Abstract
Exposure of rats to environmental heat enhances the expression of heat shock protein-72 (Hsp-72) in most of their organs proportionally to heat stress severity. Pre-induction or over-expression of Hsp-72 prevents organ damage and lethality, suggesting that heat shock proteins (Hsps) may have a pathogenic role in this condition. We investigated the expression profile of Hsps in baboons subjected to environmental heat stress until the core temperature attained 42.5°C (moderate heatstroke) or occurrence of hypotension associated with core temperature ≥43.5°C (severe heatstroke). Western blot analysis demonstrated a differential induction of Hsp-72 among organs of heat-stressed animals with the highest induction in the liver and the lowest in lung. Hsp-60 and Hsc-70 expression was similar between control and heat-stressed animals. ELISA studies indicated a marked release of Hsp-72 into the circulation of baboons with severe heatstroke with a peak at 24 h post-heatstroke onset and remained sustained up to 72 h. Hsp-72 release was not associated with core temperature or systolic blood pressure, but correlated with markers of liver, myocardium, and skeletal muscle tissue necrosis. Non-survivors displayed significantly higher Hsp-72 levels than survivors. No Hsp-60 was detected in the circulation. These findings add further evidence that increased expression of Hsp-72 may be an important component of the host response to severe heatstroke. They also suggest that extracellular Hsp-72 is a marker of multiple organs tissue damage. Whether extracellular Hsp-72 plays a role in the host immune response to heat stress merits further studies.
Keywords: Heat stress, Heatstroke, Baboon, Heat shock proteins, Hyperthermia, Multiple organ dysfunction syndrome
Introduction
Heatstroke, a life-threatening condition characterized by a rapidly increasing core temperature to more than 40°C and multiple organ dysfunction syndrome (MODS), is the leading cause of morbidity and mortality in heat waves (Bouchama and Knochel 2002; Hemon and Jougla 2004; Robine et al. 2008). The mechanisms of MODS are not fully understood but include direct tissue injury and cell death from hyperthermia, sustained organ dysfunction and damage secondary to activation of the host inflammatory and coagulation responses (Bouchama and Knochel 2002; Bouchama et al. 2005; Roberts et al. 2008). Consequently, in spite of optimal cooling and supportive treatment in intensive care, the overall mortality can exceed 60%, because as yet, there is no specific treatment available (Misset et al. 2006; Argaud et al. 2007).
Heat shock, or stress response, is a conserved host defense mechanism to protect cells and tissues against hyperthermia as well as oxidative stress, inflammation, and ischemia/reperfusion injury. Under normal conditions, most cells express a low level of specific Hsps that are increased markedly by de novo synthesis in response to stress (Hightower 1991; Welch 1992; Minowada and Welch 1995). The upregulation of Hsps enables the cells to recognize damaged proteins and either restore or eliminate them through degradation pathways (Hightower 1991; Welch 1992; Minowada and Welch 1995). In addition to this chaperone function, Hsps inhibit caspase-dependent apoptosis and NF–κB-mediated inflammation (Welch 1992; Minowada and Welch 1995; Yenari et al. 2005). Hsps can also be released into the circulation, but in contrast to their intracellular anti-inflammatory action, extracellular Hsps exert an immune-stimulatory effect by interacting with pattern recognition receptors, such as toll-like receptors, and thereby activate the host inflammatory response (Asea et al. 2002; Johnson and Fleshner 2006).
Clinical and experimental studies suggest that heat shock response is an important component in the pathogenesis of heatstroke (Flanagan et al. 1995; Moseley 1997; Welch 2001; Wang et al. 2005). Passive exposure of laboratory rats to environmental heat results in an increase in Hsp-72 expression in most of the organs proportional to the severity of the heat stress (Blake et al. 1990; Flanagan et al. 1995), while pre-induction or over-expression of Hsp-72 prevents organ damage and lethality (Yang and Lin 1999; Wang et al. 2005; Lee et al. 2006). In humans, exercise associated heat stress was shown to be a potent inducer of Hsp-72 expression and release into the circulation (Moseley 1997; Febbraio and Koukoulas 2000; Walsh et al. 2001). In human victims of heatstroke, conflicting findings were reported namely; that both low and high levels of Hsp-72 were observed and correlated with poor outcome (Wang et al. 2001; Huisse et al., 2008). To date, there are no published data that assessed tissue expression and blood release of Hsps in a clinically relevant model of heatstroke. In addition, no data are available on the time course of circulating Hsps and their potential clinical implications in heatstroke.
We have recently established that baboons subjected to environmental heat stress mimic the clinical spectrum from moderate to severe heatstroke seen in human heatstroke (Bouchama et al. 2005; Roberts et al. 2008). Baboons with moderate heatstroke display hyperthermia, neurologic alteration, and MODS, which subside after 72 h with full recovery, whereas baboons with severe heatstroke develop hypotension and rapidly progressing MODS that can culminate in death (Bouchama et al. 2005; Roberts et al. 2008). The objectives of the present study were to use these two experimental baboon models of heatstroke to: (1) examine the expression of Hsp-60 and Hsc-70, together with inducible Hsp72; (2) determine whether Hsp-60 and Hsp-72 are released into the circulation, and if so, (3) analyze their time course and relation with biomarkers of cell injury, organ dysfunction, as well as death in heatstroke. Finally, since the findings in the present study demonstrated differential expression of Hsp-72 in most baboon organs with highest in liver and lowest in lung, we investigated the effect of heat shock in vitro in liver and lung cell lines to determine whether this difference is inherent to tissue.
Materials and methods
Animals
After approval of the study protocol by the institutional review board, the baboons were handled in accordance with the American Physiological Society Guiding Principles in the Care and Use of Animals. For ethical and animal welfare concern, the study was designed to use the smallest number of animals and with parsimony the archived tissue samples. Hence, we used archived tissue samples for Western blot analysis from a first group that includes moderate (n = 3), severe heatstroke (n = 3), and sham-heated control (n = 3). Because there has been no data available on extracellular Hsp-72 and Hsp-60 in heatstroke baboons, we examined prospectively whether Hsp-60 and Hsp-72 are released into the circulation in moderate (n = 1) and severe heatstroke (n = 1) as well as sham-heated control (n = 1). The finding that Hsp-72 was predominantly detected in severe heatstroke (Fig. 1) prompted us to analyze the kinetics of Hsps release in archived plasma samples of a second group of severe heatstroke (n = 6) only. Since, the baboons with severe heatstroke from the first (n = 3) and second (n = 6) group displayed similar baseline characteristics and were subjected to the same heat stress, they were merged together in a single group (n = 9).
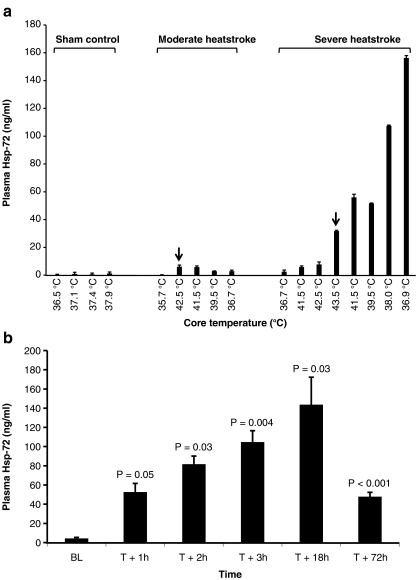
Fig. 1.
Severe heatstroke triggers the release of Hsp-72 into the circulation. a Effect of heat stress on Hsp-72 release into the circulation of sham control (n = 1), moderate (n = 1), and severe (n = 1) heatstroke baboons. Values represent plasma Hsp-72 concentration as a function of the core temperature. Error bars reflect duplicate measurements. Arrows indicate the onset of heatstroke that occurred at 42.5°C (moderate heatstroke) and 43.5°C (severe heatstroke). b Kinetics of Hsp-72 release in baboons (n = 6) subjected to severe heatstroke. Values represent mean ± SE. P values indicate the degree of significance in circulating Hsp-72 levels, when post-heat stress levels are compared with baseline (BL), using Student’s t test
Induction of moderate and severe heatstroke
On the day of the experiment, juvenile baboons (Papio hamadryas) weighing 4–6 kg were anesthetized, then intubated, and arterial and venous catheters were inserted, as described previously (Bouchama et al. 2005). The anesthesia protocol consisted of a continuous intravenous infusion of ketamine (20–25 mg/kg/h) and diazepam (0.4–0.8 mg/kg) intravenously every 2 h). The animals were subjected to environmental heat stress in a pre-warmed neonatal incubator (Isolette Infant Incubator; Air-shield, Hatboro, PA, USA) pre-set at 44–47°C to induce moderate and severe heatstroke. Vital signs were monitored continuously and recorded every 15 min for a period of 24 h using a bedside monitor (Hewlett Packard, USA). Rectal temperature was measured with a reusable, pediatric rectal thermistor probe calibrated from 0°C to 70°C with an accuracy of ±0.15°C (Yellow Springs Incorporated, Ohio, USA) that was positioned 7–8 cm above the anal sphincter. Arterial blood pressure was monitored with an intravascular catheter inserted percutaneously in the radial artery.
As described previously, moderate heatstroke occurs when core body temperature attains 42.5°C, while severe heatstroke is signaled by a drop in systolic blood pressure below 90 mmHg, and this typically takes place at core temperature exceeding 43°C (Bouchama et al. 2005). After heat exposure, the animals were allowed to cool passively at an ambient temperature of 26–29°C and were given normal saline as bolus of 10 ml to keep the mean arterial pressure ≥60 mmHg. Baboons surviving for 72 h were considered as permanent survivors. No animals in either moderate or severe heatstroke have been subjected to more than a single episode of heat stress.
In the sham-heated control group, the animals were handled in manner identical to the study group with the only difference that the incubator temperature was maintained between 26–29°C.
Blood and tissue sampling
Blood samples were collected prior to heat exposure or baseline, at the end of heat exposure (T + 0) and 1 (T + 1), 2 (T + 2), 3 (T + 3), 18 (T + 18), and 72 h later (T + 72). Plasma was prepared by centrifugation at 1,550g for 15 min and stored in aliquot at −80°C until assayed. Tissues samples from kidney, lung, jejunum, liver, spleen, adrenal, and heart were obtained at immediate autopsy in animals that died spontaneously from severe heatstroke (non-survivors). All with moderate heatstroke survived and were subjected to euthanasia at T + 72 h from the onset of heatstroke. Euthanasia was performed using an approved protocol consisting of 80 mg/kg of pentobarbital given intravenously. Tissues were snap-frozen in liquid nitrogen and stored at −80°C until assayed.
Laboratory investigations
Liver, renal, and cardiac profiles were determined on automated devices. Plasma levels of Hsps were measured by enzyme-linked immunosorbent assay (ELISA) using specific kits (Stressgen, Victoria, BC, Canada), consisting of Hsp-60 (EKS-600) and the inducible Hsp-72 (EKS-700). All the assays were performed according to the instructions of the manufacturers.
Preparation of whole tissue lysate
Snap-frozen tissues were suspended in a buffer consisting of 50 mM HEPES pH 7.3, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 200 μM sodium ortho-vanadate, 10% glycerol, 0.01% of Triton X-100, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) and then lysed by homogenization. Samples were centrifuged at 13,000 rpm for 20 min at 4°C to pellet debris. The supernatant was collected, and the protein concentration was determined by Bradford method using γ-globulin as a standard (BioRad, Hercules, CA, USA). Samples were aliquoted and stored at −80°C for Western blot analysis.
Induction of heat shock in vitro and preparation of whole cellular lysate
Cell lines used in this study consisted of human embryonic lung fibroblasts (HEL-299) and human hepatoma cells (HUH-7). They are maintained at 37°C in the presence of 5% CO2 in DMEM supplemented with 10% FBS, antibiotics, and 2 mM l-glutamine. For heat shock induction, ∼85% confluent cells were shifted from 37°C to 43°C for 1 h and then returned to 37°C and allowed to recover for different periods of time as indicated in the Fig. 4 legend. Cells were collected, washed with PBS, and the pellet was resuspended in 100 μl PBS. Cells were subsequently lysed by repeated freezing–thawing (three cycles) and then centrifuged at 13,000 rpm for 10 min. The supernatant was collected, and the protein concentration was determined as described above. Samples were aliquoted and stored at −80°C.
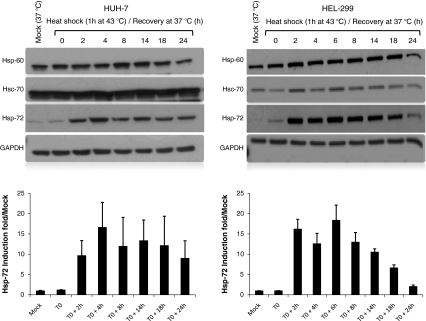
Fig. 4.
Heat stress induces Hsp-72 in vitro using cell lines. Western blots comparing the endogenous expression of Hsp-60, Hsc-70, and Hsp-72 in liver and lung cell lines. GADPH was used as internal control to correct for loading efficiency. The autoradiographs were scanned, and the relative intensity of Hsp-72 was determined after GAPDH correction. The data are presented as Hsp-72 induction fold relative to the mock. The blots shown are representative of at least three independent experiments with similar results
Western blot analysis
Western blot analysis was carried out using 30 μg of whole tissue lysate or cellular lysate. The protein samples were resolved on a 10% SDS–polyacrylamide gels and then blotted onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% non-fat dried milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) and then probed with the primary antibody for 2 h at room temperature. Subsequently, the membrane was washed with TBST for 30 min (3 × 10 min) and horseradish peroxidase-conjugated secondary antibody was added for 1 h at room temperature. Following incubation with the secondary antibody, the membrane was washed again as described above, and the protein bands were visualized by the use of an enhanced chemiluminescence detection system as recommended by the manufacturer (GE Healthcare, Piscataway, NJ, USA). The primary Hsps antibodies used in this study consisted of Hsp-60 (SPA-828), the constitutive Hsp-70 (SPA-820), and the inducible Hsp-72 (SPA-811) were obtained from Stressgen (Victoria, BC, Canada). GAPDH (sc-25778, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as internal control to monitor for loading efficiency. For densitometric analysis, the autoradiographs were scanned using a GS-800 scanner (BioRad, Hercules, CA, USA), and the intensity of the bands was determined using Quantity One Software (BioRad, Hercules, CA, USA).
Thermal calculation and statistical analysis
Thermal calculation Heat stress was quantified by determining the heat load, a product of magnitude of TC above 40.4°C and duration of hyperthermia as described previously (Hubbard et al. 1976; Bouchama et al. 2005). The temperature threshold of 40.4°C degrees represents a baseline above which mortalities occurred in experimental heat stress (Hubbard et al. 1976). Core body temperature was recorded at 15-min intervals and heat load (degree Celsius-minute) was calculated as Σ time interval (min) [TC (°C) above 40.4°C–40.4°C)]. Heating rate (degree Celsuis per minute) was calculated as [maximum TC (°C, TC max) attained during heat exposure—TC (°C) recorded before heat exposure]/time (min) to attain Tc max. Cooling rate (degree Celsius per minute) was calculated as [TC max (°C) − 40.4°C]/time (min) for passive cooling to TC of 40.4°C.
Statistical analysis Data are presented as median ± interquartile ranges (IQR 25–75th percentile), unless stated otherwise. For continuous variables, groups were compared with the Mann–Whitney test (two groups) and Kruskal–Wallis (three groups). Student’s t test was used when values follow a normal distribution. Friedman’s test was used to compare observations overtime, and Dunn’s test was used for multiple comparisons. Correlations between variables were calculated with the Spearman-rank correlation test.Differences were considered statistically significant at P values less than 0.05.
Results
Severe heatstroke triggers a marked release of Hsp-72 into the circulation
We first investigated whether Hsps-72 and Hsp-60 are released into the circulation as a function of core temperature in heat-stressed animals using ELISA. Figure 1a shows that from a single baboon in each group, Hsp-72 is released into the circulation in severe heatstroke but not in control. In moderate heatstroke, Hsp-72 was marginally induced. This finding prompted us to analyze the kinetics of Hsp-72 release in six more baboons with severe heatstroke only (Fig. 1b). Before heat stress (baseline), the circulating levels of Hsp-72 were less than 4 ng/ml (Fig. 1b). Plasma Hsp-72 levels were increased by 9-fold in the first hour after severe heatstroke onset (T + 1 h) and continued to increase during cooling and resuscitation (T + 2, and T + 3 h), reaching a zenith (34-fold baseline levels) in the recovery period (T + 24 h) before declining at T + 72 h. Under the same conditions, Hsp-60 was not detected in the circulation of severe heatstroke animals (data not shown), suggesting that the observed release was specific to Hsp-72 rather than a leakage into the circulation from damaged cells.
Severe heatstroke induces the expression of Hsp-72 in multiple tissue organs
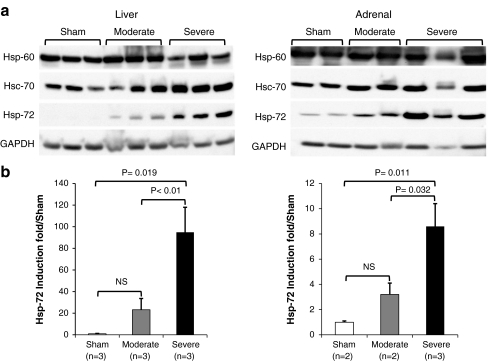
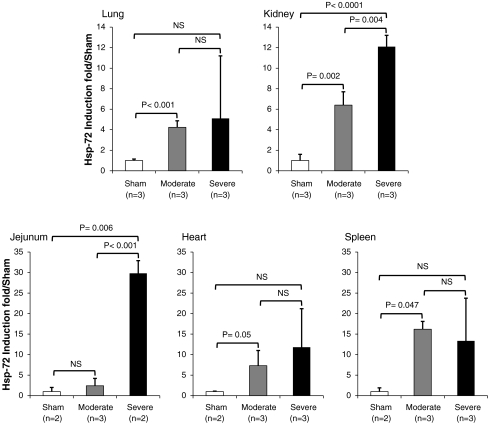
We next investigated the tissue expression of Hsp-60, Hsc-70, and Hsp-72 in liver, kidney, jejunum, adrenal, spleen, heart, and lung. The results of Western blot analysis of two representative tissues, namely liver and adrenal, show that, as predicted, the expression of Hsp-60 and Hsc-70 was similar between control and heat-stressed animals confirming the constitutive nature of these proteins (Fig. 2a). In contrast, Hsp-72 was significantly induced in heat-stressed animals both at the time of death (T + 3 h for severe) and at the time of euthanasia (T + 72 h for moderate; Fig. 2a). Densitometric analysis revealed that in severe heatstroke, Hsp-72 expression was more pronounced in the liver (95× compared with the sham; P = 0.01) than in the adrenal (9×; P = 0.01; Fig. 2b). In the other organs investigated, Hsp-72 was also induced but the magnitude of induction varied between organs and the lowest induction was observed in the lung (Fig. 3). A similar pattern of differential tissue expression of Hsp-72 was observed in moderate heatstroke except that the jejunum displayed a more marked attenuation of the heat shock response than the other organs (Figs. 2 and 3).
Fig. 2.
Heat stress induces the expression of Hsp-72. a Western blots comparing the endogenous expression of Hsp-60, Hsc-70, and Hsp-72 in two representative organs, namely liver and adrenal from sham control, moderate heatstroke, and severe heatstroke baboons. GADPH was used as internal control to correct for loading efficiency. b Densitometric analysis of Hsp-72 induction from Western blots. The autoradiographs were scanned and the relative intensity of Hsp-72 was determined after GAPDH correction. The data are presented as Hsp-72 induction fold relative to sham control. For each organ, the Western blot was done in triplicate in at least two baboons for each group. P values indicate the degree of significance of Hsp-72 induction between different groups using Student’s t test. NS not significant
Fig. 3.
Heat stress induces differential expression of Hsp-72 in multiple organs. Summary of densitometric analysis of Hsp-72 induction from Western blots from sham control, moderate heatstroke, and severe heatstroke baboons (data not shown). The relative intensity of Hsp-72 was determined after GAPDH correction and presented as Hsp-72 induction fold relative to sham control. For each organ, the Western blot was done in triplicate in at least two baboons for each group. P values indicate the degree of significance of Hsp-72 induction between different groups using Student’s t test. NS not significant
Induction of heat shock response in vitro
To determine whether the difference in Hsp-72 observed in vivo in baboon is a cell-specific response, we investigated the effect of 1 h heat shock at 43°C followed by a recovery at 37°C over 24-h period using liver and lung cell lines. Figure 4 shows that the intensity of Hsp-72 expression was comparable between liver and lung cell lines, thus differing from the in vivo findings. Kinetic studies demonstrated that the induction of Hsp-72 started 2 h after the end of heat shock and remained sustained up to 24 h (Fig. 4). There was no change in the expression profile of neither Hsp-60 nor Hsc-70 in either cell lines.
Hsp-72 expression and thermal and cardiovascular responses to heat stress
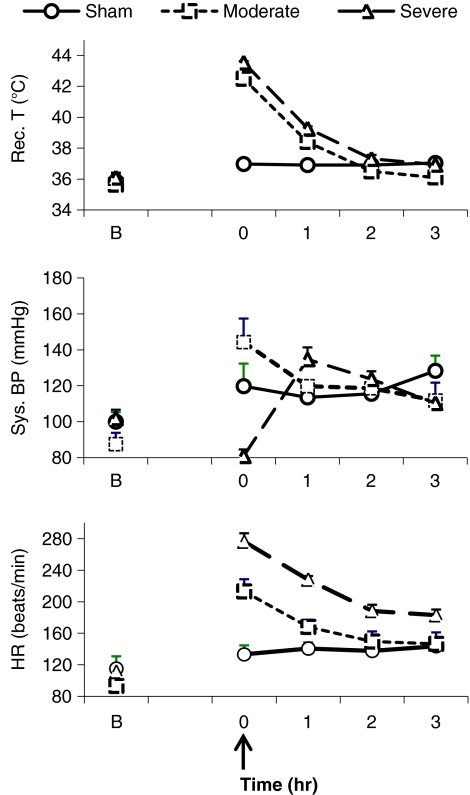
Figure 5 shows that heat stress-induced hyperthermia and hemodynamic alteration in moderate and severe heatstroke. Core temperature, systolic arterial pressure, and heart rate were significantly different between severe and moderate heatstroke. There was no significant correlation between Hsp-72 levels and core temperature or systolic blood pressure.
Fig. 5.
Thermal and cardiovascular responses in baboons subjected to moderate and severe heatstroke. Values represent mean ± SE changes in rectal temperature (rec. T; degree Celsius), systolic blood pressure (sys. BP; millimeters of Hg), and heart rate (HR; beats per minute). Arrow indicates the onset of heatstroke
Heat shock response and markers of cell injury/organ dysfunction and outcome
Severe heatstroke baboons displayed a more pronounced cell injury and multiple organs dysfunction than moderate heatstroke, whereas sham-heated animals had no remarkable changes (Table 1). Six out of nine animals with severe heatstroke died.
Table 1.
Biomarkers for cell injury and organ dysfunction in control and heat-stressed animals
| Variable | Baseline | T + 3 h | T + 18 h | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Moderate | Severe | Control | Moderate | Severe | Control | Moderate | Severe | ||
| Urea (mM) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 3 (3–3) | 4 (4–5) | 6 (6–7) | 3 (3–3) | 5 (5–6) | 9 (8–10) | 0.0015 |
| Creatinine (µM) | 54 (49–59) | 54 (48–73) | 55 (46–57) | 55 (49–60) | 102 (93–119) | 135 (120–148) | 58 (51–64) | 99 (86–111) | 133 (106–186) | 0.0002 |
| CK (U/L) | 640 (619–798) | 304 (284–722) | 553 (352–673) | 1,413 (1,225–1,601) | 973 (679–2,191) | 1,951 (1,122–2,161) | 1,288 (1,091–1,484) | 3,674 (3,050–4,297) | 6,126 (4,045–6,562) | <0.0001 |
| ALT (U/L) | 18 (15–20) | 14 (10–20) | 23 (18–29) | 20 (15–24) | 21 (18–25) | 47 (35–61) | 26 (25–26) | 32 (30–33) | 107 (86–133) | <0.0001 |
| AST (U/L) | 25 (17–28) | 28 (20–29) | 28 (25–33) | 27 (24–29) | 44 (43–59) | 169 (111–219) | 37 (32–41) | 86 (76–95) | 280 (260–390) | <0.0001 |
| Troponin (µg/L) | 0.01 (0.01–0.01) | NA | 0.01 0.01–0.01) | 0.01 (0.01–0.01) | NA | 1.07 (0.70–5.45) | 0.01 (0.01–0.01) | NA | 0.13 (0.05–0.43) | 0.0042 |
| Platelet (109/L) | 442 (397–558) | 426 (414–533) | 503 (487–529) | 478 (406–554) | 462 (360–505) | 254 (201–356) | 466 (397–574) | 379 (266–452) | 134 (96–174) | 0.0008 |
| d-dimer (µg/ml) | 124 (99–178) | 118 (87–505) | 167 (123–198) | 114 (96–161) | 405 (341–707) | 755 (400–10827) | 135 (107––195) | 603 (272–939) | 685 (582–1,516) | 0.0005 |
| Fibrinogen (g/L) | 1.10 (1.05–1.15) | 0.90 (0.90–1.80) | 1.20 (1.10–1.20) | 1.05 (1.00–1.15) | 1.30 (1.10–1.50) | 1.10 (0.85–1.45) | 1.15 (1.05–1.35) | 1.30 (1.20–1.80) | 1.40 (1.00–1.60) | 0.4298 |
| Hemoglobin (g/L) | 119 (114–128) | 127 (117–137) | 126 (121–129) | 117 (113–125) | 145 (124–156) | 137 (133–139) | 109 (106–121) | 126 (121–144) | 137 (133–139) | 0.0943 |
Values represent median±interquartile (IQR) changes in blood urea, creatinine, creatine kinase (CK), aspartic aminotransferase (AST), alanine aminotransferase (ALT), troponin, platelet, d-dimer, and hemoglobin. Friedman test was used to compare the difference between the three groups overtime (three sham-heated control, three moderate, and nine severe heatstroke). Data were considered significant at P < 0.05
As shown in Figs. 2 and 3, the magnitude of tissue expression of Hsp-72 appears to be proportional to organ injury and dysfunction manifested in moderate and severe heatstroke groups (Table 1). Likewise, plasma Hsp-72 levels correlated significantly with markers of cell injury/organ dysfunction, namely aspartic aminotransferase (AST; r = 0.83; P = 0.0008), ALT (r = 0.73; P = 0.004), creatinine (r = 0.74; P = 0.005), creatine kinase (r = 0.78; P = 0.002), and troponin (r = 0.683; P = 0.009) in severe heatstroke group.
Table 2 shows that both survivors and non-survivors were subjected to comparable heat stress as indicated by maximum rectal temperature, the heat load, and duration of heat exposure. However, non-survivors displayed more hemodynamic instability as indicated by large fluctuation of systolic blood pressure and severe tachycardia, as well as more severe multiple organ injury/dysfunction than survivors (Tables 2 and 3). The plasma Hsp-72 level was significantly higher in non-survivors than in survivors, suggesting that Hsp-72 levels reflect the magnitude of tissue injury (Table 3).
Table 2.
Thermal and cardiovascular responses to severe heatstroke in survivors and non-survivors
| Variable | Survivors | Non-survivors | P value |
|---|---|---|---|
| Heat load (°C. min) | 363 (276–402) | 319 (308–339) | 0.605 |
| Core temperature maximum (°C) | 43.7 (43.7–43.8) | 43.5 (43.3–43.8) | 1.000 |
| Duration of heat exposure (min) | 210 (200–325) | 262 (130–365) | 0.796 |
| Heating rate (°C/min) | 0.03 (0.02–0.04) | 0.03 (0.02–0.04) | 0.294 |
| Cooling rate (°C/min) | 0.02 (0.02–0.02) | 0.04 (0.02–0.06) | 0.589 |
| Systolic blood pressure (mmHg) | 111 (105–118) | 118 (98–133) | 0.018 |
| Heart rate (beats/min) | 167 (152–182) | 197 (157–233) | <0.0001 |
Values represent median±interquartile range (IQR). Mann–Whitney test was used to compare the difference between survivors (n = 3) and non-survivors (n = 6). Data were considered significant at P < 0.05
Table 3.
Biomarkers for cell injury, organ dysfunction, and Hsp-72 release according to the outcome in severe heatstroke
| Variable | Baseline | T + 3 h | P value | ||
|---|---|---|---|---|---|
| Survivors | Non-survivors | Survivors | Non-survivors | ||
| Urea (mM) | 2 (2–3) | 2 (2–4) | 6 (5–7) | 9 (8–11) | 0.009 |
| Creatinine (µM) | 55 (34–56) | 54 (53–57) | 109 (103–134) | 167 (167–184) | 0.001 |
| CK (U/L) | 535 (259–739) | 571 (445–606) | 2064 (936–2232) | 3057 (2677–4098) | 0.0008 |
| AST (U/L) | 26 (18–29) | 32 (27–33) | 190 (115–196) | 242 (239–382) | 0.0008 |
| ALT (U/L) | 18 (13–34) | 23 (23–23) | 35 (34–57) | 117 (104–186) | 0.0008 |
| Troponin (µg/L) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.70 (0.43–1.07) | 11.33 (5.45–17.22) | 0.009 |
| Platelet (109/L) | 498 (476–529) | 516 (487–545) | 271 (237–337) | 183 (182–219) | 0.0003 |
| d-dimer (µg/ml) | 145 (54–193) | 183 (123–224) | 750 (441–861) | 2793 (1796–4210) | 0.0008 |
| Hemoglobin (g/L) | 127 (124–132) | 124 (120–129) | 136 (133–140) | 133 (123–150) | 0.126 |
| Hsp–72 (ng/ml) | 5 (2–9) | 3 (0–6) | 75 (74–94) | 145 (121–169) | 0.004 |
Values represent median±IQR changes in blood urea, creatinine, creatine kinase (CK), aspartic aminotransferase (AST), alanine aminotransferase (ALT), troponin, platelet, d-dimer, hemoglobin, and Hsp-72. Friedman test was used to compare the difference overtime between survivors (n = 3) and non-survivors (n = 6). Data were considered significant at P < 0.05
Discussion
Our findings show that heatstroke in baboons induces a strong intra- and extracellular stress response. The median levels of inducible Hsp-72 in most of the tissue organs examined in moderate and severe heatstroke was as high as 90-fold sham-heated control value. Likewise, a large amount (up to 40-fold the baseline value) of Hsp-72 was released into the circulation of severe but not moderate heatstroke, and levels of Hsp-72 were highest in baboons with more severe MODS and death. In contrast, no significant difference in tissue expression or release into circulation of Hsp-60 was detected. These findings add further evidence that upregulation of heat shock proteins may be an important component of the host response to heatstroke, and circulating levels Hsp-72 may serve as a marker of heatstroke severity and outcome.
Earlier reports showed that, in rats subjected to heat stress, an elevation of core temperature to 42°C enhances Hsp-72 expression but with a time course and magnitude that differ between tissues of the same animal (Blake et al. 1990; Flanagan et al. 1995). Flanagan et al. (1995) demonstrated that the expression of Hsp-72 was tissue-specific, more in the liver, jejunum, and kidney than in brain and muscle and that the magnitude of expression was dependent on the severity of heat stress, hence suggesting that Hsp-72 synthesis reflects tissue susceptibility to early thermal damage. The findings in this study concur with these observations and strengthen them by demonstrating a tissue-specific response of Hsp-72 with the highest induction level observed in the liver, followed by the jejunum, heart, kidney, and the lowest level in the adrenal and lung. The degree of Hsp-72 expression paralleled the severity of liver injury and renal dysfunction as assessed by plasma liver enzyme activity and creatinine levels, respectively. This is also in agreement with previous clinical and experimental studies showing that in severe heatstroke; liver and jejunum are the sites of extensive damage compared with other organs, such as the lung (Bouchama et al. 2005; Roberts et al. 2008).
The exact mechanisms responsible for the differential tissue organ expression remain to be elucidated; however, one immediate explanation could be that Hsp-72 regulation varies with the intrinsic characteristics of each tissue type. Therefore, we explored this hypothesis by subjecting liver and lung cells to severe heat shock in vitro and were able to rule out this possibility by demonstrating that contrary to the in vivo findings, no differential expression was observed between these two cell types in vitro. Although, this difference between in vitro and in vivo effects of heat shock is not entirely clear, it may be in part attributable to the importance of the interaction of the cells with their environment. Thermoregulation in acute heat stress induces redistribution of blood flow that may result in variable visceral perfusion (Hales et al. 1979; Rowell 1983; Hall et al. 1999) and storage of heat (Febbraio and Koukoulas 2000; Kuboki et al. 2007; Dickson et al. 1979) among regions and organs of the body, particularly the splanchnic organs. These could have accounted for the differential Hsp-72 expression noted in our study.
Until recently, heat shock proteins were considered intracellular proteins, however, the detection of Hsp-60 and Hsp-72 in the sera of normal individuals (Pockley et al. 1999; Pockley et al. 2002) and observations of increased circulating levels of Hsp-72 in various conditions such as cardiovascular (Wright et al 2000; (Dybdahl et al. 2005), acute lung injury (Ganter et al. 2006), heat illness (Wu et al. 2001), and trauma (Wright et al. 2000; Wu et al. 2001; Pittet et al. 2002; Pockley et al. 2002) raised the question of a potential extracellular role. In classical heatstroke, two clinical studies so far have reported high circulating levels of Hsp-72 and Hsp-60 with conflicting findings (Wang et al. 2001; Huisse et al., 2008). Wang et al. (2001) detected increased serum levels of Hsp-72 in patients with moderate heatstroke and healthy individuals exposed to heat wave, but not in patients with severe heatstroke. In contrast, in a recent study of patients with severe heatstroke, circulating Hsp-72 and Hsp-60 levels were found markedly elevated and were associated with increased morbidity and mortality (Huisse et al., 2008). However, both clinical studies had serious limitations including a single measurement of plasma Hsp levels, the presence of antibodies to Hsp that may have interfered with the assay and finally, variable characteristics of the population studied, particularly the age, which is known to affect the heat shock response (Moseley 1997; Gutsmann-Conrad et al. 1998).
Using a non-human primate that mirrors human heatstroke, our findings could demonstrate that in severe heatstroke, Hsp-72 (but not Hsp-60) appears in the circulation at the onset of heatstroke, peaks at 24 h, and remains elevated at 72 h from the onset of heatstroke. The biologic half-life of Hsp-72 being reported at 18 h (Gerner et al. 2002), suggests that the persistent levels may be due to continuous release of this protein and/or delayed clearance from the circulation. The significant correlation of Hsp-72 levels with plasma creatinine levels makes the latter mechanism more likely. The time course of Hsp-72 was neither associated with core body temperature nor systolic blood pressure suggesting that heat and ischemia associated with hypotension may not have contributed significantly to its sustained release into the circulation.
The cellular sources and mechanisms of release of Hsp-72 in the circulation were not addressed in this study. Nonetheless, previous studies have shown that cell necrosis and subsequent spilling into the circulation was the main mechanism of release of Hsp (Johnson and Fleshner 2006). Thus, in the present study, the findings that plasma Hsp-72 levels correlated with markers of liver, myocardium, and skeletal muscle necrosis, namely AST, troponin, and creatine kinase concentrations lend support to this explanation. On the other hand, Hsp-60 was not detected in the circulation in our baboons with severe heatstroke, as one would expect if cell necrosis was the only mechanism of release, suggesting that other mechanisms of active release might be also at play (Johnson and Fleshner 2006). Future studies may help delineate the cellular source of Hsp-72 and the pathways of its release in heatstroke.
As in human, severe heatstroke in our experimental baboon induced liver and cardiac injury and renal dysfunction that culminated in the demise of more than 50% of the animals. The markers of cell injury and organ dysfunction were significantly correlated with Hsp-72 levels. Furthermore, non-survivors displayed higher circulating Hsp-72 levels than survivors. These observations are consistent with other reports suggesting that circulating Hsp-72 may indicate the extent of tissue damage and necrotic cell death and thereby represent a danger signal (Welch 2001; Campisi et al. 2003).
There are limitations in this study that deserve consideration. The number of animals was small with an LD50 at 3 h, thus leaving very few animals to study for a much longer follow-up. This is together with the obvious unfeasibility to carry out a kinetic study in baboons with much earlier tissue organ collection as reported in heat-stressed laboratory rats (Blake et al. 1990). Also, the study analyzed the heat shock response in juvenile baboons, and this may not be relevant to older population, as aging is known to decrease the expression of Hsp-72 (Gutsmann-Conrad et al. 1998).
Nonetheless, despite these limitations, the present study showed that heatstroke induces a massive intra- and extracellular heat shock response. The expression of Hsp-72 was tissue–organ-specific confirming its potential role as marker of susceptibility to heat injury. The source and the role of circulating Hsp-72 remains to be elucidated, however, because its plasma levels were associated with MODS and death raises the possibility that Hsp-72 could serve as a prognostic marker in heatstroke.
Acknowledgements
This work was supported by King Faisal Specialist Research Center, Riyadh, Saudi Arabia “Grant 2020 017”.
Conflicts of interest There are no conflicts of interest.
References
- Argaud L, Ferry T, et al. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177–2183. doi: 10.1001/archinte.167.20.ioi70147. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277(17):15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Blake MJ, Gershon D, et al. Discordant expression of heat shock protein mRNAs in tissues of heat-stressed rats. J Biol Chem. 1990;265(25):15275–15279. [PubMed] [Google Scholar]
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Roberts G, et al. Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J Appl Physiol. 2005;98(2):697–705. doi: 10.1152/japplphysiol.00461.2004. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, et al. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8(3):272–286. doi: 10.1379/1466-1268(2003)008<0272:SEHIAF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson JA, McKenzie A et al (1979) Temperature gradients in pigs during whole-body hyperthermia at 42 degrees C. J Appl Physiol 47(4):712–7 [DOI] [PubMed]
- Dybdahl B, Slordahl SA, et al. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91(3):299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Koukoulas I. HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J Appl Physiol. 2000;89(3):1055–1060. doi: 10.1152/jappl.2000.89.3.1055. [DOI] [PubMed] [Google Scholar]
- Flanagan SW, Ryan AJ, et al. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol. 1995;268(1 Pt 2):R28–R32. doi: 10.1152/ajpregu.1995.268.1.R28. [DOI] [PubMed] [Google Scholar]
- Ganter MT, Ware LB, et al. Extracellular heat shock protein 72 is a marker of the stress protein response in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L354–L361. doi: 10.1152/ajplung.00405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner C, Vejda S, et al. Concomitant determination of absolute values of cellular protein amounts, synthesis rates, and turnover rates by quantitative proteome profiling. Mol Cell Proteomics. 2002;1(7):528–537. doi: 10.1074/mcp.M200026-MCP200. [DOI] [PubMed] [Google Scholar]
- Gutsmann-Conrad A, Heydari AR, et al. The expression of heat shock protein 70 decreases with cellular senescence in vitro and in cells derived from young and old human subjects. Exp Cell Res. 1998;241(2):404–413. doi: 10.1006/excr.1998.4069. [DOI] [PubMed] [Google Scholar]
- Hales JR, Rowell LB et al (1979) Regional distribution of blood flow in awake heatstressed baboons. Am J Physiol 237(6):H705–12 [DOI] [PubMed]
- Hall DM, Baumgardner KR et al (1999) Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am J Physiol 276(5 Pt 1):G1195–203 [DOI] [PubMed]
- Hemon D, Jougla E. The heat wave in France in August 2003. Rev Epidemiol Sante Publique. 2004;52(1):3–5. doi: 10.1016/S0398-7620(04)99017-7. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hubbard RW, Matthew WT, et al. The laboratory rat as a model for hyperthermic syndromes in humans. Am J Physiol. 1976;231(4):1119–1123. doi: 10.1152/ajplegacy.1976.231.4.1119. [DOI] [PubMed] [Google Scholar]
- Huisse MG, Pease S, Hurtado-Nedelec M, et al. Leukocyte activation: the link between inflammation and coagulation during heatstroke. A study of patients during the 2003 heat wave in Paris. Crit Care Med. 2008;36(8):2288–2295. doi: 10.1097/CCM.0b013e318180dd43. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79(3):425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- Kuboki S, Schuster R et al (2007) Role of heat shock protein 70 in hepatic ischemiareperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 292(4):G1141–9 [DOI] [PubMed]
- Lee WC, Wen HC, et al. Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. J Appl Physiol. 2006;100(6):2073–2082. doi: 10.1152/japplphysiol.01433.2005. [DOI] [PubMed] [Google Scholar]
- Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest. 1995;95(1):3–12. doi: 10.1172/JCI117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misset B, Jonghe B, et al. Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: a national multiple-center risk-factor study. Crit Care Med. 2006;34(4):1087–1092. doi: 10.1097/01.CCM.0000206469.33615.02. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol. 1997;83(5):1413–1417. doi: 10.1152/jappl.1997.83.5.1413. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Lee H, et al. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52(4):611–617. doi: 10.1097/00005373-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Bulmer J, et al. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4(1):29–35. doi: 10.1379/1466-1268(1999)004<0029:IOHHSP>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Faire U, et al. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20(9):1815–1820. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- Roberts GT, Ghebeh H, et al. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arterioscler Thromb Vasc Biol. 2008;28(6):1130–1136. doi: 10.1161/ATVBAHA.107.158709. [DOI] [PubMed] [Google Scholar]
- Robine JM, Cheung SL, et al. Death toll exceeded 70,000 in Europe during the summer of 2003. C R Biol. 2008;331(2):171–178. doi: 10.1016/j.crvi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Rowell LB (1983) Cardiovascular aspects of human thermoregulation. Circ Res 52:367–79 [DOI] [PubMed]
- Walsh RC, Koukoulas I, et al. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6(4):386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZZ, Wang CL, et al. Autoantibody response to heat shock protein 70 in patients with heatstroke. Am J Med. 2001;111(8):654–657. doi: 10.1016/S0002-9343(01)00974-3. [DOI] [PubMed] [Google Scholar]
- Wang JL, Ke DS, et al. Heat shock pretreatment may protect against heatstroke-induced circulatory shock and cerebral ischemia by reducing oxidative stress and energy depletion. Shock. 2005;23(2):161–167. doi: 10.1097/01.shk.0000150779.47107.d5. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72(4):1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Heat shock proteins as biomarkers for stroke and trauma. Am J Med. 2001;111(8):669–670. doi: 10.1016/S0002-9343(01)01046-4. [DOI] [PubMed] [Google Scholar]
- Wright BH, Corton JM, et al. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15(1):18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- Wu T, Chen S, et al. Presence of antibody against the inducible Hsp71 in patients with acute heat-induced illness. Cell Stress Chaperones. 2001;6(2):113–120. doi: 10.1379/1466-1268(2001)006<0113:POAATI>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Lin MT. Heat shock protein expression protects against cerebral ischemia and monoamine overload in rat heatstroke. Am J Physiol. 1999;276(6 Pt 2):H1961–H1967. doi: 10.1152/ajpheart.1999.276.6.H1961. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Liu J, et al. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]