Abstract
To investigate whether individuals’ ongoing neuronal activity at resting state can affect their response to brain stimulation, fMRI BOLD signals were imaged from the human visual cortex of fifteen healthy subjects in the absence and presence of visual stimulation. It was found that the temporal correlation strength but not amplitude of baseline BOLD signal fluctuations acquired under the eyes-fixed condition is positively correlated with the amplitude of stimulus-evoked BOLD responses across subjects. Moreover, the spatiotemporal correlations of baseline BOLD signals imply a coherent network covering the visual system, which is topographically indistinguishable from the “resting-state visual network” observed under the eyes-closed condition. The overall findings suggest that the synchronization of ongoing brain activity plays an important role in determining stimulus-evoked brain activity even at an early stage of the sensory system. The tight relationship between baseline BOLD correlation and stimulus-evoked BOLD amplitude provides an essential basis for understanding and interpreting the large inter-subject BOLD variability commonly observed in numerous fMRI studies and potentially for improving group fMRI analysis. This study highlights the importance to integrate the information from both resting-state coherent networks and task-evoked neural responses for a better understanding of how the brain functions.
Keywords: functional MRI (fMRI), BOLD, resting-state fMRI, functional connectivity, ongoing brain activity, resting brain
Introduction
In the past two decades, the functional magnetic resonance imaging (fMRI) technique based on the blood oxygenation level dependent (BOLD) contrast (Bandettini et al., 1992; Kwong et al., 1992; Ogawa et al., 1990; Ogawa et al., 1992) has been widely applied to noninvasive imaging of brain activity in numerous neuroscience and psychology studies. However, a major challenge in fMRI application is the large variability in the magnitude, shape, and location of stimulus-evoked BOLD responses commonly observed within or between subjects (White et al., 2001). Such large intra- and inter-subject variations make one difficult to quantify fMRI data and interpret outcomes, and usually demand a large sample size for reaching a statistical significance level, particularly for comparative studies.
Many factors have been suggested to account for the large variability of stimulus-evoked BOLD responses, and most of them are from non-neural sources, such as the hematocrit level (Gustard et al., 2003), venous blood oxygenation level (Lu et al., 2008), vasculature (D'Esposito et al., 2003), and motion artifacts (Lund et al., 2005). This is probably because the fMRI BOLD contrast is mainly based on hemodynamic responses to brain activity changes (Ogawa et al., 1998). Nevertheless, possible contributions from neural-related factors should not be neglected. Previous electrophysiology studies have demonstrated that ongoing brain activity prior to brain stimulation may have a significant influence on stimulus-evoked brain responses (Arieli et al., 1996; Makeig et al., 2002). Therefore, the variability of ongoing brain activity could be another factor possibly relating to the variability of stimulus-evoked brain responses, including those measured with fMRI BOLD signal.
A number of recent fMRI studies have shown that baseline BOLD signals acquired in the resting state fluctuate slowly and coherently within a variety of anatomically connected and functionally specific brain networks (Biswal et al., 1995; Fox and Raichle, 2007). It has been suggested that such baseline BOLD fluctuations could result from ongoing brain activity and their spatiotemporalcorrelations reflect functional connectivity between different brain regions (Biswal et al., 1995; Fox and Raichle, 2007). Moreover, several fMRI studies have demonstrated that ongoing brain activity, reflected by pre-stimulus baseline BOLD signals, can influence behavioral responses of human subjects to external stimuli (Boly et al., 2007; Fox et al., 2007; Hesselmann et al., 2008a; Hesselmann et al., 2008b; Sapir et al., 2005). These interesting findings not only suggest an interaction between the ongoing brain activity and evoked brain responses at a behavioral level, but also demonstrate the feasibility of measuring ongoing brain activity noninvasively using the BOLD-based fMRI technique. It would be essential to examine and quantify the relationship between ongoing and evoked brain activities, if it exists, at a more mechanistic level.
The purpose of this study is to quantitatively investigate the interaction between ongoing and evoked brain activity, and more specifically to examine the relationship between baseline BOLD signal fluctuation and stimulus-evoked BOLD response in the human visual cortex using fMRI. Our working hypothesis is that individuals’ baseline BOLD fluctuation could influence the amplitude of their evoked BOLD responses to identical brain stimulation and their relationship is one of the major factors responsible for the large inter-subject variability in the stimulus-evoked BOLD amplitudes. To test this hypothesis, BOLD signals were imaged from the human visual cortex according to a two-stage paradigm design (see Fig. 1A). The temporal correlation strength and fluctuation magnitude of baseline BOLD signals acquired during the control stage (i.e., the eyes-fixed condition in the absence of visual stimulation) were used to quantify ongoing brain activity, while the amplitude of evoked BOLD responses acquired during the block-design stage (i.e., the stimulus was presented according to the conventional block-design paradigm) was used to quantify evoked brain activity. Their relationships were then examined through a regression analysis to test the hypothesis. In addition, the baseline BOLD signals were also imaged under the eyes-closed condition for a subgroup of subjects and compared with those acquired under the eyes-fixed condition.
Fig. 1.
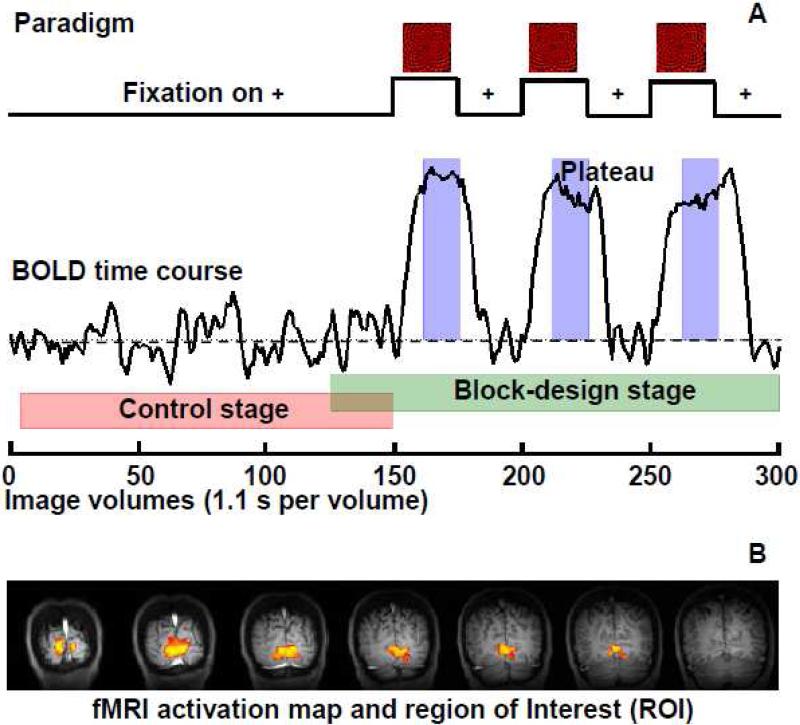
Standard experimental paradigm and an example of ROI selection. (A) The experimental paradigm for a standard fMRI run with the control (red shadow), block-design (green shadow), and BOLD plateau (blue shadow) stages being marked; (B) An example of functional activation maps generated based on the block-design stage data from a single fMRI run of a representative participant (Subject 1). The statistical threshold was adjusted to show the most activated (~10% of) brain regions, which defined the ROI utilized for quantification.
Materials and Methods
Participants
Sixteen volunteers (6 males and 10 females; age: 28 ± 13 years) participated in this study with written informed consent proved by the Institutional Review Board of the University of Minnesota. All of them were healthy without histories of neurological or psychiatric diseases. One subject was excluded from the final data analysis because of large head motion during MRI data acquisition; therefore the results from fifteen subjects were summarized and presented herein.
Stimuli and Experimental Paradigm
A full-screen (30° × 23° visual angle) red-black checkerboard (spatial frequency 1.5° per cycle) visual stimulus flashing at 8 Hz, with a small white cross in the screen center for eyes fixation, was used to activate visual cortices. The stimulus was back-projected onto a screen at a viewing distance of ~ 35 cm. A control image with only the small white cross on a black and uniform background was used for eyes fixation when the visual stimulus was off.
Each standard fMRI run consists of 300 image volumes with a total of 330 seconds of acquisition time. During each run, the visual stimulus was presented to volunteers interleaved with the control image (a white cross on a black background) according to the experimental paradigm shown in Fig. 1A: a long control block (150 fMRI volumes, 165 seconds) followed by six short blocks (25 volumes, 27.5 seconds each) switching between stimulus and control conditions. The first 150 and last 175 image volumes (25 overlapped volumes between them) were regarded as the control (Fig. 1A, red shadow) and block-design (Fig. 1A, green shadow) stages, respectively; while the 160~175th, 210~225th, and 260~275th image volumes were assigned to the stimulus-evoked BOLD plateau stage (Fig. 1A, blue shadow).
For a subgroup of five subjects, besides the standard fMRI runs, two additional fMRI runs were also acquired under the eyes-closed condition, during which the subjects were instructed to close their eyes and refrain from cognitive, language, and motor tasks as much as possible, but not to fall asleep. Each eyes-closed fMRI run consisted of 150 image volumes.
MRI Data Acquisition
All MRI experiments were performed on a 4 Tesla 90 cm bore human magnet (Oxford, UK) interfaced with the Varian INOVA console (Varian Inc., Palo Alto, CA). A single-loop radiofrequency (RF) surface coil (10 cm in diameter) was applied to detect brain MRI signals mainly from the occipital and parietal lobes and cerebrum for achieving high detection sensitivity.
At the beginning of each experiment, the T1-weighted TurboFLASH MRI method (Haase, 1990) was used to acquire a set of anatomical images in transversal, sagittal, and coronal orientations with the following parameters: field of view (FOV) = 20 × 20 cm2; repetition time (TR) = 3 s; 128 × 128 image matrix size; and slice thickness = 5 mm. For the fMRI experiments, seven consecutive coronal gradient-echo echo-planar image (GE-EPI (Mansfield, 1977)) slices were acquired (FOV = 20 × 20 cm2; TR/TE = 1100/30 ms; 64 × 64 matrix size; 5 mm slice thickness; and a nominal excitation pulse flip angle of ≈ 45°) to cover the entire calcarine fissure, with reference to the anatomical images. Five dummy scans were also added to the beginning of GE-EPI data acquisition to avoid the transient BOLD signal change at the initial acquisition period.
During fMRI data acquisition, the magnet room was kept dark and the only light source was the projector sitting in the next room. Before each standard fMRI run, the importance of eyes fixation on the white cross and minimizing head motion was re-emphasized to subjects. Four to seven standard fMRI runs were acquired for each subject and two additional eyes-closed fMRI runs were acquired for a subgroup of five subjects.
Preprocessing and Analysis of fMRI Data
Motion correction was performed on all fMRI data using the 3D registration tool (3dvolreg) of AFNI (Cox, 1996), and fMRI runs with large head motion (> 3 mm at any direction) were excluded from further analysis. All images were then spatially smoothened with a Gaussian kernel with 6 mm full width at half maximum (FWHM). The first five GE-EPI image volumes were excluded to further eliminate the transient BOLD signal complication at the initial GE-EPI acquisition period.
For each standard fMRI run, functional activation maps were generated by cross-correlating the block-design stage data (126th to 300th image volumes) with the convolution of the task paradigm and canonical hemodynamic response function (Bandettini et al., 1993). Then, based on each functional activation map, a corresponding region of interest (ROI) was drawn automatically based on three criteria: 1) to only include the activated fMRI voxels reaching a statistical significance: having high correlation with the task paradigm with a p value of < 0.05 (corrected for multiple comparison with Bonferroni correction); 2) to exclude the voxels showing an extremely large BOLD percentage increase of >15% in response to the visual stimulation, thus, to reduce the large vessel BOLD contamination; 3) to limit the total number of the activated voxels for ensuring that the ROI size was 10% of the total brain region imaged by seven fMRI slices and the selected ROI mainly covered the calcarine fissure (see an example shown in Fig. 1B). We did not use a fixed statistical threshold (p value) for determining the ROI, because it could result in considerable variations in both ROI size and large vessel BOLD contribution across subjects due to the nature of large inter-subject variability in the stimulus-evoked BOLD responses. The different ROI size over different subjects may significantly affect the quantification of evoked BOLD amplitude and lead to underestimation of the inter-subject variation. We neither applied a fixed number of activated fMRI voxels to determine the ROI, because the size of the brain as well as of the calcarine fissure also varies across subjects, even though to a much lesser extent than the evoked BOLD response. Instead, we used a fixed proportion of the brain volume we imaged, which can assure the consistence of the selected ROI size with the consideration of the inter-subject brain size variation (418 ± 58 voxels, Mean ± SD).
For each standard fMRI run acquired under the eyes-fixed condition, we calculated one quantity to quantify the amplitude of stimulus-evoked BOLD response and two quantities to quantify the magnitude and correlation strength of baseline BOLD signal fluctuation. For each fMRI run acquired under the eyes-closed condition for a subgroup of five subjects, two quantities were calculated to quantify the magnitude and correlation strength of baseline BOLD fluctuation.
To obtain the quantity of the stimulus-evoked BOLD amplitude, the mean fMRI signal averaged within the stimulus-evoked BOLD plateau stage was divided by the mean fMRI signal of the control stage for each voxel to generate a map of percentage BOLD increases, whose values were then averaged within the corresponding ROI to give the quantity.
To quantify the magnitude and correlation strength of baseline BOLD fluctuation under eyes-fixed (or eyes-closed) conditions, the fMRI signal time course acquired during the control stage of the standard fMRI run (or the eyes-closed fMRI run) were first normalized by their means and band-pass filtered (0.005~0.1 Hz) in the frequency domain to remove the DC component and very slow drift and to reduce the possible fluctuations induced by cardiac and respiratory pulsations. Next, the standard deviation (SD) of BOLD time course was calculated for each fMRI voxels to generate a SD map, and the average of the SD values within the ROI presents the magnitude of baseline BOLD fluctuation under the eyes-fixed (or eyes-closed condition). In addition, the mean of the correlation coefficients (CCs) of any pair of voxels within the corresponding ROI was calculated to quantify the correlation strength of baseline BOLD fluctuation under the eyes-fixed (or eyes-closed) condition.
For the quantification based on the standard fMRI run, the ROIs were determined for each run. While for the quantification based on the eyes-closed MRI run, which did not include the block-design stage, the ROI was therefore determined based on the averaged fMRI activation map across multiple runs of the same subject; and it is to some extent an average of the single-run ROIs, which showed small variation across multiple runs for the same subject.
A group-based regression analysis was employed instead of individual-based analysis to examine the relationships between these quantities, since it is advantageous to utilize the large inter-subject variability to increase the dynamic range of evoked BOLD responses.
Correlation Maps and Independent Component Analysis (ICA)
Correlation maps were generated based on the GE-EPI data acquired during the control stage (eyes-fixed) and block-design stage of the standard fMRI runs, as well as under the eyes-closed condition. The most activated 2-voxel × 2-voxel region was selected first, based on the averaged functional activation map in response to visual stimulation, as the reference region. For each GE-EPI dataset, then, the signal time courses of all image voxels (after preprocessing) were correlated with the averaged signal time course extracted from the reference region to create a correlation map.
Spatial Independent Component Analysis (sICA) was also performed on the same fMRI datasets including seven coronal slices of GE-EPI images covering the visual cortex. FastICA (Hyvarinen, 1999), a fixed-point ICA algorithm, was implemented on the data by using a MATLAB package downloaded from http://www.cis.hut.fi/projects/ica/fastica/. A total of 30 components, which can account for more than 90% of original variance, were decomposed for each dataset. The meaningful components, which show certain spatial pattern but are not obvious artifacts, were extracted by visual inspection. There was usually only one component (out of 30 components) showing a “meaningful” spatial pattern, which is similar to the correlation map based on the reference (or seeding) analysis approach.
All data were analyzed using MATLAB7.5 (MathWorks, Natick, MA). A p value of < 0.05 was considered statistically significant.
Results
Baseline BOLD Fluctuation versus Evoked BOLD Response
Figure 1B illustrates a typical functional activation map from a representative subject (Subject 1), which was generated based on the fMRI data (single run) acquired during the block-design stage. The activated brain regions in the primary visual cortex represent the region of interest (ROI) used for data quantification. All subjects showed robust activations in the visual cortex, and the functional activation maps and corresponding ROIs were highly consistent across multiple fMRI runs for the same subjects.
For each fMRI run, the correlation strength and fluctuation magnitude of the control stage BOLD signals and the amplitude of the plateau BOLD signals acquired during the block-design stage (see Fig. 1A) were quantified within the corresponding ROI. These three quantities were then averaged over multiple runs for the same subject and their relationships were examined through a linear regression analysis across all subjects. Figure 2 summarizes the results.
Fig. 2.
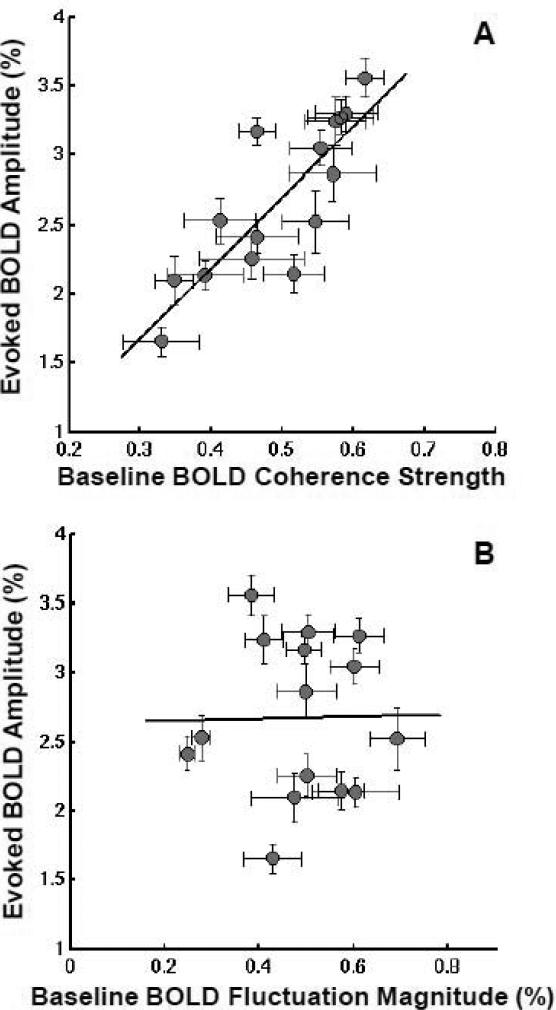
Scatter plots showing, for each subject, the amplitude of stimulus-evoked BOLD responses versus (A) the correlation strength and (B) fluctuation magnitude of baseline BOLD signals. Each gray circle in the plots represents the data from one subject with error bars standing for standard errors across multiple fMRI runs. The solid lines show the best linear fitting of experimental data (n=15) using the least-square regression method: R2 = 0.69 and p = 1.2 × 10-4 for (A); R2 = 0.0001 and p = 0.97 for (B).
Figure 2A shows a significant, positive correlation (R2 = 0.69, p = 1.2 × 10-4, n = 15) between the amplitude of subjects’ evoked BOLD responses and the correlation strength of their baseline BOLD fluctuations. The subjects with more synchronized baseline BOLD fluctuations showed stronger evoked BOLD responses to the same visual stimulus, and vice versa. Such a correlation is still statistically significant (R2 = 0.37, p = 1.1 × 10-9) if the regression analysis was based on pooled single-fMRI-run quantities without averaging them within the same subject (see Fig. S1B).
In contrast, no statistically significant correlation (R2 = 0.0001, p = 0.97, n = 15) was found between the magnitude of baseline BOLD fluctuation and the amplitude of stimulus-evoked BOLD response (Fig. 2B) in the present study. It suggests that the large inter-subject variability in evoked BOLD response observed in the present study may not be explained by the difference in subjects’ vasculature within the selected ROI. Moreover, the correlation strength and fluctuation magnitude of baseline BOLD signals were found to be not correlated with each other either (R2 = 0.053, p = 0.41, n = 15) based on the ROI analysis.
BOLD Coherent Networks under Eyes-Fixed and Eyes-Closed Conditions
Spatiotemporal correlations of baseline BOLD fluctuations have been found to imply many “resting-state brain networks” including the one covering the visual cortex (Cordes et al., 2000; Greicius et al., 2003; Hampson et al., 2002; Lowe et al., 1998; Stein et al., 2000; Vincent et al., 2007). However, the resting-state visual network was usually identified using the fMRI BOLD signals acquired under the eyes-closed condition (Lowe et al., 1998; Mansfield, 1977). In contrast, the baseline BOLD signals in the present study were acquired when the subjects fixed their eyes on a small cross on a black background: a common control condition for most fMRI applications. To compare baseline BOLD fluctuations and their associated coherent networks in the human visual cortex under these two conditions, additional fMRI BOLD measurements under the eyes-closed condition were included for a subgroup of five subjects (Subject 11 to Subject 15).
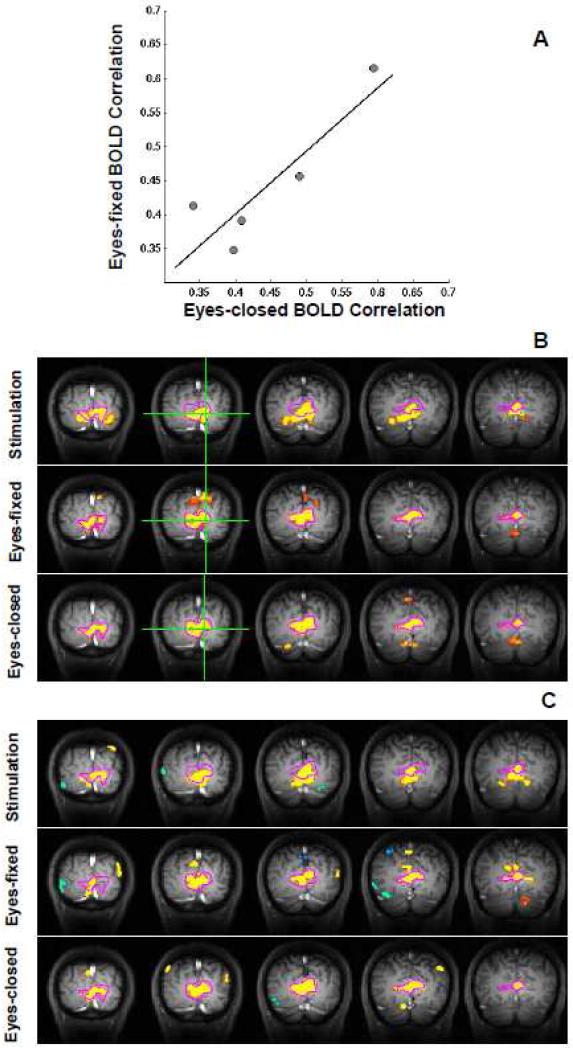
Figure 3A shows a positive correlation (R2 = 0.78, p = 0.046, n = 5) between the correlation strengths of baseline BOLD fluctuations measured under eyes-fixed and eyes-closed conditions. This result indicates a close relationship between these two baseline brain states, and this notion is further supported by correlation maps showing the spatiotemporal correlations of BOLD signals as discussed below.
Fig. 3.
Comparison of BOLD correlation strength and correlation maps between the eyes-fixed and eyes-closed conditions. (A) A scatter plot showing, for each subject, the correlation strength of baseline BOLD fluctuations under these two conditions suggests a significant association between them (R2 = 0.78, p = 0.046, n=5). (B) Correlation maps from a representative participant (Subject 12) under the eyes-fixed (middle) and eyes-closed (bottom) conditions indicate two topographically undistinguishable coherent networks, which are different from the one during stimulation (top). These maps were generated with respect to a reference region (green cross), and their statistical thresholds were adjusted to show the same amount of voxels for better comparison of spatial pattern. (C) Independent components extracted from the same dataset using sICA method confirm the results of correlation analysis. All maps shown in this figure were averaged over multiple fMRI runs. The contour of the correlation map under the eyes-closed condition (the bottom of B) was outlined with magenta color and overlapped on all other maps.
The correlation maps were generated based on the BOLD signals acquired during visual stimulation (the block-design stage), under the eyes-fixed (the control stage depicted in Fig. 1A) and eyes-closed conditions (additional fMRI run), respectively. Figure 3B compares BOLD correlation maps (five coronal images) from a representative subject (Subject 12). The BOLD correlation map during visual stimulation mainly covers the activated brain regions showing evoked responses to the visual stimulus, and it is almost identical to the conventional fMRI activation map (not shown in Fig. 3) as expected. The BOLD correlation maps under the eyes-fixed and eyes-closed conditions show almost identical patterns, which are substantially different from, even though largely overlapped with, the BOLD correlation map obtained during visual stimulation. The major difference lies in the cuneus gyrus region, which is clearly one part of the visual network obtained under either the eyes-fixed or eyes-closed condition but did not show an evoked BOLD response to the full-screen visual stimulus. This observation is consistent across all five subjects (see Fig. S2).
To further confirm the above observation, the spatial independent component analysis (sICA) was also performed on the same fMRI data to independently examine spatial coherent patterns of BOLD signals. The meaningful independent components (ICs) extracted under the three conditions (Fig. 3C) resembled the pattern of the corresponding BOLD correlation maps (Fig. 3B), supporting the results of the seed-based correlation analysis.
The similarity between the baseline BOLD signal fluctuations measured under the eyes-fixed and eyes-closed conditions suggests that the relationship shown in Fig. 2A could be further extended from the eyes-fixed (control) condition to the eyes-closed (resting-state) condition.
Discussion
Baseline BOLD Correlation Accounts for Large Inter-Subject Variability in Evoked BOLD Amplitude
Many factors have been shown to possibly contribute to the large inter-subject variability in hemodynamic responses during brain activation (D'Esposito et al., 2003; Gustard et al., 2003; Lu et al., 2008; Lund et al., 2005); a number of approaches, including spatial smoothing and motion correction, were applied in the present study to minimize possible variability induced by some of non-neural factors (Lund et al., 2005; White et al., 2001). Even so, the stimulus-evoked BOLD amplitude still exhibited considerably large variation across subjects, so did the baseline BOLD correlation strength. Interestingly, a significant association was found between these two BOLD measurements, which suggests that the inter-subject variability in the evoked BOLD amplitude can, at least substantially, be attributed to the large variation of baseline BOLD correlation across subjects.
In contrast to the large inter-subject variability, both baseline BOLD correlation and stimulus-evoked BOLD amplitude are relatively stable across multiple fMRI runs within the same subject and this is evident by the small error bars shown in Fig. 2. This result is consistent with previous observations that the intra-subject variability of both evoked BOLD responses (Aguirre et al., 1998) and baseline BOLD correlations (Zhang et al., 2007) is smaller than their inter-subject variability. For this reason, the relationship between the baseline BOLD correlation and evoked BOLD amplitude is not obvious based only on single-subject data (Fig. S1A), except for a few subjects (e.g., Subject 8 and Subject 11, Fig. S1A) showing relatively large inter-run variability in both evoked BOLD response and baseline BOLD correlation strength.
Coherent Networks Implied by Baseline BOLD Fluctuations under Eyes-Fixed and Eyes-Closed Conditions
Most studies on baseline BOLD fluctuations are conducted during a resting-state with eyes either fixed on a cross or closed (Fox and Raichle, 2007), and a number of resting-state networks have been found to remain similar under these two baseline conditions (Fox et al., 2005). Nevertheless, a few studies (Bianciardi et al., 2009a; Yang et al., 2007) have suggested that the magnitude and correlation of baseline BOLD fluctuations in the visual cortex might be different between the eyes-fixed and eyes-closed conditions. It is therefore interesting to examine this aspect quantitatively.
Our parallel correlation analyses on the baseline BOLD signals acquired under the eyes-fixed and eyes-closed conditions showed two spatially indistinguishable coherent networks mainly covering the visual cortex. They are, however, not completely overlapped with the cortical regions showing evoked BOLD responses to a full-screen visual stimulus (Fig. 3). The main difference is in the cuneus gyrus region, which belongs to visual association areas involved in the dorsal pathway and high-level visual functions and was not activated by the reversal checkerboard stimuli with full coverage of visual field. Furthermore, quantitative analysis also suggests a correlation between the correlation strengths within the visual coherent network under these two conditions (Fig. 3A). These results suggest a similar neurophysiologic basis for the baseline coherent networks under the eyes-fixed and eyes-closed conditions.
Interaction between Ongoing Brain Activity and Evoked Brain Response
The coherent visual network as observed in the present study is just one of many “resting-state networks” mapped through baseline BOLD fluctuations. Although the exact mechanism underlying the baseline BOLD fluctuation remains elusive, several studies have linked it to spontaneous neural activity (Feige et al., 2005; Goldman et al., 2002; Liu et al., 2010; Mantini et al., 2007; Shmuel and Leopold, 2008).
More interestingly, recent fMRI studies have demonstrated how human behaviors could be influenced by pre-stimulus baseline BOLD signals acquired under the eyes-fixed condition (the same as in the present study) (Boly et al., 2007; Fox et al., 2007; Hesselmann et al., 2008a; Hesselmann et al., 2008b; Sapir et al., 2005). These studies have shown, for example, that differences in the pre-stimulus BOLD signal can predict whether: a motion discrimination judgment is right or wrong (Sapir et al., 2005), a sensory stimulus will be perceived or missed (Boly et al., 2007), an ambiguous visual stimulus will be perceived as a face or object (Hesselmann et al., 2008a), or a group of dots will move coherently or randomly (Hesselmann et al., 2008b). These important findings clearly suggest an interaction between ongoing brain activity and evoked brain response at a behavioral level.
Compared to those behavioral studies, our study aims to understand the quantitative relationship between ongoing and evoked brain activities from a more mechanistic perspective by measuring and examining the relationship between baseline and evoked BOLD signals. We found that synchronization strength within a resting-state coherent network (implied by the baseline BOLD signal fluctuation associated with the underlying ongoing brain activity) has an important influence on the amplitude of its evoked response (evoked brain activity) to external stimulation, even at the early stage of the visual system.
We were unable to directly draw conclusions in regard to the quantitative relationship between the evoked BOLD amplitude in the visual cortex and associated visual behaviors, which were not measured in this study. However, several previous studies have provided evidence to link them (Buchel et al., 2002; Dehaene et al., 2001; Ress et al., 2000). For example, the amplitude of evoked BOLD responses in the primary visual cortex was found to be able to predict how well subjects performed a visual perception task (Ress et al., 2000).
The present study is also distinct from the previous studies aiming to link baseline BOLD signal with human behavior in the aspect of ongoing brain activity quantification. The majority of previous behavior studies employed an event-related fMRI paradigm, and a short period of baseline BOLD signals (or even a single image time point) prior to the stimulation (or task) onset was utilized to quantify the relative level of ongoing brain activity, which may only reflect an instantaneous status of the resting brain. In contrast, we used a relatively long period (165 seconds) of baseline BOLD signals to quantify the correlation of ongoing brain activity, which is likely to reflect a more general status of the resting brain. From this perspective, our study is closer to previous studies relying on the quantification of correlation strength (functional connectivity) of resting-state networks. For instance, the correlation strength of individuals’ resting-state default mode networks has been successfully applied to predict how well subjects perform a working memory task (Hampson et al., 2006), to distinguish schizophrenia patients from healthy subjects, or to correlate with the schizophrenia psychopathology (Whitfield-Gabrieli et al., 2009). Together with those findings, our study reveals potential roles of resting-state networks and neuronal synchronization in brain activation, function, and behavior.
Neurophysiologic Basis of Baseline BOLD Fluctuations
Converged evidence from a number of studies using simultaneous EEG-fMRI measurements (Feige et al., 2005; Goldman et al., 2002; Moosmann et al., 2003) has consistently shown that the baseline BOLD signal measured in the human visual cortex is negatively correlated with the power of occipital alpha-band EEG oscillations under the eyes-closed condition. Interestingly, even though it is known that the occipital alpha-band EEG power will decrease drastically when subjects open their eyes, its topographic distribution has not been shown to change significantly (Barry et al., 2007). To some extent, this finding is in line with our observation that the coherent networks implied by baseline BOLD fluctuations are spatially indistinguishable under the eyes-fixed and eyes-closed conditions. Moreover, the individual alpha-frequency (IAF) under the resting-state has been shown to negatively correlate with: (i) the amplitude of alpha-band EEG oscillations at rest; (ii) the amplitude of the visual evoked potential (VEP); and (iii) the amplitude of hemoglobin oxygenation level change, which was measured by the near-infrared spectroscopy (NIRS) technique, in response to visual stimulation (Koch et al., 2008). All of these findings suggest that the ongoing occipital alpha-band brain activity may be linked to the baseline BOLD fluctuations under both the eyes-fixed and the eyes-closed condition.
Besides the alpha-band activity at rest, the amplitude of stimulus-evoked BOLD responses has also been correlated with a number of other neurophysiologic measurements, for example, the stimulus-induced gamma oscillation frequency and the resting-state concentration of GABA (Muthukumaraswamy et al., 2009), which is a neurotransmitter essential for inhibitory interneurons and the generation of synchronized neuronal oscillations (Buzsaki et al., 2007). Understanding how the baseline BOLD correlation is associated with these neuro- and electro-physiology parameters should provide an important step for revealing the underlying mechanism of ongoing and evoked brain activity interaction. This remains an interesting topic for future studies.
Methodology Considerations and Perspectives
Since a correlation, but not causality, was found between the baseline BOLD correlation and stimulus-evoked BOLD amplitude in the present study, one possible argument on our results is that the visual input under the eyes-fixed condition (i.e., a white cross on a black screen), if considered as a weak “stimulus”, might evoke stronger fluctuations and thus higher correlation (due to a higher temporal contrast-to-noise ratio) of baseline BOLD signals for the subjects showing a stronger stimulus-evoked BOLD response. However, this possibility could be excluded for the following reasons. First, we minimized the effect of visual input under the eyes-fixed condition by using a very small cross-mark with moderate illumination on a dark screen with minimum illumination. The BOLD correlation maps for the eyes-fixed condition, which are almost identical to those obtained under the eyes-closed condition but different from those during visual stimulation (Fig. 3), are very unlikely to be induced by the very small fixation cross on a dark screen. Secondly, no significant correlation was found between the magnitude of the baseline BOLD fluctuation and stimulus-evoked BOLD amplitude (Fig. 2B), which means subjects showing stronger evoked BOLD responses do not necessarily have stronger baseline BOLD fluctuations in the same ROI – another line of evidence against the argument.
In fact, when the ROI was reduced to cover mainly the large-vessel GE-EPI voxels, the relationship between the magnitude of the baseline BOLD fluctuation and evoked BOLD amplitude is still not statistically significant (see Fig. S3). One implication of this finding is that the vasculature (especially in the venous side) variation is unlikely to contribute significantly to the large inter-subject variability in evoked BOLD amplitude observed in the present study, presumably due to: i) the large suppression of the large-vessel BOLD contribution at high magnetic field (Ogawa et al., 1998); ii) the further exclusion of large-vessel voxels in the ROI selection and data analysis; and iii) the narrow age distribution of the subjects (13 out of 15 subjects are aged between 20 and 30). For these reasons, the large inter-subject variability in baseline BOLD correlation and evoked BOLD amplitude cannot be explained from a vascular-origin perspective. Furthermore, our results should not conflict with a previous study (Kannurpatti and Biswal, 2008) showing a correlation between the magnitude of baseline BOLD fluctuation and hypercapnia-induced BOLD responses, in which the relationship was established on intra-subject and voxel-based analysis, and thus a strong correlation is logically expected based on the underlying BOLD mechanism (Ogawa et al., 1998).
It has been shown previously that the spontaneous BOLD signal fluctuation was not only contributed by ongoing brain activity but also other sources including physiologic noise (Bianciardi et al., 2009b). Although certain measures, for example, the combination of short TR and band-pass filter, have been taken to minimize possible contributions from these sources; some of them, for example, very low-frequency variation of respiration volume (Birn et al., 2006), cannot be removed completely from the image data. However, our conclusion is likely less affected by those noise sources for the following three reasons. First, a previous study had shown that the spontaneous BOLD signal fluctuation in the human visual cortex is contributed dominantly by ongoing brain activity if high-frequency thermal noise and low-frequency drift are removed (Bianciardi et al., 2009b). Second, the present study focused on the relative correlation strength of baseline BOLD fluctuation (inter-subject difference); therefore, if the contribution from noise sources is relatively consistent across subjects, it would not affect our conclusion. Thirdly, the relationship between the evoked BOLD amplitude and correlation strength of baseline BOLD fluctuation observed in the present study cannot be explained only from the noise perspective.
In this study, ROI was selected based on each fMRI run data. In parallel, we also tested another strategy using a ROI averaged from multiple fMRI runs for an individual subject and the results are shown in Figure S4 of supplementary materials. It is clear these two analysis strategies resulted in the same results and conclusions.
Another impact of our finding that baseline BOLD correlation accounts for large inter-subject variability in stimulus-evoked BOLD amplitude is on fMRI quantification, especially for those studies aiming to compare differences between evoked BOLD amplitudes of different groups of subjects (e.g., healthy subjects versus patients) under the same stimulation or task performance. Our analysis suggests that 69% inter-subject variation in the stimulus-evoked BOLD response could be explained by the inter-subject variability in the baseline BOLD correlation (based on R2 = 0.69). By monitoring baseline BOLD signals and removing the variation they cause, the inter-subject (within-group) variation in the stimulus-evoked BOLD amplitude could be significantly reduced, which can increase the statistical power of detecting the difference between groups and thus reduce the sample size required for such studies.
Conclusion
In the present study, we found that the spatiotemporal correlations of baseline BOLD signals acquired under the eyes-fixed condition from the human visual cortex imply an organized, coherent network that is topographically indistinguishable from the resting-state visual network observed under the eyes-closed condition. More strikingly, the correlation strength of this ongoing visual network can significantly influence the amplitude of subjects’ evoked BOLD responses to visual stimulation. The subject with a more synchronized ongoing network tends to show a stronger response to identical stimulation. The overall findings indicate a strong interaction between ongoing and evoked brain activities, and they also suggest the importance of the coherent networks implied by low-frequency baseline BOLD fluctuations under either the eyes-fixed or eyes-closed condition for linking brain activation, function and dysfunction. This study highlights the importance to integrate the information from both resting-state coherent networks and task-evoked neural responses for a better understanding of how the brain works.
Supplementary Material
Acknowledgements
This work was in part supported by NIH grants: NS041262, NS041262S1, NS057560, EB000329, NS070839, P41 RR008079 and P30 NS057091. The authors thank Dr. Kamil Ugurbil for his support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Zarahn E, D'Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn. Reson. Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol. 2007;118:2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Duyn JH. Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. Neuroimage. 2009a;45:160–168. doi: 10.1016/j.neuroimage.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Shmueli K, Duyn JH. Sources of functional magnetic resonance imaging signal fluctuations in the human brain at rest: a 7 T study. Magn Reson Imaging. 2009b;27:1019–1029. doi: 10.1016/j.mri.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci U S A. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22:970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol. 2005;93:2864–2872. doi: 10.1152/jn.00721.2004. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Jr., Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustard S, Williams EJ, Hall LD, Pickard JD, Carpenter TA. Influence of baseline hematocrit on between-subject BOLD signal change using gradient echo and asymmetric spin echo EPI. Magn Reson Imaging. 2003;21:599–607. doi: 10.1016/s0730-725x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- Haase A. Snapshot FLASH MRI. Applications to T1, T2, and chemical-shift imaging. Magn Reson Med. 1990;13:77–89. doi: 10.1002/mrm.1910130109. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G, Kell CA, Eger E, Kleinschmidt A. Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proc Natl Acad Sci U S A. 2008a;105:10984–10989. doi: 10.1073/pnas.0712043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G, Kell CA, Kleinschmidt A. Ongoing activity fluctuations in hMT+ bias the perception of coherent visual motion. J Neurosci. 2008b;28:14481–14485. doi: 10.1523/JNEUROSCI.4398-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999;10:626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. Neuroimage. 2008;40:1567–1574. doi: 10.1016/j.neuroimage.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SP, Koendgen S, Bourayou R, Steinbrink J, Obrig H. Individual alpha-frequency correlates with amplitude of visual evoked potential and hemodynamic response. Neuroimage. 2008;41:233–242. doi: 10.1016/j.neuroimage.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu XH, Zhang Y, Chen W. Neural Origin of Spontaneous Hemodynamic Fluctuations in Rats under Burst-Suppression Anesthesia Condition. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lu H, Zhao C, Ge Y, Lewis-Amezcua K. Baseline blood oxygenation modulates response amplitude: Physiologic basis for intersubject variations in functional MRI signals. Magn Reson Med. 2008;60:364–372. doi: 10.1002/mrm.21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TE, Norgaard MD, Rostrup E, Rowe JB, Paulson OB. Motion or activity: their role in intra- and inter-subject variation in fMRI. Neuroimage. 2005;26:960–964. doi: 10.1016/j.neuroimage.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Mansfield P. Multi-planar image formation using NMR spin-echos. J. Phys. C: Solid State Physics. 1977;10:L55–L58. [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee T-M, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Kim S-G, Ugurbil K. On the characteristics of functional magnetic resonance imaging of the brain. Annu. Rev. Biophys. Biomol. Struct. 1998;27:447–474. doi: 10.1146/annurev.biophys.27.1.447. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim S-G, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Sapir A, d'Avossa G, McAvoy M, Shulman GL, Corbetta M. Brain signals for spatial attention predict performance in a motion discrimination task. Proc Natl Acad Sci U S A. 2005;102:17810–17815. doi: 10.1073/pnas.0504678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am J Neuroradiol. 2000;21:1397–1401. [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- White T, O'Leary D, Magnotta V, Arndt S, Flaum M, Andreasen NC. Anatomic and functional variability: the effects of filter size in group fMRI data analysis. Neuroimage. 2001;13:577–588. doi: 10.1006/nimg.2000.0716. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Nolan TS, Larson-Prior LJ, Raichle ME. Subject variability of spontaneous BOLD functional connectivity. Soc. for Neuroscience Abstract viewer/planner, online. 2007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.