Abstract
Synthesis of heat shock proteins (HSPs) following cellular stress is a response shared by many organisms. Amongst the HSP family, the ∼70 kDa HSPs are the most evolutionarily conserved with intracellular chaperone and extracellular immunoregulatory functions. This study focused on the effects of larval excretory-secretory products (ESPs) from the parasite Schistosoma mansoni on HSP70 protein expression levels in haemocytes (defence cells) from its snail intermediate host Biomphalaria glabrata. S. mansoni larval stage ESPs are known to interfere with haemocyte physiology and behaviour. Haemocytes from two different B. glabrata strains, one which is susceptible to S. mansoni infection and one which is resistant, both showed reduced HSP70 protein levels following 1 h challenge with S. mansoni ESPs when compared to unchallenged controls; however, the reduction observed in the resistant strain was less marked. The decline in intracellular HSP70 protein persisted for at least 5 h in resistant snail haemocytes only. Furthermore, in schistosome-susceptible snails infected by S. mansoni for 35 days, haemocytes possessed approximately 70% less HSP70. The proteasome inhibitor, MG132, partially restored HSP70 protein levels in ESP-challenged haemocytes, demonstrating that the decrease in HSP70 was in part due to intracellular degradation. The extracellular signal-regulated kinase (ERK) signalling pathway appears to regulate HSP70 protein expression in these cells, as the mitogen-activated protein-ERK kinase 1/2 (MEK1/2) inhibitor, U0126, significantly reduced HSP70 protein levels. Disruption of intracellular HSP70 protein expression in B. glabrata haemocytes by S. mansoni ESPs may be a strategy employed by the parasite to manipulate the immune response of the intermediate snail host.
Keywords: Heat shock protein 70 (HSP70), Excretory-secretory products (ESPs), Extracellular signal-regulated kinase (ERK), Schistosomes, Snail haemocytes, Biomphalaria glabrata
Introduction
Cellular stress factors such as infection, inflammation, toxins or variation in temperature can lead to elevated synthesis of heat shock proteins (HSPs) which protect cells from irreversible damage and death (Mager and De Kruijff 1995). The ∼70 kDa HSPs include constitutively expressed heat shock cognate 70 (HSC70) and stress-inducible HSP70. In normal, non-stressed conditions, intracellular HSPs play a critical role in the synthesis, transport and folding of proteins (Mager and Ferreira 1993; Morimoto 1993; Yura et al.1993). Amongst the HSP family, the HSP70s are the most evolutionarily conserved proteins and have been identified in a range of organisms, including molluscs (Kourtidis et al. 2006a, b; Laursen et al. 1997; Martynova et al. 2007; Tirard et al. 1995; Yoshino et al. 1998). HSP70 can be actively secreted from cells independent of the common secretory pathway, or released during necrosis (Lancaster and Febbraio 2005; Martynova et al. 2007). Extracellularly, HSP70 has an immunoregulatory function and can interact with neurons, antigen-presenting cells and blood vessels (Boorstein et al. 1994; van Eden et al. 2005, 2007). However, the mechanisms which regulate HSP70 protein synthesis and secretion are currently not fully understood.
The snail Biomphalaria glabrata is an intermediate host for Schistosoma mansoni a platyhelminth parasite that, in the human definitive host, can cause the debilitating and chronic disease, human schistosomiasis. Once S. mansoni eggs are released in faeces of the infected human host, a free-living miracidium hatches from each egg and infects a susceptible snail host. Inside the snail, the parasite transforms into a mother and subsequently daughter sporocysts which release cercariae capable of infecting the definitive host (reviewed in Bayne 2009). As the parasite transforms from a miracidium into a mother sporocyst, it releases ciliary epidermal plates and a variety of molecules from its surface and excretes a range of molecules from its excretory pore, these being jointly referred to as excretory-secretory products (ESPs; Lodes and Yoshino 1989); the protein fraction, comprising larval transformation proteins has recently been analysed by proteomics (Wu et al. 2009). ESPs from platyhelminths generally consist of antioxidant enzymes, protease inhibitors, cysteine proteases, small HSPs, glycolytic enzymes, mucins, calcium, ion-binding proteins and other unknown proteins (Connors et al. 1991; Guillou et al. 2007; Humphries and Yoshino 2008; Lodes and Yoshino 1989; Roger et al. 2008; Wu et al. 2009; Zelck and Von Janowsky 2004). These parasite-derived molecules can influence the behaviour of B. glabrata defence cells (haemocytes) by affecting their motility, adhesion, ability to phagocytose or encapsulate large antigens and produce reactive oxygen species and intracellular nitric oxide (NO; Connors et al. 1991; Connors and Yoshino 1990; Humbert and Coustau 2001; Loker et al. 1992; Lodes and Yoshino 1990; Zahoor et al. 2009). Laboratory strains of B. glabrata exist that are either resistant or susceptible to S. mansoni infection; unlike in the susceptible strain, in the resistant strain the parasite is killed before it develops fully into a mother sporocyst. These strains are therefore invaluable for studying snail-schistosome interactions in the context of parasite survival. Larval stage ESPs are thought to assist the survival of developing sporocysts in a susceptible snail host by interacting with haemocytes via cell surface receptors and modulating the activities of cell signalling pathways, such as the extracellular signal-regulated kinase (ERK) pathway that regulates cellular responses including cell spreading, NO and hydrogen peroxide production (Bayne 2009; Humphries and Yoshino 2008; Johnston and Yoshino 1996; Johnston and Yoshino 2001; Walker 2006; Zahoor et al. 2008; Zahoor et al. 2009). In the present study, we report for the first time that intracellular HSP70 protein levels in haemocytes from schistosome-susceptible and schistosome-resistant snails are reduced following exposure to S. mansoni larval ESPs. Furthermore, HSP70 appears to be degraded intracellularly by proteasomes in response to ESP exposure. Finally, it is demonstrated that HSP70 expression is regulated by the ERK-signalling pathway. Given that ERK activity in B. glabrata haemocytes is affected by S. mansoni ESPs (Zahoor et al. 2008), the current findings have implications for survival of the parasite in the snail host.
Materials and methods
Animals
Snail strains used in this study were a B. glabrata strain resistant to S. mansoni (NHM accession number 3017), originally derived from BS90 strain snails, and a B. glabrata strain susceptible to S. mansoni (NHM accession number 1742). All snails were maintained at 26°C with a 12:12 h, light:dark cycle and were fed fresh lettuce twice weekly. The life cycle of S. mansoni (Belo Horizonte strain) was maintained in albino CD1 mice. Animal use received appropriate local ethical approval.
Collection of S. mansoni ESPs
In vitro transformation of S. mansoni miracidia into mother sporocysts and collection of S. mansoni ESPs have been described previously (Zahoor et al. 2008, 2009). Briefly, parasite eggs were isolated from the liver and spleen of S. mansoni-infected mice and left to hatch in spring water (Evian) for 3 h, before being collected, washed and concentrated using a Stericup HV filter unit with a 0.45 μm membrane (Millipore, Watford, UK). The miracidia (approximately 60,000 from seven mice) were then left to transform into mother sporocysts in 25 cm2 vented sterile tissue culture flasks (Nunc, Rochester, NY, USA) containing sterile Chernin’s balanced salt solution (CBBS) with glucose and trehalose (1 g/L each), penicillin and streptomycin (100 U/ml of each; chemicals purchased from Sigma, Poole, UK); flasks were placed in an incubator at 26°C for 36-40 h and transformation was monitored using an inverted light microscope. The culture medium containing the ESPs was removed and concentrated approximately 20 times at 4°C, in Vivapore 10 ml concentrators (Vivascience, Sartorius, Epsom, UK) with a 7,500 MW cut-off. The protein concentration of the ESP preparation was then determined with a NanoOrange fluorescence-based protein assay kit (Molecular Probes, Leiden, Netherlands) and a Fluorstar Optima microplate spectrofluorometer (BMG Labtech, Aylesbury, UK), using bovine serum albumin (BSA) as the protein standard. Finally, the ESP solution was aliquoted and stored at −20°C prior to use.
Haemocytes and haemocyte treatments
Haemolymph containing haemocytes was extracted from adult B. glabrata using the head-foot retraction method. Cell monolayers were then created in individual wells of 48-well flat bottom culture plates (Corning Costar, Schiphol-Rijk, The Netherlands) using pooled haemolymph (diluted 2:1 in CBBS, total volume of 250 μl; or approximately 1 × 105 cells/well) from same strain snails of similar age and size (1-1.5 cm diameter). After 30 min at room temperature (RT), the adherent haemocytes that formed the cell monolayer were washed twice with CBBS and then challenged for 1 h with S. mansoni ESPs. The ESP concentrations chosen (0-20 μg/ml in CBSS) were those employed in our previous studies (Zahoor et al. 2008, 2009). In some experiments, the proteasome inhibitor Z-Leu-Leu-Leu-aldehyde (MG132; 50 μM; Merck Chemicals, Nottinghamshire, UK), the MEK1/2 inhibitor, 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (U0126; 1-10 μM; Sigma), or vehicle control (0.1% (v/v) DMSO; Sigma) were added with or without ESPs. The inhibitor, U0126, has previously been used at these concentrations to block MEK1/2 activity in B. glabrata and Lymnaea stagnalis haemocytes, subsequently suppressing the phosphorylation (activation) of ERK (Plows et al. 2004; Plows et al. 2005; Wright et al. 2006; Zahoor et al.2008, 2009). The concentration chosen for MG132 was similar to that used by Kim et al. (1999). Immediately after treatment, the medium was removed and haemocyte proteins were extracted by adding hot sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer; samples were then sonicated for 30 s and boiled for 1 min. In a further set of experiments studying the effect of ESP treatment on longer term HSP70 expression, ESP-exposed haemocytes were washed once with CBBS and left in CBSS for 5 h at RT before protein extraction.
Detection of intracellular HSP70 proteins by western blotting
Haemocyte protein samples and control extracts from a mammalian cell line (HC60) were electrophoresed on 10% discontinuous SDS-PAGE gels. Separated proteins were transferred onto Hybond nitrocellulose membranes (0.45 μm; GE Healthcare, Amersham, UK) using a semi-dry electrotransfer unit (Bio-Rad, Hemel Hempstead, UK). The membrane was then blocked for 40 min at RT with 5% (w/v) non-fat dried milk in Tris-buffered saline (TBS) containing 0.1% (v/v) Tween-20 (TTBS), and incubated overnight at 4°C followed by 1 h at RT in primary anti-HSP70 monoclonal antibodies (BRM-22, Santa Cruz Biotechnology, Heidelberg, Germany, 1:1,000 in TTBS). This anti-HSP70 antibody has been used previously to detect HSP70/HSC70 in mammals and Xenopus, as well as homologues in invertebrates including the fruit fly, Drosophila melanogaster, and the zebra mussel, Dreissena polymorpha (Clayton et al. 2000; Lilja et al. 2007; Smith et al. 2005).The membrane was then washed three times in TTBS before being incubated with an anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2,000 in TTBS; Santa Cruz Biotechnology) for 3 h at RT. The immunoreactive signal was developed using SuperSignal West Pico chemiluminescent substrate (PerBioscience, Tatenhall, UK) and visualised using a GeneGnome chemiluminescence imaging system (Syngene, Cambridge, UK); band intensities were analysed using GeneTools software (Syngene). To confirm equal loading of protein between samples, blots were stripped with Restore western blot stripping buffer (PerBioscience) following the manufacturers’ guidelines and incubated with anti-actin rabbit polyclonal antibodies (1:2,500 in TTBS; Sigma) overnight at 4°C, prior to detection with anti-rabbit HRP-conjugated secondary antibodies (1:2,500 in TTBS; Sigma).
Intracellular staining of HSP70 and confocal microscopy
Pooled haemocytes in CBSS from adult schistosome-resistant or schistosome-susceptible snails were left to adhere to glass coverslips for 30 min at RT before being washed with CBSS three times and exposed to 20 μg/ml ESPs for 1 h. Challenged and unchallenged haemocytes were fixed and permeabilised in 3.7% (v/v) formaldehyde containing 0.18% (v/v) Triton X-100 in phosphate buffered saline (PBS) for 1 h and washed before being blocked in 1% (w/v) BSA for 12 min. The cells were then treated with anti-HSP70 (BRM-22) antibodies (Santa Cruz Biotechnology, 1:100 in blocking buffer) for 3 h at RT, and subsequently incubated in FITC-conjugated goat anti-mouse secondary antibodies (Sigma; 1:500 in blocking buffer) for 1 h. Finally, cells were incubated in rhodamine phalloidin and, in some experiments, 4′,6-diamidino-2-phenylindole (DAPI; 0.1 μg/ml and 15 min each; Sigma). Haemocytes were washed three times with PBS between incubations and all other steps were carried out at RT in humidified chambers. Next, coverslips were mounted onto slides using Vectashield (Vecta Laboratories, Peterborough, UK). Cells were visualised using a Leica TCS SP2 AOBS laser scanning confocal microscope with a 63× immersion objective; the laser settings were kept constant for all observations and the average fluorescence intensity of the image was captured using Leica confocal imaging software.
Effect of S. mansoni infection on haemocyte HSP70 expression in snails
Adult schistosome-susceptible B. glabrata were each individually exposed to five freshly hatched S. mansoni miracidia for 12 h in water. At 25 days post-exposure, the snails were kept in the dark (with food) for 24 h at 26°C before being placed in plastic cups under artificial light for 2 h to induce the release of cercariae. The snails were then checked for cercariae release until 35 days post-exposure; individual snails were categorised as ‘infected’ if cercariae emerged on any day. Snails that shed significant numbers of cercariae (between 10 and 50 at any one observation) were killed and haemolymph from three snails pooled. A similar volume of haemolymph was then also collected from three adult (control) snails that had not been exposed to miracidia; these snails were maintained and haemolymph extracted in a similar way to that for the schistosome-infected snails. Haemocytes were collected by brief centrifugation of haemolymph in a microfuge and cells were lysed with SDS-PAGE sample buffer. Samples were then sonicated for 30 s, heated at 90°C for 1 min and processed for western blotting using anti-HSP70 and anti-actin antibodies.
Statistical analysis
Two sample t tests and analysis of variance were performed where appropriate using the statistical software package SPSS; P ≤ 0.05 was considered significant and P ≤ 0.01 was considered highly significant. Each experiment was independently repeated three to four times, usually on separate days, using pooled haemocytes from susceptible or resistant snail strains. In most experiments, HSP70 expression levels were normalised to control values (represented by 1).
Results
HSP70 proteins in B. glabrata haemocytes
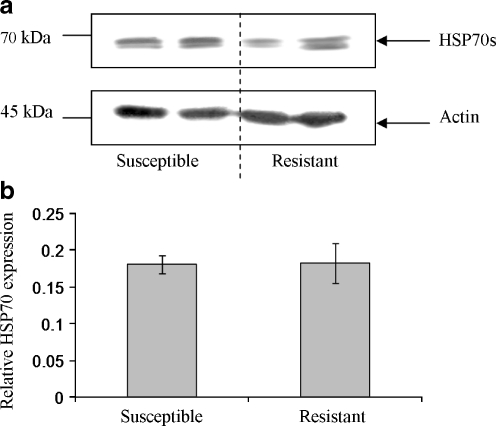
Western blotting with anti-HSP70 monoclonal antibodies (clone: BRM-22) that detect HSP70/HSC70, identified two discrete immunoreactive bands of approximately 70-75 kDa in unchallenged B. glabrata haemocytes (Fig. 1); immunoreactive bands of similar size were detected in mammalian control cells (constitutively expressed, data not shown). Basal levels of HSP70/HSC70 protein in tissue not exposed to stress have also been noted in a number of organisms including the oyster, Ostrea edulis, and the sea urchin, Paracentrotus lividus, indicating a possible housekeeping role (Matranga et al. 2000; Piano et al. 2002). For clarity, the immunoreactive proteins will be referred to as HSP70s; clearly, they could comprise HSC or HSP forms. Unchallenged schistosome-susceptible and schistosome-resistant snail haemocytes expressed HSP70 protein in approximately equal amounts (relative to actin) when an equal number of haemocytes was used for each snail strain (Fig. 1).
Fig. 1.
Basal levels of HSP70 protein in schistosome-resistant and schistosome-susceptible snail haemocytes are similar. a An equal number of haemocytes (approximately 1 × 105 cells) from each snail strain were lysed and the proteins processed for western blotting with anti-HSP70 antibodies (upper panel). Blots were subsequently stripped of antibodies with a stripping buffer and re-probed with anti-actin antibodies (lower panel), to confirm equal loading of protein between samples. b Quantitative band analysis was used to calculate mean (±SEM) HSP70 protein levels in each snail strain relative to actin levels; n = 4 for each snail strain, performed in two independent experiments
S. mansoni ESPs modulate HSP70 protein expression in host haemocytes
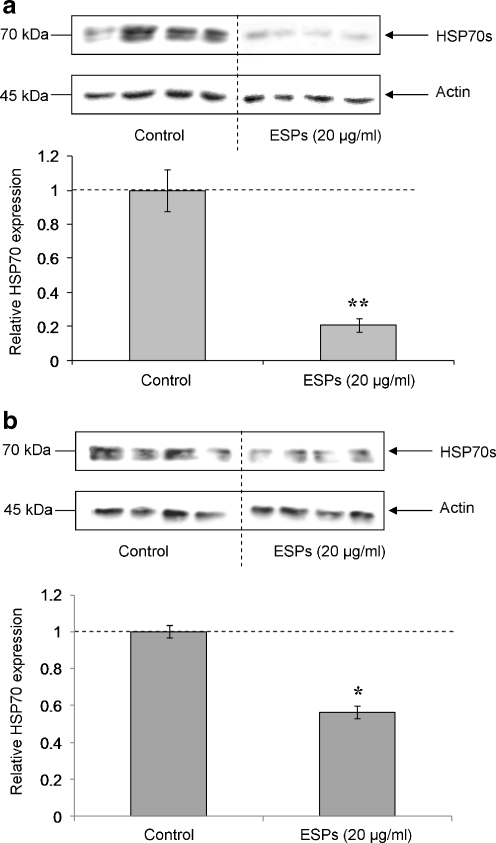
Pooled haemocytes from either schistosome-susceptible or schistosome-resistant B. glabrata strains were exposed to 20 μg/ml ESP for 1 h prior to protein extraction and HSP70 detection by western blotting. Analysis of band intensities revealed that mean HSP70 protein levels were significantly reduced (P ≤ 0.01) in susceptible snail haemocytes following ESP exposure when compared to controls (Fig. 2a). Resistant snail haemocytes also exhibited attenuated HSP70 levels following ESP challenge, but the effect was less marked (P ≤ 0.05; Fig. 2b). Overall, a 50% decrease in HSP70 was observed (Fig. 2) for schistosome-resistant snail haemocytes, compared to an 80% reduction in haemocytes from the susceptible strain; thus, a 60% difference in HSP70 levels was seen in ESP-challenged haemocytes between the two snail strains.
Fig. 2.
S. mansoni ESPs affect HSP70 protein expression in haemocytes from schistosome-susceptible (a) and schistosome-resistant (b) B. glabrata. Haemocytes were left to adhere to individual wells of culture plates before being washed with CBBS and challenged with 20 μg/ml ESPs in CBSS, or left in CBSS alone (controls), for 1 h. Western blots were probed with anti-HSP70 antibodies (upper panels), then stripped of antibodies before being re-probed with anti-actin antibodies to confirm equal protein loading (lower panels). Intensities of immuno-reactive bands were measured and mean change in HSP70 expression calculated (a and b, graphs), relative to control values having a relative value of 1 (and represented by the dotted line). *P ≤ 0.05, **P ≤ 0.01 when compared to control values (n = 4, ±SEM)
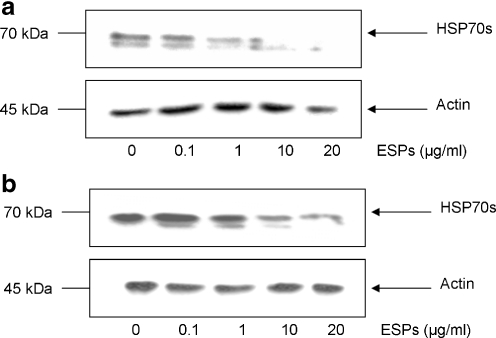
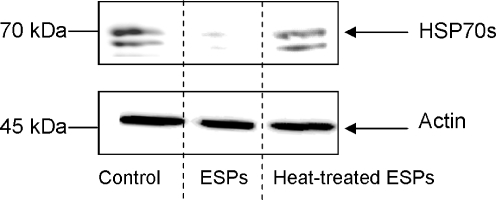
Haemocytes extracted from schistosome-susceptible or schistosome-resistant snails were then challenged with different ESP concentrations (0.1-20 μg/ml) for 1 h in order to determine the dose response of HSP70 protein levels to ESP exposure. Susceptible snail haemocytes showed a general reduction in HSP70 protein when exposed to 1 μg/ml or more ESPs (Fig. 3a). Analysis of blots revealed that 10 μg/ml ESPs decreased HSP70 protein by approximately 80% compared to controls, a 90% reduction was observed with 20 μg/ml ESPs. Haemocytes from resistant snails consistently had decreased HSP70 protein levels following exposure to 10 μg/ml ESPs, but showed the greatest reduction at the highest dose used (20 μg/ml; Fig. 3b). In both snail strains 0.1 μg/ml ESP did not affect HSP70 protein levels. To assess the effect of heat denaturation on the ability of ESPs to modulate haemocyte HSP70 expression levels, ESPs were boiled for 5 min and subsequently cooled before haemocyte exposure. When haemocytes from either snail strain were challenged with these boiled ESPs, no attenuation in HSP expression levels was observed suggesting that the modulatory factor present in the ESPs is likely to be proteinacious in nature (Fig. 4).
Fig. 3.
S. mansoni ESPs affect HSP70 expression in schistosome-susceptible and schistosome-resistant B. glabrata haemocytes in a dose-dependent manner. Susceptible (a) and resistant (b) snail haemocytes were challenged with a range of concentrations of S. mansoni ESPs in CBSS (0-20 μg/ml) for 1 h, prior to protein extraction and HSP70 protein detection (upper panels) using western blotting with anti-HSP70 antibodies. Blots were stripped and re-probed with anti-actin antibodies (lower panels) to confirm equal loading of protein. The blots are representative of two independent experiments
Fig. 4.
Heating of S. mansoni ESPs removes their ability to suppress HSP70 expression in B. glabrata haemocytes. Schistosome-susceptible snail haemocytes were challenged for 1 h with either S. mansoni ESPs (20 μg/ml) in CBSS, ESPs (in CBSS) heated at 90°C for 5 min, or CBSS alone (control). Protein extracts were processed for western blotting with anti-HSP70 antibodies (upper panel) and anti-actin antibodies (lower panel). The blots are representative of two independent experiments
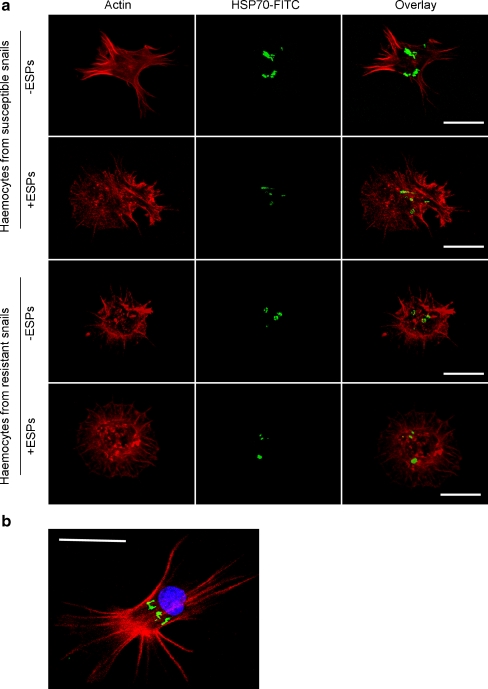
Fluorescence confocal microscopy was used to visualise the general distribution of HSP70 in haemocytes exposed, or not exposed, to 20 μg/ml ESPs for 1 h. Individual haemocytes exhibited varying degrees of HSP70 expression. However, haemocytes from susceptible snails consistently showed a general decrease in HSP70 levels following ESP challenge, while changes in HSP70 expression in haemocytes from the resistant strain were less marked (Fig. 5). In order to identify the cellular location of HSP70, haemocytes were also stained with DAPI, a DNA-binding fluorescent probe; microscopy revealed that HSP70 was consistently located close to the nucleus, but not within (Fig. 5b). Whether HSP70 was present in the cytosol and/or the endoplasmic reticulum or Golgi could not be ascertained.
Fig. 5.
Cellular distribution and levels of HSP70 in B. glabrata haemocytes exposed or not exposed to S. mansoni ESPs. a Schistosome-susceptible and schistosome-resistant snail haemocytes were challenged with (+) and without (−) 20 μg/ml ESPs in CBSS for 1 h on glass coverslips before being fixed and subsequently stained with anti-HSP70 primary antibodies and FITC-conjugated secondary antibodies (represented by green); haemocytes were also incubated in rhodamine phalloidin (red) to visualise filamentous actin. Panel b shows a typical haemocyte not exposed to ESPs, additionally stained with DAPI (blue) to visualise the nucleus. Haemocytes were observed with a Leica laser scanning confocal microscope; images show z-axis projections in average pixel brightness mode that were captured using Leica software. The results shown are characteristic of those obtained in at least three independent experiments (bar 10 μm)
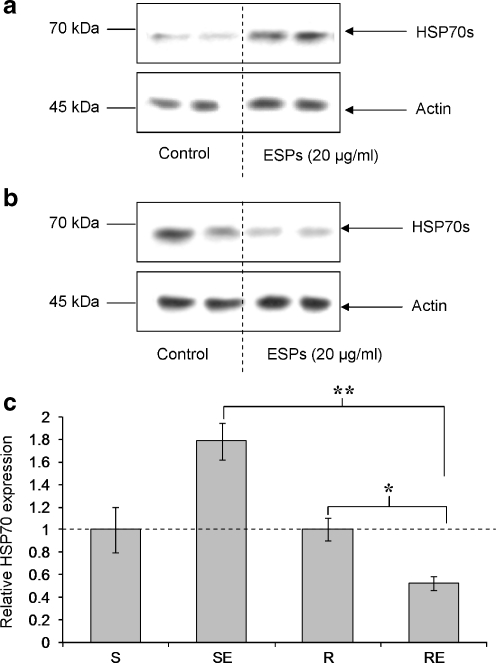
To study further the dynamics of HSP70 expression following ESP exposure, after 1 h ESPs (20 μg/ml) were removed and the challenged haemocytes were allowed to recover for 5 h at RT before protein extraction and HSP70 detection by western blotting. Resistant snail haemocytes exposed to ESPs still exhibited significantly reduced HSP70 protein levels after the 5 h recovery period (P ≤ 0.05; Fig. 6b and c) with a reduction (50%) similar to that observed after 1 h exposure (Fig. 2b). In contrast, ESP-challenged haemocytes from susceptible snails consistently showed a general increase in HSP70 levels (Fig. 6a), although this was not statistically significant (Fig. 6c). Nevertheless, a significant difference in mean haemocyte HSP70 expression was observed between the two snail strains following ESP challenge and 5-h recovery (P ≤ 0.01).
Fig. 6.
HSP70 expression levels recover after 5 h in ESP-exposed haemocytes from schistosome-susceptible B. glabrata only. Susceptible (a) and resistant (b) snail haemocytes were challenged with 20 μg/ml ESPs in CBSS, or CBSS alone (control), for 1 h followed by 5 h recovery period before haemocyte proteins were extracted and processed for western blotting. Blots were probed with anti-HSP70 antibodies (upper panels) before being stripped and re-probed with anti-actin antibodies (lower panels) to confirm equal protein loading. Intensities of immunoreactive bands were measured and mean change in HSP70 expression calculated (c), relative to control values having a relative value of 1 (and represented by the dotted line). *P ≤ 0.05, **P ≤ 0.01 (n = 6 from three independent experiments, ±SEM). S susceptible, SE susceptible snail haemocytes exposed to ESPs, R resistant, RE resistant snail haemocytes exposed ESPs
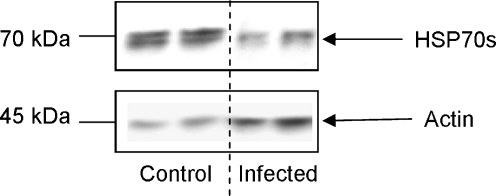
Finally, the effects of S. mansoni infection on B. glabrata haemocyte HSP70 protein levels were determined in vivo. Schistosome-susceptible snails were individually exposed to five miracidia and 35 days post-exposure snails that shed cercariae were killed and their haemolymph collected and haemocyte proteins processed for western blotting. Successful infection, development and reproduction of the parasite were associated with considerably reduced HSP70 protein expression in host haemocytes (Fig. 7); image analysis of blots from duplicate experiments revealed that HSP70 levels were attenuated by approximately 70% in S. mansoni-infected snails compared to uninfected schistosome-susceptible controls.
Fig. 7.
Infection of schistosome-susceptible B. glabrata with S. mansoni reduces HSP70 protein expression in haemocytes. Snails were exposed to miracidia and haemolymph containing haemocytes was extracted and pooled from three infected snails 35 days post-exposure; an equal volume of pooled haemolymph was also obtained from three unexposed snails (control). Haemocytes were then recovered, lysed and proteins processed for western blotting with anti-HSP70 and anti-actin antibodies
HSP70 protein degradation in haemocytes challenged with ESPs
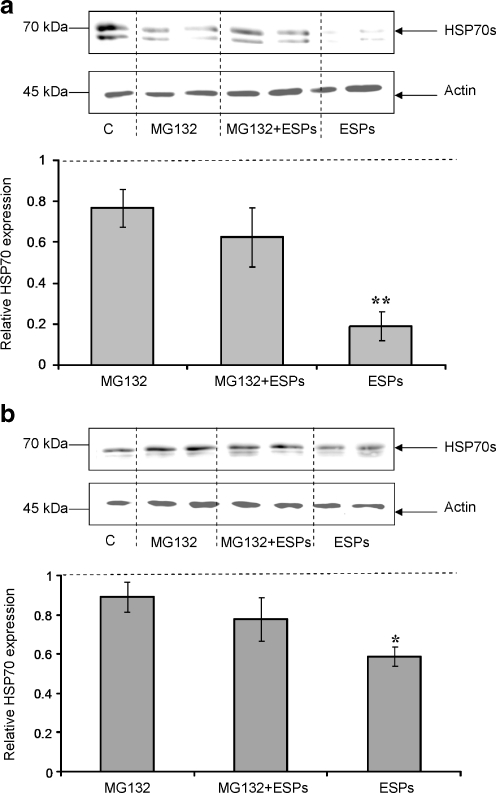
The reduction of HSP70 in B. glabrata haemocytes when challenged with ESPs raised the question as to what was happening to the protein following exposure. The protein could either be transported out of the cell by vesicles or degraded intracellularly by proteasomes (Lancaster and Febbraio 2005; Mambula et al. 2007). Active secretion of HSP70 was investigated by removing the medium from ESP-challenged or unchallenged haemocytes and processing it for gel electrophoresis and western blotting. However, the HSP70 protein could not be detected in the cell medium using this method (data not shown). The protein may have fallen below the detection limit given the larger volume of medium or HSP70 maybe degraded intracellularly by proteasomes or lysosomes. To explore this further, the 26S proteasome-specific inhibitor MG132 was used to inhibit general intracellular protein degradation (Kim et al. 1999). Susceptible and resistant snail haemocytes were exposed to MG132 (50 μM) in the presence and absence of ESPs (20 μg/ml) for 1 h, prior to protein extraction. In the presence of MG132 and ESPs, mean HSP70 levels were sustained, when compared to haemocytes exposed to the inhibitor alone, in haemocytes from either schistosome-susceptible or schistosome-resistant snail strains (Fig. 8). This contrasts with the significant reduction in HSP70 expression observed in haemocytes treated with ESPs in the absence of the inhibitor (P ≤ 0.05; Fig. 8). MG132 alone, slightly reduced mean HSP70 protein levels in haemocytes when compared to levels in haemocytes exposed to DMSO (control); however, the difference observed was not statistically significant (Fig. 8).
Fig. 8.
MG132 reduces the suppressive effects of S. mansoni ESPs on B. glabrata haemocyte HSP70 expression. Schistosome-susceptible (a) and schistosome-resistant (b) snail haemocytes were treated for 1 h with or without 20 μg/ml ESPs in CBSS containing MG132 (50 μM), or with either ESPs alone or DMSO (vehicle control, c). Blots were probed with anti-HSP70 antibodies (upper panel) and re-probed with anti-actin antibodies (lower panel). Intensities of immuno-reactive bands were measured and mean change in HSP70 expression calculated (graphs), relative to DMSO control values having a relative value of 1 (and represented by the dotted line). Blots are characteristic of those from at least three independent experiments. *P ≤ 0.05, **P ≤ 0.01 when compared to unchallenged DMSO controls (n = 4, ±SEM)
HSP70 protein reduction may be regulated by effects of ESPs on ERK signalling
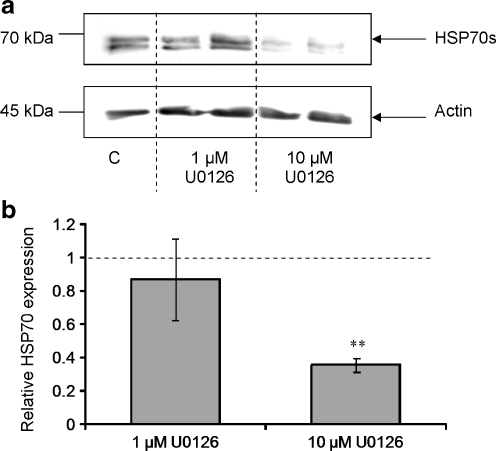
Previous research has shown that S. mansoni larval ESPs differentially affect ERK signalling in B. glabrata haemocytes with ERK attenuation occurring in haemocytes from schistosome-susceptible snails only (Zahoor et al. 2008). It was therefore considered important to establish the effects of ERK pathway inhibition on HSP70 expression in the same snail strains. Susceptible snail haemocytes were incubated for 1 h with the highly selective mitogen-activated protein-ERK kinase (MEK1/2) inhibitor, U0126 (1-10 μM), at RT. Western blotting revealed that treatment of haemocytes with 10 μM U0126 significantly attenuated haemocyte HSP70 expression (Fig. 9a); image analysis of replicate blots demonstrated that HSP70 levels were suppressed by 60%, when compared to DMSO controls (P ≤ 0.01; Fig. 9b). A lower concentration of U0126 (1 μM) was less effective at reducing HSP70 protein levels.
Fig. 9.
Inhibition of ERK signalling suppresses HSP70 expression in B. glabrata haemocytes. Haemocytes from schistosome-susceptible snails were exposed to the MEK inhibitor, U0126 (1 μM or 10 μM) or DMSO vehicle (control) for 1 h prior to protein extraction. Western blots (a) were probed with anti-HSP70 antibodies (upper panel) and anti-actin antibodies (lower panel). Intensities of immuno-reactive bands on blots from three independent experiments were measured and mean HSP70 expression levels calculated (b), with respect to control values represented by 1 and shown as the dotted line. **P ≤ 0.01 when compared to DMSO controls (n = 6, ±SEM)
Discussion
Aquatic organisms exhibit altered HSP expression when exposed to range of stressful conditions including seasonal acclimatisation, climate change (increase and decrease in temperature), competition for space, food availability, wave exposure, heavy metal exposure and laboratory adaptation (Buckley et al. 2001; Franzellitti and Fabbri 2005; Helmuth et al. 2002; Helmuth and Hofmann 2001; Kefaloyianni et al. 2005; Lund et al. 2006; Todgham et al. 2007; Tomanek and Sanford 2003). For molluscs, HSP-like molecules have been identified in the sea slug, Aplysia californica, the oysters, Crassostrea virginica, O. edulis, and Crassostrea gigas, the mussel, Mytilus galloprovincialis, and the clams Tapes philippinarum and Scapharca inaequivalvis (Kuhl et al. 1992; Piano et al. 2002, 2004; Tirard et al. 1995). Furthermore, HSP70 genes have been cloned and characterised in the B. glabrata embryonic (Bge) cell line and in M. galloprovincialis (Kourtidis et al. 2006a, b; Laursen et al. 1997).
In the present study, it is shown that intracellular HSP70 protein levels are attenuated in B. glabrata haemocytes by S. mansoni ESPs, with a greater reduction seen in haemocytes from schistosome-susceptible snails than in those from resistant snails after 1 h ESP exposure. Furthermore, a lower concentration of ESPs mediated this effect in susceptible snail haemocytes than in resistant snail haemocytes; at all doses tested (and for durations up to 5 h) ESP exposure does not affect the viability of haemocytes (Zahoor et al. 2009). These findings are important when considering in vivo interactions where high ESP concentrations should only be encountered by haemocytes in close proximity to the transforming larvae. S. mansoni ESPs are known to affect a range of haemocyte functions, including protein synthesis, especially in schistosome-susceptible snails (Yoshino and Lodes 1988). Studies on mammals and other organisms have shown that HSPs are essential in protein folding and protein transport; therefore, a decrease in intracellular HSP70 protein levels may correlate with a general decrease in protein synthesis (Tavaria et al. 1996).
Of particular note, 5 h after ESP exposure, HSP70 protein levels increased in haemocytes from schistosome-susceptible snails. In contrast, HSP70 levels remained suppressed in ESP-challenged haemocytes from the schistosome-resistant strain. Why S. mansoni ESPs failed to suppress HSP70 protein levels over 5 h in susceptible snail haemocytes is unknown; it is possible that continued attenuation in susceptible snail haemocytes requires the continual presence of ESPs. Although extracellular HSP70 could not be detected in our system after 1 h ESP exposure, HSP70 could potentially be actively secreted from haemocytes, as occurs in other immune cell types (Lancaster and Febbraio 2005); HSP70 has also been shown to exocytose from cardiac cells in the snail, Achatina fulica (Martynova et al. 2007). In mammalian cells, existing HSP70 protein can be removed by two additional mechanisms: active intracellular degradation and release during necrosis (Healy et al. 1992; Lancaster and Febbraio 2005; Mambula et al. 2007). Here, we have found that HSP70 is at least in part degraded intracellularly by proteasomes in response to S. mansoni ESPs. Proteasomes are cellular organelles with protease activities that degrade mature proteins including HSPs (Ciechanover 1994). Co-treatment of haemocytes with the proteasome inhibitor MG132 and ESPs largely restored cellular HSP70 levels. MG132 can increase heat shock factor activity in mammalian cells and thus increase intracellular HSP70 expression (Kim et al. 1999). However, this inhibitor does not induce de novo synthesis of HSP70 protein in B. glabrata haemocytes as HSP70 expression in MG132-treated cells was similar to that in untreated control haemocytes. During infection of B. glabrata by S. mansoni, short-term effects of parasite ESPs on host haemocytes might be considered more relevant to success of infection as the schistosome transforms over several hours to the mother sporocyst stage in susceptible snails, but is usually killed relatively quickly in resistant snails. Interestingly, in susceptible snails infected with S. mansoni for 35 days haemocyte HSP70 protein expression was approximately 70% less than that in haemocytes from susceptible snails not exposed to the parasite. Thus, it appears that S. mansoni infection, development and reproduction leads to a general downregulation of haemocyte HSP70 in vivo.
HSP70 protein is usually localised in the cytoplasm, endoplasmic reticulum, mitochondria or the plastids of cells (Boorstein et al. 1994). Confocal microscopy not only revealed that susceptible snail haemocytes exposed to S. mansoni ESPs showed a general decrease in HSP70 protein, in agreement with the western blotting results, but also highlighted the localisation of HSP70 within cells. In all cases, HSP70 was localised near the nucleus and in some cells was found in association with cortical filamentous actin almost forming a ring around the nucleus.
Haemocytes from the molluscs Haliotis tuberculata and C. gigas exposed to noradrenaline or the α-adrenoceptor agonist, phenylephrine, expressed increased levels of inducible HSP70, which may have been promoted by phospholipase C, protein kinase C or phosphoinositide kinase-3 signalling pathways (Lacoste et al. 2001a, b). Furthermore, a study using zebrafish fibroblast cells demonstrated correlation between increased HSP70 mRNA transcription after heat shock and ERK pathway activation (Keller et al. 2008). Results of the present study are consistent with the ERK cell signalling pathway playing a role in regulating HSP70 production in B. glabrata haemocytes, as ERK pathway inhibition by U0126 resulted in significantly reduced HSP70 protein. S. mansoni ESPs can down-regulate ERK signalling in schistosome-susceptible snail haemocytes, but not in those from the resistant strain (Zahoor et al.2008). Taking the current results into consideration, the ERK pathway seems to be required to maintain basal levels of HSP70 protein. Thus, modulation of ERK by S. mansoni ESPs, particularly in schistosome-susceptible snails, might be relevant to the outcome of infection.
Previously, a number of studies have focused on the expression of HSP70 mRNA during snail-schistosome interactions. Lockyer et al. (2004) demonstrated that production of HSP70 mRNA was up-regulated in schistosome-resistant B. glabrata mantle and brain tissue after in vivo S. mansoni infection using fluorescent-based differential display. The same researchers used a B. glabrata cDNA microarray to confirm that HSP70 mRNA expression was differentially up-regulated within 24 h in resistant S. mansoni-infected snails when compared to the susceptible strain (Lockyer et al. 2008). In contrast, Ittiprasert et al. (2009) demonstrated that juvenile susceptible B. glabrata exposed to S. mansoni miracidia for 24 h had increased HSP70 mRNA, but after 48 h expression levels decreased. Furthermore, resistant and non-susceptible snails presented low or slightly attenuated levels of HSP70 mRNA when challenged with irradiated or non-irradiated S. mansoni miracidia (Ittiprasert et al. 2009). These studies used different techniques, snail tissues and developmental stages of B. glabrata to investigate HSP70 gene expression following infection by schistosomes. Importantly, changes in mRNA expression only suggest possible changes in protein levels or function; in a biological context, characterisation of protein levels is required. Here, we have shown that resistant and susceptible snail haemocytes both display reduced HSP70 protein levels following S. mansoni larval ESP exposure for 1 h; and after 5 h, HSP70 levels begin to increase in ESP-challenged susceptible snail haemocytes only. Moreover, analysis of haemocytes from snails infected by S. mansoni for 35 days revealed that parasitism results in suppressed haemocyte HSP70 levels. Clearly, it is difficult to unravel fully the interaction between the parasite (intact, irradiated, or parasite ESPs) and the haemocytes in respect of HSP70 expression without further study. However, the present work is the first to explore effects of parasite exposure on HSP70 protein levels and thus is important in our quest to understand schistosome-mediated HSP70 modulation in a biological context.
To conclude, the present work has demonstrated that S. mansoni ESPs significantly influence HSP70 protein expression in B. glabrata haemocytes and that this effect can vary over time and between schistosome-susceptible and schistosome-resistant snail strains. Parasite components such as ESPs play an important role in suppressing snail host immune defence mechanisms, mainly by interfering with the physiology and behaviour of host haemocytes (reviewed by Humphries and Yoshino 2003). Our previous study showed that S. mansoni ESPs are capable of attenuating ERK signalling in susceptible snail haemocytes (Zahoor et al. 2008); in the current study, we have found that the inhibition of the ERK pathway also influences HSP70 protein levels in these cells. Interestingly, resistant snail haemocytes also have reduced HSP70 protein levels when challenged with S. mansoni ESPs, even though it has been shown previously that ESPs do not affect ERK activity in these haemocytes (Zahoor et al. 2008). That HSP70 levels in haemocytes from resistant snails were affected less than in susceptible snails may be due to the differential effects of ESPs on ERK signalling. Clearly, HSP70 expression in response to ESPs could also be influenced by the activities of additional cell signalling pathways or through complex interactions of cell signalling pathways yet to be explored. Finally, addressing the important question of whether HSP70 is actively being secreted even at low levels from haemocytes during schistosome infection, and whether such secretion can stimulate haemocyte defence activity, will provide important insights into snail–schistosome interactions and possibly the outcome of infection. Whether or not the parasite has evolved additional strategies to manipulate haemocyte intracellular HSP70 protein expression also warrants investigation.
Acknowledgments
This project was funded by The School of Life Sciences, Kingston University, London. We are indebted to Anne Lockyer of Aberdeen University and the Natural History Museum (NHM), London, for her general advice on the project. We would like to thank Aidan Emery, Mike Anderson and Jayne King also of the NHM for providing S. mansoni-infected mice for this study, Paulusaramge De Saram of Kingston University (KU) for his help in culturing the snails for this study and Suzanne Newton also of KU for supplying control mammalian HC60 cells.
References
- Bayne CJ. Successful parasitism of vector snail Biomphalaria glabrata by the human blood fluke (trematode) Schistosoma mansoni: a 2009 assessment. Mol Biochem Parasitol. 2009;165:8–18. doi: 10.1016/j.molbiopara.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorstein WR, Ziegelhoffer T, Craig EA. Molecular evolution of the HSP70 multigene family. J Mol Evol. 1994;38:1–17. doi: 10.1007/BF00175490. [DOI] [PubMed] [Google Scholar]
- Buckley BA, Owen ME, Hofmann GE. Adjusting the thermostat: The threshold induction temperature for the heat shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol. 2001;204:3571–3579. doi: 10.1242/jeb.204.20.3571. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Clayton ME, Steinmann R, Fent K. Different expression patterns of heat shock proteins hsp 60 and hsp 70 in zebra mussels (Dreissena polymorpha) exposed to copper and tributyltin. Aquat Toxicol. 2000;47:213–226. doi: 10.1016/S0166-445X(99)00022-3. [DOI] [Google Scholar]
- Connors VA, Yoshino TP. In vitro effect of larval Schistosoma mansoni excretory-secretory products on phagocytosis-stimulated superoxide production in hemocytes from Biomphalaria glabrata. J Parasitol. 1990;76:895–902. doi: 10.2307/3282811. [DOI] [PubMed] [Google Scholar]
- Connors VA, Lodes MJ, Yoshino TP. Identification of a Schistosoma mansoni sporocyst excretory-secretory antioxidant molecule and its effect on superoxide production by Biomphalaria glabrata hemocytes. J Invert Pathol. 1991;58:387–395. doi: 10.1016/0022-2011(91)90185-S. [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Fabbri E. Differential HSP70 gene expression in the mediterranean mussel exposed to various stressors. Biochem Biophys Res Comm. 2005;336:1157–1163. doi: 10.1016/j.bbrc.2005.08.244. [DOI] [PubMed] [Google Scholar]
- Guillou F, Roger E, Mone Y, Rogno A, Grunau C, Theron A, Mitta G, Coustau C, Gourbal BE. Excretory-secretory proteome of larval Schistosoma mansoni and Echinostoma caproni, two parasites of Biomphalaria glabrata. Mol Biochem Parasitol. 2007;155:45–56. doi: 10.1016/j.molbiopara.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Healy AM, Mariethoz E, Pizurki L, Polla BS. Heat shock proteins in cellular defense mechanisms and immunity. Ann N Y Acad Sci. 1992;663:319–330. doi: 10.1111/j.1749-6632.1992.tb38675.x. [DOI] [PubMed] [Google Scholar]
- Helmuth BS, Hofmann GE. Microhabitats, thermal heterogeneity, and patterns of physiological stress in the rocky intertidal zone. Biol Bull. 2001;201:374–384. doi: 10.2307/1543615. [DOI] [PubMed] [Google Scholar]
- Helmuth B, Harley CD, Halpin PM, O'Donnell M, Hofmann GE, Blanchette CA. Climate change and latitudinal patterns of intertidal thermal stress. Science. 2002;298:1015–1017. doi: 10.1126/science.1076814. [DOI] [PubMed] [Google Scholar]
- Humbert E, Coustau C. Refractoriness of host haemocytes to parasite immunosuppressive factors as a putative resistance mechanism in the Biomphalaria glabrata-Echinostoma caproni system. Parasitology. 2001;122:651–660. doi: 10.1017/S003118200100782X. [DOI] [PubMed] [Google Scholar]
- Humphries JE, Yoshino TP. Cellular receptors and signal transduction in molluscan hemocytes: connections with the innate immune system of vertebrates. Integ and Comparative Biology. 2003;43:305–312. doi: 10.1093/icb/43.2.305. [DOI] [PubMed] [Google Scholar]
- Humphries JE, Yoshino TP. Regulation of hydrogen peroxide release in circulating hemocytes of the planorbid snail Biomphalaria glabrata. Dev Comp Immunol. 2008;32:554–562. doi: 10.1016/j.dci.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittiprasert W, Nene R, Miller A, Raghavan N, Lewis F, Hodgson J, Knight M. Schistosoma mansoni infection of juvenile Biomphalaria glabrata induces a differential stress response between resistant and susceptible snails. Exp Parasitol. 2009;123:203–211. doi: 10.1016/j.exppara.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Yoshino TP. Analysis of lectin- and snail plasma-binding glycopeptides associated with the tegumental surface of the primary sporocysts of Schistosoma mansoni. Parasitology. 1996;112(Pt 5):469–479. doi: 10.1017/S0031182000076939. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Yoshino TP. Larval Schistosoma mansoni excretory-secretory glycoproteins (ESPs) bind to hemocytes of Biomphalaria glabrata (gastropoda) via surface carbohydrate binding receptors. J Parasitol. 2001;87:786–793. doi: 10.1645/0022-3395(2001)087[0786:LSMESG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kefaloyianni E, Gourgou E, Ferle V, Kotsakis E, Gaitanaki C, Beis I. Acute thermal stress and various heavy metals induce tissue-specific pro- or anti-apoptotic events via the p38-MAPK signal transduction pathway in Mytilus galloprovincialis (lam.) J Exp Biol. 2005;208:4427–4436. doi: 10.1242/jeb.01924. [DOI] [PubMed] [Google Scholar]
- Keller JM, Escara-Wilke JF, Keller ET. Heat stress-induced heat shock protein 70 expression is dependent on ERK activation in zebrafish (Danio rerio) cells. Comp Biochem Physiol A Mol Integr Physiol. 2008;150:307–314. doi: 10.1016/j.cbpa.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim SH, Li GC. Proteasome inhibitors MG132 and lactacystin hyperphosphorylate HSF1 and induce hsp70 and hsp27 expression. Biochem Biophys Res Comm. 1999;254:264–268. doi: 10.1006/bbrc.1998.9840. [DOI] [PubMed] [Google Scholar]
- Kourtidis A, Drosopoulou E, Nikolaidis N, Hatzi VI, Chintiroglou CC, Scouras ZG. Identification of several cytoplasmic HSP70 genes from the mediterranean mussel (Mytilus galloprovincialis) and their long-term evolution in mollusca and metazoa. J Mol Evol. 2006;62:446–459. doi: 10.1007/s00239-005-0121-4. [DOI] [PubMed] [Google Scholar]
- Kourtidis A, Drosopoulou E, Pantzartzi CN, Chintiroglou CC, Scouras ZG. Three new satellite sequences and a mobile element found inside HSP70 introns of the mediterranean mussel (Mytilus galloprovincialis) Genome. 2006;49:1451–1458. doi: 10.1139/G06-111. [DOI] [PubMed] [Google Scholar]
- Kuhl D, Kennedy TE, Barzilai A, Kandel ER. Long-term sensitization training in Aplysia leads to an increase in the expression of BiP, the major protein chaperon of the ER. J Cell Biol. 1992;119:1069–1076. doi: 10.1083/jcb.119.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste A, Cian MC, Cueff A, Poulet SA. Noradrenaline and alpha-adrenergic signaling induce the hsp70 gene promoter in mollusc immune cells. J Cell Sci. 2001;114:3557–3564. doi: 10.1242/jcs.114.19.3557. [DOI] [PubMed] [Google Scholar]
- Lacoste A, Malham SK, Cueff A, Poulet SA. Stress-induced catecholamine changes in the hemolymph of the oyster Crassostrea gigas. Gen Comp Endocrinol. 2001;122:181–188. doi: 10.1006/gcen.2001.7629. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- Laursen JR, Liu H, Wu XJ, Yoshino TP. Heat-shock response in a molluscan cell line: characterization of the response and cloning of an inducible HSP70 cDNA. J Invert Pathol. 1997;70:226–233. doi: 10.1006/jipa.1997.4686. [DOI] [PubMed] [Google Scholar]
- Lilja T, Aihara H, Stabell M, Nibu Y, Mannervik M. The acetyltransferase activity of Drosophila CBP is dispensable for regulation of the dpp pathway in the early embryo. Dev Biol. 2007;305:650–658. doi: 10.1016/j.ydbio.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Lockyer AE, Noble LR, Rollinson D, Jones CS. Schistosoma mansoni: resistant specific infection-induced gene expression in Biomphalaria glabrata identified by fluorescent-based differential display. Exp Parasitol. 2004;107:97–104. doi: 10.1016/j.exppara.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Lockyer AE, Spinks J, Kane RA, Hoffmann KF, Fitzpatrick JM, Rollinson D, Noble LR, Jones CS. Biomphalaria glabrata transcriptome: cDNA microarray profiling identifies resistant and susceptible-specific gene expression in haemocytes from snail strains exposed to Schistosoma mansoni. BMC Genomics. 2008;9:634. doi: 10.1186/1471-2164-9-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes MJ, Yoshino TP. Characterization of excretory-secretory proteins synthesized in vitro by Schistosoma mansoni primary sporocysts. J Parasitol. 1989;75:853–862. doi: 10.2307/3282863. [DOI] [PubMed] [Google Scholar]
- Lodes MJ, Yoshino TP. The effect of schistosome excretory-secretory products on Biomphalaria glabrata hemocyte motility. J Invert Pathol. 1990;56:75–85. doi: 10.1016/0022-2011(90)90147-X. [DOI] [PubMed] [Google Scholar]
- Loker ES, Cimino DF, Hertel LA. Excretory-secretory products of Echinostoma paraensei sporocysts mediate interference with Biomphalaria glabrata hemocyte functions. J Parasitol. 1992;78:104–115. doi: 10.2307/3283696. [DOI] [PubMed] [Google Scholar]
- Lund SG, Ruberte MR, Hofmann GE. Turning up the heat: the effects of thermal acclimation on the kinetics of hsp70 gene expression in the eurythermal goby, Gillichthys mirabilis. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:435–446. doi: 10.1016/j.cbpa.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Mager WH, Kruijff AJ. Stress-induced transcriptional activation. Microbiol Rev. 1995;59:506–531. doi: 10.1128/mr.59.3.506-531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager WH, Ferreira PM. Stress response of yeast. Biochem J. 1993;290:1–13. doi: 10.1042/bj2900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43:168–175. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynova MG, Bystrova OA, Shabelnikov SV, Margulis BA, Prokofjeva DS. Hsp70 in the atrial neuroendocrine units of the snail, Achatina fulica. Cell Biol Int. 2007;31:413–419. doi: 10.1016/j.cellbi.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Matranga V, Toia G, Bonaventura R, Muller WE. Cellular and biochemical responses to environmental and experimentally induced stress in sea urchin coelomocytes. Cell Stress Chaperones. 2000;5:113–120. doi: 10.1379/1466-1268(2000)005<0113:CABRTE>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Piano A, Asirelli C, Caselli F, Fabbri E. Hsp70 expression in thermally stressed Ostrea edulis, a commercially important oyster in Europe. Cell Stress Chaperones. 2002;7:250–257. doi: 10.1379/1466-1268(2002)007<0250:HEITSO>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano A, Valbonesi P, Fabbri E. Expression of cytoprotective proteins, heat shock protein 70 and metallothioneins, in tissues of Ostrea edulis exposed to heat and heavy metals. Cell Stress Chaperones. 2004;9:134–142. doi: 10.1379/483.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plows LD, Cook RT, Davies AJ, Walker AJ. Activation of extracellular-signal regulated kinase is required for phagocytosis by Lymnaea stagnalis haemocytes. Biochim Biophys Acta. 2004;1692:25–33. doi: 10.1016/S0167-4889(04)00042-4. [DOI] [PubMed] [Google Scholar]
- Plows LD, Cook RT, Davies AJ, Walker AJ. Carbohydrates that mimic schistosome surface coat components affect ERK and PKC signalling in Lymnaea stagnalis haemocytes. Int J Parasitol. 2005;35:293–302. doi: 10.1016/j.ijpara.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Roger E, Gourbal B, Grunau C, Pierce RJ, Galinier R, Mitta G. Expression analysis of highly polymorphic mucin proteins (sm PoMuc) from the parasite Schistosoma mansoni. Mol Biochem Parasitol. 2008;157:217–227. doi: 10.1016/j.molbiopara.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Smith GB, Umbach JA, Hirano A, Gundersen CB. Interaction between constitutively expressed heat shock protein, hsc 70, and cysteine string protein is important for cortical granule exocytosis in Xenopus oocytes. J Biol Chem. 2005;280:32669–32675. doi: 10.1074/jbc.M501806200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:AHSGTT>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirard CT, Grossfeld RM, Levine JF, Stroskopf SK. Effect of hyperthermia in vitro on stress protein synthesis and accumulation in oyster haemocytes. Fish Shellfish Immunol. 1995;5:9–25. doi: 10.1016/S1050-4648(05)80003-8. [DOI] [Google Scholar]
- Todgham AE, Hoaglund EA, Hofmann GE. Is cold the new hot? Elevated ubiquitin-conjugated protein levels in tissues of antarctic fish as evidence for cold-denaturation of proteins in vivo. J Comp Physiol. 2007;177:857–866. doi: 10.1007/s00360-007-0183-2. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Sanford E. Heat-shock protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (genus: Tegula) Biol Bull. 2003;205:276–284. doi: 10.2307/1543291. [DOI] [PubMed] [Google Scholar]
- Eden W, Zee R, Prakken B. Heat shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007;1113:217–237. doi: 10.1196/annals.1391.020. [DOI] [PubMed] [Google Scholar]
- Walker AJ. Do trematode parasites disrupt defence cell signalling in their snail hosts? Trends Parasitol. 2006;22:154–159. doi: 10.1016/j.pt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Wright B, Lacchini AH, Davies AJ, Walker AJ. Regulation of nitric oxide production in snail (Lymnaea stagnalis) defence cells: a role for PKC and ERK signalling pathways. Biol Cell. 2006;98:265–278. doi: 10.1042/BC20050066. [DOI] [PubMed] [Google Scholar]
- Wu X, Sabat G, Brown JF, Zhang M, Taft A, Peterson N, Harms A, Yoshino TP. Proteomic analysis of Schistosoma mansoni proteins released during in vitro miracidium-to-sporocyst transformation. Mol Biochem Parasitol. 2009;164:32–44. doi: 10.1016/j.molbiopara.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino TP, Lodes MJ. Secretory protein biosynthesis in snail hemocytes: in vitro modulation by larval schistosome excretory-secretory products. J Parasitol. 1988;74:538–547. doi: 10.2307/3282169. [DOI] [PubMed] [Google Scholar]
- Yoshino TP, Wu XJ, Liu HD. Transfection and heat-inducible expression of molluscan promoter-luciferase reporter gene constructs in the Biomphalaria glabrata embryonic snail cell line. Am J Trop Med Hyg. 1998;59:414–420. doi: 10.4269/ajtmh.1998.59.414. [DOI] [PubMed] [Google Scholar]
- Yura T, Nagai H, Mori H. Regulation of the heat shock response in bacteria. Annu Rev Microbiol. 1993;47:321–350. doi: 10.1146/annurev.mi.47.100193.001541. [DOI] [PubMed] [Google Scholar]
- Zahoor Z, Davies AJ, Kirk RS, Rollinson D, Walker AJ. Disruption of ERK signalling in Biomphalaria glabrata defence cells by Schistosoma mansoni: implications for parasite survival in the snail host. Dev Comp Immunol. 2008;32:1561–1571. doi: 10.1016/j.dci.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Zahoor Z, Davies AJ, Kirk RS, Rollinson D, Walker AJ. Nitric oxide production by Biomphalaria glabrata haemocytes: effects of Schistosoma mansoni ESPs and regulation through the extracellular signal-regulated kinase pathway. Parasites Vectors. 2009;2(18):1–10. doi: 10.1186/1756-3305-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelck UE, Janowsky B. Antioxidant enzymes in intramolluscan Schistosoma mansoni and ROS-induced changes in expression. Parasitology. 2004;128:493–501. doi: 10.1017/S0031182004004895. [DOI] [PubMed] [Google Scholar]