terminal 75 residues of DnaJ constitute an evolutionarily highly conserved motif, the J domain, which, together with the adjacent region, rich in glycine and phenylalanine, is essential for DnaJ’s interactions with Hsp70 chaperone. Third domain, rich in cysteine residues, together with the least conserved C-terminal region, functions to bind substrate proteins (Qiu et al. 2006; Han et al. 2007; Fig. 1). Of human Hsp40, the Hdj1, Hdj2, and Hdj3 proteins are best characterized (Terada and Mori 2000). Hdj2 and Hdj3 belong to the class I of Hsp40, possessing all the domains characteristic for DnaJ; Hdj1 belonging to the class II does not have the cysteine-rich domain (Cheetham and Caplan 1998; Terada and Mori 2000).
Fig. 1.
Schematic outline of the wild-type structure of E. coli DnaJ protein. Black lines below the E. coli DnaJ schematic protein represent the mutant proteins containing the N- and C-terminal domains of DnaJ and human Hdj2. Deleted amino acids are indicated in parentheses (Krzewski et al. 2003, modified)
The presence of antibodies against the E. coli DnaJ, in RA and juvenile rheumatoid arthritis (JIA), has been shown previously (Albani et al. 1994; Albani et al. 1995; Chukwuocha et al. 1999) as well as overexpression of human Hsp40s in the synovial tissue of patients with RA (Kurzik-Dumke et al. 1999). We have previously shown, using a set of poly- and monoclonal antibodies directed to bacterial DnaJ, that human Hsp40 (Hdj1, Hdj2 and Hdj3) and bacterial DnaJ are immunologically similar, as the antibodies against DnaJ cross-react with these human proteins (Krzewski et al. 2003 and unpublished observations). However, the humoral response against human Hsp40 antigens in RA is not known. Recently, it has been demonstrated that T cells from patients with JIA respond differentially to peptides derived from bacterial and human Hsp40s and that Hsp40s modulate autoimmune inflammation (Massa et al. 2007). This study points to Hsp40s as potential targets for immune therapy of various inflammatory diseases. At the same time it makes research on immunomodulatory effects of Hsp40 in other than JIA arthritic diseases important.
In this study, we have focused on characterization of humoral and cellular immune responses directed to bacterial DnaJ protein, and its human counterparts Hdj1, Hdj2, and Hdj3 proteins, as well as to separated N- and C-terminal domains of bacterial DnaJ and human Hdj2 in RA patients.
Material and methods
Patients and controls
All rheumatoid arthritis patients were diagnosed according to American Rheumatism Association criteria (Arnett et al. 2008). Sera were obtained from 48 (46 females and two males) patients (mean age 46 ± 16.7, range 20–80). The patients were divided into two groups according to their disease duration: those with the early onset (n = 12; mean age 39.5 ± 19.6, range 20–80), in which the disease lasted up to 1 year and those with advanced RA (n = 36), in which the disease lasted longer than 1 year (mean age 49 ± 15.4, range 21–74). Peripheral blood mononuclear cells (PBMCs) obtained from 22 RA patients were used for T-cell proliferation assay (mean age 43.5 ± 15.2, range 22–70) and 14 for cytokine measurements (mean age 43 ± 14.7, range 22–61). For controls, we used sera and PBMCs of sex- and age-matched healthy volunteers: 50 sera for antibody assays, 15 PBMCs for proliferation assay and 11 PBMCs for cytokine measurements (Table 1).
Table 1.
Selected characteristics of the study RA population
| RA (antibody assay) | RA (T-cell proliferation) | RA (cytokine assay) | |
|---|---|---|---|
| No. of patients | 48 | 22 | 14 |
| Age (years) | 46 ± 16.7 (20–80) | 43.5 ± 15.2 (22–70) | 43 ± 14.7 (22–61) |
| Disease duration (years) | 7.9 ± 8.5 (0.2–32) | 8.9 ± 9.5 (0.5–32) | 8.3 ± 6.5 (1–21) |
| Steinbrocker’s criteria | |||
| Stage I | 10 | 2 | 1 |
| Stage II | 10 | 6 | 5 |
| Stage III + IV | 16 | 8 | 3 |
| Unknown | 12 | 6 | 5 |
| RF-positive patients | 24 | 11 | 6 |
| ESR (mm/h) | 29.63 ± 24.33 | 21.57 ± 16.26 | 17.15 ± 10.57 |
| Disease activity (DAS28) | 3.81 ± 1.04 | 3.72 ± 0.89 | 3.41 ± 0.089 |
Consent
The study was approved by the Local Committee for Biomedical Research Ethics at the Medical University of Gdansk. All subjects were informed of the details of the experiment prior to the taking of a sample of 20-ml peripheral venous blood.
Antigens
The DnaJ, DnaJΔ1-199 (C-DnaJ), and Hdj1 proteins were overproduced in E. coli B178 cells transformed with plasmids pTTQ18/dnaJ (Zylicz et al. 1985), pAED4/dnaJΔ1-199 (Doering 1992), pET21d(+)/hdj1 (Freeman et al. 1995), respectively and purified as described previously (Zylicz et al. 1985; Krzewski et al. 2003). The DnaJΔ107-375 (N-DnaJ) was overproduced in E. coli BL21(DE3)ΔdnaJ cells transformed with pKL51/dnaJΔ107-375 (received from Prof. K. Liberek, University of Gdansk–Medical University of Gdansk, Poland) and purified as described previously (Karzai and McMacken 1996). Overproduction of Hdj3, Hdj2 proteins, and of the N- and C-terminal domains of Hdj2 (N-Hdj2 and C-Hdj2) was carried out in E. coli BL21(DE3) cells transformed with plasmids pT7-7/His6Hdj3 (Terada and Mori 2000), pET24(+)/His6hdj2 (our collection), pET24(+)His6Hdj2Δ106-397 (our collection), and pET24(+)His6Hdj2Δ1-215 (our collection), respectively, and purified by affinity chromatography technique on Ni–NTA column according to the manufacturers' instructions (Qiagen). All Hsp40s purification procedures included hydroxyapatite chromatography (Bio-Rad). This procedure ensured that the human Hsp40s overproduced in bacterial cells were DnaJ-free (Hdj1 was eluted by 90 mM phosphate buffer, DnaJ—by 140 mM, and Hdj2—by 300 mM). His6-tagged E. coli HtrA heat shock protein without proteolytic activity (Skórko-Glonek et al. 1995) overproduced in bacterial cells was purified as described before (Lipinska et al. 1990). Proteins were analyzed by SDS polyacrylamide gel electrophoresis and were at least 95% pure. Western blotting analysis, with the use of the anti-DnaJ, anti-Hdj1, anti-Hdj2, anti-Hdj3, anti-DnaK, and anti-HtrA rabbit polyclonal sera (prepared as described previously in Krzewski et al. 2003) confirmed that the Hsp40 preparations were free of contaminations with other Hsps. Protein concentration was estimated by the Bradford method (Sambrook et al. 1989). Lipopolysaccharide (LPS) content in protein preparations were assayed using the Limulus Amebocyte Lysate (LAL) Endotoxin Assay Kit (#L00350, GenScript, USA) and did not exceed 0.02 endotoxin units (EU)/μg.
ELISA assay
Enzyme-linked immunosorbent assay (ELISA) was performed as described previously by Krzewski et al. (2003) with minor modifications; 96-well medium binding ELISA plates (Costar 3590) were coated with 50 μl of each Hsp40 antigen (40 μg/ml) in phosphate buffer saline (PBS). After incubation at 37°C overnight, plates were washed in PBS and blocked with PBS containing 1% bovine serum albumin (Fluka) for 1 h at room temperature. Following the washing, the sera of RA patients or healthy subjects, diluted in PBS (1:500), were added to the wells and incubated for 1 h at 37°C. After washing, the secondary mouse-anti-human Fc IgG antibodies coupled with horseradish peroxidase (HRP; Sigma) were added to the wells (1:2,000). After 1 h of incubation at 37°C and washing step, the HRP substrate, tetramethylbenzidine (Sigma), was added. After 15 min of incubation at room temperature, the reaction was stopped with the addition of 1 M H2SO4. The optical density was measured at 450 nm using an ELISA plate reader (Asys Hitech GmbH, Austria).
Proliferation assay
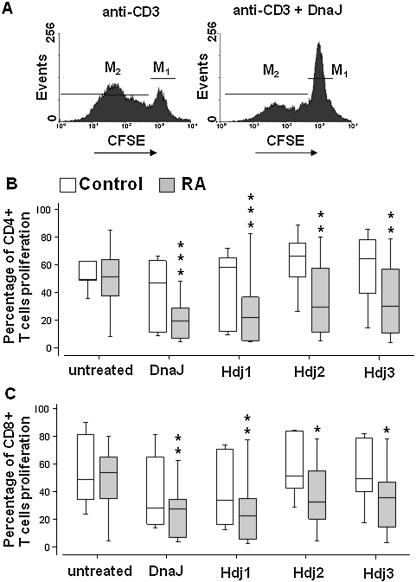
Peripheral blood mononuclear cells were isolated from venous blood by Histopaque™ (Sigma, Poland) density centrifugation. PBMCs were washed and stained with 10 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, Sigma, Poland) according to Hasbold et al. (1999). Labeled PBMCs were resuspended to 1 × 106 per 2 ml of medium (RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and penicillin/streptomycin antibiotics—all components from Sigma, Poland), and cultured and stimulated with 0.25 μg immobilized anti-CD3 (DAKO Cytomation, Poland) in 24-well culture plates in 5% CO2 at 37°C, with or without 1 μg/ml of antigen (DnaJ, Hdj1, Hdj2, and Hdj3). After 72 and 120 h of incubation, CFSE-labeled PBMCs were collected and stained with phycoerythrin (PE)-conjugated anti-CD4 and PE-Cy5-conjugated anti-CD8 monoclonal antibodies (DAKO, Poland). The proliferation of PBMCs was analyzed by flow cytometry (FACScan Becton Dickinson) and interpreted by WinMDI 2.8 software. Percentage of proliferation was defined as the fraction of cells which have divided at least once, as described previously (Verhoef et al. 2005) and explained in Fig. 3a.
Fig. 3.
Proliferative responses of CD4 T (b) and CD8 T (c) cells from patients with RA (n = 22) and age-matched healthy controls (n = 15) in the presence of Hsp40 proteins: bacterial DnaJ and human Hdj1, Hdj2 and Hdj3. PBMCs were stained with CFSE, cultured for 72 h in the presence or absence of an antigen, labeled with anti-CD4+ or CD8+ antibodies and assayed by flow cytometry. An example of the flow cytometry result showing T CD4+ cells of a representative RA patient untreated with antigen or treated with DnaJ (a). M1 marks cells which have not divided while M2 marks cells which have divided at least once. A ratio of the M2 cells to the total cells (M1 + M2), expressed in %, was used for further analyses and is shown in the graphs b and c. Graphs present median values of the percentage of dividing cells; the bottom and the top of boxes represent the 75th and 25th percentiles, respectively, and the ends of the vertical lines show min–max. Responses of the antigen-treated T cells are compared to those of the antigen-untreated cells. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
Cytokine measurements
Supernatants of the PBMCs cultures (obtained in the proliferation assays, see above) were collected after the incubation for 120 h with or without an antigen (bacterial DnaJ or human Hdj2). Cytokines (INFγ, TNFα, IL-6, IL-4, IL-10 and IL-2) were measured by the flow cytometric bead array, using human Th1/Th2 cytokine kit CBA™ (Becton Dickinson, Poland).
Measurement of rheumatoid factor was done using the latex method
Statistical analysis
Statistical analysis was performed using the SPSS 12.0 for Windows program. The normality distribution was analyzed using the Shapiro–Wilk test. Non-normal data were analyzed using the Mann–Whitney U test for unpaired samples or the Wilcoxon signed-rank test for related samples and Spearman’s rank correlation test. P values less than 0.05 were considered significant.
Results
The levels of antibodies against bacterial Hsp40 DnaJ and human Hsp40 (Hdj2 and Hdj3) proteins are increased in the sera of RA patients
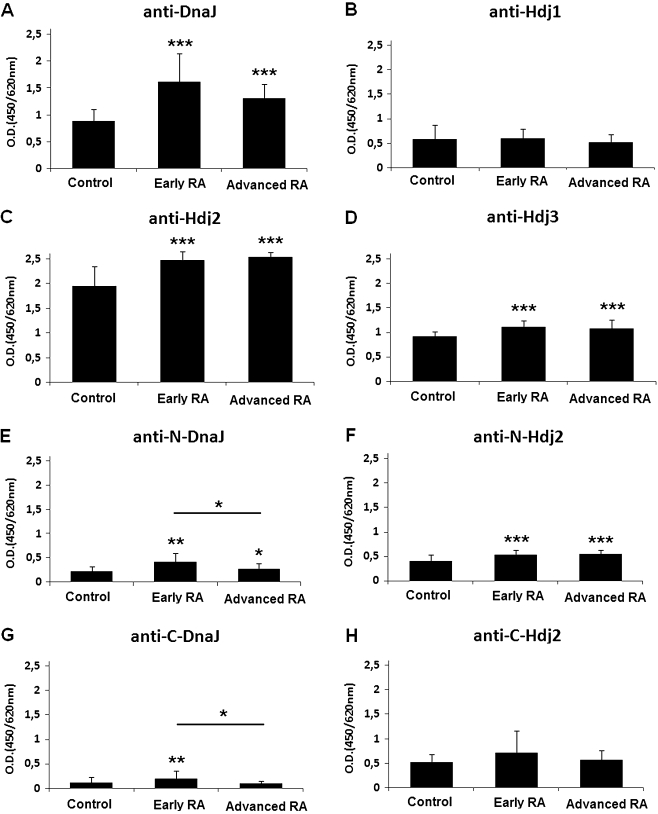
Using ELISA test we found that the sera of the RA patients contained a significantly elevated levels of the anti-DnaJ (Fig. 2a), anti-Hdj2 (Fig. 2c), and anti-Hdj3 (Fig. 2d) antibodies, compared to the sera of healthy control individuals (P ≤ 0.001). There was no significant difference between the levels of the anti-Hdj1 autoantibodies of the RA and control group (Fig. 2b). The above findings applied to both early and advanced stages of the disease.
Fig. 2.
The levels of anti-Hsp40 antibodies (IgG) in sera of rheumatoid arthritis (RA) patients (n = 48) and age-matched healthy controls (n = 50), assayed by ELISA test. The E. coli DnaJ (a) and human Hdj1 (b), Hdj2 (c) and Hdj3 (d) proteins or N-terminal J domains of DnaJ (N-DnaJ) (e) and Hdj2 (N-Hdj2) (f), and the C-terminal domains of DnaJ (C-DnaJ) (g) and Hdj2 (C-Hdj2) (h) were used as antigens. The results are presented as mean values (±SD). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
To investigate whether the humoral responses to the highly evolutionarily conserved J domains and to the poorly conserved C-terminal domains of bacterial and human Hsp40 proteins differed, we used as antigens mutant DnaJ and Hdj2 proteins, containing either the J domain or the C-terminal domain (Fig. 1). Out of the human Hsp40 group of proteins we have chosen Hdj2 because of the high level of anti-Hdj2 antibodies in both RA and control groups (Fig. 2c). The levels of the antibodies against the J domains of both DnaJ (Fig. 2e) and Hdj2 (Fig. 2f) were significantly increased in the RA patients’ sera compared to healthy controls (P ≤ 0.01 and P ≤ 0.001, respectively). In the case of the C-terminal domains, a significant increase was observed for C-DnaJ (P ≤ 0.01) in early RA patients (Fig. 2g) but not for C-Hdj2 (Fig. 2h). In the case of DnaJ, there was a markedly higher response to the J domain than to the C domain both in the RA and control sera (Fig. 2e, g). Interestingly, we did not observe such phenomenon for Hdj2, where responses to its both domains were similar (Fig. 2f, h); they also were higher compared to the anti-J-DnaJ and anti-C-DnaJ (Fig. 2e, g).
We found that humoral response of RA patients directed to DnaJ and C-Hdj2 positively correlated (r = 0.374, P = 0.019 and r = 0.350, P = 0.029 respectively) with the stages of joint damage (Steinbrocker’s criteria). The level of anti-C-Hdj2 antibodies correlated positively (r = 0.349, P = 0.023) with the erythrocyte sedimentation rate (ESR). Additionally, the level of autoantibodies against Hdj1 positively correlated (r = 0.309, P = 0.047) with the disease activity according to Disease Activity Score (DAS28) index (Table 2).
Table 2.
Correlation of the levels of anti-Hsp40 antibodies (IgG) in sera of RA patients with DAS28, Steinbrocker’s rtg staging (I–IV), and erythrocyte sedimentation rate
| Anti-DnaJ | Anti-Hdj1 | Anti-Hdj2 | Anti-Hdj3 | Anti-N-DnaJ | Anti-C-DnaJ | Anti-N-Hdj2 | Anti-C-Hdj2 | |
|---|---|---|---|---|---|---|---|---|
| DAS28 | 0.119 | 0.309 | −0.186 | 0.157 | 0.222 | 0.205 | 0.050 | 0.297 |
| P = 0.453 | P = 0.047 | P = 0.238 | P = 0.322 | P = 0.159 | P = 0.193 | P = 0.755 | P = 0.056 | |
| Steinbrocker’s rtg staging | 0.374 | 0.042 | 0.173 | 0.045 | 0.173 | 0.066 | 0.186 | 0.350 |
| P = 0.019 | P = 0.801 | P = 0.291 | P = 0.787 | P = 0.292 | P = 0.691 | P = 0.257 | P = 0.029 | |
| ESR | −0.011 | 0.065 | −0.054 | 0.132 | 0.038 | 0.038 | 0.126 | 0.349 |
| P = 0.945 | P = 0.684 | P = 0.735 | P = 0.404 | P = 0.812 | P = 0.810 | P = 0.426 | P = 0.023 |
We observed that levels of antibodies against both J and C-terminal domains of DnaJ were lower in the advanced RA patients compared to the early onset ones (Fig. 2e, g; P ≤ 0.05) and the response against the whole DnaJ antigen also tended to be decreased at the advanced stage of RA (Fig. 2a). We did not find any significant differences in reaction of early and advanced RA sera with Hdj2 or its separate domains (Fig. 2c, f, h). These results indicate that while humoral response against DnaJ is higher at the early stage of RA as compared to the advanced stage of the disease, the antibody production against Hdj2 remains high independently of the RA stage.
Fifty percent of the RA patients involved in this study were rheumatoid factor (IgM)-positive (Table 1) but there were no differences between the reactivity of the RF-positive and the RF-negative RA sera with any of the investigated Hsp40 antigens (results not shown).
Bacterial DnaJ and human Hdj1, Hdj2, and Hdj3 proteins inhibit proliferation of the peripheral blood T cells from RA patients
We analyzed proliferation of the T CD4+ and T CD8+ cells in response to the DnaJ, Hdj1, Hdj2, and Hdj3 proteins and found that none of these Hsp40 antigens alone stimulated proliferation of the CD4+ or CD8+ cells after 72 h of culturing, compared to the cells not challenged with antigen (not shown).
Furthermore, all Hsp40 antigens significantly inhibited proliferation of the CD4+ and CD8+ lymphocytes of the RA patients stimulated with anti-CD3 in vitro while there was no significant inhibition of the healthy controls’ lymphocytes stimulated with anti-CD3 and exposed to Hsp40 (Fig. 3a, b, c).
In the first experiments, we have included His6-tagged HtrA heat shock protein of E. coli as a control non-Hsp40 protein and, since it had no significant effect on T cells (not shown), we assumed that the observed inhibition of T-cell proliferation was specific for Hsp40s.
There were no significant differences in the proportion of proliferating, antigen-untreated T (CD4+ and CD8+) cells of the RA patients and healthy controls (Fig. 3b, c).
Immunomodulatory effect of bacterial DnaJ and human Hdj2 on the cytokine secretion by PBMCs of RA patients
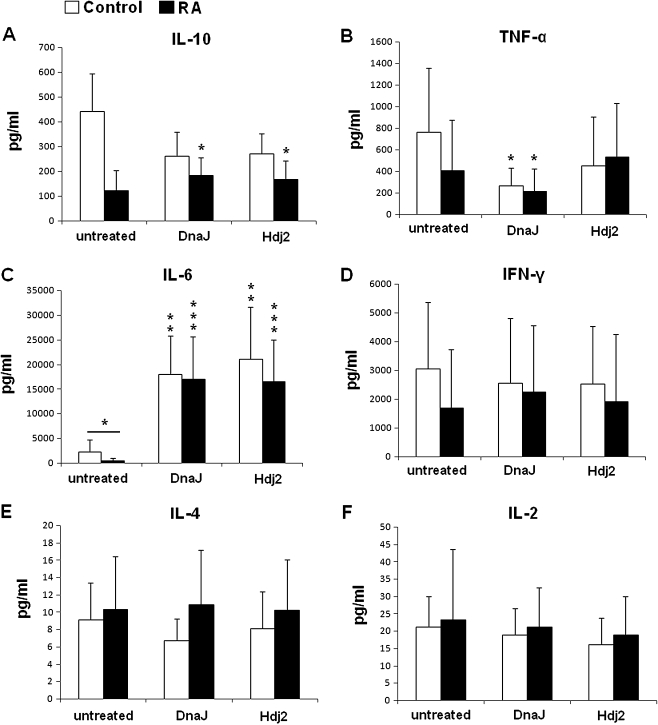
We measured effect of Hsp40 proteins on secretion of cytokines by the PBMCs from RA patients and healthy controls. Cytokines were assayed in supernatants of PBMCs’ cultures exposed for 120 h to DnaJ or Hdj2. The presence of DnaJ or Hdj2 considerably induced secretion of IL-6 in both the RA (P = 0.001) and control cultures (P = 0.005) compared to the cells untreated with antigen (Fig. 4c). The PBMCs from RA patients (13 out of 14 tested), cultured with DnaJ or Hdj2, secreted a significantly higher amount (P ≤ 0.05) of IL-10 compared to the untreated cultures (Fig. 4a). Contrary to this, there was no significant effect of DnaJ or Hdj2 on IL-10 secretion by the control PBMCs (Fig. 4a); however, the level of IL-10 tended to be decreased in response to the antigens.
Fig. 4.
Evaluation of IL-6, IL-10, TNFα, IL-2, IL-4, and IFNγ secretion by PBMCs from patients with RA and healthy control. The results are presented as mean values (±SD). The PBMCs’ responses to Hsp40 antigens are compared to those of the untreated cells, unless otherwise indicated. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
TNFα secretion by the PBMCs of RA patients and healthy controls was reduced in response to DnaJ (P ≤ 0.05; Fig. 4b)
We found no significant influence of the Hsp40 on secretion of INFγ (Fig. 4d), IL-4 (Fig. 4e), or IL-2 (Fig. 4f) in both the RA and control cultures. Similar results were obtained for cultures exposed to antigens for a shorter time period (72 h; not shown).
While comparing the cytokine levels of the RA patients and healthy controls, the only significant difference was found in the case of PBMCs not challenged with Hsp40 and IL-6 (P = 0.036; Fig. 4c).
Discussion
We found that sera of the early and advanced RA patients contained a significantly elevated levels of the anti-DnaJ, anti-Hdj2, and anti-Hdj3 antibodies, compared to the sera of control individuals, while there was no significant difference between the levels of the anti-Hdj1 autoantibodies in the RA and control group. These results suggest involvement of the anti-DnaJ, anti-Hdj2, and anti-Hdj3 responses in RA. To the best of our knowledge, this is the first report showing an increase of the humoral response against human Hsp40 Hdj2 and Hdj3 in RA. Furthermore, we found that the levels of the antibodies against the evolutionarily conserved J domains of both DnaJ and Hdj2 were significantly increased in the RA patients’ sera compared to healthy control, which suggests involvement of the Hsp40 J domains in the humoral immune response in RA. In the case of the C-terminal domains, an increase was observed for C-DnaJ in early RA patients but not for C-Hdj2. In the case of DnaJ, there was a markedly higher response to the J domain compared to anti-C domain both in the RA and control sera, suggesting that the J domain is more immunogenic. Interestingly, in RA patients, the responses to both Hdj2 domains were similar and also higher compared to the anti-DnaJ response. This suggests that, in case of Hdj2, both J and C domains are highly immunogenic.
The enhanced levels of anti-Hsp40 antibodies in RA patients could result from increased expression of Hsp40 at the sites of inflammation. On the other hand, the humoral immune response directed to Hsp40 in RA patients could be a regulatory mechanism which participates in RA limitation, as described previously in the model of adjuvant-induced arthritis in rats where a specific group of anti-Hsp65 antibodies induced suppression of arthritis or disease resistance (Ulmansky et al. 2002).
Our findings concerning anti-DnaJ response are in agreement with the early observations of Albani (Albani et al. 1995), which showed that the synthetic dnajP1 peptide containing a 15-amino acid sequence of the J domain of E. coli DnaJ (including the “shared epitope” sequence QKRAA), reacted preferentially with sera of the RA patients compared to the healthy controls. It should be noted that the human Hsp40s do not contain the “shared epitope” sequence; however, the identity of the J domains of DnaJ and Hdj2 is approximately 50%.
We observed several positive correlations between the anti-Hsp40 humoral response and RA manifestations, namely between (1) anti-DnaJ or anti-C-Hdj2 and stages of joint damage (Steinbrocker’s criteria) and (2) anti-C-Hdj2 and ESR. The above correlations support suggestion that Hsp40s may participate in the pathomechanisms of RA in humans. A correlation between the level of humoral response and Steinbrocker’s stages was demonstrated previously for another class of Hsps, the Hsp70 (Zlacka et al. 2006).
Our results indicated that while the anti-DnaJ response is lower at the advanced compared to the early RA stage, the anti-Hdj2 response is maintained at a high level at both stages. It is tempting to speculate that the anti-DnaJ response could be due to a bacterial infection occurring at the onset of the disease; this might trigger events leading to response against autologous Hsp40 and the latter would be continued through the later stages of RA while the anti-DnaJ response would decline when the infection has been eliminated. The development of the response against autologous Hsp40 could be generated via the epitope spreading mechanism. Such phenomenon has been described recently for citrullinated antigens in collagen-induced arthritis in mice (Kidd et al. 2008).
To find out if bacterial and human Hsp40 have an immunomodulatory effect in RA, we investigated their influence on T cell response and found that all tested Hsp40s (bacterial DnaJ and human Hdj1, Hdj2, and Hdj3) inhibited proliferation of stimulated CD4+ or CD8+ T cells in PBMCs cultures of RA patients but not those of healthy controls. We believe that this is the first description of the regulatory effect of human Hsp40s on T cells from RA patients. Previous study on T-cell response to bacterial Hsp40 showed that the dnaJP1 peptide stimulated T cells of the early onset RA patients (Albani et al. 1995). The reason why we failed to observe a similar result could be due to the difference in methods used to assay T-cell proliferation (we applied flow cytometry to determine the proportion of dividing cells in either CD4+ or CD8+ population while in the earlier work DNA replication was assayed by [3H] thymidine incorporation) or to the fact that we tested whole antigens, not a selected epitope. Our results however are more consistent with the findings of McColl et al. (1997) who did not observe increase in T-cell response to dnaJ peptides encompassing the “shared epitope”. These discrepancies show the importance of the antigen/epitope used for the proliferation assays.
In the past, reactivity to heat shock proteins has been thought to be associated with development of autoimmune diseases (Albani et al. 1992, 1994, 1995). However, recent studies (Prakken et al. 2002a, b; Kamphuis et al. 2005; Massa et al. 2007) indicate that recognition of peptides derived from heat shock proteins by the immune system can have an anti-inflammatory effect and down-regulate chronic state of inflammation. The reduction of T-cell proliferation in the presence of Hsp40s, observed in our study, indicates that a down-regulation of immune response by Hsp40 may occur in RA. We assume that the down-regulation is antigen-specific, since a non-Hsp40 protein (His6-HtrA of E. coli) had no significant effect on the T-cell proliferation. However, the underlying mechanism of the observed suppression remains to be elucidated. It could be either the result of direct interactions of the Hsp40s with the T cells or the effect of Hsp40s acting via T cells with regulatory phenotype (Treg CD4+CD25+high) (reviewed by Wieten et al. 2007). Treg cells induced by a peptide derived from human Hsp40 have been shown previously to down-regulate proliferation of synovial fluid mononuclear cells (SFMCs) of JIA patients (Massa et al. 2007). In order to determine whether the response of T cells of RA patients to Hsp40s might be mediated by Treg cells, the percentage of CD4+CD25+high cells following incubation with antigen should be assayed, as well as FoxP3 and IL-10 expression of the Tregs. Finally, effects of addition of FACs-sorted Tregs to the PMBCs cultures should be monitored. Paralelly, proliferation of the sorted CD4+CD25− cells exposed to the antigens should be tested. Such experiments are planned to be performed. There is also a possibility that Hsp40s action could be mediated through innate receptors. Such regulation has been demonstrated in the case of Hsp60 which were found to enhance Tregs to down-regulate CD4+CD25− or CD8+ target T cells via innate TLR signaling (Zanin-Zhorov et al. 2006).
The suppressive action of Hsp40s on T-cell proliferation of the RA patients and, at the same time, high levels of anti-Hsp40 antibodies in their sera, pose an intriguing problem. However, influence of Hsp40s on proliferation of B cells, responsible for antibody production, has not been tested, as it was beyond scope of this work. It is possible that proliferation of B cells is stimulated by the Hsp40 induced at the sites of inflammation, in spite of the lack of T-cells proliferation. As shown in this work, PBMCs are stimulated by Hsp40s to secrete cytokines, including IL-6, known to promote B-cell maturation. This stimulation is much higher in RA patients compared to the controls. Therefore, it is feasible that intensified production of cytokine(s) by T-cells makes up for the lower number of the latter and anti-Hsp40 antibodies are produced.
Massa et al. (2007) observed that peptides derived from human Hsp40s had an anti-inflammatory effect in juvenile idiopatic arthritis manifested by increasing IL-10 secretion in SFMCs culture. To investigate the potential regulatory role of Hsp40 proteins in rheumatoid arthritis, which is an arthritic disease different from JIA, we assayed cytokine production by PBMCs cultures exposed to DnaJ or Hdj2. Both antigens significantly stimulated secretion of the main anti-inflammatory cytokine IL-10 by the PBMCs of RA patients, but not of the healthy control group, suggesting the anti-inflammatory character of both bacterial and human Hsp40s in RA. It should be noted that in the earlier work (Massa et al. 2007) the peptides derived from bacterial Hsp40 (DnaJ) had an opposite, pro-inflammatory effect. Observed difference in reaction to DnaJ in Massa et al. (2007) and our studies could be due to different regulatory mechanisms in RA and JIA, to the fact that we used whole antigens, not single epitopes, or to different reactions of SFMCs and PBMCs. Irrespective of these differences, both studies point to the anti-inflammatory character of human Hsp40 proteins in arthritic diseases.
We noted that secretion of the pro-inflammatory cytokine TNFα, by the PBMCs of both RA patients and healthy control group, was reduced in response to DnaJ. The observed decrease of TNFα as well as increase of the IL-10 secretion by PBMCs are consistent with the hypothesis that Hsp40s have an anti-inflammatory effect in RA.
We found that presence of DnaJ or Hdj2 drastically induced secretion of IL-6 by the RA and control PBMCs. One of the main roles of IL-6, belonging to the Th2 group of cytokines, is to participate in B cells maturation to produce antibodies (Lafaille 1998; Diehl and Rincón 2002; Taylor and Feldmann 2004). There is a possibility that the observed increase in humoral anti-Hsp40 response in RA patients could be associated with stimulation of Th2 cells by Hsp40s. The idea that Hsp40s stimulate Th2 cells in RA is also supported by the described increase of IL-10 secretion in the presence of DnaJ or Hdj2.
Significant difference between the cytokine secretion levels in PBMCs cultures (not challenged with Hsp40) of patients and controls were found only for IL-6, whose level was decreased in RA. This does not reflect the situation in peripheral blood, where concentration of IL-6 was increased in RA patients compared to healthy controls (not shown). However, it is worth noticing that stimulation of IL-6 secretion by Hsp40 was much higher in the case of the RA PBMCs (approximately 40-fold) than in the case of control PBMCs (eight- to ninefold stimulation)—such high stimulation by antigens could explain the observed discrepancy regarding IL-6 between the cultured PBMCs and blood serum.
Since the PBMCs cultures contain not only T cells but also other lymphocytes, monocytes, and macrophages, it is not possible at this stage to state which cells are responsible for the Hsp40-modulated production of cytokines. This problem will be an object of further investigations.
Recently, the presence of LPS contaminations in preparations of Hsp60 (Osterloh et al. 2004) or Hsp70 (Motta et al. 2007) has been shown to affect immunomodulatory influence of the Hsps on macrophages, T cells and dendritic cells. Generally, LPS acted as a pro-inflammatory agent and the major effect was an increase in TNF-α production. Since we observed that our Hsp40 preparations caused either a decrease of TNF-α secretion (DnaJ) or had no effect on TNF-α level (Hdj2) in PBMCs cultures, we assume that possible contaminations with LPS did not have a significant effect on our results. Furthermore, using the LAL Endotoxin Assay, we found that LPS levels were below 0.02 EU/ml. Such low levels were probably due to the fact that we included hydroxyapatite chromatography as one of the purification steps, the procedure known to efficiently remove endotoxins (Gagnon 2009). According to Osterloh et al. (2004), the threshold amount of LPS activating TNF-α secretion by macrophages was 0.1–1.0 EU/ml.
In conclusion, our results show for the first time that the RA patients have an increased humoral response to human Hsp40 proteins Hdj2 and Hdj3. This is also the first description of immunomodulatory effect of human Hsp40 proteins on T cells in RA, manifested by the reduction of T cells’ proliferation and stimulation of the main anti-inflammatory cytokine IL-10, suggesting that Hsp40s act as natural anti-inflammatory agents in RA. Thus, we assume that Hsp40s could be considered as a potential target for designing new anti-inflammatory therapies in RA.
Acknowledgments
We would like to express our thanks to Dr. J. Skokowski of the Medical University of Gdansk for his help with collecting sera from control individuals. This work was supported by grants from Polish Committee for Scientific Research (KBN, No. 1074/P04/2004/26), from Polish Ministry of Science and Higher Education (No. 1610/B/P01/2008/35), and from University of Gdansk (BW/1460-5-0334-6, BW/1460-5-0096-7, BW/1460-5-0380-8).
Abbreviations
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- ELISA
Enzyme-linked immunosorbent assay
- Hsp
Heat shock proteins
- PBMC
Peripheral Blood Mononuclear Cells
- RA
Rheumatoid arthritis
References
- Albani S, Carson DA, Roudier J. Genetic and environmental factors in the immune pathogenesis of rheumatoid arthritis. Rheum Dis Clin North Am. 1992;18:729–740. [PubMed] [Google Scholar]
- Albani S, Keystone EC, Nelson JL, et al. Positive selection in autoimmunity: abnormal immune responses to a bacterial dnaJ antigenic determinant in patients with early rheumatoid arthritis. Nat Med. 1995;1:448–452. doi: 10.1038/nm0595-448. [DOI] [PubMed] [Google Scholar]
- Albani S, Ravelli A, Massa M, et al. Immune responses to the Escherichia coli dnaJ heat shock protein in juvenile rheumatoid arthritis and their correlation with disease activity. J Pediatr. 1994;124:561–565. doi: 10.1016/S0022-3476(05)83134-8. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 2008;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bryl E, Daca A, Jozwik A, Witkowski JM. Human CD4(low)CD25(high) regulatory T cells indiscriminately kill autologous activated T cells. Immunology. 2009;128:287–295. doi: 10.1111/j.1365-2567.2008.02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham ME, Caplan AJ (1998) Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones 3:28–36 [DOI] [PMC free article] [PubMed]
- Chukwuocha RU, Zhang B, Lai CJ, et al. Isolation of an IgG monoclonal anti-dnaJ antibody from an immunoglobulin combinatorial library from a patient with rheumatoid arthritis. Rheumatol. 1999;26:1439–1445. [PubMed] [Google Scholar]
- Cobelens PM, Kavelaars A, Zee R, Eden W, Heijnen CJ. Dynamics of mycobacterial HSP65-induced T-cell cytokine expression during oral tolerance induction in adjuvant arthritis. Rheumatology (Oxford) 2002;41:775–779. doi: 10.1093/rheumatology/41.7.775. [DOI] [PubMed] [Google Scholar]
- Kleer IM, Wedderburn LR, Taams LS, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63:1318–1326. doi: 10.1136/ard.2003.017798. [DOI] [PubMed] [Google Scholar]
- Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–536. doi: 10.1016/S0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- Doering JR (1992) Functional and structural studies of small F-actin binding domain. PhD thesis, Massachusetts Institute of Technology, Boston, 815–821.
- Fournier C. Where do T cells stand in rheumatoid arthritis? Joint Bone Spine. 2005;72:527–532. doi: 10.1016/j.jbspin.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon P. Monoclonal antibody purification with hydroxyapatite. N Biotechnol. 2009;25:287–293. doi: 10.1016/j.nbt.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Han C, Chen T, Li N, Yang M, Wan T, Cao X. HDJC9, a novel human type C DnaJ/HSP40 member interacts with and cochaperones HSP70 through the J domain. Biochem Biophys Res Commun. 2007;353:280–285. doi: 10.1016/j.bbrc.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Hasbold J, Gett AV, Rush JS, et al. Quantitative analysis of lymphocyte differentiation and proliferation in vitro using carboxyfluorescein diacetate succinimidyl ester. Immunol Cell Biol. 1999;77:516–522. doi: 10.1046/j.1440-1711.1999.00874.x. [DOI] [PubMed] [Google Scholar]
- Kamphuis S, Kuis W, Jager W, et al. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet. 2005;366:50–56. doi: 10.1016/S0140-6736(05)66827-4. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzai AW, McMacken R. A bipartite signaling mechanism involved in DnaJ-mediated activation of the Escherichia coli DnaK protein. J Biol Chem. 1996;271:11236–11246. doi: 10.1074/jbc.271.19.11236. [DOI] [PubMed] [Google Scholar]
- Kidd BA, Ho PP, Sharpe O, et al. Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination. Arthritis Res Ther. 2008;10:119. doi: 10.1186/ar2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewski K, Kunikowska D, Wysocki J, et al. Characterization of the anti-DnaJ monoclonal antibodies and their use to compare immunological properties of DnaJ and its human homologue HDJ-1. Cell Stress Chaperones. 2003;8:8–17. doi: 10.1379/1466-1268(2003)8<8:COTAMA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzik-Dumke U, Schick C, Rzepka R, Melchers I. Overexpression of human homologs of the bacterial DnaJ chaperone in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:210–220. doi: 10.1002/1529-0131(199902)42:2<210::AID-ANR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Lafaille JJ. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 1998;9:139–151. doi: 10.1016/S1359-6101(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- Lipinska B, Zylicz M, Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990;172:1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa M, Passalia M, Manzoni SM, et al. Differential recognition of heat-shock protein dnaJ-derived epitopes by effector and Treg cells leads to modulation of inflammation in juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:1648–1657. doi: 10.1002/art.22567. [DOI] [PubMed] [Google Scholar]
- Motta A, Schmitz C, Rodrigues L, Ribeiro F, Teixeira C, Detanico T, Bonan C, Zwickey H, Bonorino C. Mycobacterium tuberculosis heat-shock protein 70 impairs maturation of dendritic cells from bone marrow precursors, induces interleukin-10 production and inhibits T-cell proliferation in vitro. Immunology. 2007;121:462–472. doi: 10.1111/j.1365-2567.2007.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl GJ, Hammer J, Harrison LC. Absence of peripheral blood T cell responses to "shared epitope' containing peptides in recent onset rheumatoid arthritis. Ann Rheum Dis. 1997;56:240–246. doi: 10.1136/ard.56.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh A, Meier-Stiegen F, Veit A, Fleischer B, Bonin A, Breloer M. Lipopolysaccharide-free heat shock protein 60 activates T cells. J Biol Chem. 2004;279:47906–47911. doi: 10.1074/jbc.M408440200. [DOI] [PubMed] [Google Scholar]
- Prakken B, Kuis W, Eden W, Albani S. Heat shock proteins in juvenile idiopathic arthritis: keys for understanding remitting arthritis and candidate antigens for immune therapy. Curr Rheumatol Rep. 2002a;4:466–473. doi: 10.1007/s11926-002-0052-7. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Roord S, Kooten PJ, et al. Inhibition of adjuvant-induced arthritis by interleukin-10-driven regulatory cells induced via nasal administration of a peptide analog of an arthritis-related heat-shock protein 60 T cell epitope. Arthritis Rheum. 2002b;46:1937–1946. doi: 10.1002/art.10366. [DOI] [PubMed] [Google Scholar]
- Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006;63:2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Shahrara S, Huang Q, Mandelin AM, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;2:4–14. doi: 10.1186/ar1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorko-Glonek J, Wawrzynów A, Krzewski K, Kurpierz K, Lipińska B. Site-directed mutagenesis of the HtrA (DegP) serine protease, whose proteolytic activity is indispensable for Escherichia coli survival at elevated temperatures. Gene. 1995;163:47–52. doi: 10.1016/0378-1119(95)00406-V. [DOI] [PubMed] [Google Scholar]
- Taylor P, Feldmann M. Rheumatoid arthritis: pathogenic mechanisms and therapeutic targets. Drug Discov Today. 2004;1:289–295. doi: 10.1016/j.ddmec.2004.10.006. [DOI] [Google Scholar]
- Terada K, Mori M. Human DnaJ homologs dj2 and dj3, and bag-1 are positive cochaperones of hsc70. J Biol Chem. 2000;275:24728–24734. doi: 10.1074/jbc.M002021200. [DOI] [PubMed] [Google Scholar]
- Ulmansky R, Cohen CJ, Szafer F, et al. Resistance to adjuvant arthritis is due to protective antibodies against heat shock protein surface epitopes and the induction of IL-10 secretion. J Immunol. 2002;168:6463–6469. doi: 10.4049/jimmunol.168.12.6463. [DOI] [PubMed] [Google Scholar]
- Eden W, Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007;1113:217–237. doi: 10.1196/annals.1391.020. [DOI] [PubMed] [Google Scholar]
- Verhoef A, Alexander C, Kay AB, Larché M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med. 2005;2(3):e78. doi: 10.1371/journal.pmed.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling U, Paul L, Zee R, Prakken B, Singh M, Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–2717. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- Wieten L, Broere F, Zee R, Koerkamp EK, Wagenaar J, Eden W. Cell stress induced HSP are targets of regulatory T cells: a role for HSP inducing compounds as anti-inflammatory immuno-modulators? EBS Lett. 2007;581:3716–3722. doi: 10.1016/j.febslet.2007.04.082. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zlacka D, Vavrincova P, Hien Nguyen TT, Hromadnikova I. Frequency of anti-hsp60, -65 and -70 antibodies in sera of patients with juvenile idiopathic arthritis. J Autoimmun. 2006;27:81–88. doi: 10.1016/j.jaut.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Zylicz M, Yamamoto T, McKittrick N, Sell S, Georgopoulos C. Purification and properties of the dnaJ replication protein of Escherichia coli. J Biol Chem. 1985;260:7591–7598. [PubMed] [Google Scholar]