Abstract
Social exclusion inherently involves an element of expectancy violation, in that we expect other people to follow the unwritten rule to include us in social interactions. In this functional magnetic resonance imaging (fMRI) study, we employed a unique modification of an interactive virtual ball-tossing game called “Cyberball” (Williams et al., 2000) and a novel paradigm called “Cybershape”, in which rules are broken in the absence of social exclusion, to dissociate brain regions that process social exclusion from rule violations more generally. Our Cyberball game employed an alternating block design and removed evoked responses to events when the participant was throwing the ball in inclusion to make this condition comparable to exclusion, where participants did not throw. With these modifications, we replicated prior findings of ventral anterior cingulate cortex (vACC), insula, and posterior cingulate cortex activity evoked by social exclusion relative to inclusion. We also identified exclusion-evoked activity in the hippocampi, left ventrolateral prefrontal cortex, and left middle temporal gyrus. Comparing social exclusion and rule violation revealed a functional dissociation in the active neural systems as well as differential functional connectivity with vACC. Some overlap was observed in regions differentially modulated by social exclusion and rule violation, including the vACC and lateral parietal cortex. These overlapping brain regions showed different activation during social exclusion compared to rule violation, each relative to fair play. Comparing activation patterns to social exclusion and rule violation allowed for the dissociation of brain regions involved in the experience of exclusion versus expectancy violation.
Keywords: Social Exclusion, Rule Violation, Anterior Cingulate Cortex, Functional Connectivity, Default Mode Network
Introduction
Ostracism, the exclusion of one member from a group, is a social phenomenon that threatens relationships, especially the security offered by friendships and peer networks. Williams et al. (2000) developed an experimental paradigm modeling social exclusion in an interactive computer-based program called Cyberball. In Cyberball, two other ostensibly real players may throw the ball with the participant, or only amongst themselves. Behavioral studies using Cyberball (Boyes & French, 2009; Masten et al., 2009; Sebastian et al., 2009; Zadro et al., 2004, 2005) have found that exclusion elicits a more negative overall mood and decreases feelings of belongingness, control, meaningful existence, and self-esteem (Williams et al., 2000, 2007; Zadro et al., 2004). These effects occur even if participants know that the other players are computerized (Zadro et al., 2004).
Cyberball has been used in fMRI studies to investigate the brain response to social exclusion (Eisenberger et al., 2003; Masten et al., 2009; Onoda et al., 2009, in press). Brain regions found active during exclusion versus fair play include ventrolateral prefrontal cortex (vlPFC) and anterior insula (Eisenberger et al., 2003; Masten et al., 2009), ventral anterior cingulate cortex (vACC, Masten et al., 2009; Onoda et al., 2009), dorsal anterior cingulate cortex (dACC, Eisenberger et al., 2003), and posterior cingulate cortex (Onoda et al., 2009). Several studies reported correlations between dACC activity during exclusion and self-reported social pain (Eisenberger et al., 2003; Onoda et al., 2009, in press). However, the precise psychological correlates of these neural effects remain unclear. Exclusion in Cyberball potentially involves the emotional state of rejection, cognitive reactions to violated expectations of inclusion, and cognitive efforts to assess the situation. This complexity has likely contributed to inconsistent findings across fMRI studies.
We used two different virtual ball-toss games to functionally dissociate brain mechanisms involved in processing social exclusion and rule violation. Social exclusion was elicited by a modification of the original Cyberball paradigm. In prior studies, inclusion always preceded one period of exclusion. Our Cyberball design used alternating blocks of inclusion and exclusion to eliminate the potential confounds of scanner drift, changes in participant motion and attention, and fatigue occurring over the course of a scan session. In addition, the alternating block design decreases the likelihood of participant disengagement from the task in long periods of exclusion. The alternating blocks also model important aspects of naturally occurring ostracism, where exclusion can be transient and relatively ambiguous at times. Note that ambiguous ostracism, where the participant still occasionally receives the ball during periods of perceived exclusion, has been shown in past behavioral and imaging Cyberball studies to elicit strong feelings of exclusion (Boyes & French, 2009; Chernyak & Zayas, 2010; Onoda et al., 2009; Williams et al., 2000). As an additional methodological adjustment, we modeled and removed evoked responses to events when the participant was throwing the ball, to account for potential confound that participants are actively playing during fair play but not exclusion.
Cyberball was compared to a new ball tossing game, “Cybershape”, in which the ball takes on a new shape on each toss, which specifies the player to whom the ball should be thrown. In Cybershape, one of the computerized players breaks the shape rule, eliciting a non-exclusive expectancy violation. Unlike social exclusion, however, rule violations do not threaten one's social relationships. A past study comparing rejection and expectancy violation found that dorsal and ventral ACC were recruited differentially by these two conditions (Somerville et al., 2006). Past Cyberball studies have found both dACC and vACC activation in exclusion, yet the psychological correlates of this activation remains unclear. Comparing patterns of activation during rule violation in Cybershape and social exclusion in Cyberball, two similar interactive, social paradigms, allowed for a functional dissociation of brain regions involved in the experiences of exclusion and expectancy violation more generally. Further, psychophysiological interaction analyses in the two games aimed to provide illumination of regional functional connectivity involved in the experience of social exclusion, as compared to connectivity in rule violation.
Materials and Methods
Participants
We studied a group of 26 healthy young adults (13 male, mean age = 24.15 years ± 4.07). Twenty-five of the participants played Cyberball and Cybershape consecutively in the same scanning session with a counterbalanced order (one participant played Cyberball only). All participants were asked upon completion of both games if they felt excluded during Cyberball, and if they noticed rules being broken in Cybershape. If the answer to either of these questions was no, the participant was excluded from analysis. The average number of errors made (shape thrown to the wrong player by the participant) in Cybershape was 0.57 (± 0.84; maximum: 3), where 13 of the participants made no errors. If a participant made more than 3 errors, they were excluded from analyses. Two participants were excluded from all analyses, one for not noticing rules being broken, the other for purposefully breaking the rules and failing to throw the ball on more than two occasions. A third participant was left out of the Cyberball analysis only for not reporting experiencing exclusion during the game. Of the remaining 22 participants that played both games, 12 played Cyberball first. In sum, 23 participants (11 male, mean age 24.0 years ± 3.81) were included in the Cyberball study and 23 subjects (11 male, mean age 24.04 years ± 3.77) were included in the Cybershape study. Twenty-two of these participants overlapped. Two participants included in the analysis believed that they were playing against real people, while the rest suspected that the online players were not real. Written informed consent was obtained from each participant according to a protocol approved by the Yale University Human Investigation Committee.
Experimental Design
Cyberball
A game of Cyberball began with a sham Google® search engine screen where the participant was told that they were being logged into the game by the experimenter. Once the experimenter clicked on the link to “Cyberball” a loading screen appeared followed by a screen where the participant could choose, from 6 possible selections, the catching glove he or she wanted to use during the game. After the glove choice, instructions on how to play the game were given visually and auditorily, and the participants practiced playing the game for 16 throws. The participants were given two button boxes, one in each hand, which allowed them to throw the virtual ball to either the right or left. Once their understanding of the game was confirmed, the scan began and they continued to play the Cyberball game for 5 minutes. An image of the Cyberball game screen is shown in Figure 1. The first and last 10 seconds of the game were an inactive play screen with no ball present. Participants played Cyberball in 10 continuous, alternating blocks of fair play and exclusion (e.g. fair play, exclusion, fair play, exclusion, etc.). Each block consisted of 12 throws, completed in a total of 30 seconds. In fair play, participants received the ball on one-third of the throws; in exclusion, participants never received the ball. If the participant did not throw the ball within 3 seconds of receiving it, the ball was thrown automatically (to a random player). Participants who failed to throw more than twice during a game were excluded from analysis (this excluded one participant). The computer players’ pictures were matched on gender and ethnicity to each participant, as playing with members of a perceived “in group” has been demonstrated to intensify negative emotional reactions to Cyberball exclusion (Wirth and Williams, 2009). Computer players also varied between the two cyber games. To further validate participants’ reported feelings of exclusion, a ten-item questionnaire was given to a subset of participants (n = 7) in the present study immediately after playing Cyberball. The questionnaire had not been prepared in time for administration to all participants. The questionnaire was a sample of questions from the Needs Threat Scale (van Beest & Williams, 2006), used to assess distress following Cyberball exclusion (Eisenberger et al., 2003; Williams, 2007). Participants were asked to rate statements about feelings of control, meaningful existence, belongingness, and self-esteem on a likert scale from 1 = “not at all” to 5 = “extremely”. The questions used are listed in Table 1.
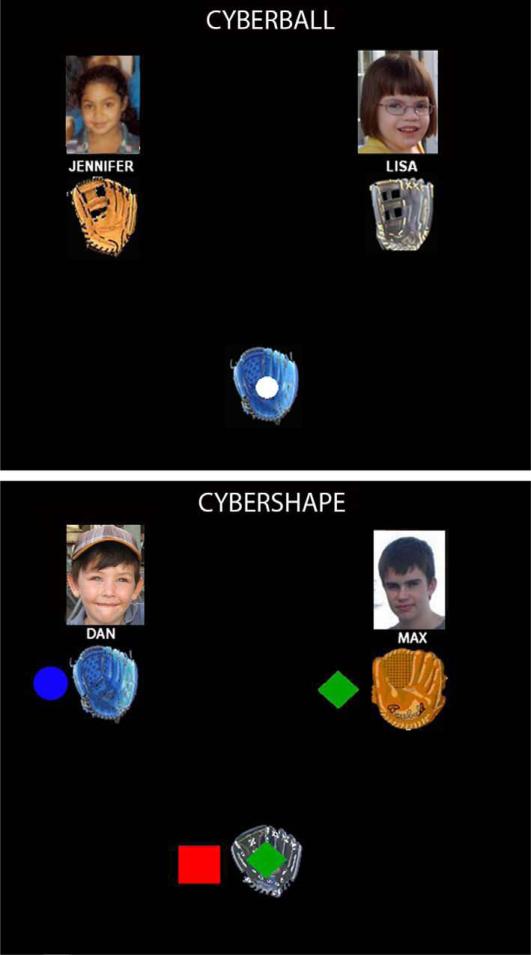
Figure 1.
Cyberball and Cybershape game screens. Above: Image of the game screen in Cyberball. The participant (bottom center glove) currently has the ball and can choose to throw the ball to either of the other players. Below: Image of the game screen in Cybershape. In this game the participant chose the red square as their shape. The participant currently has the ball in his/her glove, which is in the shape of a green diamond, indicating that the participant must throw to the player on the right with the matching green diamond next to his glove. When that player receives the green diamond it will change to a different shape (either a red square or a blue circle) dictating the next appropriate throw. Player pictures displayed in the figure were not used in the actual paradigm, as those pictures were not consented for publication.
Table 1.
Social exclusion and rule violation questionnaire items. Items were administered visually and auditorily after Cyberball or Cybershape, respectively. Responses were given on a 1 to 5 likert scale from “not at all” to “extremely”. Questions with a * were reverse coded.
| Social exclusion questionnaire items: | Rule violation questionnaire items: |
|---|---|
| I felt Rejected | I was annoyed when players didn't follow the rules |
| I felt like an outsider | I didn't care when rules were broken* |
| I felt like the other players were interacting with me a lot* | I wanted the other players to throw the shapes to the right people |
| I felt liked* | We had a nice game going but sometimes the other players messed it up |
| I felt unsure of myself | I felt upset when something unexpected happened in the game |
| I felt like an outsider | I wondered why they were throwing to the wrong person |
| I felt powerful* | I didn't want to play once the other players broke the rules |
| I felt the other players decided everything | I lost interest in following the rules |
| I felt invisible | I tried my best to throw the ball to the correct player* |
| I felt good about myself* | I felt unsure of myself |
Cybershape
A game of Cybershape began in the same manner as Cyberball, but after selecting the catching glove, the participant was asked to choose which shape they wanted to correspond to their glove (out of 4 possible selections). After the glove and shape choice, instructions on how to play the game were given visually and auditorily. An explanation of the shape matching rule in which they were asked to throw the shape in their glove to the person with the matching shape next to their picture also appeared on the screen. The participants practiced playing the game for 16 throws. Once their understanding of the game was confirmed, the scan began and they continued to play the Cybershape game for 5 minutes. An image of the Cybershape game screen is shown in Figure 1. The first and last 10 seconds of the game were an inactive play screen with no shape present. Each participant played Cybershape in 10 continuous, alternating blocks of fair play (rule consistent) and rule violation (e.g. fair play, rule violation, fair play, rule violation, etc.). Again, each block was 30 seconds long and consisted of 12 throws. In fair play, participants received the shape one-third of the time, and the shape rule was never broken. In rule violation, participants still received the shape one-third of the time, but one of the confederate virtual players consistently violated the shape rule by throwing the shape to the wrong person. The rule violations occurred both in favor of the participant (getting the ball when it was not his or her shape) and in disfavor of the participant (not getting the ball when it was his or her shape). The player who broke the shape rule alternated between rule violation blocks in the pattern ABBAB (where A and B are the two confederate Cybershape players). Only one rule violator per violation block was employed to ensure that participants did not feel excluded being the only player to follow the rules, or suspect a rule change if both computer players broke the rules together. In contrast to the self-report questionnaire administered following Cyberball to assess exclusion-related distress, a different ten item questionnaire to assess rule violation-related distress was created with questions pertaining specifically to the experience of rule violation, and given to the same subset of participants as assessed on the social exclusion questionnaire (n = 7) immediately following the completion of Cybershape. Like the social exclusion questionnaire, this questionnaire had not been prepared in time for administration to all participants. The questions used are listed in Table 1.The order of presentation of Cyberball and Cybershape was counterbalanced across participants.
Imaging Protocol
Images were collected on a Siemens 3T Tim Trio scanner located in the Yale University Magnetic Resonance Research Center. Whole-brain T1-weighted anatomical images were acquired using an MPRAGE sequence (TR = 1900 ms; TE = 2.96 ms; flip angle = 9°; FOV = 256 mm; image matrix 2562; voxel size =1 × 1 × 1 mm; 160 slices; NEX = 1). Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 642; voxel size = 3.4 × 3.4 × 4 mm; 34 slices) sensitive to BOLD contrast.
Data Analysis
Data were preprocessed and analyzed using the BrainVoyager QX 2.0 software package (Brain Innovation, Maastricht, The Netherlands). Preprocessing of the functional data included slice time correction (using sinc interpolation), 3-dimensional rigid-body motion correction (using trilinear-sinc interpolation), spatial smoothing with a FWHM 4-mm Gaussian kernel, linear trend removal, and temporal high-pass filtering (fast Fourier transform based with a cutoff of 3 cycles/time course). Functional datasets were coregistered to within-session anatomical images, which were in turn normalized to Talairach space. Estimated motion plots and cine loops were examined for each participant in order to identify movement and eliminate runs with head motion greater than 2 mm of translation in any direction or 2 degrees of rotation about any axis (for which no runs were eliminated).
To identify brain regions activated by exclusion in Cyberball and rule violation in Cybershape, a random-effects multi-subject general linear model (GLM)-based analysis was performed for each game. Regressors were defined as boxcar functions peaking during each condition, convolved with a double-gamma hemodynamic response function (HRF). To eliminate the confound of the lack of decision making and motor response in the exclusion blocks, modeled activation from events when the participant was throwing the ball were removed from the data in a regression performed prior to the main regression analysis. Regressors for this analysis were defined as boxcar functions peaking at ball throw events, defined as the period from when the participant received the ball until the participant's throw response, and convolved with a double-gamma HRF; subsequent analyses were performed on residuals from this regression. For consistency, the same was done in Cybershape even though the potential confound did not exist in this game because participants threw the ball equally frequently in fair play and rule violation blocks.
For Cyberball, a multi-subject analysis was performed and brain activation in the contrast exclusion > fair play was assessed at an uncorrected statistical threshold of p < .05. To correct for multiple comparisons, we used a cluster threshold of 35 contiguous voxels (Forman et al., 1995; Xiong et al., 1995). This cluster threshold was calculated to correspond to a corrected threshold of α < .05 using a Brain Voyager QX Cluster-level Statistical Threshold Estimator plugin. After 5,000 iterations of a Monte-Carlo simulation, an alpha value is assigned to each cluster size based on its relative frequency. Considering the number of active voxels in this Cyberball analysis, the chances of finding a cluster of 35 or more voxels was less than 5%. For Cybershape, a multi-participant GLM analysis was performed, and the contrast of rule violation > fair play was assessed at the same statistical threshold of p < .05, with a cluster threshold of 35 functional voxels, again estimated by the BrainVoyager QX plugin with α < .05.
Following analyses in each game individually, we performed a paired samples t-test on whole brain contrast maps from each game derived from each subject, thereby constituting a random effects analysis. We compared the differences (social exclusion – fair play) and (rule violation – fair play) in each participant with functional data from both games (22 participants). This allowed us to localize regions showing statistically different activation in social exclusion and rule violation when compared to fair play in each game. The results of this whole brain analysis were assessed at a statistical threshold of p < .01.This threshold was chosen for its allowance of a meaningful separation of regions of activation. A cluster size of 9 contiguous voxels was used, corresponding to a threshold of α < .05 as calculated by the BrainVoyager QX Cluster-level Statistical Threshold Estimator plugin.
We defined anatomically a region of interest that captured the vACC. This structural ROI was modified from the anterior cingulate cortex region as defined in the Talairach Database (Lancaster et al., 1997, 2000) by excluding all voxels above the plane z = 9 in Talairach space, around the tip of the genu (see left panel of Figure 4). ROI averages of beta values for the contrasts social exclusion – fair play and rule violation – fair play were computed for each participant and statistically assessed using a two-tailed t-test.
Figure 4.
Structural Region of Interest (ROI) analysis of the vACC. A structural ROI (shown in purple) was used to compare vACC activity between the two games. Average contrast beta values for this ROI in each game were calculated for each participant using (social exclusion – fair play, rule violation – fair play). These average beta values are shown in the bar graph, with error bars depicting standard error.
Furthermore, a psychophysical interaction (PPI) analysis (Friston et al., 1997) was performed on each data set to determine regions of greater functional connectivity with ventral anterior cingulate cortex (vACC) in social exclusion or rule violation compared to fair play. Prior to connectivity analysis, the global mean (average signal across voxels) was removed from each volume, as a surrogate method for physiological artifact removal (Fox et al., 2005). Using a seed region of the vACC functionally defined by differential activation in social exclusion compared to fair play in our random-effects whole brain analysis, PPI regressors were created by multiplying the difference of the two task regressors by the preprocessed, normalized vACC time course for each participant. This PPI function along with the task regressors and vACC time course were used as regressors in a multi-participant random-effects GLM analysis. The PPI function was used as the only predictor of interest and assessed at a statistical threshold of p < .05, corrected with a cluster threshold of 34 voxels to α < .05.
Results
The social exclusion questionnaire given to a subset of participants (n = 7) confirmed that Cyberball elicited feelings of distress following exclusion. The average response to each question was 2.89 (on a scale from 1 = “not at all” to 5 = “extremely”). The average total score on this questionnaire was 28.86 (± 2.64), This score was significantly greater than the minimum score of 10 reflecting no exclusion-related distress (t(6) = 7.14, p < .001). On the questionnaire consisting of ten different questions aimed to assess distress due to rule violation, the average response to each question was 2.09. The average total score in the same subset of participants was 20.86 (± 1.10), again significantly greater than a minimum score of 10 reflecting no rule violation-related distress (t(6) = 9.87, p < .001).
As illustrated in Figure 2, we identified a network of brain regions that were differentially active in either social exclusion (left panel of Figure 2) or rule violation (right panel of Figure 2) compared to fair play in Cyberball and Cybershape. Peak coordinates, statistical values, size, and anatomical labels for the regions of differential activation in Cyberball and Cybershape are displayed in Tables 2 and 3, respectively. In examining regions that were more active in social exclusion compared to fair play (social exclusion > fair play), we identified three areas that have been implicated in past Cyberball imaging studies: left PCC extending into retrosplenial cortex (t = 5.79, p < .001), right insula (t = 3.75, p < .01), and vACC (t = 3.85, p < .001). Additional areas of interest included bilateral hippocampus (left (posterior): t = 3.80, p < .001, left (anterior): t = 3.42, p < .01, right: t = 4.91, p < .001), left MTG (t = 3.93, p < .001), subgenual ACC (t = 3.62, p < .01), and left ventrolateral PFC (t = 4.22, p < .001). Regions that were more active in fair play compared to exclusion (fair play > social exclusion) that have also been identified in past Cyberball studies included bilateral cerebellum (right: t = -3.65, p < .01, left: t = -3.94, p < .01). Other areas of interest in this contrast included large expanses of parietal cortex (t = -5.78, p < . 001), bilateral precentral gyrus (left: t = -3.86, p < .001, right: t = -4.97, p < .001), and bilateral orbitofrontal cortex (left: t = -4.14, p < .001, right: t = -5.16, p < .001). Addressing the potential concern that the beginning of the Cyberball game yielded stronger responses to exclusion than the end, average activation from the first four blocks (two fair play and two exclusion) was compared to the last four blocks in each participant using a paired-sample t-test in each region of differential activation in exclusion > fair play identified from the whole-game analysis. None of these regions showed significantly different activation in the first and last four blocks of Cyberball (p > .05, corrected for multiple comparisons).
Figure 2.
Left column: whole-brain comparison of social exclusion and fair play. Regions in orange showed greater activation in social exclusion compared to fair play. Regions in blue showed greater activation in fair play compared to social exclusion (p < .05, cluster threshold of 35 contiguous voxels). Right Column: whole-brain comparison of rule violation and fair play. Regions in orange showed greater activation in rule violation compared to fair play. Regions in blue showed greater activation in fair play compared to rule violation (p < .05, cluster threshold of 35 contiguous voxels).
Table 2.
Activation in Cyberball. Regions identified in a full brain contrast of social exclusion to fair play. Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest.
| Brain Region | X | Y | Z | t | Size | p |
|---|---|---|---|---|---|---|
| Social Exclusion > Fair Play | ||||||
| Right insula | 38 | 13 | -12 | 3.75 | 995 | 0.00111 |
| Right cerebellum | 35 | -74 | -36 | 4.58 | 971 | 0.00015 |
| Left hippocampus (posterior) | -37 | -29 | -9 | 3.80 | 2312 | 0.00099 |
| Left hippocampus (anterior) | -19 | -8 | -15 | 3.42 | 1122 | 0.00246 |
| Right hippocampus | 20 | -35 | 15 | 4.91 | 4134 | 6.6E-05 |
| Right middle occipital cortex | 8 | -95 | 12 | 3.63 | 2366 | 0.00147 |
| Left middle occipital cortex | -4 | -71 | -6 | 3.82 | 1400 | 0.00093 |
| Left MTG | -58 | -5 | -12 | 3.93 | 1424 | 0.00072 |
| SubACC | -1 | 10 | 0 | 3.62 | 1167 | 0.00152 |
| vACC | -7 | 25 | -9 | 3.85 | 3189 | 0.00086 |
| Left vlPFC | -34 | 4 | -33 | 4.22 | 4650 | 0.00035 |
| Left PCC | -19 | -38 | 21 | 5.79 | 9440 | 8E-06 |
| Fair Play > Exclusion | ||||||
| parietal cortex | -25 | -8 | 54 | -5.78 | 75444 | 8E-06 |
| Right inferior occipital cortex | 41 | -71 | -6 | -3.37 | 995 | 0.00274 |
| Right cerebellum | 32 | -41 | -27 | -3.65 | 1124 | 0.0014 |
| Left cerebellum | -43 | -80 | -3 | -3.94 | 9085 | 0.00071 |
| Left OFC | -34 | 61 | 3 | -4.14 | 5592 | 0.00042 |
| Right OFC | 38 | 58 | 0 | -5.16 | 7365 | 3.5E-05 |
| Left precentral gyrus | -49 | -2 | 36 | -3.86 | 5056 | 0.00086 |
| Right precentral gyrus | 29 | 1 | 60 | -4.97 | 22948 | 5.6E-05 |
Abbreviations: Posterior cingulate cortex (PCC), ventral anterior cingulate cortex (vACC), orbital frontal cortex (OFC), medial temporal gyrus (MTG), ventrolateral prefrontal cortex (vlPFC), subgenual ACC (subACC).
Table 3.
Activation in Cybershape. Regions identified in a full brain contrast of rule violation to fair play. Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest.
| Brain Region | X | Y | Z | t | Size | p |
|---|---|---|---|---|---|---|
| Rule Violation > Fair Play | ||||||
| Right anterior MTG | 42 | 8 | -17 | 4.24 | 2903 | 0.000334 |
| Dorsal medial and lateral PFC | 42 | 32 | -5 | 5.75 | 70402 | 0.000009 |
| Right cerebellum | 36 | -67 | -17 | 3.87 | 4480 | 0.000827 |
| Right MOC | 12 | -91 | 1 | 3.52 | 1330 | 0.001928 |
| Left cerebellum | -33 | -73 | -20 | 4.97 | 9210 | 0.000056 |
| Left vlPFC | -39 | 53 | 13 | 5.27 | 6699 | 0.000027 |
| Left MOC | -36 | -85 | 13 | 4.43 | 1129 | 0.000212 |
| Left STS | -48 | -34 | 1 | 3.35 | 1018 | 0.002914 |
| Right STS | 69 | -46 | 4 | 3.84 | 6679 | 0.000889 |
| Parietal cortex | 63 | -52 | 28 | 5.47 | 64051 | 0.000017 |
| Fair Play > Rule Violation | ||||||
| Right insula | 39 | -13 | 1 | -5.67 | 14320 | 0.000011 |
| Left insula | -42 | -7 | 13 | -6.57 | 23455 | 0.000001 |
| Paracentral lobule | -6 | -16 | 55 | -5.68 | 35837 | 0.00001 |
| Left hippocampus | -33 | -37 | -2 | -4.31 | 1675 | 0.000281 |
| Right anterior cerebellum | 24 | -40 | -20 | -3.86 | 1920 | 0.000849 |
| Left anterior cerebellum | -18 | -43 | -14 | -4.09 | 1916 | 0.000487 |
| Ventral ACC | 3 | 14 | 1 | -5.76 | 11071 | 0.000009 |
Abbreviations: Anterior cingulate cortex (ACC), medial temporal gyrus (MTG), ventrolateral prefrontal cortex (vlPFC), prefrontal cortex (PFC), middle occipital cortex (MOC), superior temporal sulcus (STS).
As shown in the right column of Figure 2, in Cybershape, regions that were more active in rule violation compared to fair play (rule violation > fair play) included large areas of parietal cortex (t = 5.47, p < .001), right anterior MTG (t = 4.24, p < .001), contiguous regions of dorsal medial and lateral PFC (t = 5.75, p < .001), left ventrolateral prefrontal cortex (t = 5.27, p < .001), and bilateral superior temporal sulcus (right: t = 3.84, p <.001, left: t = 3.35, p <.005). Regions that were more active in fair play compared to rule violation (fair play > rule violation) included bilateral insula (left: t = -6.57, p < .001, right: t = -5.67, p < .001), ventral/subgenual ACC (t = -5.76, p < .001), bilateral anterior cerebellum (right: t = -3.86, p < .001, left: t = -4.09, p < .001), left hippocampus (t = -4.31, p = < .001), and paracentral lobule (t = -5.68, p < .001).
As shown in Figure 3, regions more active in the Cyberball contrast (social exclusion – fair play) included bilateral posterior insula (right: t = 5.40, p < .001, left: t = 4.83, p < .001), bilateral posterior cingulate cortex (right: t = 4.97, p < .001, left: t = 5.97, p < .001), right hippocampus (t = 3.73, p < .01), left parahippocampal gyrus (t = 5.00, p < .001), paracentral lobule extending into dorsal ACC (t = 5.00, p < .001), and ventral ACC (t = 6.67, p < .001). Regions more active in the Cybershape contrast (rule the violation – fair play) included bilateral superior frontal gyrus (right: t = -4.75, p < .001, left: t = -5.64, p < .001), bilateral dorsolateral PFC (right: t = -5.31, p < .001, left: t = -5.98, p < .001), and parietal cortex (t = -6.48, p < .001).
Figure 3.
Between game comparison of differential activation in Cyberball and Cybershape, using a paired samples t-test of social exclusion – fair play and rule violation –fair play. Regions in orange showed greater activation in the Cyberball contrast (social exclusion – fair play), while regions in blue showed greater activation in the rule violation contrast (rule violation – fair play) (p < .01, cluster threshold of 9 contiguous voxels). All contrasts were performed after the removal of activation modeled from events when the participant was throwing the ball. Activations are displayed on a Talairach-transformed template brain in radiological orientation.
Responses to exclusion and rule violation in the structurally-defined vACC are plotted in the bottom panel of Figure 4. Activation in this region significantly differed as a function of paradigm (t = 3.29, p < .01), characterized by entirely opposite directions of significant activation. The vACC exhibited increased activation to social exclusion versus fair play, yet this same region produced decreased activation in rule violation compared to fair play.
As shown in Figure 5, in a PPI analysis of activation during Cyberball, regions more functionally connected to the vACC in exclusion compared to fair play included right MTG (right: t = 4.64, p < .001), medial PFC (t = 4.07, p < .005), right inferior parietal lobule (t = 3.15, p < .005), and precuneus (t = 3.59, p < .005). In a PPI analysis using the same vACC seed region in Cybershape, regions more functionally connected in rule violation compared to fair play included bilateral precentral gyrus extending into paracentral lobule (right: t = 4.21, p < .001, left: t = 5.75, p < .001), bilateral dorsolateral prefrontal cortex (dlPFC; right: t = 5.24, p < .001, left: t = 5.05, p < .001), bilateral insula (right: t = 3.81, p < .001, left: t = 4.82, p < .001), right ventrolateral PFC (t = 5.53, p < .001), and bilateral lingual gyri (right: t = 3.80, p < .001, left: t = 4.13, p < .001). Peak coordinates, statistical values, size, and anatomical labels for the regions of differential functional connectivity (Exclusion > Fair Play and Rule Violation > Fair Play) in Cyberball and Cybershape are provided in Table 4.
Figure 5.
PPI analysis of differential connectivity to vACC in Cyberball (left) and Cybershape (right). In both connectivity analyses the seed region used was functionally defined by greater activation in Cyberball social exclusion compared to fair play (3189 voxels, shown in green). In Cyberball (left), areas of activation in orange are regions that showed more functional connectivity to the seed region in social exclusion compared to fair play (p < .05, cluster threshold of 34 contiguous voxels). In Cybershape (right), areas of activation in orange are regions that showed more functional connectivity to the seed region in rule violation compared to fair play (p < .05, cluster threshold of 34 contiguous voxels). Activations are displayed on a Talairach-transformed template brain in radiological orientation.
Table 4.
Psychophysiological interaction (PPI) analyses. Two PPI analyses, performed for each game, identified regions of statistically reliable functional connectivity. Regions of functional connectivity in social exclusion > fair play (Cyberball) and rule violation > fair play (Cybershape) were identified (p < .05). Talairach coordinates and statistics refer to the voxel with the maximum signal change in each ROI.
| Brain Region | X | Y | Z | t | Size | p |
|---|---|---|---|---|---|---|
| Social Exclusion > Fair Play | ||||||
| right MTG | 42 | 11 | -29 | 4.64 | 2801 | 0.000128 |
| right IPL | 45 | -64 | 34 | 3.15 | 1565 | 0.004636 |
| mPFC | 6 | 65 | 16 | 4.07 | 4977 | 0.000508 |
| Precuneus | -6 | -55 | 31 | 3.59 | 2797 | 0.001649 |
| Rule Violation > Fair Play | ||||||
| Right precentral gyrus | 39 | -37 | 61 | 4.21 | 14775 | 0.000358 |
| Right vlPFC | 39 | 50 | 10 | 5.53 | 4072 | 0.000015 |
| Right insula | 36 | -1 | 13 | 3.81 | 2642 | 0.00095 |
| Right dlPFC | 36 | 35 | 34 | 5.24 | 5492 | 0.000029 |
| Right lingual gyrus | 12 | -64 | -8 | 3.80 | 982 | 0.000981 |
| Left lingual gyrus | -18 | -49 | -5 | 4.13 | 2101 | 0.000437 |
| Left dlPFC | -36 | 44 | 34 | 5.05 | 6775 | 0.000047 |
| Left precentral gyrus/paracentral lobule | -3 | -19 | 43 | 5.75 | 41188 | 0.000009 |
| Left insula | -48 | -10 | 13 | 4.82 | 6478 | 0.000081 |
Abbreviations: middle temporal gyrus (MTG), dorsolateral prefrontal cortex (dlPFC), medial prefrontal cortex (mPFC), ventrolateral prefrontal cortex (vlPFC), inferior parietal lobule (IPL).
Discussion
The present study demonstrated a functional dissociation in the neural responses to social exclusion and rule violation. The finding of statistically different regional activation between games (Figure 3) supports the conclusion that the two games, though very similar in design, recruit dissociable neural systems as a function of the experience of social exclusion versus rule violation. Further, contrasting patterns of functional connectivity to the vACC were found between these two conditions. Thus, social exclusion and rule violation not only engaged different brain regions, but also differentially affected connectivity patterns in large-scale neural networks.
Our novel alternating block Cyberball paradigm elicited nearly identical self-reported feelings of exclusion to a past study using the original Cyberball design that also assessed distress with the Needs Threat Scale. The mean score per question in the present study was 2.89, compared to the previously reported mean of 2.90 (both on a 1 to 5 likert scale; Masten et al., 2009). Because the alternating block design has not been used in past Cyberball studies, a concern existed that the repetitive nature of the block design may make the exclusion blocks less salient over time. The comparability of present distress scores to past Cyberball studies without the alternating block design suggests that even at the end of all 10 blocks, the participants felt distress caused by the exclusion periods. This finding supports the conclusion that alternating blocks of exclusion do not become less upsetting over the course of the game. This conclusion was further supported by statistical comparisons of brain activation in the first and last portions of the game. An ROI analysis using each region of differential activation identified in the whole-game contrast (exclusion > fair play) compared average activation from the first four blocks (two fair play and two exclusion) to the last four blocks in each participant using a paired-sample t-test. None of these regions showed significantly different activation in the first and last four blocks of Cyberball (p > .05, corrected for multiple comparisons). In a whole brain post-hoc analysis of Cyberball exclusion > fair play comparing the first four blocks to the last four, only 2 regions showed significantly higher activation in the first four blocks (p < .05, k = 35 voxels). These regions were inferior temporal gyrus (peak voxel: 63, -7, -26; t = 4.65; p < .001) and left anterior insula (peak voxel: -39, 14, -11; t = 4.25; p < .001). At a more lenient cluster threshold of 10 voxels, bilateral insula showed greater activation in the first four blocks, along with ventromedial and dorsolateral PFC.
Our imaging results replicated some, but not all, aspects of previous social exclusion studies. Specifically, we found activation in the PCC, vACC, and right insula during social exclusion, replicating prior Cyberball studies (Krill & Platek, 2009; Masten et al., 2009; Onoda et al., 2009). The vACC has often been implicated in studies of emotion, especially those evoking sadness (Levesque et al., 2003; Liotti et al., 2000; Phan et al., 2002; Shafritz et al., 2006). Based on these findings, we speculate that the vACC activity observed here during social exclusion may reflect negative emotion induced by social exclusion relative to fair play. In addition, we found activation in the hippocampi, and left middle temporal gyrus, both areas implicated in aspects of emotional experience (Blood et al., 1999; Blair et al., 2007; Britton et al., 2006; Davis et al., 2008; Farrow et al., 2001, 2005; Garrett & Maddock, 2006; Gosselin et al., 2006; Maddock, 1999; Small et al., 2001; Winston et al., 2002; Wood et al., 2005; Zahn et al., 2009). Three regions that showed activation in social exclusion, vACC, bilateral hippocampus, and retrosplenial cortex, are functionally connected and are considered a medial temporal subnetwork of the default mode network (Andrews-Hanna et al., 2010; Buckner et al., 2008). Our findings highlight a potential emotional role for this subnetwork.
To further explore the role of the vACC in social exclusion, a PPI analysis allowed for a contrast of functional connectivity between this and other regions in social exclusion versus fair play. This region was chosen as a seed for the PPI analysis because of its modulation by both social exclusion and rule violation, as well as its implicated role in emotion processing, specifically negative emotion. We found greater functional connectivity of the vACC to medial prefrontal cortex, right inferior parietal lobule, and precuneus during social exclusion. These three regions form the central components of the default mode network (DMN; Buckner et al., 2008). The DMN is a system of brain regions active in the absence of task performance, which has been proposed to subserve reflective, stimulus-independent thought processes (Buckner et al., 2008; Fox et al., 2005). In contrast, we used the same region as a seed in a connectivity analysis in Cybershape, where another PPI analysis showed greater connectivity between vACC and areas of precentral gyrus, bilateral insula, and paracentral lobule in rule violation (Figure 5). The finding of increased connectivity between vACC and midline DMN regions during social exclusion (and not rule violation) could result from the engagement of reflective processing during exclusion blocks, when the participant is no longer engaged in playing the game and may begin to question the motives of other players, or to ruminate generally on the situation. DMN functional connectivity to vACC during rest has also been demonstrated to be stronger in people with depression compared to controls, highlighting a potential relationship between vACC-DMN connectivity, negative emotion, and rumination (Greicius et al., 2007).
A whole-brain contrast of rule violation and fair play revealed a largely different pattern of activation compared to the social exclusion contrast. Regions differentially active in rule violation, unlike those discussed in relation to social exclusion, have been implicated in various dimensions of tasks involving rule use. Regions found to be more active in rule violation as compared to fair play include the parietal cortex, right MTG, dorsal medial and lateral PFC and left ventrolateral PFC. All of these regions have demonstrated activity during the performance of rule-governed tasks in previous studies, and are related to such processes as response selection and preparation when rules are changed spontaneously, rule learning and online maintenance, storage of rule knowledge, and rule switching (Brass & Yves von Cramon, 2004; Bunge, 2004; Bunge et al., 2005; Crone et al., 2005; Donohue et al., 2005; Mulette-Gillman & Huettel, 2009). Additionally, right lateral parietal activation extended into a region of the posterior superior temporal sulcus which has been shown to be differentially active in incorrect (compared to correct) goal-directed actions (Pelphrey et al., 2004). Considering the previously demonstrated roles in rule use, it is possible that the regional activity found in rule violation corresponds to an increase in cognitive processing associated with attention to and implementation of the present study's shape-matching rule when the rule is unexpectedly broken by the other players.
In contrast to prior work, we did not observe dACC activation in whole brain analyses in Cyberball after correcting for multiple comparisons. However, at an uncorrected threshold of p < .05, a region of dACC was found to be more active in social exclusion than fair play (peak Talairach coordinate: 0, -1, 28). This region showed differential activation in Cyberball but not in Cybershape (corrected or uncorrected), supporting previous claims of a role for dACC in the experience of exclusion (Eisenberger et al., 2003; Masten et al., 2009; Onoda et al., in press). Our results suggest that both ventral and dorsal ACC serve roles in the psychological response to exclusion. Contextualizing these findings in terms of past evidence for a dorsal versus ventral distinction in cognitive and emotional processing in the medial PFC (Bush et al., 2000; Steele & Lawrie, 2004) may be aided by an interpretation of ventral and dorsal ACC activations in Cyberball as primary and secondary emotional responses to exclusion. Damasio first described this distinction, hypothesizing that primary emotions are innate and preorganized, involving the brain's limbic system, while secondary emotions reflect more cognitive, evaluative processes (Damasio, 1994, 1995). In consideration of this model of emotion, vACC activation in exclusion might reflect a primary emotional reaction to ostracism while dACC activation might reflect a secondary emotional reaction. A role of dorsal ACC in cognitive evaluation of the exclusion experience is consistent with its previously reported correlation with self-reported distress and rejection sensitivity in exclusion (Eisenberger et al., 2003, Masten et al., 2009). The present study, with its slightly modified version of Cyberball, may have also decreased the opportunity for cognitive evaluation in participants by making periods of exclusion substantially shorter (30 seconds) and thus transient.
While a cognitively evaluative role for the dACC might explain the absence of this region from our corrected whole brain Cyberball analyses, one might expect to see differential activation in this region in Cybershape based on the previous finding of dACC in expectancy violation (e.g. Somerville et al., 2006). We did not find greater dACC activity in rule violation compared to fair play. However, we did find extensive activation of dorsal mPFC extending into pre-supplementary motor area in this contrast. In meta-analyses, dorsal mPFC activity has been associated with action monitoring, error detection and negative feedback (Amodio & Frith, 2006; Ridderinkhof et al., 2004). This evidence, along with the study by Somerville et al. (2006), suggests that dorsal mPFC is involved in monitoring expectancies and error detection, but may also be part of a larger functional region including pre-supplementary motor area.
An area of the vACC found to be less active in rule violation than fair play overlaps substantially with a region that is more active in social exclusion (see Figures 3 and 4). It is possible that decreased activation of the vACC in rule violation is due to an inverse functional relation between activity in emotional and cognitive regions of the brain (Bush et al., 2000; Butler et al., 2007; Phan et al., 2005). Like the vACC, bilateral insula and supplementary motor area (SMA) also showed less activation in rule violation than in fair play. Functional connectivity between posterior insula and SMA has been found in resting state connectivity analyses (Taylor et al., 2009). Additionally, in our PPI analysis, vACC, insula, and SMA showed greater functional connectivity in rule violation compared to fair play. However, these three regions showed decreased activation in the same contrast. These findings raise important questions for further investigations on the roles of these regions.
The present study included some limitations. The collection of self-report data from only 7 participants, though illuminating in its validation of the effectiveness of the elicitation of distress from exclusion and rule violation, does not allow for correlations between behavioral data and brain activation as has been done in previous Cyberball studies. In addition, the method of removing activation to ball throws, while essential in removing a confound in Cyberball, may not perfectly remove activity resulting from decision-making processes, as the precise timing of these events is unknown. Our approach assumes that decisions are made in the period from when the participants receive the ball to when they throw it, but it is possible that they make a decision prior to receiving the ball. We time-locked ball throws to the period from when the participant received the ball to when they pressed the button to throw it, but presumably the participant could be making throw decisions before they receive the ball. Our method is effective in consistently removing activation corresponding to the act of pressing a button to throw the ball.
In sum, our results provide evidence for a functional dissociation of brain networks involved in the experiences of social exclusion and rule violation, two scenarios in which expectancies of others’ actions are violated. In Cyberball, an implicit, social rule of inclusion is violated in the social scenario of an internet game, while in Cybershape an explicit non-social rule of shape matching is violated in a comparable social setting. Thus, comparing the two games allows for the comparison of neural activation to a social versus non-social rule violation. The observation that both exclusion and rule violation were distressing to participants, yet each modulated a different set of brain regions supports the hypothesis that the distressing experience of social exclusion is unique from the distress evoked by expectancy violation in Cybershape. The robust findings in our novel Cybershape game support its validity as a contrast to Cyberball to assess sensitivity to rule-related expectancy violations as distinct from expectancy violations inherent in social exclusion. Our new Cybershape paradigm will be valuable in studying populations with clinical disorders that show differential sensitivities to expectancy violations, such as obsessive-compulsive disorder or autism spectrum disorder.
Table 5.
Between game comparison of differential activation in Cyberball and Cybershape. A paired samples t-test compared social exclusion – fair play in Cyberball to rule violation – fair play in Cybershape. Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest.
| Brain Region | X | Y | Z | t | size | p |
|---|---|---|---|---|---|---|
| Social Exclusion - Fair Play > Rule Violation - Fair Play | ||||||
| Right hippocampus | 30 | -37 | 1 | 3.73 | 342 | 0.00123 |
| White matter (CC) | 30 | -55 | 10 | 3.47 | 307 | 0.002304 |
| Right posterior cingulate cortex | 18 | -43 | 22 | 4.97 | 974 | 0.000065 |
| Right paracentral lobule | 15 | -25 | 43 | 5.26 | 804 | 0.000032 |
| Left Posterior Insula | -42 | -16 | 1 | 4.83 | 2345 | 0.000089 |
| Right posterior insula | 39 | -19 | 4 | 5.40 | 2810 | 0.000023 |
| Ventral ACC | 0 | 5 | -2 | 6.67 | 8836 | 0.000001 |
| Paracentral Lobule / Dorsal ACC | 6 | -4 | 46 | 5.00 | 1801 | 0.000059 |
| Left paracentral lobule | -6 | -34 | 46 | 4.06 | 504 | 0.000568 |
| Left posterior cingulate cortex | -33 | -49 | 7 | 5.98 | 8353 | 0.000006 |
| Left anterior cerebellum | -15 | -52 | -11 | 3.98 | 334 | 0.000683 |
| Left parahippocampal gyrus | -18 | -25 | -11 | 5.00 | 323 | 0.00006 |
| Left middle insula | -39 | -1 | 10 | 3.57 | 298 | 0.001796 |
| Rule Violation - Fair Play > Social Exclusion - Fair Play | ||||||
| Right dorsolateral PFC | 42 | 5 | 43 | -5.31 | 22245 | 0.000029 |
| Right superior frontal gyrus | 30 | 65 | -2 | -4.75 | 818 | 0.000109 |
| Left superior frontal gyrus | -33 | 62 | 7 | -5.64 | 8329 | 0.000014 |
| Right fusiform gyrus | 36 | -67 | -17 | -3.97 | 375 | 0.000692 |
| Left cerebellum | -21 | -64 | -32 | -4.01 | 2648 | 0.000633 |
| Left dorsolateral PFC | -48 | 8 | 31 | -5.95 | 11504 | 0.000007 |
| Left middle occipital gyrus | -36 | -85 | 13 | -4.71 | 731 | 0.00012 |
| Left fusiform gyrus | -36 | -70 | -17 | -4.50 | 1017 | 0.000197 |
| Right superior temporal gyrus | 63 | -28 | -8 | -3.68 | 896 | 0.001396 |
| Parietal cortex | -18 | -64 | 52 | -6.48 | 47309 | 0.000002 |
Abbreviations: prefrontal cortex (PFC), corpus callosum (CC), anterior cingulate cortex (ACC).
Acknowledgements
The research presented herein was supported by grants from the National Institute of Mental Health, the John Merck Scholars Fund, and the Simons Foundation. Kevin Pelphrey was supported by a Career Development Award from the National Institutes of Health (NIMH Grant MH071284). Linda Mayes was also supported by a Career Development Award (NIDA K05 DA020091). Naomi Pitskel was supported by a grant from the Doris Duke Charitable Foundation to Yale University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DGV, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJR. Modulation of emotion by cognition and cognition by emotion. NeuroImage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2:382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- Boyes ME, French DJ. Having a Cyberball: Using a ball-throwing game as an experimental social stressor to examine the relationship between neuroticism and coping. Pers Individ Dif. 2009;47:396–401. [Google Scholar]
- Brass M, Yves von Cramon D. Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Brass M, Ullsperger M, Knoesche TR, Yves von Cramon D, Phillips NA. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J Cogn Neurosci. 2005;17:1367–1375. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- Britton JC, Luan Phan K, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. NeuroImage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schachter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: A review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wallis JD, Parker A, Brass M, Crone EA, Hoshi E, Sakai K. Neural circuitry underlying rule use in humans and nonhuman primates. J Neurosci. 2005;25:10347–10350. doi: 10.1523/JNEUROSCI.2937-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Butler T, Imperato-McGinley J, Pan H, Voyer D, Cunningham-Bussel AC, Chang L, Zhu Y, Cordero JJ, Stern E, Silbersweig D. Sex specificity of ventral anterior cingulate cortex suppression during a cognitive task. Hum Brain Mapp. 2007;28:1206–1212. doi: 10.1002/hbm.20340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2005;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' error: emotion, reason, and the human brain. Grosset/Putnam; New York: 1994. [Google Scholar]
- Damasio AR. Toward a neurobiology of emotion and feeling: operational concepts and hypotheses. Neuroscientist. 1995;1:19–25. [Google Scholar]
- Davis H, IV, Liotti M, Ngan ET, Woodward TS, Van Snellenberg JX, van Anders SM, Smith A, Mayberg HS. fMRI BOLD signal changes in elite swimmers while viewing videos of personal failure. Brain Imaging Behav. 2008;2:84–93. [Google Scholar]
- Donohue SE, Wendelken C, Crone EA, Bunge SA. Retrieving rules for behavior from long-term memory. NeuroImage. 2005;26:1140–1149. doi: 10.1016/j.neuroimage.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow TFD, Zheng Y, Wilkinson ID, Spence SA, Deakin JFW, Tarrier N, Griffiths PD, Woodruff PWR. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12:2433–2438. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- Farrow TFD, Hunter MD, Wilkinson ID, Gouneea C, Fawbert D, Smith R, Lee K, Mason S, Spence SA, Woodruff PWR. Quantifiable change in functional brain response to empathic and forgivability judgments with resolution of posttraumatic stress disorder. Psychiatry Res. 2005;140:45–53. doi: 10.1016/j.pscychresns.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci, U.S.A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Maddock RJ. Separating subjective emotion from the perception of emotion-inducing stimuli: An fMRI study. NeuroImage. 2006;33:263–274. doi: 10.1016/j.neuroimage.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gosselin N, Samson S, Adolphs R, Noulhiane M, Roy M, Hasboun D, Baulac M, Peretz I. Emotional responses to unpleasant music correlates with damage to the parahippocampal cortex. Brain. 2006;129:2585–2592. doi: 10.1093/brain/awl240. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krill A, Platek SM. In-group and out-group membership mediates anterior cingulate activation to social exclusion. Front Evol Neurosci. 2009;1:1–7. doi: 10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kotchunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux J, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biol Psychiatry. 2000;30:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullette-Gillman OA, Huettel SA. Neural substrates of contingency learning and executive control: dissociating physical, valuative, and behavioral changes. Front in Hum Neurosci. 2009;3:1–9. doi: 10.3389/neuro.09.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc Neurosci. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Yoshimura S, Yamawaki S, Yamaguchi S, Ura M. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nsq002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: The perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci. 2004;10:1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore S. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage. 2006;31:468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Steele JD, Lawrie SM. Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. NeuroImage. 2004;21:868–875. doi: 10.1016/j.neuroimage.2003.09.066. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LF, Heslenfeld DJ, Koole SL. Tuning down the emotional brain: An fMRI study of the effects of cognitive load on the processing of affective images. NeuroImage. 2009;45:1212–1219. doi: 10.1016/j.neuroimage.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: Effects of being ignored over the internet. J Pers Soc Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD. Ostracism. Annu Rev Psychol. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Wirth JH, Williams KD. ‘They don't Like our kind’: Consequences of being ostracized while possessing a group membership. Group Process Intergroup Relat. 2009;12:111–127. [Google Scholar]
- Wood JN, Romero SG, Knutson KM, Grafman J. Representation of attitudinal knowledge: role of prefrontal cortex, amygdala and parahippocampal gyrus. Neuropsychologia. 2005;43:249–259. doi: 10.1016/j.neuropsychologia.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:287–301. [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. J Exp Soc Psychol. 2004;40:560–567. [Google Scholar]
- Zadro L, Williams KD, Richardson R. Riding the ‘O’ train: Comparing the effects of ostracism and verbal dispute on targets and sources. Group Process Intergroup Relat. 2005;8:125–143. [Google Scholar]
- Zahn R, de Oliveira-Souza R, Bramati I, Garrido G, Moll J. Subgenual cingulate activity reflects individual differences in empathic concern. Neurosci Lett. 2009;457:107–110. doi: 10.1016/j.neulet.2009.03.090. [DOI] [PubMed] [Google Scholar]