Abstract
Long-term heat acclimation (AC, 30d/34°C) is a phenotypic adaptation leading to increased thermotolerance during heat stress (HS, 2 h 41°C). AC also renders protection against ischemic/reperfusion (I/R, 30′ global ischemia/40′ reperfusion) insult via cross-tolerance mechanisms. In contrast to the protected AC phenotype, the onset of acclimation (34°C, AC2d) is characterized by cellular perturbations, suggesting increased susceptibility to HS and I/R insults. In this investigation, we tested the hypothesis that apoptosis resistance is part of the AC repertoire and that, at the initial phase of acclimation (AC2d), cytoprotection is impaired. TUNEL staining and caspase 3 levels in HS and I/R insulted hearts affirmed this hypothesis. To examine the role of the mitochondria in life/death decision in AC2d and 30d AC settings vs. control hearts, we studied the Bcl-2 apoptotic cascade and found increased levels of the anti-apoptotic Bcl-XL and decreased levels of the pro-apoptotic death promoter Bad in hearts from AC2d and AC animals. In these groups, cytochrome c (cyt c) was elevated in the mitochondria and remained unchanged in the cytosol. This adaptation was insufficient to negate apoptosis in AC2d rats. At this early acclimation phase (and in controls), increased caspase 8 activity confirmed activation of the extrinsic (Fas ligand) apoptosis pathway. In conclusion, the elevated Bcl-XL/Bad ratio and decreased cyt c leakage to the cytosol are insufficient to protect the heart and interactions with additional cytoprotective pathways involved in acclimation (elevated HSP70, ROS, and sarcolemmal adaptations to abolish extrinsic apoptosis pathways) are required to induce the apoptosis-resistant AC phenotype.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-010-0178-x) contains supplementary material, which is available to authorized users.
Keywords: Heat acclimation-mediated cross-tolerance, Apoptosis, Heat stress, Mitochondria, Ischemia/reperfusion
Introduction
Heat acclimation (AC) is an evolutionarily conserved feature leading to the generation of a metabolically efficient, thermotolerant phenotype (Horowitz 2002, 2007). The acclimation process is biphasic. The initial, transient phase is characterized by impaired cellular processes (Horowitz 1998; Horowitz and Meiri 1985), and increased excitability of the autonomic nervous system compensates for impaired cellular processes in order to achieve thermoregulation. In the AC phenotype, efficient metabolic and molecular processes replace the need for enhanced autonomic excitability. The development of acclimatory homeostasis depends on a continuum of temperature adaptive shifts in gene expression during the entire acclimation regimen; however, during the initial acclimation phase (2–5 days), the transcriptional program of acclimation is activated. Important changes occur in the expression pattern of stress-associated genes including those responsible for the heat shock response and anti-apoptotic and anti-oxidative networks (Horowitz 2007; Horowitz et al. 2004). The buildup of large heat shock protein (HSP) (HSP72 and HSP90) cellular reserves when acclimation homeostasis has been achieved is noteworthy. Up-regulation of Bcl-XL and down-regulation of Bad transcripts (anti- and pro-apoptotic members of the Bcl superfamily; linked with the release of the mitochondrial apoptotic signals) may indicate promotion of an anti-apoptotic cellular environment. This hypothesis is supported by the fact that HSPs inhibit specific steps of the mitochondrial apoptotic cascade (Beere 2005). Even though the high Bcl-XL/Bad transcript ratio and up-regulation of hsp70 transcript are already detectable during the initial acclimation phase, greater HSP reserves are only found when acclimatory homeostasis has been achieved.
An inseparable outcome of acclimation is protection from acute novel stressors via cross-tolerance mechanisms (heat acclimation-induced cross-tolerance [HACT]; Horowitz 2007). HACT is a long-standing effect that is committed to memory, i.e., returns rapidly following its decline (Tetievsky et al. 2008). Due to the fact that the mechanisms underlying AC depend on enhanced constitutive reserves of cytoprotective molecules (Horowitz 2007), we suggested that HACT relies on the activation of “on-call” cytoprotective pathways shared by many stressors (Horowitz 2007). These reserves are absent following short acclimation when adequate performance depends primarily on enhanced autonomic excitability. HACT mechanisms have been well established in the ischemic AC heart and confirmed by reduced infarct size, improved hemodynamics post-global ischemic events (vs. non-acclimated) and greater cytoprotective protein reserves (Levy et al. 1997; Maloyan et al. 1999, 2005).
Apoptosis is a highly regulated cellular process, triggered in diverse physiological and pathological contexts to facilitate the removal of cells with minimal damage to the surrounding tissue (Gustafsson and Gottlieb 2007, 2008; Kroemer et al. 2007). It is initiated via two major pathways, extrinsic and intrinsic, both ultimately activating the “executioner” caspase 3. The intrinsic pathway is tightly regulated by the Bcl-2 protein family and is also known as the “mitochondrial pathway”. When lethal signals predominate, Bcl-2 family members interact with the mitochondria to induce apoptosis via increasing outer-mitochondrial membrane permeabilization (Chipuk and Green 2008), leading to the release of cytochrome c (cyt c) and other mitochondrial constituents, e.g., Ca2+, into the cytosol, which cause conformational changes in Apaf-1 and activate the caspase 9–caspase 3 cascade (Bratton et al. 2001). The Bcl-2 proteins are upstream to “the point of no return” in the intrinsic pathway of cellular apoptosis (Kutuk and Basaga 2006). Pathways, independent of caspase 9 activation (Milleron and Bratton 2007) and cross-talk between the extrinsic (e.g., Fas ligand and TNF cascades) and the intrinsic apoptotic pathway via Bcl-2 (Han et al. 2006) are well documented.
Given our previous data showing an augmented Bcl-XL/Bad transcript ratio and overexpression of HSP70 in the acclimated phenotype, the aim of this investigation was to test our hypotheses that: (1) apoptosis resistance is part of the AC repertoire; (2) at the initial phase of acclimation phase (short-term AC), cytoprotection is impaired; (3) adaptation in the Bcl-2 family plays a role in the attenuation of death signals. Using heat stress (HS) and ischemic/reperfusion (I/R) as inducers of death, we demonstrated that long-term AC conveys resistance to apoptosis; furthermore, apoptosis increased when short-term AC rats were exposed to stressors. Attenuation of intrinsic mitochondrial death signals can already be noted during short acclimation, suggesting that the Bcl-2 apoptosis resistance adaptive modality is rapid, but insufficient to negate other death signals. The stress induced up-regulation of caspase 8 implies that the extrinsic, Fas ligand pathway triggers apoptosis at the early acclimation phase. In contrast, sarcolemmal adaptation abolishes the extrinsic apoptosis pathway in the fully acclimated phenotype. Additional cytoprotective networks, which develop throughout the acclimation period, leading to apoptosis tolerance are yet to be discovered.
Materials and methods
Animals Male, Rattus norvegicus (Sabra strain, albino var.), initially 3 weeks old, weighing 80–90 g, and fed Ambar laboratory chow with water ad libitum were used. The animals were assigned to AC for 30 days heat-acclimated for 2 days (AC2d), and control—normothermic (C)—groups. The effect of acclimation on the response of the intrinsic mitochondrial apoptotic pathway was studied in AC hearts (1) following subjection of the animals to acute HS and (2) in isolated hearts following I/R insult. To examine our hypothesis that susceptibility to injury is increased during the first acclimation phase, the effect of AC2d on the apoptotic response to the above stressors was also studied. We measured apoptosis (TUNEL and the executioner active caspase 3) as well as key steps along the intrinsic pathway including Bcl-XL/Bad ratio, cyt c, and caspase 9 level and activity. Although there are many proteins in the Bcl-2 family, Bcl-XL and Bad were chosen because our previous studies using a stress cDNA array demonstrated significant changes in these two members of the Bcl family. Apoptosis kinetics over the course of 48 h post-insult was followed in the HS-treated groups whereas I/R-mediated apoptosis was only measured at one time point (Maloyan et al. 2005). In order to determine whether the Fas ligand extrinsic apoptotic pathway is also activated, we measured the transcription and activity of caspase 8, a known marker of this pathway. The detailed experimental scheme is presented in Fig. 1. The Ethics Committee for Animal Experimentation of The Hebrew University, Jerusalem, Israel, approved all experimental protocols.
Fig. 1.
a Experimental scheme. b Analyses scheme performed in heart (left ventricle) tissues. C normothermic controls (at 24°C); AC2d short-term heat acclimation; AC long-term heat acclimation, HS heat stress, I/R ischemic/reperfusion insult, cyt c cytochrome c, qPCR real-time PCR, WB Western immunoblot, RFU relative fluorescent units. HS-induced apoptosis was studied in three independent series of 11 rats each (three animals each time point); I/R insult was studied in four independent series of six experimental treatments each (four per treatment)
Experimental conditions The C group was maintained at an ambient temperature of 24 ± 1°C for 30 days; long-term AC was achieved by continuous exposure to 34 ± 1°C and 30–40% relative humidity in a light-cycled room (12:12 h) for 30 days; short-term heat acclimation was achieved by exposure to 24 ± 1°C for 28 days followed by an exposure to 34 ± 1°C and 30–40% relative humidity for 2 days as previously described (Horowitz 1976). This experimental setup assured that animals from all treatment groups were of the same age. The HS or I/R experiments were conducted at the end of each acclimation phase. For characterization of the effects of HS on the intrinsic apoptotic pathway in C and AC rats, the animals were subjected to HS at 41°C for 2 h (Maloyan et al. 1999; Schwimmer et al. 2004). Schwimmer et al. (2004) and Tetievsky et al. (2008) demonstrated that, during HS, colonic temperature (Tc) rises and then plateaus. The temperature at the plateau (Tc-pl) is significantly higher in AC and somewhat lower in AC2d than in controls (P < 0.05). Hence, the Tc-pl of the rat during HS is a reliable criterion for acclimation status (Maloyan et al. 1999; Schwimmer et al. 2004; Tetievsky et al. 2008). On termination of HS, the animals were allowed to recover under control conditions for different intervals (from 0 to 48 h) and then euthanized by cervical dislocation (Maloyan et al. 1999; Tetievsky et al. 2008). The hearts were removed and the left ventricles carefully excised and stored at −80°C.For the cross-tolerance experiments, animals were anesthetized using a mixture of ketamine and xylazine (8.5 mg/100 g body weight ketamine in 0.5% xylazine, IP); the hearts were rapidly removed, mounted on a Langendorff perfusion system and retrogradely perfused with Krebs–Henseleit buffer containing (in millimolars) 120 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 1.25 CaCl2, 25 NaHCO3, and 11 glucose, at pH 7.4, and aerated with a mixture of 95% O2–5% CO2 at 37°C (Maloyan et al. 2005; Tetievsky et al. 2008) at a perfusion pressure of 100 cmH2O. After 10 min of equilibration, perfusion was stopped (global ischemia) for 30 min and the hearts were then reperfused for 40 min. This time protocol enhances the likelihood of visualizing apoptosis. Hearts were frozen (−80°C) until analysis (Maloyan et al. 2005).
Cell fractionation For cytosolic and mitochondrial fraction separation lysates, the left ventricle of the heart was homogenized with 20 mM Tris (pH 7.6), 1.5 mM MgCl2, 1 mM EDTA, 0.25 M sucrose, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, and protease inhibitor cocktail. The homogenate was centrifuged for 10 min at 5,000 rpm, at 4°C. The supernatant was further centrifuged for 30 min at 12,000 rpm at 4°C; the cytosolic fraction was removed and stored at −80°C until analysis, and the pellet [crude, mitochondria-enriched fraction, (Xilouri and Papazafiri 2006; Umschwief et al. 2009)] was resuspended and stored. Protein concentration of both cytosolic and mitochondrial suspensions was determined using Bradford reagent (Bio-Rad Laboratories, Richmond, CA).
Western blot analysis Total protein (cytosolic and mitochondrial fractions; 50 μg/lane) was fractionated by electrophoresis on 9% or 14% polyacrylamide gels under denaturing conditions (Laemmli 1970), transferred onto nitrocellulose membranes, blocked for 1 h in phosphate-buffered saline containing 5% dried skimmed milk powder and then probed overnight at 4°C with primary antibody. After repeated washings, the membranes were incubated at room temperature for 1 h with horseradish peroxidase-conjugated goat anti-rabbit IgG (Jackson Lab., West Grove, PA).The following antibodies were used: rabbit polyclonal anti-Bad (1:1,000), rabbit polyclonal anti-Bcl-XL (1:500), rabbit polyclonal anti-caspase 9 [1:200; Delta Biolabs, Gilroy, CA: (DB003, DB002, DB081, respectively)], rabbit polyclonal anti-cytochrome c (1:1,000), rabbit polyclonal anti-caspase 3 (1:500), rabbit monoclonal anti-cleaved caspase 3 (1:500; Cell Signaling Technology, Beverly, MA: C-4272, C-9662, C-9664, respectively), rabbit polyclonal β-actin (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, sc-1616-R) was used as a loading control (Xilouri and Papazafiri 2006; Umschwief et al. 2009). Specific antibody binding was detected using enhanced chemiluminescence (Beit Haemek Biological Industries, Israel) and visualized by exposing an X-ray film to the membrane. The densities of the scanned protein bands were calculated using TINA software (Raytest, Straubenhardt, Germany). For further details, see Maloyan et al. (1999, 2005) and Tetievsky et al. (2008).
Caspase-9 and caspase-8 activity test Caspase 9 activity was measured in heart homogenates using a commercially available kit according to the manufacturer's protocol (Biovision, CA). In brief, the left ventricle was homogenized in caspase lysis buffer. The homogenate was centrifuged at 3,000g for 4 min to remove connective tissue. The supernatant (100 μg total protein) was mixed with 50 µl of 2× reaction buffer in the presence of substrate [LEHD-AFC (7-amino-4-trifluoromethyl coumarin [AFC]), 50 µM final concentration]. The mixture was incubated at 37°C for 2 h, and fluorescence was measured using a 505-nm emission filter. Caspase activity was expressed in raw fluorescence units. Caspase 8 activity was measured as above, using IETD-AFC as a substrate (Biovision, CA).
mRNA detection Changes in mRNA transcripts were detected using semi-quantitative RT-PCR and validated by quantitative real-time PCR (qPCR). RT-PCR was performed as previously described (Maloyan et al. 2005). Briefly, total RNA was extracted from the left ventricle homogenate, using Tri-Reagent (Molecular Research Center, OH). Total RNA (1 μg) was reverse-transcribed in a 20-μl reaction mixture containing 1 μl of oligo(dT) as primer, 10 mM dNTP, together with 200 U of Moloney murine leukemia virus RT and 40 U of ribonuclease inhibitor according to the manufacturer's protocol (Fermentas, Hanover, MD). For the PCR, 0.8 μl of the cDNA mixture was added to 7.5 μl of a master mix, with 0.06 μg of each specific primer. To ensure equal amounts of initial mRNA, we performed parallel β-actin amplification. The PCR products were resolved on 1% agarose gel, stained with ethidium bromide, and visualized under UV light. Band density was analyzed using TINA software. For qPCR, an ABI Prism 7000 Sequence Detection System (Applied Biosystems) was used. Reaction volumes of 20 μl contained 10 μl of SYBR Green Master Mix (Applied Biosystems), 500 nM each of the forward and reverse primers, and 5 μl of diluted cDNA. The appropriate cDNA dilutions were determined using calibration curves established for each primer pair. The thermal profile for SYBR Green qPCR was 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The primers for the qPCR were designed using Primer Express software (Applied Biosystems; Table 1).
Table 1.
Real-time PCR (qRT) and RT PCR (RT) primer sequences
| Gene | Number | Forward | Reverse | Method |
|---|---|---|---|---|
| Bcl-XL | U72350 | AGGCTGGCGATGAGTTTGAACTGCG | GGCTCTAGGTGGTCATTCAGGTAGG | RT, qRT |
| Bad | AF003523 | CTGGGACTATGGAGACCCGGAGTC | TTGTCGCATCTGTGTTGCAGTGCC | RT, qRT |
| Cytochrome c | NM_012839 | AGGCAAGCATAAGACTGGACC | GTTAGCCATTCATGATCTGA | RT |
| Caspase 9 | NM_031632 | CACTGCCTCATCATCAACAACGTG | TGAGAGAGGATGACCACCACGAAG | RT |
| Caspase 9 | NM_031632 | TCTGGCAGAGCTCATGATGTCT | TCACGTTGTTGATGATGAG | qRT |
| Caspase 3 | NM_012922 | AGGCGACTACTGCCGGAGTCTGAC | GCAAAGTGACTGGATGAACCATGACC | RT |
| Caspase 3 | NM_012922 | TGGAGAGAAGATGGTTTGAGCC | ATCATCCACACAGACCAGTGCT | qRT |
| Caspase 8 | NM_022277 | TCCCAGATGAGGCAGACTTTCT | GCTCTGGCAAAGTGACTGGATA | RT, qRT |
| β-actin | NM_031144 | TGTGGCATCCATGAAACTAC | ATTTGCGGTGCACGATGGAG | RT, qRT |
In situ cell death detection Cardiomyocyte apoptosis was analyzed using a terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) staining kit (Roche Applied Science, Mannheim, Germany). The 7.5-μm heart sections were fixed in 4% paraformaldehyde at room temperature for 4 h, rinsed in a 20% sucrose solution for 30 min, and then left in the sucrose solution overnight at 4°C. The sections were then stored in Tissue Tek O.C.T. compound at −80°C. Following removal from the freezer, the sections were washed, permeabilized, and subjected to the TUNEL assay according to the manufacturer's instructions. Nuclei were stained using 0.25 μg/ml propidium iodide (red). The nucleotide incorporation into the sample was stained in green, and when co-localized with nuclei stain (red), it appeared yellow (apoptotic nuclei). The sections were visualized using confocal microscopy (10 × 40), and for each animal, 20 random fields were scored for TUNEL-positive cells. Only nuclei that were clearly located in cardiomyocytes were considered.
Statistical analysis One-way and two-way ANOVAs with appropriate post hoc tests (Tukey–Kramer or Dunnett's tests) were performed using commercially available software (SigmaStat 2.03). Duration of acclimation (C, AC2d, and AC) and the acute stressors (HS, I/R) were taken as independent categorical variables, and individual animals or hearts were considered a random sample from the population. One-way ANOVA was used to test the effects of each acclimation phase as the only factor on the basal values or on the values following superimposed HS or I/R of the dependent expressed variables. For post hoc pairwise comparisons between the control (non-acclimated) and the various treatment time point groups, Dunnett's test was applied unless otherwise specified. To test whether duration of acclimation affected the response, two-way ANOVA was used. If significant interactions were indicated, a multiple-comparison test was conducted to detect whether the origin of significance stemmed from the insult or acclimation phase. Comparisons between HS or I/R and basal groups within matched acclimation regimens were also conducted using Student's t test. Additional details are specified in the figure legends. The data are expressed as mean ± SE; values of P < 0.05 were considered significant.
Results
Body weights and colonic temperatures (Tc) before and at the end of heat stress are shown in Table 2. As previously reported, body weights of AC rats were lower than controls (P < 0.001) (Horowitz 1976; Maloyan et al. 1999; Tetievsky et al. 2008). Basal Tc and hyperthermic plateau (Tc-pl) values also conformed to previous data (Horowitz 1976; Maloyan et al. 1999; Tetievsky et al. 2008). The mean Tc-pl in the 30 day AC group differed significantly from controls (P < 0.001); however, the AC2d group mean was unchanged.
Table 2.
Basal and hyperthermic plateau temperatures of rats undergoing 2 and 30 days of heat acclimation
| Groups | Body weight (g) | Tc-basal (°C) | Tc-pl (°C) |
|---|---|---|---|
| C | 251 ± 8 | 37.70 ± 0.08 | 40.00 ± 0.01 |
| AC2d | 242 ± 2 | 37.90 ± 0.03 | 39.92 ± 0.02 |
| AC | 206 ± 2a | 38.00 ± 0.05 | 40.78 ± 0.03a |
Values are means ± SE and were derived from representative groups of five to seven animals. Control (C), maintained at 24 ± 1°C. Heat-acclimated for 2 (AC2d) and 30 (AC) days were maintained at 34°C and 35% relative humidity. Tc basal colonic temperature before heat stress, Tc-pl Tc plateau; average Tc at time points 80, 100, and 120 min exposure to heat stress at 41°C. For significance, 2-way ANOVA followed by Dunnett's test were conducted
aSignificant difference from the C group, P < 0.001
Only long-term acclimation reduces apoptosis
Apoptosis resistance was evaluated following HS and I/R insults by cleaved caspase 3 levels and TUNEL staining. The levels of the active (cleaved) form of caspase 3 at all time points post-HS (0–48 h) were significantly lower in AC than in C hearts, with the greatest difference at 6–10 h post-HS (C, 2.29 ± 0.66 vs. AC, 0.38 ± 0.12; P < 0.001; Fig. 2a). In I/R C hearts, levels of active caspase 3 were elevated by 228% (P < 0.001) and AC2d by 204% (P < 0.001). Caspase 3 levels remained unchanged in the AC hearts (Fig. 2b). For TUNEL staining, non-treated tissues representing baseline levels, I/R tissues, and tissues from HS animals after 10 h of recovery were examined. Ten hours post-HS was chosen because the greatest elevations in cleaved caspase 3 protein levels were observed at this time (Fig. 2). AC hearts were protected from apoptosis following both HS and I/R insults, whereas C and AC2d hearts had significantly higher numbers of apoptotic cells following both insults [CI/R 29.3 ± 2.9%, AC2dI/R 39.8 ± 1.75%, ACI/R 10.7 ± 2.4% (significance vs AC: P < 0.027; and P < 0.001, respectively, Fig. 3)].
Fig. 2.
Active caspase 3 levels prior to and during recovery from HS and I/R. a Cleaved (active) caspase 3 was significantly lower in the AC vs. C group (2W ANOVA P < 0.001). Top: Protein bands. Bottom: Bar graph representative sets of protein bands. Values are means ± SE, asterisk significant difference from C (Tukey–Kramer P < 0.03–0.001), circumflex accent significant difference from basal (B) within the group (Dunnett's P < 0.05). b Cleaved caspase 3 post-I/R (top: protein bands; bottom: bar graphs). Both C and AC2d rat hearts showed significant increases in cleaved caspase 3 fragment (apoptosis executer) upon I/R while caspase 3 was unchanged in AC hearts. Values are means ± SE, Asterisk significant difference from C, circumflex accent significant difference from basal (B) within the group (Tukey–Kramer test, P < 0.001), number sign significant difference from AC (Tukey–Kramer, P < 0.001)
Fig. 3.
TUNEL stained heart slices. a Apoptotic nuclei of C, AC2d, and AC animals 10 h post-HS and following I/R insult. bBar graphs of apoptotic nuclei counts in the experimental groups. Significant increases in apoptotic nuclei in AC2d following HS and I/R suggest that the AC2d state is more susceptible to damage. Values are means ± SE, Asterisk significant difference from C (P < 0.027–0.001, Tukey–Kramer), circumflex accent significant difference from basal (B) within the group (P < 0.001, Tukey–Kramer), number sign significant difference from AC (P < 0.001, Tukey–Kramer), dollar sign significant difference between two treatments in the group (P < 0.007, Tukey–Kramer). For abbreviations see Fig. 1 legend
Long-term acclimation and heat stress affect the Bcl-XL/Bad ratio
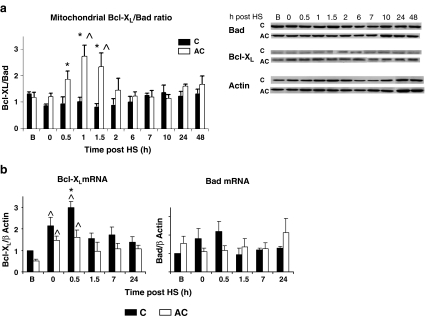
To investigate the effects of long-term AC on the intrinsic mitochondrial apoptotic pathway following HS, we measured anti-apoptotic Bcl-XL and pro-apoptotic Bad levels in cytosolic and mitochondrial fractions. The cross-talk between these proteins on the mitochondrial outer membrane plays a pivotal role in the control of trafficking harmful ions and proteins into the cytosol during stress (Kutuk and Basaga 2006). HS increased Bcl-XL expression in AC mitochondria with no change in Bad levels [Electronic Supplementary Material (ESM) Fig. 1], thereby significantly increasing the Bcl-XL/Bad ratio in AC mitochondria (vs. C), most profoundly at 1 h post-HS (AC, 2.74 ± 0.41 vs. C, 1.03 ± 0.15; P < 0.001; Fig. 4a). No significant changes in cytosolic Bcl-XL and Bad levels were detected (data not shown).
Fig. 4.
Anti-apoptotic Bcl-XL and pro-apoptotic Bad protein levels in C and AC rats. a Representative protein bands from mitochondrial fractions (right) and mitochondrial Bcl-Xl/Bad ratio (left) prior to and during recovery from HS. Bcl-XL/Bad ratios in each time point were calculated for bands analyzed on the same blots. Collectively, the Bcl-XL/Bad ratio in AC rats was significantly higher than in C (2W ANOVA P < 0.001). b qRT-PCR analyses of Bcl-XL and Bad mRNAs prior to (B), at the termination of HS (0), and at selected time points during recovery from HS. mRNA levels were normalized to β-actin. The bars represent the relative levels of PCR products normalized to the basal control. Values are means ± SE, asterisk significant difference from C, circumflex accent significant difference from basal (B) within the group (Tukey–Kramer test), p < 0.05–0.001. For abbreviations, see Fig. 1 legend
To determine whether the differences in protein expression profiles were due to transcriptional changes, Bcl-XL and Bad mRNA levels were also measured. The complete profile was obtained using semi-quantitative RT-PCR and selected time points were validated using quantitative real-time RT-PCR (Fig. 4b). Following HS, the Bcl-XL transcript was significantly up-regulated in C and AC groups (at peak levels, 2.99 ± 0.23 and 1.6 ± 0.33 fold P < 0.001 for C and AC groups, respectively). No significant changes in Bad levels were observed.
Long-term acclimation and heat stress affect mitochondrial and cytosolic cytochrome c levels
Increased levels of cyt c in the cytosol suggest opening of the mitochondrial outer membrane megachannel. We therefore measured cyt c levels before, at the termination of, and following HS in mitochondrial and cytosolic fractions (Fig. 5). In the AC hearts, mitochondrial cyt c levels increased with time, whereas the cytosolic levels remained unchanged. The initial peak coincided with the highest Bcl-XL/Bad protein ratio in this group. Additionally, delayed cyt c elevation was measured 10–48 h post-HS, despite the return of the Bcl-XL/Bad protein ratio to baseline levels (Fig. 4a). In contrast, cyt c levels in the mitochondria of C hearts were unaffected by HS but were significantly elevated in the cytosolic fractions. At the termination of HS, cytosolic cyt c levels were significantly higher in controls than in the AC group (C, 1.04 ± 0.13 vs. AC, 0.74 ± 0.08; P = 0.026; Fig. 5b). At that time point, the cyt c transcript was significantly higher in AC than in C hearts (cyt c/β-actin ratio: AC, 0.75 ± 0.11 vs. C, 0.35 ± 0.19; P < 0.024; ESM Fig. 2). No other post-HS fluctuations within the treatment groups were observed.
Fig. 5.
Cytochrome c protein in C and AC rats prior to and during recovery from HS. a Representative sets of protein bands (top) and mitochondrial protein fraction (bottom). Cyt c expression profile in AC mitochondria demonstrated marked up-regulation after subjection to HS and differed significantly from that of C (2W ANOVA P < 0.001). b Cytosolic cyt c protein increased significantly in C but not in AC hearts. Values are means ± SE, asterisk significant difference from C, P < 0.05–0.001, circumflex accent significant difference from basal (B) within the group (Tukey–Kramer test), P < 0.05. For abbreviations, see Fig. 1 legend
Long-term acclimation delays and attenuates caspase cascade activation post-heat stress
When Apaf-1 is bound to cyt c, it can recruit procaspase 9 and undergo oligomerization into the active apoptosome. Procaspase 9 is then cleaved, and active caspase 9 propagates the death signal by proteolytic processing and activating downstream caspases (Bratton et al. 2001; Srinivasula et al. 1998). Representative sets of protein bands, caspase 9 activity, and caspase 9 mRNA levels are presented in Fig. 6 and ESM Fig. 3. We demonstrated that (1) procaspase 9 levels in AC hearts were lower than in C hearts (p < 0.03); (2) caspase 9 activity at selected post-HS time points was also significantly lower in AC vs. C hearts; (3) caspase 9 mRNA levels immediately and 30 min post-HS (where a cytosolic cyt c elevation was noted in C rats) were significantly higher in C vs. AC hearts. Caspase 3 protein showed a different profile. Although the procaspase 3 level was higher in AC than in C hearts (Fig. 7), the levels of the active (cleaved) form of caspase 3 at all time points post-HS were significantly lower in AC than in C hearts (Fig. 2). No significant differences were observed in caspase 3 transcripts (data not shown).
Fig. 6.
Caspase 9 protein levels and activity prior to and during recovery from HS in AC and C rats. a Representative sets of protein bands (top) and procaspase 9 protein levels (bottom). b Caspase 9 activity (relative fluorescent units [RFU]). Both procaspase and active caspase 9 were decreased in AC, as compared with C, hearts (2W ANOVA P < 0.003 and 0.009, respectively). For abbreviations, see Fig. 1 legend
Fig. 7.
Procaspase 3 protein levels prior to and during recovery from HS in the AC and C hearts. Top: representative sets of protein bands. Bottom: averaged data of the protein, normalized by β-actin. Procaspase 3 protein levels were significantly higher in the AC vs. C group. Values are means ± SE, asterisk significant difference from C (Tukey–Kramer P < 0.03–0.001), circumflex accent significant difference from basal (B) within the group (Dunnett's P < 0.05). For abbreviations, see Fig. 1 legend
Ischemic/reperfusion-mediated changes in Bcl-XL, Bad, and cyt c during the course of heat acclimation
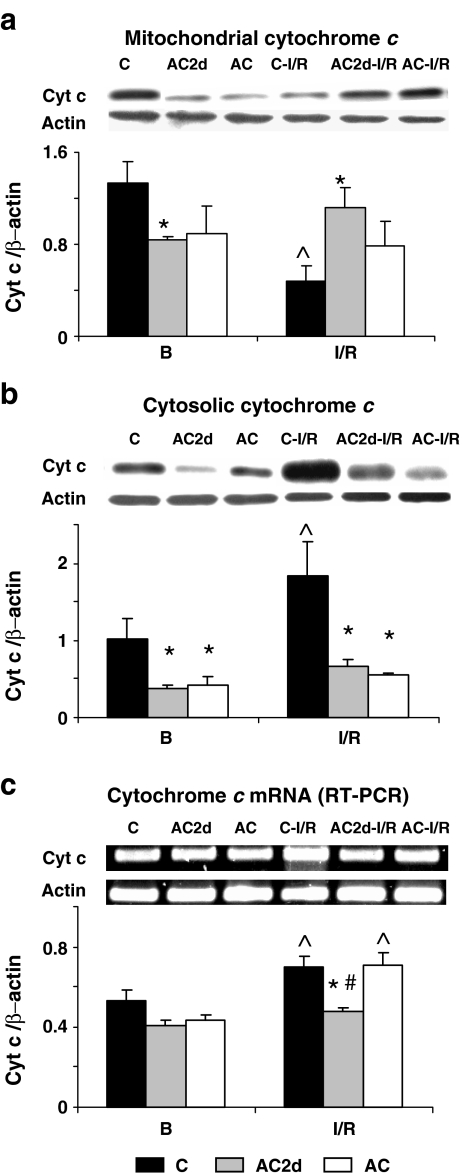
Given our finding of decreased apoptosis following I/R in AC but not in AC2d hearts (Figs. 2b and 3), we investigated Bcl-XL, Bad, and cyt c in these groups post-insult. Figure 8a demonstrates that under basal-normoperfusion, Bcl-XL levels were higher in both AC2d and AC than in control mitochondria by approximately 35% and 30%, (C, 0.48 ± 0.02; AC2d, 1.37 ± 0.11; AC, 1.04 ± 0.14; P < 0.001 and 0.03, respectively) with a significantly higher Bcl-XL/Bad protein ratio in the mitochondria of the AC2d group. I/R decreased Bad levels in C mitochondria but did not significantly affect the protein levels in the short- and long-term acclimated groups. Bcl-XL showed an increase, although not statistically significant in the control group (Fig. 8a), and thus, I/R did not affect the mitochondrial Bcl-XL/Bad protein ratio. Transcriptional changes were noted in both genes: Bcl-XL mRNA and Bad levels decreased in all I/R insulted groups; Bad mRNA transcript decreased significantly also in the AC2d group under basal conditions (C, 0.64 ± 0.01 vs. AC2d, 0.46 ± 0.03; P < 0.01; Fig. 8b). Mitochondrial cyt c levels of C animals decreased significantly post-I/R insult (CI/R, 0.48 ± 0.12 vs. CBasal, 1.33 ± 0.18; P < 0.003; Fig. 9a), while cytosolic cyt c levels were significantly higher (CBasal, 1.02 ± 0.25 vs. CI/R, 1.83 ± 0.4; P < 0.016; Fig. 9b). There were no differences in mitochondrial or cytoplasmic cyt c levels, under basal or post-I/R conditions, between the AC2d and AC hearts. Cyt c mRNA levels were significantly higher in the C and AC insulted groups (Fig. 9c).
Fig. 8.
Bcl-XL and Bad protein and mRNA levels in C, AC2d, and AC rats under basal conditions and following I/R insult. a Bcl-XL and Bad levels and Bcl-XL/Bad protein ratio in mitochondrial fractions. b Bcl-XL and Bad mRNA levels. Values are means ± SE, asterisk significant difference from C (P < 0.034–0.001), circumflex accent significant difference from basal (B) within the group (P < 0.043–0.003), number sign significant difference from AC (P < 0.01–0.003; Tukey–Kramer test). For abbreviations, see Fig. 1 legend
Fig. 9.
Cytochrome c protein levels in mitochondrial (a) and cytosolic (b) fractions and cyt c mRNA levels (c) under basal conditions and following an I/R insult in hearts from C, AC2d, and AC30d animals. Top: protein/mRNA bands. Bottom: bar graphs. Values are means ± SE, asterisk significant difference from C (P < 0.02–0.002), circumflex accent significant difference from basal (B) within the group (P < 0.01–0.003), number sign significant difference from 30d AC (P < 0.002; Tukey–Kramer test). For abbreviations, see Fig. 1 legend
Ischemic/reperfusion insult affects caspase 9 and caspase 3 transcripts and protein levels during the course of heat acclimation
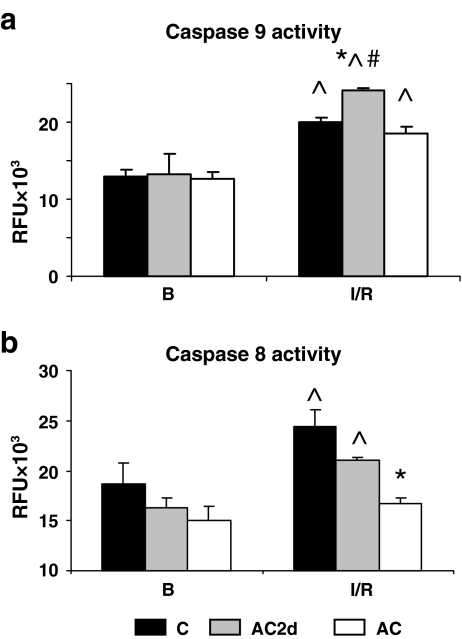
Procaspase 9 remained unchanged in all the groups except for a significant decrease in the AC2d group (data not shown). However, caspase 9 activity increased significantly in all treatment groups following I/R insult, with the AC2d group showing the greatest increase (C 155%, P < 0.001; AC2d 194%, P < 0.001; AC 148%, P < 0.003; Fig. 10a). These death signals only activated caspase 3 in C and AC2d groups as discussed above (Fig. 2b). Our finding that the caspase 9 transcript in AC was significantly lower than those from the C and AC2d groups is noteworthy. For additional transcriptional changes, see ESM Fig. 4.
Fig. 10.
Caspase 9 and caspase 8 activities under basal conditions and following I/R insult in C, AC2d, and AC rats. a Caspase 9 activity assay (relative fluorescent units [RFU]). Caspase 9 activity increased post-I/R insult in all treatment groups. b Caspase 8 activity assay (relative fluorescent units [RFU]). Caspase 8 activity increased in C and AC2d groups post-I/R, but not in AC. Values are means ± SE, asterisk significant difference from C, circumflex accent significant difference from basal (B) within the group (Tukey–Kramer test, P < 0.001), number sign significant difference from AC (Tukey–Kramer, P < 0.001). For abbreviations, see Fig. 1 legend
Is caspase 8 activated?
Despite the resemblance in cytosolic cyt c levels and caspase 9 activity in the two acclimated groups (AC2d and AC), only the AC group had an anti-apoptotic phenotype. Hence, we measured caspase 8 transcript and activity to clarify whether the extrinsic pathway was activated and contributes to the apoptotic events detected in the AC2d group. Caspase-8 activity increased by 30% in both the C and AC2d groups following I/R (P < 0.032) and remained unchanged following I/R insult in the AC group (Fig. 10b), thereby excluding the activation of the extrinsic apoptotic pathway in the 30d acclimated group. Notably, I/R AC2d hearts demonstrated significant caspase 8 transcript up-regulation [(AC2dI/R 12.9 ± 3.7 vs. CI/R 3.4 ± 0.65 and ACI/R 4.2 ± 1.04 (P < 0.005)], confirming a dominant role of this pathway in the AC2d group. For further details, see ESM Fig. 5.
Discussion
We are the first to report that heat acclimation in homeotherms (1) induces an apoptosis-resistant cardiac phenotype and (2) causes adaptive changes in mitochondrial outer membrane permeability that attenuate propagation of mitochondrial death signaling. Mitochondrial adaptation is already apparent after 2 days of acclimation. Nevertheless, the apoptosis-resistant phenotype is only found following 30 days of AC, suggesting that additional, more slowly developing adaptations contribute to forming the protected/resistant phenotype. Our observation of abolishment of extrinsic death signals in the 30d AC hearts points on cell membrane adaptations as likely candidates. Additionally, our previous studies on the heart show that heat acclimation increases reserves of numerous cytoprotective mediators (Horowitz 2007). These may interact with the anti-apoptotic arm of the Bcl-2 superfamily to promote the apoptosis-resistant phenotype and will be discussed below.
The 2-day, short acclimation period is associated with exacerbated apoptotic processes, thus confirming our hypothesis that a host of cellular and membranal perturbations at the onset of heat acclimation (Horowitz 2007) increase the susceptibility to injury following exposure to stressors.
Apoptosis dynamics following acute heat stress: does elevated Bcl-XL play a central role?
We previously reported that heat acclimation alters the anti/pro-apoptotic transcript ratio following heat stress and also following I/R, exercise, and traumatic brain injury (Horowitz 2007; Horowitz et al. 2004; Shein et al. 2007; Umschwief et al. 2009; and Kodesh and Horowitz unpublished observations). In this investigation, we demonstrated that 30 days of heat acclimation increases levels of Bcl-XL (the mitochondrial anti-apoptotic member of the Bcl-2 superfamily) and down-regulates Bad (the pro-apoptotic death promoter). Following HS, Bcl-XL was further up-regulated, with no significant changes in Bad. The Bcl-XL–Bad complex (in combination with other Bcl-2 proteins) is involved in opening mitochondrial permeability pores (Borutaite et al. 2003; Cheng et al. 2001), with Bcl-XL serving as the “guard”. Consequently, our finding that mitochondrial Bcl-XL/Bad kinetics in the AC group was associated with decreased cyt c release implies reduced megachannel opening. Decreased cleaved caspase 3 levels and TUNEL staining in this group confirmed enhanced cell survival (i.e., less apoptosis). This was not the case in the non-acclimated–control HS rats. In the latter group, no changes in Bcl-XL/Bad ratio and cyt c release post-HS were observed. Based on our cleaved caspase 3 observations, peak apoptosis in C hearts was 6–10 h post-HS. These data are in agreement with those of Qian et al. (2004). Changes in Bcl-XL plasticity seem to be essential to the alterations noted in intrinsic apoptotic signaling in the AC group. The significant, HS-induced up-regulation of Bcl-XL transcript (almost 4-fold), implies transcriptional regulation of this protein.
The Bcl-2-induced apoptotic pathway is mediated by post-translational modifications initiated by AKT (Serine/threonine protein kinase AKT) phosphorylation (AKT-P; Cheng et al. 2001; Chiang et al. 2008). Although this step was not investigated in the current study, we have previously observed increased basal and post-insult AKT phosphorylation in AC brains (Shein et al. 2007) and hearts (Assayag and Horowitz unpublished). These findings are consistent with our current results of a higher Bcl-XL/Bad ratio in the AC heart, not only because of augmented Bcl-XL but also because of less Bad trafficking to the mitochondria (enhanced AKT-P attenuates Bad translocation to the mitochondria; Chiang et al. 2008).
The marked increase in mitochondrial cyt c levels can be partially explained by the up-regulated transcription of cyt c in acclimated (but not in C) hearts, immediately and 1 h after HS. Increased cyt c transcription in C hearts lagged behind that of the AC hearts and was not reflected in the mitochondrial levels of this protein. Greater leaking of this molecule to the cytosol can be inferred from our finding that cytosolic cyt c levels were significantly higher immediately after HS in the C group.
Additional confirmation of the role of the mitochondrial axis in forming the anti-apoptotic phenotype was achieved by comparing the “caspase 9–caspase 3” targeted pathway in the hearts of HS C and AC rats by measuring procaspase 9 levels and caspase 9 activity. There were marked differences in cleaved caspase 9 profiles between the AC and the C groups: C hearts, which demonstrated HS-induced apoptosis, maintained a relatively high level of active caspase 9 1 h post-HS, whereas there was a marked drop in this protease in the AC group. Despite the greater basal Bcl-XL/Bad ratio in AC2d, cleaved caspase 3 and TUNEL staining demonstrated that this favorable ratio did not abolish or diminish apoptosis in this group. Apoptosis tolerance downstream to caspase 9 was studied by exposing the animals to a novel stress. Given that AC renders cross-tolerance to oxygen deprivation, I/R insult was selected as the stressor (Horowitz 2007). In contrast to the HS series, the protein levels in this experimental series were measured at one time point and do not cover early I/R events or temporal changes post-insult.
Long- but not short-term acclimation diminishes ischemic/reperfusion-mediated apoptosis
Marked elevation of cytosolic cyt c in C rat hearts following I/R insult implies involvement of the intrinsic mitochondrial apoptotic cascade. This finding is consistent with results reported in other studies (e.g., Correa et al. 2007; Hausenloy and Yellon 2003; Schwimmer et al. 2004). The maintenance of lowered cytosolic cyt c in I/R AC2d and AC hearts (Fig. 9) implies that death signal propagation from the mitochondria was blocked. However, the decreased cyt c levels were associated with significantly lower levels of cleaved caspase 3 and positive TUNEL staining in the AC group only, thus confirming that long-term heat acclimation promotes the emergence of an apoptotic-resistant phenotype but that decreased cytosolic cyt c is not the main source of protection. There is a discrepancy between the low cytosolic cyt c levels and the elevated active caspase 9 in these groups. Currently, we have no adequate explanation for this phenomenon, since our studies were not designed to detect immediate post-insult events. C and AC2d, but not AC, demonstrated activation of the extrinsic apoptotic cascade (namely, elevated activation of caspase 8). The blockade of this pathway in the AC heart implies cell membrane upstream adaptation in this group. Shmeeda et al. (2002) and Schwimmer et al. (2006), indeed, report on heat acclimation-mediated changes in the lipid composition of AC membranes in the brain and the heart. A major difference between the long-term AC group and the other treatment groups is the presence of large constitutive HSP70, HSP90, and low-molecular-weight antioxidant reserves, as well as low ROS production upon anoxia (Horowitz et al. 2006; Maloyan et al. 1999). These cytoprotective elements shield the cell from apoptosis upstream to caspase 3 and downstream of the mitochondrial cascade. Hence, their contribution to the AC apoptosis resistance is unequivocal.
A possible additional explanation which was not studied here is the involvement of the “inhibitor of apoptosis proteins” (IAPs; Bergmann et al. 2003; Shiozaki and Shi 2004). Anti-apoptotic IAPs including XIAP can inhibit enzymatic activity of initiator and effector caspases (Sanna et al. 2002; Shiozaki et al. 2003). Datta et al. 2000 showed that XIAP may inhibit caspase-3 activation despite caspase-9 activation. Choi et al. (2009) provided another mechanism for caspase 3 inhibition. Specifically, they described the presence of a prodomain and cleavage of the large and small subunits not involving the classical IAP-binding motif. Therefore, it is possible that effector caspase inhibition is among the plethora of cellular changes caused by long-term heat acclimation.
In sum, in this study, we provide evidence that heat acclimation induces a continuum of adaptive changes leading to generation of apoptosis-resistant cardiac phenotype. There are changes in mitochondrial outer membrane permeability to attenuate propagation of mitochondrial death signaling. This adaptation is rapid (AC2d), but insufficient, to develop apoptosis resistance if multiple apoptotic cascades are activated. There is an inference of adaptation in the sarcolemma to attenuate propagation of extrinsic death signals. This process is slow and brought into play when acclimation homeostasis has been achieved (AC). Data from our previous studies implicate additional potential anti-apoptotic pathways, HSPs, antioxidation, and also HIF-1-mediated (Maloyan et al. 2005) cascades. Concerted effect of these factors contributes to anti-apoptotic environment; however, the exact mechanisms and the share of each of these factors remain to be studied.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Bad and Bcl-XL protein expression in the mitochondrial fraction following recovery from HS in the C and AC groups. Bad expression did not change significantly between the groups. Bcl-XL was increased post-HS in the AC group and was significantly different from the C at three time points: HS+1, HS+1.5, and HS+24. Asterisk indicates significant difference from Con (P < 0.01–0.002), Tukey–Kremer (PPT 148 kb)
Cytochrome c mRNA levels following HS. The only significant difference was immediately post-HS, when there was an up-regulation in the AC group and down-regulation in the C group. * - significant difference from Con (P < 0.024), Tukey-Kremer (PPT 127 kb)
Caspase 9 mRNA levels (qRT-PCR) prior to and at selected time points during recovery from HS. Values are means±SE; mRNA levels were normalized to β-actin. Procaspase 9 was decreased in AC, as compared with C, hearts (2W ANOVA P < 0.003). In contrast, caspase 9 transcript was up-regulated post-HS in C but not in AC groups (2W ANOVA P < 0.001). Values are means±SE; asterisk indicates significant difference from C (P < 0.03–0.001) (PPT 126 kb)
Caspase 9 and caspase 3 transcripts in left ventricle of the hearts of C, AC2d, and AC rats before and following I/R insult. Basal caspase 9 transcript in AC was significantly lower than that of C and AC2d. Basal caspase 3 transcripts (except for slight up-regulation on day 2 of the acclimation) did not differ significantly in all groups. I/R insult induced minor changes only in caspase 9 transcripts but not in caspase 3. Asterisk indicates significant difference from C (P < 0.003); circumflex accent indicates significant difference from basal (B) within the group (P < 0.002); number sign indicates significant difference from AC (P < 0.03; Tukey–Kramer) (PPT 200 kb)
Caspase 8 transcripts in left ventricle of C, AC2d, and AC rats following I/R insult vs. basal normoxic C hearts. AC2d caspase 8 level was significantly higher than that of C and AC I/R heart, as well as vs. basal normoxic levels. Basal normoxic transcript levels did not differ significantly among the groups. Asterisk indicates significant difference from basal (P < 0.005); number sign indicates significant difference from C and AC (P < 0.02–0.04), 1W ANOVA (P < 0.005) followed by Tukey test (PPT 119 kb)
Acknowledgement
This study was supported by the USA–Israel Binational Fund BSF Grant 2003-298 and (in part) by the Intramural Research Program of the NIH National Institute on Aging.
References
- Beere HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115(10):2633–2639. doi: 10.1172/JCI26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann A, Yang AY, Srivastava M. Regulators of IAP function: coming to grips with the grim reaper. Curr Opin Cell Biol. 2003;15(6):717–724. doi: 10.1016/j.ceb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Jekabsone A, Morkuniene R, Brown GC. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J Mol Cell Cardiol. 2003;35(4):357–366. doi: 10.1016/S0022-2828(03)00005-1. [DOI] [PubMed] [Google Scholar]
- Bratton SB, Walker G, Srinivasula SM, Sun XM, Butterworth M, Alnemri ES, Cohen GM. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001;20(5):998–1009. doi: 10.1093/emboj/20.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-XL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–711. doi: 10.1016/S1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chiang CW, Yan L, Yang E. Phosphatases and regulation of cell death. Methods Enzymol. 2008;446:237–257. doi: 10.1016/S0076-6879(08)01614-5. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18(4):157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YE, Butterworth M, Malladi S, Duckett CS, Cohen GM, Bratton SB. The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processing. J Biol Chem. 2009;284(19):12772–12782. doi: 10.1074/jbc.M807550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa F, Soto V, Zazueta C. Mitochondrial permeability transition relevance for apoptotic triggering in the post-ischemic heart. Int J Biochem Cell Biol. 2007;39(4):787–798. doi: 10.1016/j.biocel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Datta R, Oki E, Endo K, Biedermann V, Ren J, Kufe D. XIAP regulates DNA damage-induced apoptosis downstream of caspase-9 cleavage. J Biol Chem. 2000;275(41):31733–31738. doi: 10.1074/jbc.M910231199. [DOI] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292(1):C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77(2):334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- Han J, Goldstein LA, Gastman BR, Rabinowich H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. JBC. 2006;281(15):10156–10163. doi: 10.1074/jbc.M510349200. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol. 2003;35(4):339–341. doi: 10.1016/S0022-2828(03)00043-9. [DOI] [PubMed] [Google Scholar]
- Horowitz M. Acclimatization of rats to moderate heat: body water distribution and adaptability of the submaxillary salivary gland. Pflugers Arch. 1976;366(2–3):173–176. doi: 10.1007/BF00585874. [DOI] [PubMed] [Google Scholar]
- Horowitz M. Do cellular heat acclimation responses modulate central thermoregulatory activity? News Physiol Sci. 1998;13(5):218–225. doi: 10.1152/physiologyonline.1998.13.5.218. [DOI] [PubMed] [Google Scholar]
- Horowitz M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp Biochem Physiol A Mol Integr Physiol. 2002;131(3):475–483. doi: 10.1016/S1095-6433(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Horowitz M (2007) Heat acclimation and cross-tolerance against novel stressors: genomic-physiological linkage. In: Shanker H (ed) Progress in brain research: Elsevier, Amsterdam, 162:373–392. doi:10.1016/S0079-6123(06)62018-9 [DOI] [PubMed]
- Horowitz M, Meiri U. Thermoregulatory activity in the rat: effects of hypohydration, hypovolemia and hypertonicity and their interaction with short-term heat acclimation. Comp Biochem Physiol A Comp Physiol. 1985;82(3):577–582. doi: 10.1016/0300-9629(85)90436-0. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Eli-Berchoer L, Wapinski I, Friedman N, Kodesh E. Stress-related genomic responses during the course of heat acclimation and its association with ischemic-reperfusion cross-tolerance. J Appl Physiol. 2004;97(4):1496–1507. doi: 10.1152/japplphysiol.00306.2004. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Cannana H, Kohen R (2006) ROS generated in the mitochondria may be linked with heat stress mediated HIF-1 transcriptional activation in the heart. APS Conference Comparative Physiology, 8–11 Oct 2006, Virginia Beach, VA
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kutuk O, Basaga H. Bcl-2 protein family: implications in vascular apoptosis and atherosclerosis. Apoptosis. 2006;11(10):1661–1675. doi: 10.1007/s10495-006-9402-7. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy E, Hasin Y, Navon G, Horowitz M. Chronic heat improves mechanical and metabolic response of trained rat heart on ischemia and reperfusion. Am J Physiol. 1997;272(5):H2085–H2094. doi: 10.1152/ajpheart.1997.272.5.H2085. [DOI] [PubMed] [Google Scholar]
- Maloyan A, Palmon A, Horowitz M. Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am J Physiol. 1999;276(5):R1506–R1515. doi: 10.1152/ajpregu.1999.276.5.R1506. [DOI] [PubMed] [Google Scholar]
- Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. HIF-1alpha-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics. 2005;23(1):79–88. doi: 10.1152/physiolgenomics.00279.2004. [DOI] [PubMed] [Google Scholar]
- Milleron RS, Bratton SB. ‘Heated’ debates in apoptosis. Cell Mol Life Sci. 2007;64(18):2329–2333. doi: 10.1007/s00018-007-7135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Song X, Ren H, Gong J, Chang S. Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress Chaperones. 2004;9(3):281–293. doi: 10.1379/CSC-20R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna MG, Silva Correia J, Ducrey O, Lee J, Nomoto K, Schrantz N, Deveraux QL, Ulevitch RJ. IAP suppression of apoptosis involves distinct mechanisms: the TAK1/JNK1 signaling cascade and caspase inhibition. Mol Cell Biol. 2002;22(6):1754–1766. doi: 10.1128/MCB.22.6.1754-1766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer H, Gerstberger R, Horowitz M. Heat acclimation affects the neuromodulatory role of AngII and nitric oxide during combined heat and hypohydration stress. Brain Res Mol Brain Res. 2004;130(1–2):95–108. doi: 10.1016/j.molbrainres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Schwimmer H, Eli-Berchoer L, Horowitz M. Heat-Acclimation phase specificity of gene expression during the course of heat acclimation and superimposed hypohydration in the rat hypothalamus. J Appl Physiol. 2006;100(6):1992–2003. doi: 10.1152/japplphysiol.00850.2005. [DOI] [PubMed] [Google Scholar]
- Shein NA, Tsenter J, Alexandrovich AG, Horowitz M, Shohami E. Akt phosphorylation is required for heat acclimation-induced neuroprotection. J Neurochem. 2007;103(4):1523–1529. doi: 10.1111/j.1471-4159.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- Shiozaki EN, Shi Y. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem Sci. 2004;29(9):486–494. doi: 10.1016/j.tibs.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11(2):519–527. doi: 10.1016/S1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Shmeeda H, Kaspler P, Shleyer J, Honen R, Horowitz M, Barenholz Y. Heat acclimation in rats: modulation via lipid polyunsaturation. Am J Physiol Regul Integr Comp Physiol. 2002;283:R389–R399. doi: 10.1152/ajpregu.00423.2001. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1(7):949–957. doi: 10.1016/S1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- Tetievsky A, Cohen O, Eli-Berchoer L, Gerstenblith G, Stern MD, Wapinski I, Friedman N, Horowitz M. Physiological and molecular evidence of heat acclimation memory: a lesson from thermal responses and ischemic cross-tolerance in the heart. Physiol Genomics. 2008;34(1):78–87. doi: 10.1152/physiolgenomics.00215.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umschwief G, Shein NA, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Heat acclimation provides sustained improvement in functional recovery and attenuates apoptosis after traumatic brain injury. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Papazafiri P. Anti-apoptotic effects of allopregnanolone on P19 neurons. Eur J NeuroSci. 2006;23(1):43–54. doi: 10.1111/j.1460-9568.2005.04548.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Bad and Bcl-XL protein expression in the mitochondrial fraction following recovery from HS in the C and AC groups. Bad expression did not change significantly between the groups. Bcl-XL was increased post-HS in the AC group and was significantly different from the C at three time points: HS+1, HS+1.5, and HS+24. Asterisk indicates significant difference from Con (P < 0.01–0.002), Tukey–Kremer (PPT 148 kb)
Cytochrome c mRNA levels following HS. The only significant difference was immediately post-HS, when there was an up-regulation in the AC group and down-regulation in the C group. * - significant difference from Con (P < 0.024), Tukey-Kremer (PPT 127 kb)
Caspase 9 mRNA levels (qRT-PCR) prior to and at selected time points during recovery from HS. Values are means±SE; mRNA levels were normalized to β-actin. Procaspase 9 was decreased in AC, as compared with C, hearts (2W ANOVA P < 0.003). In contrast, caspase 9 transcript was up-regulated post-HS in C but not in AC groups (2W ANOVA P < 0.001). Values are means±SE; asterisk indicates significant difference from C (P < 0.03–0.001) (PPT 126 kb)
Caspase 9 and caspase 3 transcripts in left ventricle of the hearts of C, AC2d, and AC rats before and following I/R insult. Basal caspase 9 transcript in AC was significantly lower than that of C and AC2d. Basal caspase 3 transcripts (except for slight up-regulation on day 2 of the acclimation) did not differ significantly in all groups. I/R insult induced minor changes only in caspase 9 transcripts but not in caspase 3. Asterisk indicates significant difference from C (P < 0.003); circumflex accent indicates significant difference from basal (B) within the group (P < 0.002); number sign indicates significant difference from AC (P < 0.03; Tukey–Kramer) (PPT 200 kb)
Caspase 8 transcripts in left ventricle of C, AC2d, and AC rats following I/R insult vs. basal normoxic C hearts. AC2d caspase 8 level was significantly higher than that of C and AC I/R heart, as well as vs. basal normoxic levels. Basal normoxic transcript levels did not differ significantly among the groups. Asterisk indicates significant difference from basal (P < 0.005); number sign indicates significant difference from C and AC (P < 0.02–0.04), 1W ANOVA (P < 0.005) followed by Tukey test (PPT 119 kb)