Abstract
Palmitic acid is a saturated fat found in foods that lead to obesity, cardiovascular disease, and Type II diabetes. It is linked to the development of resistance to insulin stimulation in muscle, liver and other organs involved in glucose metabolism, which, in turn, underlines the onset of Type II diabetes. The cellular and molecular mechanisms of this insulin resistance are complex and not completely understood. This article is focused on the role of palmitic acid as a precursor in the synthesis of sphingolipids, a class of lipid molecules that participate in cellular stress response. Recent evidence had indicated that increased dietary supply of palmitate can stimulate the rate of sphingolipid synthesis in “lean” tissues and generate excessive amounts of sphingolipid metabolites that have a negative effect on the insulin signaling cascade. Many experimental results point to the existence of a causative link between sphingolipid synthesis, insulin response, and hyperglycemia. It is not yet clear, however whether ceramides or glycosphingolipids are involved as both have been implicated to be inhibitors of the insulin signaling cascade. Evidence for a coordinated regulation of sphingolipid and tri/diacylglycerol metabolism complicates further the delineation of a single mechanism of sphingolipid effect on glucose homeostasis.
Keywords: ceramide, liver, triacylglycerols, serine palmitoyltransferase, acid sphingomyelinase
Introduction
“Metabolic syndrome” is a cluster of metabolic disorders including obesity, insulin resistance, hyperglycemia, dyslipidemia, hypertension, and cardiovascular disease. The consumption of food with high fat content and the resulting pathology in lipid metabolism is causatively linked to metabolic syndrome. In a healthy state, the excess fatty acids accumulate in the adipose tissues in the form of triacylglycerol (TAG); however, in conditions characterized by the metabolic syndrome, TAG droplets form in the “lean” organs such as liver, muscles, and pancreas. The accumulation of excessive fat, mostly neutral lipids, in the parenchymal cells of these organs is known as steatosis. The development of hepatic steatosis in particular, is a strong predictor for the onset of insulin resistance and hyperglycemia in obese individuals [1]. The precursor of TAG synthesis, diacylglycerol (DAG), has been identified as the bioactive metabolite that can inhibit insulin signaling pathway in liver and muscle, thus leading to a decreased cellular response to insulin stimulation and to the onset of hyperglycemia; however it is hard to accept that this is the sole of even the main mechanism, as increasing number of studies provide evidence that the different types of fats have complex effects on cell lipid homeostasis. Insulin resistance in particular is associated with high intake of saturated and monounsaturated fats and low intake of polyunsaturated fats. Epidemiological studies consistently suggest that the fatty acid pattern that is associated with insulin resistance includes high proportion of palmitic acid (a saturated fat) and palmitoleic acid (a monounsaturated fat), but low content of linoleic acid (a polyunsaturated fat) [2]. These specific fatty acids can induce changes at different level of cellular lipid metabolism and properties, including but not limited to changes in membrane fluidity and biophysical characteristics, the lipid microenvironement of integral membrane proteins, the rate of fatty acid oxidation, and gene expression via PPAR or other receptors that bind lipids (i.e. zTLR-4)[3].
A growing body of evidence however, shows that steatosis is also associated with an increased synthesis of sphinolipids and an accumulation of ceramide, a bioactive sphingolipid metabolite [4–7]. These findings had outlined a novel concept for the development of insulin resistance in lean tissues because the accumulation of sphingolipids and ceramide, in particular, during the consumption of high fat diet is linked solely to the increased up-take of palmitate, but not other fats.
Sphingolipids are class of lipids that contain a characteristic 18-carbon amino-alcohol, sphingosine, or its saturated analog, sphinganine. The rate-limiting step in the synthesis of sphingolipids is catalyzed by serine palmitoyltransferase (SPT) [8], which exhibits a high degree of specificity for the CoA-thioester of palmitic acid [9, 10]. The flux through the sphingolipid synthetic pathway depends upon the availability of palmitate [11, 12]. Substantial increases in sphingolipid synthesis have been observed in C2C12 myotubes, adipocytes, and hepatocytes when the culture medium is supplemented with palmitate, as well as in muscles and liver of mice, when the animals are fed food with elevate content of saturated fats, mainly palmitate. These additions have also lead to the accumulation of ceramide, a bioactive lipid molecule, and glycosphingolipids and sphingomyelin (SM), ceramide metabolites that are the major components of the lipid rafts. The latter are specialized microdomains on the plasma membrane that seems to play essential role in cell signaling by facilitating activation of various receptors by their respective ligands. The membrane microdomains have been recognized as critical for proper compartmentalization of insulin signaling [13].
A number of studies have suggested that palmitate-induced accumulation of ceramide might be responsible for the onset of insulin resistance in lean tissues [14, 15]. This evidence can be briefly summarized as follows:
Palmitate-independent increases in ceramide in various cells are sufficient to blunt cellular insulin response in vitro. Cell-permeable ceramide analogs have been shown to antagonize insulin signaling in myotubes [7, 16] and adipocytes [17]. The mechanisms of these effects of ceramide involve an inhibition of Akt-1, key mediator of the insulin signaling cascade. Ceramide efficiently blocks the translocation of Akt-1 to the plasma membrane [7] in muscle cells [16, 18, 19], in adipocytes [19] and in the cells of the vasculature [20, 21]. Increases in cellular ceramide content induce Akt dephosphorylation in C2C12 myotubes [14], adipocytes [22, 23], and PC12 cells [24] through the activation of protein phosphatase 2A.
Myriocin, cycloserine, or fumonisin B1 that specifically inhibit the de novo synthesis of sphingolipids are shown to promote normal phosphorylation and translocation of Akt-1 in response to insulin, even in the presence of excess palmitate. Inhibition of SPT in animals similarly blocks the flux of palmitate through the de novo pathway, leading to less lipotoxicity, improved insulin response, and better glucose regulation [25, 26]. Together, these data indicate that elevation in ceramide biosynthesis is required for the onset of insulin resistance; it is less certain however, whether ceramide effects are direct or mediated by other bioactive lipids.
Mechanisms of stimulation of de novo ceramide synthesis by palmitic acid
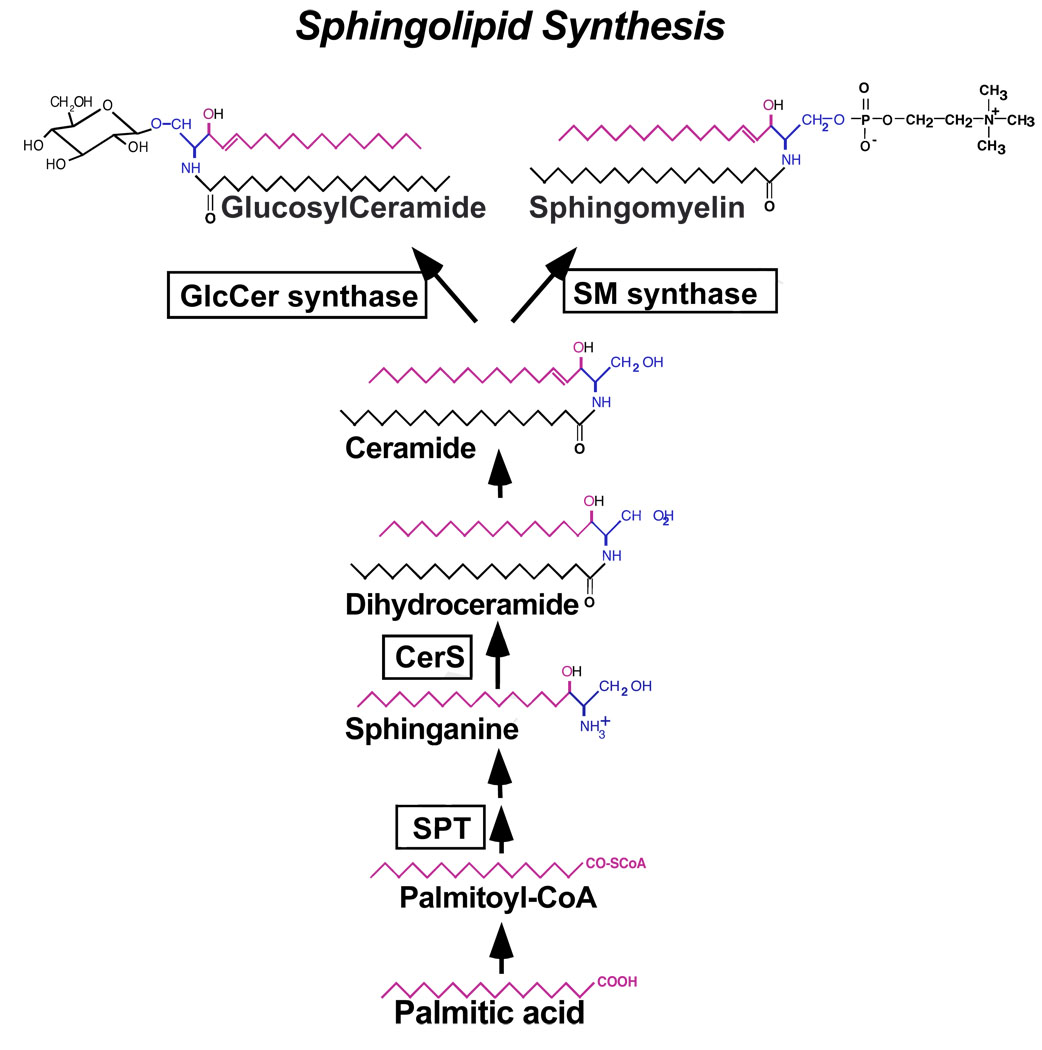
The sphingolipid biosynthesis (Fig. 1) starts in the endoplasmic reticulum with the condensation of L-serine and palmitoyl CoA, a reaction catalyzed by SPT. The product, 3 ketosphinganine, is first reduced to sphinganine and then it is acylated at the amide group by dihydroceramide synthase (CerS) forming dihydroceramide [27]. Over the past few years, six mammalian CerS (formerly known as Lass (longevity assurance)) genes were described, with each generating dihydroceramides with specific acyl chain lengths [28, 29]. Dihydroceramides however are biologically inactive and are converted to the bioactive form, ceramides, after the introduction of a characteristic 4,5-double bond in the sphingoid portion of the molecule.
Fig. 1.
Schematic representation of the de novo synthesis of sphingolipids.
A rate-limiting step in this pathway (often referred to as the de novo pathway) is the activity of SPT (reviewed in [8]) which is affected by the concentration of the available palmitate [11, 12]. Increases in the amount of palmitate (but not other free fatty acids) stimulate the formation of sphingolipids in a variety of cells. In cultured primary hepatocytes, for example, the addition of palmitic acid at a concentration as low as 0.4 mM induces sphingolipid biosynthesis within 4 h [30]. Similarly, HepG2 cells exhibit 3 to 6 fold higher rate of incorporation of radiolabeled palmitate when the latter is added at high (1 mM), as compared to low (0.1 mM) concentration [31]. Rat hepatoma H4IIE cells synthesize more ceramide when incubated with 0.5 mM palmitate but not when treated with oleate instead [32]. Increased biosynthesis of ceramide as a consequence of supplementation with palmitic acid has been shown also in long-term cultures of isolated islets [33], in muscle cells including C2C12 myotybes [14, 15, 34], L6 skeletal muscle cells [18, 35, 36], human vastus lateralis myoblasts [37], cardiac myocytes [38] and isolated rat soleus muscle [26], in human umbilical vein endothelial cells (HUVECs) [39], in cultured bovine retinal pericytes [40] and in astroglia [41]. Therefore, the upregulation of ceramide by elevated palmitate appears to be a common response in mammalian cells in culture.
Increased dietary intake of palmitate has similar effect on sphingolipid biosynthetic pathway in vivo. Rodents maintained on experimental diets enriched in saturated fats (with palmitate being the most abundant saturated fatty acid) have higher levels of ceramide in the plasma [42], liver [31], heart [43], soleus muscle and in the red, but not the white, section of gastrocnemius muscle [44]. Dihydroceramide and sphinganine, precursors of ceramide generation via the de novo pathway, are also elevated [31], consistent with a role of SPT in the diet-induced increases in ceramide content.
The mechanisms for this stimulation of the de novo sphingolipid synthesis have been extensively studied and seem to be more complex than anticipated. In addition to increasing the flux of the substrate palmitate through the pathway, diets enriched in saturated fats seems to affect the activity, the transcription, and the post-translational processing of SPT. An increase in mRNA levels of SPT1, one of the two subunits of SPT, was observed in developing hypothalamus [45], liver, [46], and adipose tissues of C57BL/6J mice fed diets enriched in fats [47]. mRNA levels of SPT1 and SPT2 are markedly elevated in adipose tissues [4] and myocardium of obese patients [48], and in different animal models of obesity such as ob/ob mice [4] and fa/fa rats [49]. Our recent study found no change in SPT mRNA levels in the liver of C57/Bl6 mice fed saturated fat-enriched diet, although SPT1 protein levels, as well as SPT specific activity were markedly increased [31].
At least in liver, the flux through the de novo pathway is also regulated by a negative-feedback mechanism reflecting the rate of sphingolipid clearance in the lysosomes. Our recent study found that the de novo sphingolipid synthesis is stimulated by palmitate more potently in mice and cells lacking acid sphingomyelinase, the enzyme that catalyzes the first step in the turnover of sphingomyelin in the endosomal/lysosomal subcellular compartment, as compared to animals and cells with functional acid sphingomyelinase [31]. The differences were abrogated by the SPT inhibitor, myriocin, which supports the existence of a negative-feed back mode of regulation of the de novo synthesis of sphingolipids.
Palmitate-induced stimulation of de novo ceramide synthesis is required, but not sufficient, for the onset of insulin resistance
Myriocin (ISP-1) is an atypic amino acid antibiotic derived from fungal strains such as Mycelia sterilia and Isaria sinclairii, which inhibits specifically SPT [50]. Since its discovery, it has been routinely used to test the role of the de novo sphingolipid synthesis in a variety of experimental systems. Myriocin treatment prevents palmitate-induced accumulation of ceramide in human myoblasts and C2C12 myotubes [37] [51] [14]. More importantly, it also seemed to restore normal insulin sensitivity of these cells evidenced by an improved phosphorylation of Akt-1 and GSK3β.
Administration of inhibitors of the de novo sphingolipid synthesis in animals reveals a diverse role of the pathway in the development of metabolic syndrome. Application of L-cycloserine, another SPT inhibitor, completely blocks the formation of ceramide in isolated pancreatic islets and β-cell lipoapoptosis in prediabetic Zucker fa/fa rats [49]. Treatment with myriocin lowers the levels of TAG and cholesterol in the plasma of ApoE knockout mice, a model of atherosclerosis [52] and decreases ceramide levels in plasma, liver and soleus muscle of Zucker fa/fa rats while improving overall glucose homeostasis [26]. Decreases in plasma ceramide levels, improved glucose homeostasis, and restoration of insulin stimulated Akt-1 phosphorylation in the liver and muscle were also achieved in myriocin-treated high-fat diet fed or ob/ob mice [42]. However, the above studies did not provide data in regards to the levels of sphingolipids in insulin sensitive tissues, which may not correlate with the plasma sphingolipid content [4]. Therefore, it is uncertain whether the improved whole body insulin sensitivity was a direct result of the blocked ceramide production in the liver and muscle or a general effect.
Recent reports began to suggest that although required, palmitate-induced stimulation of ceramide biosynthesis might not be the only mechanisms for the onset of insulin resistance. Several studies have showed restoration of insulin sensitivity in diet-induced hyperglycemic animals while ceramide content remained elevated. These data had suggested either that ceramide is not the direct lipid regulator, or that its metabolism is coordinately regulated with that of another bioactive metabolite. These two options are discussed below.
Possible role of the complex sphingolipids, glycocerbrosides and gangliosides
Once ceramide is synthesized in the endoplasmic reticulum, it is carried to the Golgi apparatus and converted to SM or glycosphingolipids (glucocerebrosides and gangliosides) by the addition of a head group to its primary hydroxyl. Ceramide is transferred in a non-vesicular manner by ceramide transfer protein CerT1, which contains an N-terminal plextrin homology (PH) domain and a C-terminal steroidogenic acute regulatory protein (StAR)-related (START) domain [53]. Some recent studies seem to indicate that CerT1-transferred ceramide is utilized more efficiently in the SM rather then glucosylceramide synthesis. According to recently proposed model, CerT1 extracts newly synthesized ceramide from the endoplasmic reticulum (ER), depending on its START domain and targets the Golgi apparatus, depending on its PH domain recognizing phosphatidylinositol-4-phosphate (PI4P). After the release of ceramide at the Golgi apparatus, CERT might bind diacylglycerol generated during SM synthesis and transfer it back to ER. SM synthase 1 and 2 (SMS1) and (SMS2) function as the key Golgi- and plasma membrane-associated SM synthases by catalyzing the transfer of phosphorylcholine head group from a phosphatidylcholine to the primary hydroxy group in ceramide [54]. In turn, glucosylceramide synthase (GCS) is a Golgi-localized membrane bound glycosyl transferase, which catalyzes the glycosylation of ceramide using UDP-glucose as a donor to produce glycosylceramide. The latter is a key player in the biosynthesis of more complex sphingolipid that are generated through stepwise addition of galactose, glucose, N-acetylglucosamine, or N-galactosamine at either the 3-O-positon or the 4-O-position of the sugar moiety [55]. Golgi-synthesized complex glucosphingolipids and SM undergo vesicular trafficking to the plasma membrane, where they are localized mainly at the outer leaflet. In the liver, SM and glycosphingolipids can also be incorporated into lipoprotein particles and secreted in the plasma.
The synthesis of these complex sphingolipids increases following palmitate-induced stimulation of ceramide generation. The resulting accumulation of glucosylceramide and complex glycosphingolipid metabolites including ganlioside GM3 is proposed to have a deleterious effect on insulin response and glucose regulation. Patients with type I Gaucher disease, an inheritable glycosphingolipid storage disorder caused by mutations in glucosylceramidase gene, accumulate glucosylceramide in the lysosomes of many tissues and organs and have impaired peripheral insulin sensitivity [56]. The levels of GM3 synthase mRNA in adipose tissue of obese Zucker fa/fa rat and ob/ob mouse are higher than in tissues from their lean counterparts [57]. Moreover, the addition of exogenous GM3 to adipocytes has been found to suppress insulin-stimulated tyrosine phosphorylation of the insulin receptor, the binding of insulin receptor substrate-1, and respectively, insulin-stimulated glucose uptake [57], suggesting that diet-induced elevation in glycosphingolipids might be causatively linked to the onset of insulin resistance. Indeed, an increase in glucosylceramide content in the retina of diabetic rats has been shown to accelerate local insulin resistance resulting in neuronal cell death and the development of diabetic retinopathy [58]. Pharmacological inhibition of GCS however restores insulin sensitivity in cultured retinal neurons [58] and improves the overall glucose homeostasis and insulin sensitivity in ob/ob mice, Zucker fa/fa rats and mice fed diet high in fat [59, 60]. The treatment also prevents the loss of insulin secretion by the pancreatic β-cells, and restores insulin signaling in the muscles of Zucker fa/fa rats [60]. Suppression of glycosphingolipid biosynthesis in ob/ob mice by feeding with AMP-DNM iminosugar, restores the ex vivo measured insulin signaling in adipocytes, improves the overall adipogenesis, GLUT-4 translocation, and lessens inflammation in adipose tissue [61]. The same GCS inhibitor prevents hepatomegaly, decreases the development of steatosis and dramatically improves insulin signaling in the liver of ob/ob mice [62]. Oddly enough, these beneficial effects take place while ceramide levels are not changed, or even in some occasions are increased [60, 62]. In a very recent study, glucosylceramide biosynthesis in hepatocytes was effectively blocked using the Cre/loxP system to delete GCS in liver [63]. However, no alteration in glucose tolerance after intraperitoneal application of glucose and insulin nor protection against high-fat diet steatosis appeared in the mutant animals [64]. Seemingly, the dilemma of which bioactive metabolite, ceramide or a higher glycocerebroside, is directly involved in the onset of insulin resistance remains unsolved.
Coordinated partitioning of exogenous palmitate into sphingolipid and TAG metabolic pools
Several recent studies have suggested that palmitate-induced ceramide accumulation may play a more diverse and complex role in the onset of insulin resistance. These studies have provided compelling evidence that the de novo sphingolipid pathway is metabolically linked to TAG synthesis and may have adverse effect on DAG and TAG accumulation in tissues.
The accumulation of fat in the cells of lean tissue, mainly liver and muscle is obesity-related pathology referred to as steatosis which correlates with the development of insulin resistance. The excess fatty acids can enter three lipid pools that are metabolically interconnected but have different impact on cellular responsiveness to insulin. DAG, the common precursor for TAG and glycerophospholipid synthesis is considered the culprit that impairs insulin signaling during steatosis. However, evidence have indicated that the different DAG pools play a different role in impairing insulin responsiveness. Two DGATs, DGAT1 and DGAT2 can convert DAG into TAG for storage, or into glycerophospholipids for membrane biogenesis. Deletion of DGAT1 has only a minor effect on TAG synthesis. Its overexpression is sufficient to elevate synthesis of TAG however, surprisingly, it also reduces that of glycerophospholipids, suggesting that the overexpressed enzyme uses a pool of DAG initially designed for phospholipid synthesis [65]. In contrast, overexpression of DGAT2 has much greater effect on TAG accumulation than DGAT1, and its silencing inhibits hepatic TAG synthesis [66]. Notably, suppression of DGAT2, but not DGAT1, can reverse diet-induced hepatic steatosis and insulin resistance [67]. Thus, the two DGAT forms seem to use two distinct and competitive pools of DAG that are specifically designated for TAG or phospholipids synthesis while only DGAT2-accessible pool seems to impair insulin signaling [65].
Recently published work has suggested that similar interaction exists between TAG and sphingolipid metabolic pathways. Initial indications to that effect have emerged from the intriguing observation that only short-term inhibition of SPT ameliorated palmitate-induced insulin resistance of L6 muscle cells, while sustained loss of SPT activity brought about by gene silencing or prolonged pharmacological inhibition failed to do so. Experiments aimed at understanding the reason of this bimodal effect revealed that in the cells with constitutively reduced de novo sphingolipid synthesis, the flux of exogenous palmitate was redirected preferentially towards TAG synthetic pathway leading to overaccumulation of TAG and the TAG precursor, DAG [68]. Apparently, in spite of the successful elimination of palmitate-induced ceramide increases, TAG/DAG overproduction is sufficient to impair insulin signaling. This observation also suggests that the sphingolipid and TAG synthetic pathways most likely compete for the available palmitate.
Further evidence for competitive partitioning of exogenous palmitate between TAG an sphingolipid pools had come from work in our lab. Deletion of the gene for acid sphingomyelinase, whose activity seems to inhibit the de novo sphingolipid synthesis, in mice leads to chronic up-regulation of the pathway and excessive hepatic accumulation of sphinganine, ceramide and more complex sphingolipids, which is especially pronounced when the animals were placed on diet rich in saturated fats for up to 12 weeks. Notably, this is paralleled by a complete elimination of diet-induced TAG accumulation and by an improved insulin response in vivo. Apparently, in this experimental model, dietary palmitate again partitioned into TAG and sphingolipid pool in a competitive manner, dependent on the rate of sphingolipid synthesis.
The interaction(s) between TAG and sphingolipid metabolic pathways during diet-induced obesity however may be quite complex. This is implied by the somewhat underappreciated role of SM synthase (SMS1) as generator of DAG in an unique compartment of the cell, the Golgi apparatus [69]. The activity of SM synthase results in the generation of one molecule of DAG for each molecule of SM. Increases in the synthesis of ceramide, including those ensued from a diet, cause reciprocal stimulation of synthesis of SM, and consequently leads to an increased generation of DAG in the Golgi. The fate of the SMS-generated DAG is unclear. It is of interest that neither of the two DAG-Acyl transferases, DGAT1 and DGAT2, are localized in the Golgi [70], and one possible explanation is that excessive stimulation of SM synthase may cause re-distribution of DAG pools away from TAG synthesis and towards phospholipid synthesis.
Concluding Remarks
It is well known that accumulation of TAG in lean tissue is a strict predictor of the development of insulin resistance; however, it is also clear that TAG is not the active mediator. Instead, the TAG precursor, DAG, is considered the culprit because it stimulates PKC, which in turn down-regulates the insulin signaling cascade. Data discussed in the review summarize some of the published evidence that consumption of diet rich in saturated fats, palmitate in particular, causes not only TAG accumulation but also stimulation of the de novo synthesis of ceramide, SM and complex sphingolipids that is causatively linked to the off-set of insulin responses in muscle, liver, and adipose tissue. In contrast to the TAG metabolic pathway, which exhibits no preference for a particular fatty acid in the diet, the stimulation of sphingolipid synthesis is specifically linked to the presence of palmitic acid, the most deleterious fat for the pathophysiology of obesity. The flux through the sphingolipid pathway seems also to regulate the extent of TAG and may be DAG accumulation, suggesting interplay between the two pathways, and respectively the two bioactive lipids, DAG and ceramide, in the onset of insulin resistance and hyperglycemia.
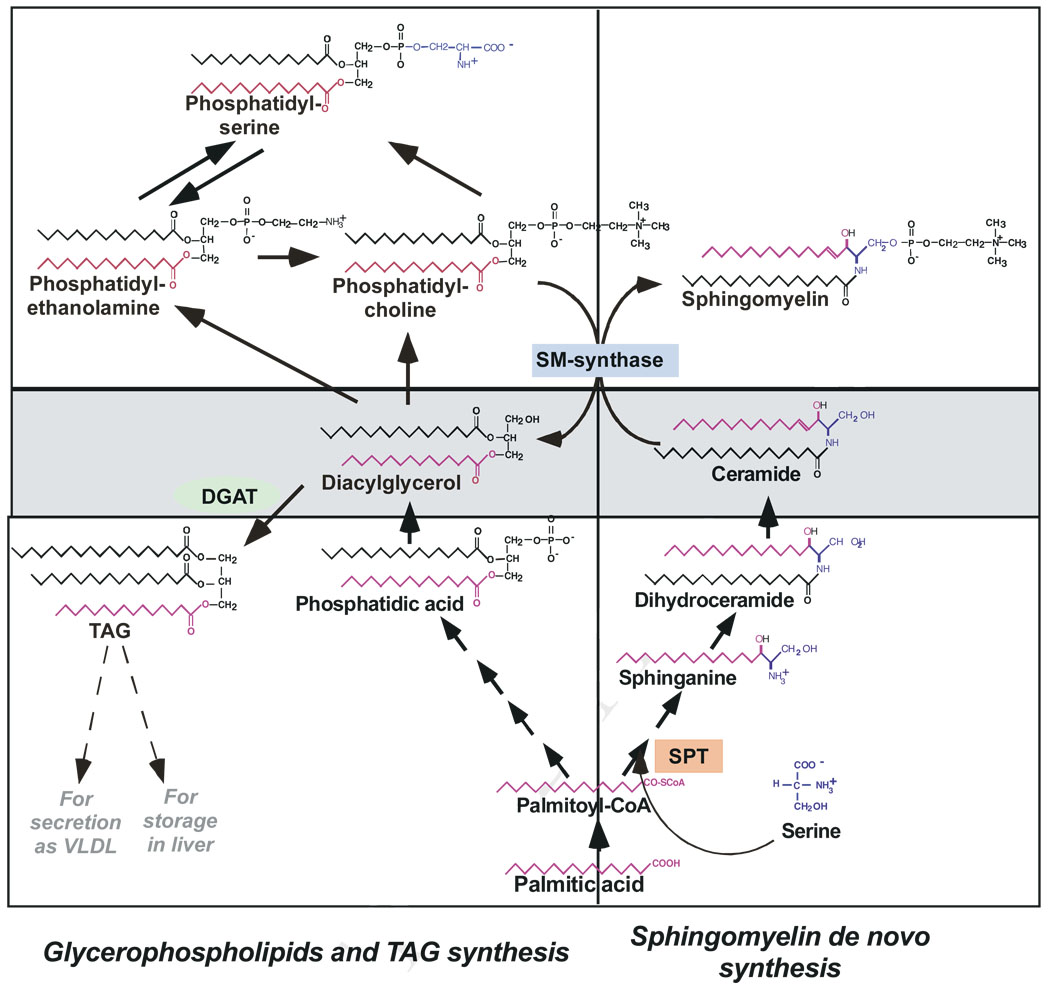
Fig. 2.
Proposed interplay between TAG, glycerophospholipids and sphingolipid metabolism. De novo synthesis of sphingolipids occurs via SPT in the endoplasmic reticulum. A ceramide transfer protein transports ceramide to the Golgi apparatus, where complex sphingolipids, like SM and glycosphingolipids (not shown) are synthesized. Complex sphingolipids are then transferred to the plasma membrane, which contains more then 75% of the total cellular sphingolipids.
The synthesis of both TAG and glycerophospholipid from activated fatty acids follows a common pathway to the formation of DAG. DAG can be used either for TAG synthesis, storage, and secretion or for synthesis of the two main glycerophospholipid classes, phosphatidylcholine (PC) or phosphatidylserine (PS). Metabolically, the sphingolipid, glycerophosoholipid and TAG pathways are linked through SM synthase, shown in the middle of the diagram. Exogenous palmitic acid partitions in a competitive fashion between sphingolipid and TAG pools.
DAG and ceramide (boxed in the middle) are bioactive metabolites implicated in down-regulation of insulin signaling cascade. As the diagram indicates, they are in the crossroad of mulltiple pathways and their levels are subject to complex regulation.
Acknowledgments
This work was supported in parts by grants AG026711 and AG 019223
Abbreviations
- DAG
diacylglycerol
- DGAT
DAG acyltransferase
- CerS
Dihydroceramide synthase
- ER
endoplasmic reticulum
- GlcCer
glucosylceramide
- GCS
glucosylceramide synthase
- PA
palmitic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- SPT
serine palmitoyltransferase
- SM
sphingomyelin
- SMS
sphingomyelin synthase
- TAG
triacylglycerides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic Steatosis: A Mediator of the Metabolic Syndrome. Lessons From Animal Models. Arterioscler Thromb Vasc Biol. 2004;24:644–649. doi: 10.1161/01.ATV.0000116217.57583.6e. [DOI] [PubMed] [Google Scholar]
- 2.Riserus U. Fatty acids and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 2008;11:100–105. doi: 10.1097/MCO.0b013e3282f52708. [DOI] [PubMed] [Google Scholar]
- 3.Galgani JE, Uauy RD, Aguirre CA, Díaz EO. Effect of the dietary fat quality on insulin sensitivity. British Journal of Nutrition. 2008;100:471–479. doi: 10.1017/S0007114508894408. [DOI] [PubMed] [Google Scholar]
- 4.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55:2579–2587. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- 5.Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–2373. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- 6.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, Hundal HS. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001;44:173–183. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- 8.Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. 2003;1632:16–30. doi: 10.1016/s1388-1981(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 9.Williams RD, Wang E, Merrill AH., Jr Enzymology of long-chain base synthesis by liver: characterization of serine palmitoyltransferase in rat liver microsomes. Arch Biochem Biophys. 1984;228:282–291. doi: 10.1016/0003-9861(84)90069-9. [DOI] [PubMed] [Google Scholar]
- 10.Merrill AH., Jr Characterization of serine palmitoyltransferase activity in Chinese hamster ovary cells. Biochim Biophys Acta. 1983;754:284–291. doi: 10.1016/0005-2760(83)90144-3. [DOI] [PubMed] [Google Scholar]
- 11.Merrill AH, Jr, Wang E, Mullins RE. Kinetics of long-chain (sphingoid) base biosynthesis in intact LM cells: effects of varying the extracellular concentrations of serine and fatty acid precursors of this pathway. Biochemistry. 1988;27:340–345. doi: 10.1021/bi00401a051. [DOI] [PubMed] [Google Scholar]
- 12.Messmer TO, Wang E, Stevens VL, Merrill AH., Jr Sphingolipid biosynthesis by rat liver cells: effects of serine, fatty acids and lipoproteins. J Nutr. 1989;119:534–538. doi: 10.1093/jn/119.4.534. [DOI] [PubMed] [Google Scholar]
- 13.Inokuchi J. Membrane microdomains and insulin resistance. FEBS Lett. 584:1864–1871. doi: 10.1016/j.febslet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 16.Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miura A, Kajita K, Ishizawa M, Kanoh Y, Kawai Y, Natsume Y, Sakuma H, Yamamoto Y, Yasuda K, Ishizuka T. Inhibitory effect of ceramide on insulin-induced protein kinase Czeta translocation in rat adipocytes. Metabolism. 2003;52:19–24. doi: 10.1053/meta.2003.50011. [DOI] [PubMed] [Google Scholar]
- 18.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, Dugail I, Hundal HS. Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J. 2008;410:369–379. doi: 10.1042/BJ20070936. [DOI] [PubMed] [Google Scholar]
- 20.Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 21.Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem. 2007;282:12450–12457. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 22.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 23.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 24.Salinas M, Lopez-Valdaliso R, Martin D, Alvarez A, Cuadrado A. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol Cell Neurosci. 2000;15:156–169. doi: 10.1006/mcne.1999.0813. [DOI] [PubMed] [Google Scholar]
- 25.Holland WL, Summers SA. Sphingolipids, Insulin Resistance, and Metabolic Disease: New Insights from in Vivo Manipulation of Sphingolipid Metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Merrill AH, Jr, Wang E. Biosynthesis of long-chain (sphingoid) bases from serine by LM cells. Evidence for introduction of the 4-trans-double bond after de novo biosynthesis of N-acylsphinganine(s) J Biol Chem. 1986;261:3764–3769. [PubMed] [Google Scholar]
- 28.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When Do Lasses (Longevity Assurance Genes) Become CerS (Ceramide Synthases)? Journal of Biological Chemistry. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 29.Pewzner-Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, Erez-Roman R, Brügger B, Sachsenheimer T, Wieland F, Prieto M, Merrill AH, Futerman AH. A Critical Role for Ceramide Synthase 2 in Liver Homeostasis. Journal of Biological Chemistry. 2010;285:10902–10910. doi: 10.1074/jbc.M109.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merrill AH, Jr, Lingrell S, Wang E, Nikolova-Karakashian M, Vales TR, Vance DE. Sphingolipid biosynthesis de novo by rat hepatocytes in culture. Ceramide and sphingomyelin are associated with, but not required for, very low density lipoprotein secretion. J Biol Chem. 1995;270:13834–13841. doi: 10.1074/jbc.270.23.13834. [DOI] [PubMed] [Google Scholar]
- 31.Deevska GM, Rozenova KA, Giltiay NV, Chambers MA, White J, Boyanovsky BB, Wei J, Daugherty A, Smart EJ, Reid MB, Merrill AH, Jr, Nikolova-Karakashian M. Acid Sphingomyelinase Deficiency Prevents Diet-induced Hepatic Triacylglycerol Accumulation and Hyperglycemia in Mice. J Biol Chem. 2009;284:8359–8368. doi: 10.1074/jbc.M807800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 33.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278:30015–30021. doi: 10.1074/jbc.M302548200. [DOI] [PubMed] [Google Scholar]
- 34.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J. 2006;399:473–481. doi: 10.1042/BJ20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JS, Pinnamaneni SK, Eo SJ, Cho IH, Pyo JH, Kim CK, Sinclair AJ, Febbraio MA, Watt MJ. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol. 2006;100:1467–1474. doi: 10.1152/japplphysiol.01438.2005. [DOI] [PubMed] [Google Scholar]
- 37.Pickersgill L, Litherland GJ, Greenberg AS, Walker M, Yeaman SJ. Key role for ceramides in mediating insulin resistance in human muscle cells. J Biol Chem. 2007;282:12583–12589. doi: 10.1074/jbc.M611157200. [DOI] [PubMed] [Google Scholar]
- 38.Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2000;279:H2124–H2132. doi: 10.1152/ajpheart.2000.279.5.H2124. [DOI] [PubMed] [Google Scholar]
- 39.Xiao-Yun X, Zhuo-Xiong C, Min-Xiang L, Xingxuan H, Schuchman EH, Feng L, Han-Song X, An-Hua L. Ceramide mediates inhibition of the AKT/eNOS signaling pathway by palmitate in human vascular endothelial cells. Med Sci Monit. 2009;15:BR254–BR261. [PubMed] [Google Scholar]
- 40.Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–1845. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 41.Patil S, Melrose J, Chan C. Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur J Neurosci. 2007;26:2131–2141. doi: 10.1111/j.1460-9568.2007.05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, Tserng KY, Hoit BD, Ernsberger P, Young ME, Stanley WC. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–H44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 44.Zendzian-Piotrowska M, Baranowski M, Zabielski P, Gorski J. Effects of pioglitazone and high-fat diet on ceramide metabolism in rat skeletal muscles. J Physiol Pharmacol. 2006;57 Suppl 10:101–114. [PubMed] [Google Scholar]
- 45.Rotta LN, Da Silva CG, Perry ML, Trindade VM. Undernutrition decreases serine palmitoyltransferase activity in developing rat hypothalamus. Ann Nutr Metab. 1999;43:152–158. doi: 10.1159/000012781. [DOI] [PubMed] [Google Scholar]
- 46.Lyn-Cook LE, Jr, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, Mark P, Wands JR, Xu H, de la Monte SM. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;16:715–729. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem. 2008;283:13538–13548. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baranowski M, Blachnio-Zabielska A, Hirnle T, Harasiuk D, Matlak K, Knapp M, Zabielski P, Gorski J. Myocardium of type 2 diabetic and obese patients is characterized by alterations in sphingolipid metabolic enzymes but not by accumulation of ceramide. J Lipid Res. 2009 doi: 10.1194/jlr.M900002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 50.Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem Biophys Res Commun. 1995;211:396–403. doi: 10.1006/bbrc.1995.1827. [DOI] [PubMed] [Google Scholar]
- 51.Sabin MA, Stewart CE, Crowne EC, Turner SJ, Hunt LP, Welsh GI, Grohmann MJ, Holly JM, Shield JP. Fatty acid-induced defects in insulin signalling, in myotubes derived from children, are related to ceramide production from palmitate rather than the accumulation of intramyocellular lipid. J Cell Physiol. 2007;211:244–252. doi: 10.1002/jcp.20922. [DOI] [PubMed] [Google Scholar]
- 52.Park TS, Panek RL, Rekhter MD, Mueller SB, Rosebury WS, Robertson A, Hanselman JC, Kindt E, Homan R, Karathanasis SK. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis. 2006;189:264–272. doi: 10.1016/j.atherosclerosis.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 53.Hanada K. Intracellular trafficking of ceramide by ceramide transfer protein. Proceedings of the Japan Academy, Series B. 2010;86:426–437. doi: 10.2183/pjab.86.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tafesse FG, Huitema K, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, Somerharju P, Holthuis JC. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem. 2007;282:17537–17547. doi: 10.1074/jbc.M702423200. [DOI] [PubMed] [Google Scholar]
- 55.Wennekes T, van†den†Berg RJBHN, Boot RG, van†der†Marel GA, Overkleeft HS, Aerts JMFG. Glycosphingolipids†-†Nature, Function, and Pharmacological Modulation. Angewandte Chemie International Edition. 2009;48:8848–8869. doi: 10.1002/anie.200902620. [DOI] [PubMed] [Google Scholar]
- 56.Langeveld M, Ghauharali KJ, Sauerwein HP, Ackermans MT, Groener JE, Hollak CE, Aerts JM, Serlie MJ. Type I Gaucher disease, a glycosphingolipid storage disorder, is associated with insulin resistance. J Clin Endocrinol Metab. 2008;93:845–851. doi: 10.1210/jc.2007-1702. [DOI] [PubMed] [Google Scholar]
- 57.Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, Sakaue S, Igarashi Y. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 58.Fox TE, Han X, Kelly S, Merrill AH, 2nd, Martin RE, Anderson RE, Gardner TW, Kester M. Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes. 2006;55:3573–3580. doi: 10.2337/db06-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aerts JM, Ottenhoff R, Powlson AS, Grefhorst A, van Eijk M, Dubbelhuis PF, Aten J, Kuipers F, Serlie MJ, Wennekes T, Sethi JK, O'Rahilly S, Overkleeft HS. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–1349. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, Komarnitsky S, Yew NS, Cheng SH. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes. 2007;56:1210–1218. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- 61.van Eijk M, Aten J, Bijl N, Ottenhoff R, van Roomen CP, Dubbelhuis PF, Seeman I, Ghauharali-van der Vlugt K, Overkleeft HS, Arbeeny C, Groen AK, Aerts JM. Reducing glycosphingolipid content in adipose tissue of obese mice restores insulin sensitivity, adipogenesis and reduces inflammation. PLoS One. 2009;4:e4723. doi: 10.1371/journal.pone.0004723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bijl N, Sokolovic M, Vrins C, Langeveld M, Moerland PD, Ottenhoff R, van Roomen CP, Claessen N, Boot RG, Aten J, Groen AK, Aerts JM, van Eijk M. Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology. 2009;50:1431–1441. doi: 10.1002/hep.23175. [DOI] [PubMed] [Google Scholar]
- 63.Jennemann R, Rothermel U, Wang S, Sandhoff R, Kaden S, Out R, van Berkel TJ, Aerts JM, Ghauharali K, Sticht C, Grone HJ. Hepatic glycosphingolipid deficiency and liver function in mice. Hepatology. 51:1799–1809. doi: 10.1002/hep.23545. [DOI] [PubMed] [Google Scholar]
- 64.Jennemann R, Rothermel U, Wang S, Sandhoff R, Kaden S, Out R, Berkel TJv, Aerts JM, Ghauharali K, Sticht C, Grˆne H-J. Hepatic glycosphingolipid deficiency and liver function in mice. Hepatology. 2010;51:1799–1809. doi: 10.1002/hep.23545. [DOI] [PubMed] [Google Scholar]
- 65.Bagnato C, Igal RA. Overexpression of diacylglycerol acyltransferase-1 reduces phospholipid synthesis, proliferation, and invasiveness in simian virus 40-transformed human lung fibroblasts. J Biol Chem. 2003;278:52203–52211. doi: 10.1074/jbc.M305760200. [DOI] [PubMed] [Google Scholar]
- 66.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and Skin Barrier Abnormalities in DGAT2-deficient Mice. J. Biol. Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 67.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 68.Watson ML, Coghlan M, Hundal HS. Modulating serine palmitoyl transferase (SPT) expression and activity unveils a crucial role in lipid-induced insulin resistance in rat skeletal muscle cells. Biochem J. 2009;417:791–801. doi: 10.1042/BJ20081149. [DOI] [PubMed] [Google Scholar]
- 69.Villani M, Subathra M, Im YB, Choi Y, Signorelli P, Del Poeta M, Luberto C. Sphingomyelin synthases regulate production of diacylglycerol at the Golgi. Biochem J. 2008;414:31–41. doi: 10.1042/BJ20071240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carrasco S, MÈrida I. Diacylglycerol, when simplicity becomes complex. Trends in Biochemical Sciences. 2007;32:27–36. doi: 10.1016/j.tibs.2006.11.004. [DOI] [PubMed] [Google Scholar]