Abstract
Carboxy terminus of Hsc70-interacting protein (CHIP) is thought to be a cytoprotective protein with protein quality control roles in neurodegenerative diseases and myocardial ischemia. This study describes the localization of CHIP expression in normal rodent brain and the early CHIP response in primary cultures of cortical neurons following ischemic stress models: heat stress (HS) and oxygen–glucose deprivation (OGD). CHIP was highly expressed throughout the brain, predominantly in neurons. The staining pattern was primarily cytoplasmic, although small amounts were seen in the nucleus. More intense nuclear staining was observed in primary cultured neurons which increased with stress. Nuclear accumulation of CHIP occurred within 5–10 min of HS and decreased to baseline levels or lower by 30–60 min. Decrease in nuclear CHIP at 30–60 min of HS was associated with a sharp increase in delayed cell death. While no changes in cytoplasmic CHIP were observed immediately following OGD, nuclear levels of CHIP increased slightly in response to OGD durations of 30 to 240 min. OGD-induced increases in nuclear CHIP decreased slowly during post-ischemic recovery. Nuclear CHIP decreased earlier in recovery following 120 min of OGD (4 h) than 30 min of OGD (12 h). Significant cell death first appeared between 12 and 24 h after OGD, again suggesting that delayed cell death follows closely behind the disappearance of nuclear CHIP. The ability of CHIP to translocate to and accumulate in the nucleus may be a limiting variable that determines how effectively cells respond to external stressors to facilitate cell survival. Using primary neuronal cell cultures, we were able to demonstrate rapid translocation of CHIP to the nucleus within minutes of heat stress and oxygen–glucose deprivation. An inverse relationship between nuclear CHIP and delayed cell death at 24 h suggests that the decrease in nuclear CHIP following extreme stress is linked to delayed cell death. Our findings of acute changes in subcellular localization of CHIP in response to cellular stress suggest that cellular changes that occur shortly after exposure to stress ultimately impact on the capacity and capability of a cell to recover and survive.
Keywords: Heat shock, Heat shock proteins, Chaperones, Ischemia, Acute stress, Stroke, Ubiquitin
Introduction
Survival of cells following stress depends heavily on the balance of protein quality control mechanisms that, on one hand, make functional proteins and, on the other hand, eliminate defective proteins (Hohfeld et al. 2001). During ischemic stress, the rapid loss of energy production disables protein folding mechanisms, resulting in unfolded or misfolded proteins. These damaged proteins trigger the unfolded protein response, which upregulates the expression of heat shock proteins (HSPs), activates HSP transcription factors, and promotes both protein refolding and degradation. As a result of these cytoprotective mechanisms, cells are able to maintain homeostasis and survive a variety of stressors.
HSPs have been widely studied in protection from stroke and other CNS insults. In particular, studies of the 70-kD family of HSPs support that the chaperone and folding functions of these proteins are essential for recovery following cerebral ischemia. This family includes heat shock cognate 70 (HSC70, the constitutively expressed form) and HSP70 (the inducible form). HSP70 is synthesized at especially high levels in the CNS in response to ischemia and appears to be highly neuroprotective (Sharp et al. 1999; Yenari et al. 1999b). Induction of HSP70 was seen in models of ischemic tolerance (Chen and Simon 1997; Kirino 2002; Yenari et al. 1999a), and inhibition of HSP70 led to partial inhibition of tolerance (Kirino 2002). Virally mediated HSP70 overexpression in cultured neurons protected these cells from heat stress (Fink et al. 1997). Viral delivery of HSP70 pre-ischemia was neuroprotective in rat models of stroke (Kelly et al. 2002; Yenari et al. 1998). Transgenic mice overexpressing HSP70 were protected against cerebral infarction in models of focal ischemia (Rajdev et al. 2000). Conversely, reduced HSP70 expression led to increased cellular damage after focal ischemia in HSP70 knockout mice (Lee et al. 2001).
Protein degradation may be just as important as folding since recent evidence supports a cytoprotective role for the carboxyl-terminus of heat shock cognate 70 interacting protein (CHIP) in models of heat stress and cardiac ischemia. CHIP (-/-) mice and cells cultured from these mice undergo temperature-sensitive apoptosis in response to thermal and proteotoxic stress (Dai et al. 2003). CHIP (-/-) mice subjected to a model of cardiac ischemia and reperfusion had larger infarcts and increased rates of arrhythmia and mortality when compared with wild-type littermates (Zhang et al. 2005).
CHIP was initially described as a co-chaperone of the heat shock proteins that promotes protein degradation via the ubiquitin–proteosome system. When bound to HSPs, CHIP functions as a chaperone-dependent ubiquitin ligase via its U-box region (Jiang et al. 2001; Murata et al. 2001). Other co-chaperones work with HSPs to refold proteins, and the balance between protein folding and degradation is likely critical to cellular homeostasis (McClellan and Frydman 2001). In vitro studies suggest that, at baseline, the HSP machinery favors protein folding. However, even small increases in CHIP appear to reconfigure the HSP machinery to favor the ubiquitination pathway (Meacham et al. 2001). Thus, under conditions of severe cellular stress, the CHIP-mediated degradation pathway may compensate when the refolding pathway is overwhelmed.
In addition to its ubiquitin ligase activity, CHIP possesses other functional properties that may be cytoprotective. Rosser et al. demonstrated that CHIP has intrinsic chaperone activity that allows it to selectively recognize and bind to non-native substrates, thereby suppressing aggregation of these proteins. Both protein binding activity and suppression of aggregation were significantly enhanced by heat treatment of CHIP (Rosser et al. 2007). Tripathi et al. (2007) have proposed CHIP as a direct chaperone of p53. In their studies, CHIP prevented thermal inactivation of p53 in an HSP-independent fashion. In addition, CHIP recovered the DNA binding activity of heat-denatured p53 (Tripathi et al. 2007).
CHIP has also been shown to stabilize HSF1 in its active form, which binds to and activates promoter regions of stress response genes (Dai et al. 2003; Kim et al. 2005). Overexpression of CHIP leads to upregulation of several chaperone proteins, especially and most profoundly HSP70. CHIP overexpression strongly activated HSP70 promoter activity in reporter gene assays in an HSF1-dependent manner. The induction of HSP70 by CHIP required the TPR domain, which mediates CHIP binding to HSPs, but not the U-box domain (responsible for ubiquitin ligase activity; Dai et al. 2003).
Our laboratory has been interested in a role for CHIP in response to cerebral ischemia. We therefore undertook studies to determine the expression of CHIP in the brain and to determine changes in neuronal patterns of expression and localization of CHIP in response to acute cellular stressors. We present evidence for widespread neuronal expression of CHIP and for rapid translocation of CHIP to the nucleus in response to both heat stress and oxygen–glucose deprivation (OGD) in primary cortical cell culture models.
Materials and methods
Cell culture and in vitro stress models
Cultured cortical neurons from E17 Long-Evans rat fetuses were maintained 8–14 days at 37°C and 5% CO2 in Minimum Essential Medium (MEM, Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (Hyclone, Logan, UT) and 20 µg/ml gentamicin (Invitrogen, Carlsbad, CA). Cells were seeded at a density of 105/cm2 on poly-d-lysine-coated plates or glass coverslips to provide a high density of neurons. These cultures contained approximately 90% or greater neurons at the time of seeding.
Cells were subjected to heat stress in a 42°C water bath for durations between 0 and 60 min in artificial cerebral spinal fluid (concentrations in millimolars, 137NaCl, 5.0KCl, 2.3CaCl2, 1.3MgCl2, 10 HEPES, 20 glucose, pH 7.37–7.40, osmolality 290) or OGD medium: glucose-free MEM salt solution (in millimolars, 1.3CaCl2·2H2O, 5.3KCl, 0.81 MgSO4, 116.4NaCl, 26.2NaHCO3, 1.0NaH2PO4 × H20) + 2-deoxy-d-glucose (5 mM, Sigma, D8375) + 1× amino acid solution (Invitrogen, Carlsbad, CA) + 1× vitamin solution (Invitrogen, Carlsbad, CA) in an anaerobic chamber (Modular Incubator Chamber, Billups-Rothenberg, Del Mar, CA) gassed with 95% argon/5% CO2, purged for 7 min and sealed for durations between 0 and 240 min. Cells were returned to normal cell culture conditions for durations between 0 and 24 h following OGD to assess recovery.
Immunostaining for CHIP
Brains from 10-week-old male Sv129 mice or 10–12 week-old male Long-Evans rats were perfused with 4% paraformaldehyde in 0.01 M phosphate buffered saline (PBS: in millimolars, 9.4NaH2PO4×H2O, 12.1Na2HPO4×7H2O, 140NaCl, pH 7.4–7.5) and harvested. Brains were paraffin-embedded and cut into 5-µm coronal sections. Sections were unmasked in heated citrate buffer (2% C6H807×H20 + 9.1% C6H5Na3O7×2H20) by boiling for 3 min, permeabilized with 0.1% Triton in PBS, blocked with horse serum (1:100), then incubated with antibody. Representative coronal sections were stained using a rabbit anti-CHIP polyclonal antibody (PA1-015, Affinity Bioreagents, Golden, CO) diluted 1:100 in PBS, and visualized with an Alexa-594 conjugated donkey anti-rabbit secondary (A21207, Invitrogen, Carlsbad, CA) or Alexa-488 conjugated donkey anti-rabbit secondary (A21206, Invitrogen, Carlsbad, CA) diluted 1:100. Coverslips of cultured cortical neurons were fixed in 4% paraformaldehyde. Cells were permeabilized with 0.1% Triton in PBS, blocked with 5% horse serum then incubated with the polyclonal CHIP antibody followed by anti-rabbit-Alexa 594 conjugate (21207, Invitrogen, CA) for visualization. Some sections and cultures were double-stained with a neuronal marker, goat anti-MAP2 (sc-12012, Santa Cruz, Santa Cruz, CA) diluted 1:100, and visualized using Alexa 488 conjugated donkey anti-goat secondary (A11055, Invitrogen, Carlsbad, CA) or Alexa-594 conjugated donkey anti-goat secondary (A11058, Invitrogen, Carlsbad, CA) diluted 1:100.
Western blot and cell death experiments
Stressed and control cells grown at a density of 105/cm2 on 100-mm plates were harvested after 4 min on ice immediately or following recovery intervals of 4–24 h. Whole-cell lysates were extracted using RIPA buffer [20 mM Tris, pH 8.0, 137 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 10% glycerol, 1 mM PMSF, 0.5% protease inhibitor cocktail (P8340, Sigma)]. Cytoplasmic and nuclear fractions were extracted using the NE-PER Nuclear and Cytoplasmic Extraction Kit (78833, Pierce, Rockford, IL) per kit instructions. Protein concentrations were determined using the BCA Assay (Bio-Rad, Hercules, CA). Whole-cell lysates from CHIP transgenic mice were gifts from Dr. Cam Patterson (Chapel Hill, NC). Equal amounts of lysate were mixed with sodium dodecyl sulfate sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blotting was performed using the appropriate antibodies.
The following primary antibodies were used for immunoblotting: CM67 (see anti-CHIP monoclonal production, below), goat anti-HSC70 diluted 1:1,000 (sc-1059, Santa Cruz, Santa Cruz, CA), goat anti-HSP70 diluted 1: 1,000 (SPA-812, Stressgen, Ann Arbor, MI), and mouse-anti-β-actin diluted 1:5,000 (A5441, Sigma, St. Louis, MO). Peroxidase-conjugated antibodies diluted 1: 10,000, for Westerns were all from Calbiochem (Gibbstown, NJ): rabbit anti-mouse (402335), goat anti-rabbit (401315), and rabbit anti-goat (401515). Primary antibody incubations were performed overnight at 4°C and secondary antibody incubations for 1 h at room temperature. Non-specific nuclear counterstains were performed with bisbenzimide (Hoechst H33258, 1 µM, B1155, Sigma, St. Louis, MO). Fluorescent sections and coverslips were mounted using Fluoromount-G (0100-01, Southern Biotech, Birmingham, AL).
Cell death was assayed by Sytox Green using modifications of published procedures (Degterev et al. 2005; Jones and Singer 2001; Pozzolini et al. 2003; Tauskela et al. 2003). For cell death experiments, cell cultures seeded at105 cells/cm2 in poly-d-lysine-coated 48-well plates were subjected to stress and allowed to recover for 24 h in normal cell culture conditions. The cells were then stained with 1 µM Sytox Green (Invitrogen, Carlsbad, CA) to stain nuclei of dead cells, and the fluorescence intensity of the dead cells were measured on a plate reader. Intensity values of dead cell nuclei were normalized and expressed as a percentage relative to the intensity reading at 0 min of stress. One-way ANOVA was performed to determine significance at a p < 0.001.
For the OGD plus recovery studies, cultures were also analyzed by plate reader after Sytox Green staining. In addition, representative images of cultures were captured, and Sytox Green-positive cells were counted to determine the dead cells resulting from OGD. The remaining live cells were then killed and fixed using 70% ethanol and also stained using Sytox Green. Representative images of the completely dead cultures were captured, and Sytox Green-positive cells were counted to determine the total cells in culture. The percentage of dead cells following OGD plus recovery were calculated as the number of Sytox Green-positive cells before fixing divided by positive cells after fixing.
Anti-CHIP antibody production
Anti-CHIP monoclonal antibodies were produced in our laboratory against a purified glutathione-S-transferase (GST)-CHIP fusion protein (Ballinger et al. 1999) using published techniques (Ausubel et al. 1987). Conditioned media from hybridoma lines were screened via enzyme-linked immunosorbent assay (ELISA) for high immunoreactivity against GST-CHIP and absence of immunoreactivity against GST. Product from hybridoma clone 67 (CM67) was selected for high immunoreactivity against CHIP in ELISA and for specificity on Western blot screens. On Western blot, CM67 recognized a single band of approximately 35 kDa molecular weight present in fibroblast whole-cell lysate from wild-type (+/+) mice, and specificity was confirmed by the absence of immunoreactivity in lysates from CHIP -/- mice (data not shown).
Results
CHIP expression in rodent brain and mixed primary cultures
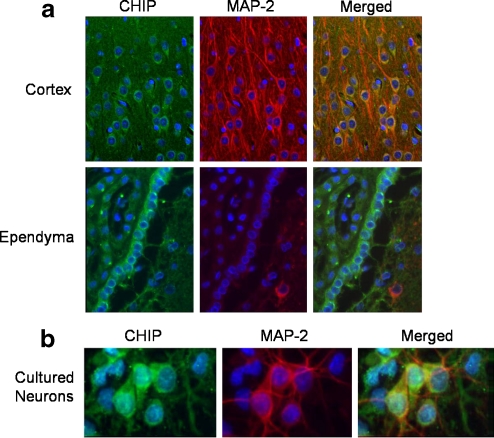
CHIP immunoreactivity was observed in the rat brain (Fig. 1a, green immunofluorescence), most notably in cortical and ependymal cells. The staining pattern was primarily cytoplasmic, as there appeared to be little or no overlap of CHIP immunostaining with the nuclear counterstain, bisbenzimide (blue). CHIP immunostaining also co-localized with MAP2-immunoreactivity (red immunofluorescence) in merged images.
Fig. 1.
CHIP immunoreactivity in brain sections from adult male Long-Evans rats (a). Coronal sections were taken from the cortical and ependymal cell regions. The sections were stained with anti-CHIP antibody (green) and anti-MAP2 antibody (red) along with the nuclear counterstain bisbenzimide (blue). Merged images are shown in panels on the far right. CHIP immunocytochemical staining of cultured neurons shows reactivity in both neurons and astrocytes (b). Cultured neurons (10 days in vitro) were stained for CHIP (green) and MAP-2 (red) along with nuclear counterstain, bisbenzimide (blue). A merged image is shown in the panel on the far right
CHIP immunoreactivity was also observed in primary cultures of rat neurons and astrocytes, with neurons (as determined by MAP2 immunoreactivity, red immunofluorescence) displaying much more intense CHIP immunoreactivity (Fig. 1b, green immunofluorescence). In addition, when the images were merged, the nuclei of cultured cells displayed more intense CHIP immunoreactivity, and the staining pattern was more granular than was observed in the brain sections. This granularity increased with the age of the cultures (data not shown). Of note, a similar granular staining pattern has been reported in COS7 cells after heat shock (Dai et al. 2003).
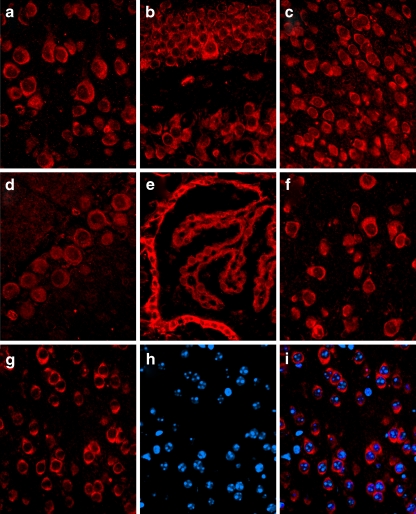
CHIP immunoreactivity was also seen in sections throughout adult mouse brain (Fig. 2a–i, red immunofluorescence), most notably in the cortex (2a), hippocampus (2b), hypothalamus (2c), Purkinje neurons (2d), choroid plexus and ependymal cells (2e), and thalamus (2f). Similar to what was seen in the immunostaining of rat brain sections, the staining pattern in mouse brain appeared to be primarily cytoplasmic, as there appeared to be little overlap of CHIP immunoreactivity (2g)with bisbenzimide staining (2h and 2i blue) in merged images (2i).
Fig. 2.
CHIP immunoreactivity in brain sections from adult male Sv129 mice. Immunoreactivity in the mouse brain was observed in the cortex (a), hippocampus (b), hypothalamus (c), Purkinje neurons (d), choroid plexus and ependymal cells (e), and thalamus (f). The lack of co-localization of cortical CHIP staining (g) and the nuclear counterstain bisbenzimide (h) in a merged image (i) suggests a primarily cytoplasmic localization of CHIP
CHIP and HSP expression following heat stress
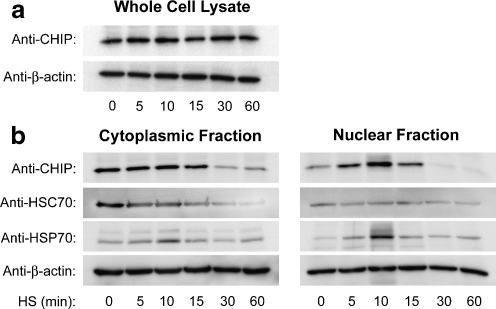
We hypothesized that the greater nuclear localization of CHIP in our cultured cells was the response to the external stresses of tissue culture. This notion was supported by observations in our laboratory and by others that heat stress induces nuclear increases in CHIP in immortalized cells (Tripathi et al. 2007). Heat stress did not induce appreciable immediate changes in overall CHIP levels, as determined by whole-cell lysate preparations obtained immediately following the stress (Fig. 3a). We therefore sought to determine if stressors would, as expected, increase nuclear CHIP levels in primary neurons; cultured cortical neurons were subjected to varying durations of heat stress at 42°C for 5 to 60 min. Cells were harvested immediately following the heat stress, and cell lysates were fractionated into soluble cytoplasmic and nuclear fractions. Levels of CHIP, HSC70, and HSP70 in the fractions were analyzed by Western blot. CHIP immunoreactivity was observed in both the cytoplasmic and nuclear fractions at baseline (Fig. 3b, 0 min). In the nuclear fraction, a rapid increase in CHIP was seen after 5 min of heat stress and peaked at 10 min. CHIP levels then decreased below baseline by 30 min of heat stress (Fig. 3b, nuclear fraction).
Fig. 3.
Total CHIP levels did not change immediately following increasing duration of heat stress (a). Equal protein concentrations from whole-cell lysate of primary mixed cultures exposed to heat stress were probed for CHIP. β-actin is shown as a loading control. Nuclear CHIP decreases following extended durations of heat stress (b). Equal protein concentrations from subcellular fractions of primary mixed cultures exposed to heat stress were probed for CHIP, HSC70, and HSP70 by Western blot. β-actin is shown as a loading control
In the cytosol, CHIP remained stable until 15 min of heat stress, when the relative amounts of CHIP decreased in parallel with nuclear CHIP (Fig. 3b, cytoplasmic fraction). The chaperone HSC70 showed cytoplasmic localization and expression patterns in response to heat stress that were similar to CHIP, however, there was no comparable increase in the nuclear fraction. Localization and expression patterns for HSP70 showed early increases in both cytoplasmic and nuclear fractions at 5 and 10 min before decreasing at 30 and 60 min. The nuclear increases for HSP70 paralleled the increases in nuclear CHIP.
Cellular death following heat stress
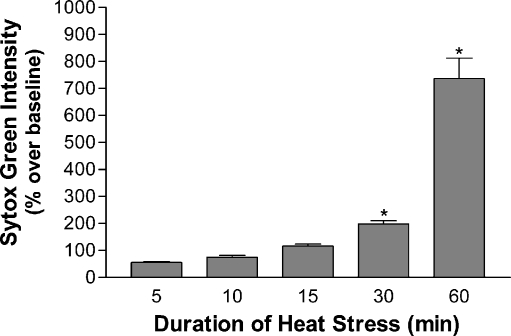
To determine the effects of our heat stress model on cellular death, we surveyed for cell death in cultures immediately after heat stress as well as after 24 h of recovery using Sytox Green nuclear stain for dead cells. No significant cellular death was seen immediately following heat stress for durations up to 60 min. However, cells subjected to heat stress followed by 24 h of recovery showed increased delayed death with increased periods of stress. Cell death was progressive with the duration of heat stress, with the first significant increase seen at 30 min, 198.3% ± 12.14 above controls. Sixty minutes of heat stress followed by the recovery period showed a level of cell death that was a 735.41% ± 76.84 increase over control (Fig. 4). The duration of heat stress that gave rise to sharp increases in delayed cell death correlated closely with the post-stress loss of nuclear and cytoplasmic CHIP. Taken with the CHIP localization data, we note that decreases in both nuclear and cytoplasmic CHIP levels immediately after heat shock roughly correlated with increases in delayed cell death at 24 h.
Fig. 4.
Cell death after prolonged heat stress is increased after a 24-h recovery period. Cultures subjected to durations of heat stress between 0 and 60 min, recovered in normal culture conditions for 24 h and stained with Sytox Green (1 µM). Sytox Green intensity was measure by plate reader. Relative intensity was analyzed as percent increase over baseline ± SEM at 24 h of recovery after 5 (55.45% ± 3.51), 10 (74.85 ± 6.35), 15 (115.78% ± 8.36), 30 (198.3% ± 12.14), and 60 min (735.41% ± 76.84) of heat stress. Measurements following 30 and 60 min were significant (*p < .001, n = 47)
Cellular death and CHIP expression following oxygen–glucose deprivation
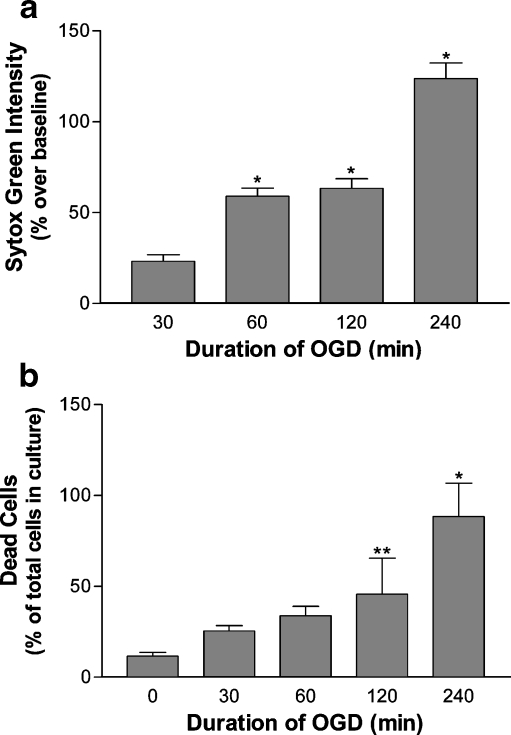
Cardiac ischemia data from CHIP -/- mice suggested that CHIP is involved in the ischemic stress response (Zhang et al. 2005). We therefore investigated CHIP levels and localization in cultured neurons following hypoxic–ischemic stress. Cultured primary cortical neurons were subjected to an oxygen–glucose deprivation (OGD) model of hypoxia ischemia using glucose-free MEM and argon gas oxygen displacement. For cells assayed immediately following the stress, no significant cellular death above baseline (0 min of OGD) was seen after any duration of OGD up to 240 min (data not shown). OGD followed by a 24-h recovery period resulted in an increase in delayed cell death at 24 h of recovery that was progressive with the duration of OGD (Fig. 5a). Sytox Green intensity over baseline was significantly increased at 24 h of recovery following 60 min of OGD (58.95% ± 4.48) and increased to 63.36% ± 5.32 and 123.74% ± 8.62 at 24 h following 120 and 240 min, respectively. Similarly, increases in the number of dead cells in the cultures following OGD and 24 h of recovery were observed (5b). 11.6% ± 0.113 of cells in the cultures were dead following 24 h of recovery with no treatment which increased to 25.3% ± 0.194 at 24 h of recovery following 30 min of OGD and 45.7% ± 0.166 at 24 h of recovery following 120 min of OGD. Delayed cell death following 120 min of OGD developed between 12 and 24 h of recovery (data not shown).
Fig. 5.
Cell death after increasing periods of OGD and a 24-h recovery period. Cultures subjected to durations of OGD between 0 and 240 min, recovered in normal culture conditions for 24 h and stained with Sytox Green (1 µM). Sytox Green intensity was measured by plate reader. Sytox Green intensity was analyzed as percent increase over baseline 24 h (a) after 30 (23.06% ± 3.75), 60 (58.95 ± 4.48), 120 (63.36% ± 5.32), and 240 min (123.74% ± 8.62) of OGD. Measurements following 60 to 240 min were significant (*p < .001, n = 47). Sytox Green-positive cells were analyzed as a percentage of all cells in culture after the 24-h recovery period (b) following 0 (11.6% ± 0.113), 30 (25.3% ± 0.194), 60 (33.8% ± 0.167), 120 (45.7% ± 0.166), and 240 min (88.3% ± 0.113) of OGD *p < .001, **p < .05
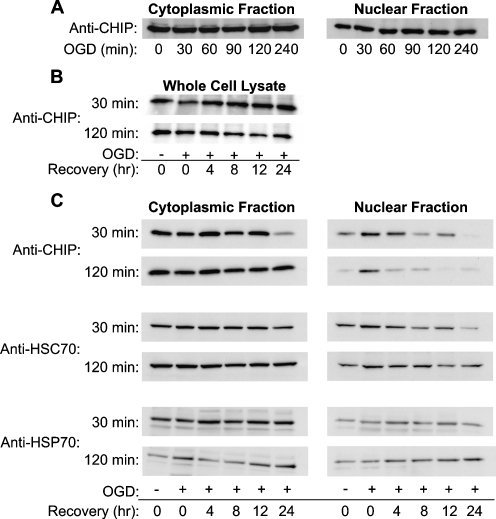
For CHIP localization experiments following OGD, cultured cortical cells were stressed for periods from 0 to 240 min, and cytoplasmic and nuclear fractions from these cells were analyzed for CHIP by Western blot immediately after the stress. Nuclear levels of CHIP increased minimally in response to OGD by 30 min, and levels remained increased at 240 min (Fig. 6a). No significant changes in cytoplasmic CHIP were seen. In contrast to the results with heat stress, no correlation was seen between CHIP and delayed death in these conditions. Additionally, total CHIP levels did not change significantly during a 24-h recovery following 30 or 120 minutes of OGD (6B), as determined by Western blots of whole-cell lysates indicating that CHIP is not degraded.
Fig. 6.
Relative amounts of subcellular CHIP are slightly altered immediately following OGD (a). Total CHIP levels did not change significantly during recovery following 30 and 120 minutes of OGD (b). Equal protein concentrations from whole-cell lysate of primary mixed cultures were probed for CHIP. Equal protein concentrations from subcellular fractions of primary mixed cultures exposed to OGD were probed for CHIP by Western blot (c). No changes are observed in the cytoplasmic fraction with increasing durations of OGD. In the nuclear fraction, a slight increase is observed following 30 min of OGD is sustained, but not otherwise altered following increasing durations of OGD. Elevated levels of nuclear CHIP are maintained during recovery after 30 min of OGD. Equal protein concentrations from subcellular fractions of primary mixed cultures exposed to mild (30 min) and severe (120 min) OGD were probed for CHIP, HSC70, and HSP70 by Western blot
However, analysis of CHIP levels in subcellular fractions following the 24-h recovery period indicated that nuclear CHIP, in particular, was lost during the post-ischemic recovery. If cells were subjected to OGD and allowed to recover for periods ranging from 4 to 24 h, differences in nuclear CHIP were seen during the recovery period. Following a period of OGD that resulted in low/moderate delayed cell death (30 min), nuclear CHIP levels were elevated in recovery for as long as 12 h (Fig. 6c, 30 min) and then declined. If cells were exposed to a period of OGD that led to significantly greater cell death (120 min), nuclear CHIP was briefly elevated immediately following the insult but consistently returned to baseline levels or below by 4 h of recovery. No consistent changes in cytoplasmic CHIP were seen, nor were there significant changes in HSP70 or HSC70. When our OGD delayed cell death data is compared with the nuclear localization data, it appeared that the post-OGD loss of nuclear CHIP correlated with delayed cell death.
Discussion
The intracellular distribution of CHIP appears to be determined by the intensity of external stress. Our in vivo immunostaining of rat and mouse brain indicated that CHIP is primarily a cytoplasmic protein at baseline, and similar observations have been made by others (Ballinger et al. 1999; Dai et al. 2005; Imai et al. 2002; Kampinga et al. 2003; Meacham et al. 2001). Expression is also similar across the two species. The pattern of CHIP localization in our primary cell cultures was also primarily cytoplasmic, but nuclear staining was more prominent in cultures than in brain tissue sections, thus supporting the notion that the conditions of tissue culture are sufficient to promote the translocation of some CHIP into the nucleus. Nuclear translocation as a homeostatic response to the tissue culture process is not a previously documented characteristic of CHIP or other HSPs. This idea of stress-associated movement of CHIP into the nucleus is further supported by increases in nuclear CHIP induced by the application of HS in our experiments and those of others (Dai et al. 2003, 2005; Galigniana et al. 2004; Kampinga et al. 2003; Meacham et al. 2001).
In our experiments, nuclear accumulation of CHIP was seen as early as 5 min after the introduction of heat shock indicating that the response is rapid. CHIP was nearly undetectable in nuclear fractions with heat shock longer than 30 min further suggesting that the elevation of CHIP is transient under conditions of extreme stress. The speed of CHIP translocation to the nucleus in our experiments was faster than previously reported by others. Dai et al. (2003) demonstrated accumulation of CHIP in the nuclear fraction of COS7 cells exposed to 90 min of 42°C heat shock, and Tripathi et al. (2007) made similar observations in KB cells. We attribute the differences, in part, to the enhanced vulnerability of primary cortical cells relative to immortalized cells lines. When the nuclear translocation of CHIP was compared with cell death a strong inverse relationship was seen. Importantly, nuclear CHIP levels began to decline at 15 min just prior to durations that resulted in increases in delayed cell death between 30 and 60 min. This time course indicated that the loss of CHIP was probably not secondary to cell death. Given the close temporal relationship between CHIP decline and delayed cell death, we hypothesized that the absence of nuclear CHIP in primary cells exposed to more severe stress may be a key to why these cells undergo delayed cell death in our assays.
Our hypothesis was supported by similar observations in cells exposed to OGD. The slower time courses for the loss of CHIP and cell death following OGD versus HS was most likely due to the fact that protein unfolding following ischemia is much slower than the rapid denaturation of proteins after heat shock. CHIP did not drop significantly below control levels until 12–24 h of recovery from OGD. Of note, significant delayed cell death developed between 12 and 24 hours after OGD, again suggesting that death follows closely behind the disappearance of nuclear CHIP. The lack of a similar relationship with cytosolic CHIP suggested there was a unique relationship between extended nuclear localization of CHIP and survival.
Since CHIP is a negative regulator of HSP-mediated protein folding, it is unlikely that increased protein folding is occurring in the nucleus in relation to CHIP. This is consistent with our observations of small and delayed increases in nuclear Hsp70 that occurred later in recovery than increases in nuclear CHIP. The importance of increases in Hsp70 for cell survival following stress are well established. In particular, Hsc70 and Hsp70 have been shown to accumulate in the nucleus after heat shock and oxidative stress (Tsukahara and Maru 2004). Others have proposed that CHIP translocates to the nucleus in complex with HSF1 (Dai et al. 2003) as part of the mechanism that upregulates the heat shock response (Kim et al. 2005). The delayed increase we observed in nuclear Hsp70 during the recovery phase is consistent with the hypothesis that Hsp70 is part of a second phase of the stress response.
The loss of CHIP relative to other stress-related proteins and the temporal relationship between CHIP loss and delayed cell death suggest that CHIP availability may play a critical role in the ability of the cell to maintain homeostasis. The majority of the literature on the HSPs focuses on more chronic time frames, but the co-chaperone of interest, CHIP, and its strong correlation in the acute time frames following stress suggests the acute time frame may be of equal interest as the more chronic time frames. The intracellular factors that regulate CHIP availability are not well understood. One possibility is that CHIP may become trapped within protein aggregates. Both HSC70 and HSP70 are components of insoluble aggregates following stress (Liu et al. 2005 and Zhang et al. 2006), and CHIP in similar insoluble aggregates may explain the decreases in CHIP observed in our studies. Liu et al. (2005) observed CHIP within protein aggregates during recovery periods of 4 h or greater in CA1 neurons that were destined to die following transient cerebral ischemia. These aggregates were mainly associated with intracellular vesicles and the nuclear membrane. Aggregates were seldom seen in neurons that were resistant to the ischemic challenge. We propose that aggregates might interfere with CHIP function in a consumptive fashion, with possible mechanisms including: (1) overwhelming CHIP with too much substrate, (2) incorporating CHIP into dysfunctional protein complexes, and (3) inhibiting CHIP translocation acutely (following heat shock) and during recovery (following oxidative stress).
We demonstrate here that nuclear accumulation of CHIP after heat shock can occur very rapidly, within minutes. The time course suggests that translocation of cytoplasmic CHIP is the initial mechanism for the nuclear accumulation of CHIP following acute stress. Translocation is further supported in the corresponding decrease in cytoplasmic CHIP seen in these experiments and those by Dai et al. (2003). CHIP movement is probably tied to its binding to other proteins. In particular, both Hsc70 and Hsp70 have been shown to accumulate in the nucleus after heat shock and oxidative stress in immortalized cells (COS7) following 2 h of heat stress (Tsukahara and Maru 2004). In this study, COS7 cells were used, in part, because they have lower cell mortality following heat stress compared with primary cultures. However, if similar changes are observed following a milder stress (OGD), the changes in CHIP may be a better predictor of delayed cell death than increases in HSP70, because part of the HSP70 response is dependent on the presence of CHIP (Dai et al. 2003, EMBO J). Others have proposed that CHIP translocates to the nucleus in complex with HSF1 (Dai et al. 2003) as part of the mechanism that upregulates the heat shock response. Thirdly, CHIP may translocate with other proteins in the capacity of a direct chaperone. Tripathi et al. have proposed that CHIP is a direct chaperone for p53 during the heat shock response. Their study demonstrated binding of CHIP to wild-type p53 as well as synchronous increases of CHIP and p53 in the nuclei of KB cells following HS. Both CHIP and p53 were present together on the DNA binding sites of the p21 and p53 promoters. It is also possible that CHIP functions as a ubiquitin ligase in the nucleus. Ubiquitinated protein and proteosomal machinery have been demonstrated in the nucleus.
Our results and the observations of other investigators raise the possibility that E3 ligases, like CHIP, participate in the nuclear regulation of the stress response. Very little is known about these potential interactions, and significant investigation is required to understand the potential role of CHIP in neuroprotection. Our finding of acute changes in subcellular localization of CHIP in response to cellular stress suggests that changes that occur shortly after exposure to stress ultimately impact on whether or not a cell has the capacity and capability to recover.
Acknowledgements
This work was funded by grants from the National Institute of Neurologic Disorders and Stroke/National Institutes of Health K08NS050164 (D.Y.H.) and the American Heart Association 0435011N (D.Y.H).
Abbreviations
- CHIP
carboxy terminus of Hsc70-interacting protein
- CNS
central nervous system
- HS
heat stress
- HSC70
heat shock cognate 70
- HSPs
heat shock proteins
- OGD
oxygen–glucose deprivation
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York: John Wiley & Sons; 1987. [Google Scholar]
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Simon R. Ischemic tolerance in the brain. Neurology. 1997;48:306–311. doi: 10.1212/wnl.48.2.306. [DOI] [PubMed] [Google Scholar]
- Dai Q, Zhang C, Wu Y, McDonough H, Whaley RA, Godfrey V, Li HH, Madamanchi N, Xu W, Neckers L, Cyr D, Patterson C. CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J. 2003;22:5446–5458. doi: 10.1093/emboj/cdg529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Qian SB, Li HH, McDonough H, Borchers C, Huang D, Takayama S, Younger JM, Ren HY, Cyr DM, Patterson C. Regulation of the cytoplasmic quality control protein degradation pathway by BAG2. J Biol Chem. 2005;280:38673–38681. doi: 10.1074/jbc.M507986200. [DOI] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Fink SL, Chang LK, Ho DY, Sapolsky RM. Defective herpes simplex virus vectors expressing the rat brain stress-inducible heat shock protein 72 protect cultured neurons from severe heat shock. J Neurochem. 1997;68:961–969. doi: 10.1046/j.1471-4159.1997.68030961.x. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Harrell JM, Housley PR, Patterson C, Fisher SK, Pratt WB. Retrograde transport of the glucocorticoid receptor in neurites requires dynamic assembly of complexes with the protein chaperone hsp90 and is linked to the CHIP component of the machinery for proteasomal degradation. Brain Res Mol Brain Res. 2004;123:27–36. doi: 10.1016/j.molbrainres.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Hohfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001;2:885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Soda M, Hatakeyama S, Akagi T, Hashikawa T, Nakayama KI, Takahashi R. CHIP is associated with Parkin, a gene responsible for familial Parkinson's disease, and enhances its ubiquitin ligase activity. Mol Cell. 2002;10:55–67. doi: 10.1016/S1097-2765(02)00583-X. [DOI] [PubMed] [Google Scholar]
- Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Hohfeld J, Patterson C. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- Jones LJ, Singer VL. Fluorescence microplate-based assay for tumor necrosis factor activity using SYTOX Green stain. Anal Biochem. 2001;293:8–15. doi: 10.1006/abio.2001.5116. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Kanon B, Salomons FA, Kabakov AE, Patterson C. Overexpression of the cochaperone CHIP enhances Hsp70-dependent folding activity in mammalian cells. Mol Cell Biol. 2003;23:4948–4958. doi: 10.1128/MCB.23.14.4948-4958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, Yenari MA, Steinberg GK. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol. 2002;52:160–167. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
- Kim SA, Yoon JH, Kim DK, Kim SG, Ahn SG. CHIP interacts with heat shock factor 1 during heat stress. FEBS Lett. 2005;579:6559–6563. doi: 10.1016/j.febslet.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/00004647-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim M, Yoon BW, Kim YJ, Ma SJ, Roh JK, Lee JS, Seo JS. Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke. 2001;32:2905–2912. doi: 10.1161/hs1201.099604. [DOI] [PubMed] [Google Scholar]
- Liu CL, Ge P, Zhang F, Hu BR. Co-translational protein aggregation after transient cerebral ischemia. Neuroscience. 2005;134:1273–1284. doi: 10.1016/j.neuroscience.2005.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AJ, Frydman J. Molecular chaperones and the art of recognizing a lost cause. Nat Cell Biol. 2001;3:E51–E53. doi: 10.1038/35055162. [DOI] [PubMed] [Google Scholar]
- Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3:100–105. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- Murata S, Minami Y, Minami M, Chiba T, Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2:1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzolini M, Scarfi S, Benatti U, Giovine M. Interference in MTT cell viability assay in activated macrophage cell line. Anal Biochem. 2003;313:338–341. doi: 10.1016/S0003-2697(02)00631-0. [DOI] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. doi: 10.1002/1531-8249(200006)47:6<782::AID-ANA11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rosser MF, Washburn E, Muchowski PJ, Patterson C, Cyr DM. Chaperone functions of the E3 ubiquitin ligase CHIP. J Biol Chem. 2007;282:22267–22277. doi: 10.1074/jbc.M700513200. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Massa SM, Swanson RA. Heat-shock protein protection. Trends Neurosci. 1999;22:97–99. doi: 10.1016/S0166-2236(98)01392-7. [DOI] [PubMed] [Google Scholar]
- Tauskela JS, Mealing G, Comas T, Brunette E, Monette R, Small DL, Morley P. Protection of cortical neurons against oxygen-glucose deprivation and N-methyl-d-aspartate by DIDS and SITS. Eur J Pharmacol. 2003;464:17–25. doi: 10.1016/S0014-2999(03)01371-2. [DOI] [PubMed] [Google Scholar]
- Tripathi V, Ali A, Bhat R, Pati U. CHIP chaperones wild type p53 tumor suppressor protein. J Biol Chem. 2007;282:28441–28454. doi: 10.1074/jbc.M703698200. [DOI] [PubMed] [Google Scholar]
- Tsukahara F, Maru Y. Identification of novel nuclear export and nuclear localization-related signals in human heat shock cognate protein 70. J Biol Chem. 2004;279:8867–8872. doi: 10.1074/jbc.M308848200. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Fink SL, Sun GH, Chang LK, Patel MK, Kunis DM, Onley D, Ho DY, Sapolsky RM, Steinberg GK. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann Neurol. 1998;44:584–591. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70) Mol Med Today. 1999;5:525–531. doi: 10.1016/S1357-4310(99)01599-3. [DOI] [PubMed] [Google Scholar]
- Zhang C, Xu Z, He XR, Michael LH, Patterson C. CHIP, a cochaperone/ubiquitin ligase that regulates protein quality control, is required for maximal cardioprotection after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2005;288:H2836–H2842. doi: 10.1152/ajpheart.01122.2004. [DOI] [PubMed] [Google Scholar]
- Zhang F, Liu CL, Hu BR. Irreversible aggregation of protein synthesis machinery after focal brain ischemia. J Neurochem. 2006;98:102–112. doi: 10.1111/j.1471-4159.2006.03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]