Abstract
Background
Brain iron promotes neurodegeneration in Parkinson’s disease (PD). While hemoglobin (Hb) is the most abundant source of peripheral iron in humans, its relationship with PD is uncertain. This report examines the association between Hb in late-life and PD incidence.
Methods
From 1991-1993, Hb was measured in 3,507 men in the Honolulu-Asia Aging Study. Men were aged 71-93 years and without PD. Participants were followed until 2001 for incident PD.
Results
Hb levels declined markedly with age. For men aged 71-75 years, 14.8% had levels <14 g/dL versus 53.6% in those aged 86 and older (p<0.001). During follow-up, 47 men developed PD (19.8/10,000 person-years). After age-adjustment, PD incidence rose significantly from 10.3 to 34.9/10,000 person-years as Hb increased from <14 to ≥16 g/dL (p=0.024, relative hazard 3.2, 95% CI 1.2-8.9). Associations persisted after accounting for early mortality and adjustments for concomitant risk factors.
Conclusions
While Hb declines with advancing age, evidence suggests that Hb that remains high in elderly men is associated with an increased risk of PD.
Keywords: Hemoglobin, iron, Parkinson’s disease, epidemiology
1. Introduction
Iron deposition occurs in the substantia nigra with normal aging but its accumulation is markedly higher in the brains of patients with Parkinson’s disease (PD) [Bartzokis et al., 2007; Kaur and Andersen, 2004]. At toxic levels, brain iron can exacerbate neurodegeneration by promoting oxidative stress, altered myelin synthesis, aggregation of α-synuclein, and neuronal death that is associated with PD and related syndromes. Some have proposed that iron chelation or screening for brain iron ferritin could have a role in the development of novel strategies for primary intervention and the treatment of PD [Bartzokis et al., 2007; Kaur et al., 2009].
As the most significant source of peripheral iron in humans, the role of hemoglobin (Hb) could be important in the presence of ongoing dysregulation in iron homeostasis [Kaur and Andersen, 2004]. Some evidence suggests that brain iron levels can be modulated by peripheral iron [Bartzokis et al., 2007; Beard and Connor, 2003; Hallgren and Sourander, 1958; Pinero et al., 2000]. A combined role with α-synuclein may also be important. Nearly all α-synuclein in human blood is found in red blood cells (which are also largely composed of Hb) [Barbour et al, 2008]. Through metal binding and aggregation of α-synuclein, iron rich Hb could have an important role in formation of Lewy bodies, the hallmark pathology of PD [Wolozin and Golts, 2002]. The presence of Hb formations (nitrosyl Hb) in the substantia nigra could be especially meaningful in promoting brain iron dysregulation, early declines in nigral glutathione levels, and increasing dopaminergic nitric oxide [Cammack et al., 1998; Chinta et al., 2007; Shergill et al., 1996]. Mechanisms and interactions that involve iron and Hb in PD neurodegeneration, however, remain obscure, particularly in late-life. The purpose of this report is to examine the association between late-life Hb levels and the incidence of PD.
2. Methods
2.1. Study design
From 1965 to 1968, the Honolulu Heart Program began following 8,006 men of Japanese ancestry living on the island of Oahu, Hawaii for the development of cardiovascular disease [White et al., 1996]. Beginning with examinations that were given from 1991 to 1993, the Honolulu-Asia Aging Study was created as an expansion of the Honolulu Heart Program to study neurodegenerative diseases and cognitive function in the elderly [White et al., 1996]. Subjects included 3,741 men aged 71 to 93 years (approximately 80% of the survivors in the original Honolulu Heart Program cohort). Procedures were in accordance with institutional guidelines and approved by an institutional review board. Written informed consent was obtained from study participants at all examinations.
For this report, follow-up began when late-life Hb levels were measured at the beginning of the Honolulu-Asia Aging Study (1991-1993). Men with prevalent PD (n=66) were excluded as were an additional 168 with missing Hb data. The remaining sample consisted of 3,507 elderly men with follow-up for incident PD that was identified during three repeat neurologic examinations occurring from 1994 to 2001.
2.2. Measurement of Hb and confounding data
Hematology measures made at the beginning of study follow-up (1991-1993) utilized the Technicon H-1 automated hematology analyzer [Bollinger et al., 1987]. For Hb, the Technicon system used a modified manual cyanmethemoglobin method. Cells were lysed, Hb was converted to cyanmethemoglobin, and the absorbance was measured at 546 nm. For this laboratory, the normal range for Hb was 14 to 18 g/dL.
To help isolate the independent association between Hb and the future risk of PD, other factors were considered as possible sources of confounding. They included age, mid-life pack-years of cigarette smoking and coffee intake, constipation, excessive daytime sleepiness, and prevalent cancer (other than non-melanoma skin cancer). Mid-life pack-years of cigarette smoking and coffee intake were measured at the time of initiation of the Honolulu Heart Program (1965-1968) as markers of typical lifetime exposures to these factors [Ross et al, 2000]. Late-life coffee intake was not determined at the time when follow-up began (1991-1993) and current cigarette smoking was too uncommon to allow for its careful assessment. Determination of the other characteristics coincided with the measurement of Hb (1991-1993).
Constipation was defined as having <1 bowel movement on a typical day [Abbott et al, 2001]. Excessive daytime sleepiness was assessed through the use of a questionnaire administered by a trained research technician. Those who reported being sleepy most of the day were defined as having excessive daytime sleepiness [Abbott et al, 2005]. Prevalent cancer was defined as cancer that predated the beginning of follow-up or was prevalent at the time when follow-up began. Diagnoses of cancer were ascertained from a comprehensive system of surveillance by a panel of physician investigators with access to medical records, hospital discharges, and tumor registry data.
2.3. PD case finding and diagnosis
At the time when Hb was measured (1991-1993) and during the course of follow-up (1994-2001), all subjects were questioned about a diagnosis of PD and the use of PD medications by a structured interview. Study participants received further screening by a technician trained by a study neurologist to recognize the clinical symptoms of parkinsonism (including gait disturbance, tremor, and bradykinesia). Participants with a history of PD or clinical signs of parkinsonism were referred to a study neurologist who administered standardized questions about the symptoms and onset of parkinsonism, previous diagnoses, and medication use, followed by a comprehensive and standardized neurologic examination. All diagnoses of PD were made by study neurologists according to published criteria without access to risk factor data examined in this report [Ward and Gibb, 1990]. These required that the subject have the following: (1) parkinsonism (any two of bradykinesia, rest tremor, rigidity, or postural reflex impairment); (2) a progressive disorder; (3) any two of a marked response to levodopa, asymmetry of signs, asymmetry at onset, or initial onset tremor; and (4) absence of any etiology known to cause similar features. Cases of parkinsonism that were related to progressive supranuclear palsy, multiple system atrophy, cerebrovascular disease, drug induced parkinsonism, post-encephalitic parkinsonism, or post-traumatic parkinsonism were excluded from follow-up based on a thorough screen for medications associated with parkinsonism, a comprehensive system of stroke surveillance since 1965, and access to medical records in a highly cooperative community where outmigration is rare [Abbott et al, 2007; Ross et al, 2000].
2.4. Statistical methods
The intent of the current report is to assess the association between Hb and the risk of PD across ranges of observed Hb values without regard to clinical definitions of normal Hb, anemia, or polycythemia. For the purpose of showing how the incidence of PD changes with Hb levels, crude and age-adjusted incidence rates of PD in person-years were estimated across ranges of Hb (<14, 14-15.9, and ≥16 g/dL) based on standard analysis of covariance methods [Lane and Nelder, 1982]. Although the selection of these cut-points is arbitrary, their use was meant to provide a description of overall associations. In contrast, as a test for trend (and possible curvature) formal statistical testing was based on the more objective use of Hb as a continuous variable. Average and percentages of other factors, along with deaths/100 person-years, were also derived and age-adjusted across the Hb ranges.
To assess the effect of Hb (and other factors) on the incidence of PD, proportional hazards regression models were used [Cox, 1972]. When analyses were based on a small number of PD cases, logistic regression models were examined using exact testing methods [Mehta and Patel, 1995]. The logistic models were further adapted for a survival analysis where parameter estimates are known to be similar to those in a proportional hazards regression model, particularly in the instance when event counts are low [Abbott, 1985]. Adjustments were made for age and the other study characteristics. Relative hazards of PD (and 95% confidence intervals) between Hb ranges were also estimated with the Hb strata modeled as discrete indicator variables. All reported p-values were based on two-sided tests of significance.
3. Results
3.1. Hb distribution
The average age of the sample of 3,507 men was 77 years (range: 71-93) at the time when follow-up began and when Hb was assessed (1991-1993). Overall, 820 men (23.4%) had Hb levels <14 g/dL, 1,953 (55.7%) had levels that ranged from 14 to 15.9 g/dL, and 734 (20.9%) had levels ≥16 g/dL. The number of men with anemia (<13 g/dL) was 313 (8.9%). Although levels of Hb as high as 18 g/dL were within the normal range for our laboratory, only 180 men (5.1%) had Hb levels ≥17 g/dL. Forty-three (1.2%) had levels ≥18 g/dL.
Table 1 further describes how Hb levels vary with age in this elderly sample. Among this group, Hb declined consistently from an average of 15.2 g/dL in men aged 71 to 75 years to 13.8 g/dL in those who were aged 86 years and older (p<0.001). The percent of men with Hb levels ≥16 g/dL also declined with age (p<0.001), while the percent with Hb levels <14 and 14-15.9 g/dL increased (p<0.001).
Table 1.
Distribution of Hb by baseline age in elderly men in the Honolulu-Asia Aging Study
| Age (y) | Sample size | Average Hb‡ (g/dL) | Percent of men within an Hb range (g/dL) |
||
|---|---|---|---|---|---|
| <14§ | 14-15.9§ | ≥16‡ | |||
| 71-75 | 1421 | 15.2 ± 1.3* | 14.8 (210)† | 58.6 (833) | 26.6 (378) |
| 76-80 | 1251 | 14.9 ± 1.4 | 22.1 (277) | 57.6 (720) | 20.3 (254) |
| 81-85 | 553 | 14.5 ± 1.4 | 32.9 (182) | 52.8 (292) | 14.3 (79) |
| 86-93 | 282 | 13.8 ± 1.6 | 53.6 (151) | 38.3 (108) | 8.2 (23) |
| Overall | 3507 | 14.9 ± 1.4 | 23.4 (820) | 55.7 (1953) | 20.9 (734) |
Mean ± standard deviation
Number of men
Average Hb levels and the percent of men with Hb levels ≥16 g/dL declined significantly with age (p<0.001).
Percent of men with Hb levels <14 and 14-15.9 g/dL increased significantly with age (p<0.001).
3.2. Relationship with other features
Table 2 describes the relationship of Hb with other features that were observed during mid-life and at the baseline examination (1991-1993) when Hb was assessed. Among the baseline features, the percent of men with constipation and prevalent cancer declined significantly with rising Hb levels (p<0.001). Deaths/100 person years also declined with rising Hb levels (p<0.001). For constipation and mortality, patterns of association were largely due to an excess of each event that occurred in the lowest Hb range (<14 g/dL). Although mid-life pack-years of cigarette smoking rose significantly with increasing Hb levels (p=0.019), the relationship was similar for levels <16 g/dL. An association between Hb and mid-life coffee intake and excessive daytime sleepiness was less apparent.
Table 2.
Age-adjusted percentages and average levels of concomitant risk factors and age-adjusted mortality across ranges of Hb in the elderly men in the Honolulu-Asia Aging Study.
| Hb range (g/dL) |
|||
|---|---|---|---|
| <14 | 14-15.9 | ≥16 | |
| Sample size | 820 | 1953 | 734 |
| Mid-life cigarette smoking† (pack-years) | 26.7 ± 29.8* | 25.5 ± 26.4 | 30.6 ± 27.2 |
| Mid-life coffee intake (oz/d) | 13.5 ± 12.8 | 13.3 ± 12.5 | 14.6 ± 13.6 |
| Percent constipated‡ (N) | 10.8 (82) | 5.3 (96) | 5.7 (38) |
| Percent with excessive daytime sleepiness (N) | 8.8 (60) | 8.3 (146) | 9.4 (61) |
| Percent with prevalent cancer‡ (N) | 19.1 (164) | 12.3 (238) | 10.2 (73) |
| Total deaths/100 person-years‡ (N) | 7.6 (447) | 4.7 (627) | 5.0 (230) |
Mean ± standard deviation
Pack-years of cigarette smoking increases with rising Hb level (p=0.019).
Percent of men with constipation and prevalent cancer, and total deaths/100 person-years decline significantly with rising Hb level (p<0.001).
3.3. Hb relationships with PD incidence
In the course of follow-up, 47 of the 3,507 men developed PD (19.8/10,000 person-years). The average age at the time of diagnosis was 80 years (range: 73-90), and the average time to diagnosis was 3.8 years (range: 4 months-7.9 years).
Table 3 further describes the incidence of PD across ranges of Hb. After age-adjustment, the incidence of PD rose significantly from 10.3 to 34.9/10,000 person-years as Hb increased from <14 to ≥16 g/dL (p=0.024). The association between Hb and the incidence of PD persisted after additional adjustment for other concomitant features (p=0.022).
Table 3.
Incidence and risk factor adjusted relative hazard of PD comparing ranges of Hb in elderly men in the Honolulu-Asia Aging Study.
| Hb (g/dL) | Incidence/10,000 person-years |
Relative hazard* | |
|---|---|---|---|
| Unadjusted | Age-adjusted | ||
| <14 | 10.3 (5/820)† | 10.3 | reference |
| 14-15.9 | 17.5 (24/1953) | 17.5 | 1.5 (0.6,4.0)‡ |
| ≥16 | 34.9§ (18/734) | 34.9∥ | 3.1¶ (1.1,8.7) |
| Test for trend (p-value) | 0.021 | 0.024 | 0.022 |
| Overall | 19.8 (47/3507) | ||
Adjusted for age, mid-life pack-years of cigarette smoking, mid-life coffee intake, constipation, excessive daytime sleepiness, and prevalent cancer.
Number of PD cases/sample at risk
95% confidence interval
Significant PD excess versus Hb levels <14 g/dL (p=0.020)
Significant PD excess versus Hb levels <14 g/dL (p=0.023)
Significant PD excess versus Hb levels <14 g/dL (p=0.028)
Although sample sizes are small in the tails of the Hb distribution, men with anemia (Hb <13 g/dL) experienced the lowest incidence of PD (6.2/10,000 person-years). Here, there was one case of PD among 313 men at risk. Sample sizes were also small for those with Hb ≥17 g/dL (5 PD cases in 180 at risk) where PD incidence was highest (41.0/10,000 person-years) as compared to the other Hb strata.
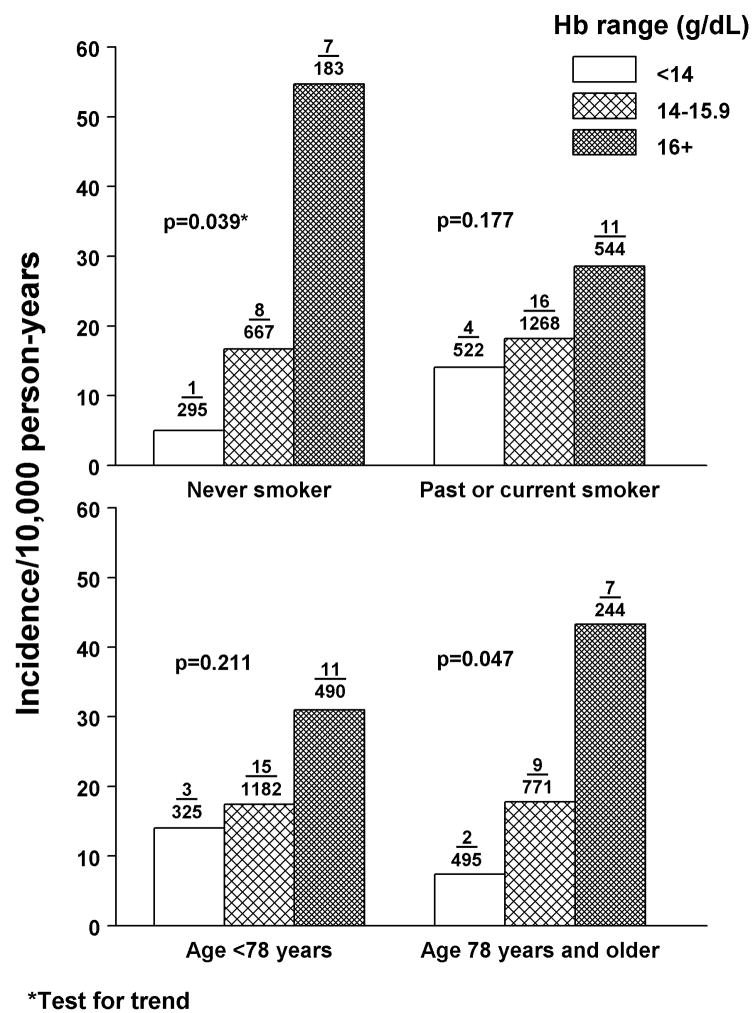
Associations were also examined within strata of confounders that are known to have relationships with both Hb and incident PD. As examples, the relationship between Hb and PD is similar between strata of mid-life smoking status and baseline age (Figure 1). Although sample sizes are reduced, the association between Hb and incident PD remains statistically significant in those who were never smokers during mid-life (p=0.039) and in men above the median age of 77 years when follow-up began (p=0.047).
Figure 1.
Age adjusted incidence of PD by mid-life smoking status (1965-1968) and baseline age (1991-1993). Numbers above the bars are the number of PD cases/sample at risk.
Early mortality could also be an important confounder in the relationship between Hb and PD. For those with Hb levels <14 g/dL, the chance of a diagnosis of PD could be reduced through the higher incidence of death versus other Hb ranges (see table 2). The effect of early mortality, however, was modest. After excluding men who died within the first 3 years of follow-up, the association between Hb and the incidence of PD persisted (p=0.042). In addition, had the men who died in the course of follow-up without a diagnosis of PD survived and gone on to develop PD at a rate that assumes that Hb is unrelated to PD, the incidence of PD for the lowest Hb range (<14 g/dL) would increase to 13.1/10,000 person years, while for Hb levels ≥16 g/dL, the incidence would become 32.9/10,000 person years. The difference remains statistically significant (p=0.020).
4. Discussion
The substantia nigra in PD contains an excessive amount of iron that is often considered neurotoxic [Bartzokis et al., 2007; Kaur and Andersen, 2004]. Findings in the current report provide evidence that higher levels of Hb, the most abundant source of peripheral iron in humans, can predate the clinical manifestation of PD in elderly men. It remains to be determined if high Hb concentrations are a cause of PD. An association between Hb and PD could be a secondary response to ongoing mechanistic derangements that disrupt the close regulation of iron in brain viability. While brain iron homeostasis is tightly regulated, severe dysregulation could be a distinctive feature in processes that lead to PD. Some evidence suggests that brain iron can be modulated by peripheral iron status [Bartzokis et al., 2007; Beard and Connor, 2003; Hallgren and Sourander, 1958; Pinero et al., 2000]. Whether it is more easily altered in PD is unknown. The presence of modified Hb formations (nitrosyl Hb) in the substantia nigra could be especially important in brain iron homeostasis, in early declines in nigral glutathione levels, and in increased dopaminergic nitric oxide in vitro and in vivo [Cammack et al., 1998; Chinta et al., 2007; Shergill et al., 1996].
Promotion of α-synuclein aggregation, a critical factor in several neurodegenerative diseases, may also be important [Uversky, 2007]. Within the blood, red blood cells (which are largely composed of Hb) are a major source of α-synuclein [Barbour et al., 2008]. In addition, as a metal binding protein, α-synuclein is aggregated by iron, which in turn contributes to the formation of Lewy pathology in PD processes [Wolozin and Golts, 2002; Uversky, 2007]. Expression of α-synuclein is closely correlated with 3 key heme metabolizing genes, likely due to common transcription factors in erythrocyte and brain precursors (GATA-1 and GATA-2) [Scherzer et al., 2008].
Whether late-life Hb levels are more important than levels that exist in middle adulthood warrants consideration. The one time finding of a relatively high Hb level in an elderly man, especially when Hb levels are expected to decline with age, could also be a marker of a life-time of accumulation of iron deposition that exceeds neuroprotective thresholds. While an earlier measurement if Hb was not available in our elderly cohort, hematocrit was determined when the men were aged 45 to 68 years at initiation of the Honolulu Heart Program (1965-1968). Hematocrit was also measured in late-life at the time when Hb was assessed as part of a complete blood count when the current follow-up began (1991-1993). The association between Hb and the risk of PD was independent of hematocrit measured in mid-life (1965-1968) and the concomitant late-life (1991-1993) measures of red blood cell distribution, mean cell volume, mean cell Hb concentration, and mean cell Hb.
Other than hematocrit and Hb that were measured in late life, none of the other blood count measures had a significant relationship with incident PD. As might be expected, because of high multicolinearity, the significant associations that hematocrit and Hb have with PD no longer persist when both factors appear in the same model. This also occurs for models that include red blood cell count, even though red blood cell count is not a significant predictor of PD. This suggests that there is a high level of redundancy among these measures and that mechanistic relationships involving the effect of hematocrit and Hb on the risk of PD may be similar or equally important.
Other than iron, there are several alternative explanations for the association between late-life Hb and PD incidence. Possible mechanisms could involve exposures to metals, pesticides, and solvents [Andre et al., 2005; Uversky, 2007]. In the current study, only data on mid-life exposure to these latter factors are available, none of which had an affect on the association between Hb and PD. Measures in later life, however, could be important as a marker of long-term accumulation of neurotoxins in vulnerable regions of the brain. As an improved correlate of life-time exposure to cigarette smoking, spirometric testing was performed in late-life at the time of Hb measurement. Neither forced vital capacity nor the first second of forced expiratory volume offered additional insight into the finding of a relationship of Hb with PD.
Whether diet has a role also needs to be considered. Dietary iron deficiency can deplete brain iron concentrations, while it can be restored with supplementation [Beard and Connor, 2003; Pinero et al., 2000]. The possibility exists that brain iron accumulation can be modified [Bartzokis et al., 2007]. In case-control studies, descriptions of an association between dietary iron and PD are equivocal and complex, sometimes involving interactions with other risk factors [Logroscino et al., 1998; Logroscino et al., 2008; Powers et al., 2003]. In the current sample, after adjustment for total kilocalories, intake of dietary iron in mid-life at the time of initiation of the Honolulu Heart Program (1965-1968) was positively associated with an increased risk of PD (p=0.034). After further adjustment for age, pack-years of cigarette smoking, and coffee intake, the association was no longer significant. Findings were unaltered within strata of manganese, vitamin C, and total fat intake. The intake of these nutrients, including carbohydrates, also failed to alter the reported association between Hb and PD. As noted by others, inadequate intake of dietary antioxidants and generalized poor nutrition in the elderly could offer partial explanation for findings of a cross-sectional excess of PD in the presence of anemia [Ferrucci et al., 2007].
In contrast to findings reported in our elderly sample, data from a recent case-control study suggest that anemia in early-life can predate the diagnosis of PD by 20 years and longer [Savica et al., 2009]. An association with late-life anemia, however, was absent. The distribution of Hb and its relationship with PD in the higher Hb ranges were also not provided. When pooled with common levels of normal Hb (where PD risk is low), a high incidence of PD in a comparatively small group of elderly men with elevated Hb could have been missed. Questions also remain about those who were selected as controls and those who were excluded because of early mortality. In the current report, follow-up began at a standardized protocol examination for all participants in a free-living population-based sample with common baseline exclusions. Recall and selection bias are less of an issue, and measures of absolute risk are easily derived.
Besides diet, there are other pathways that might explain the association between Hb and PD. Increased susceptibility to neurotoxity from iron dysregulation and overload could be due to polymorphisms in genes coding for proteins that remove free Hb from the circulation and tissues. Such mechanisms might involve genotypes of the Hb binding protein haptoglobin with distributions that are different in subjects with idiopathic PD versus normal controls [Costa-Mallen et al., 2008]. Other gene mutations or errors in protein processing could contribute to dysregulation in iron metabolism and homeostasis, neurodegeneration from brain iron deposition, aceruloplasminemia, and an increased risk of PD [Rhodes and Ritz, 2008].
A neuroprotective effect from cigarette smoking may also be important. While the association between Hb and PD was similar in men who were never versus past and current smokers during mid-life (Figure 1), it seemed stronger for those who were never smokers. Based on preliminary findings from a series of 33 autopsied members in the current sample (where death occurred within 5 years of Hb measurement) [Ross et al., 2004], total substantia nigra neuron density in never smokers declined consistently from 20.4 neurons/mm2 in 17 men with Hb <14 g/dL to 14.0 neurons/mm2 in 7 men with Hb ≥16 g/dL (p=0.016). Levels of Hb were unrelated to neuron density for men who were past or current smokers.
As is the case in any large-scale epidemiologic study, there are potential limitations in the current report. Because of the advanced age of our cohort when follow-up began, the mean age at PD onset is latter than in other studies. Diagnostic misclassification is also possible. For this to affect our results, there would need to be a higher likelihood for missed cases in the low versus high Hb strata. There is no evidence that this should occur. In addition, although efforts were made to identify everyone with PD, early cases of PD could still have been missed in the screening process. Incidence rates, however, are similar to other population-based samples, suggesting this may not be a major factor [Morens et al., 1996].
While observations in the current report are consistent with neuropathologic evidence of a relationship between iron and neurodegeneration in PD [Bartzokis et al., 2007; Kaur and Andersen, 2004], further confirmation and studies of mechanistic pathways are needed. Findings are consistent with age related increases in brain ferritin iron and an increased risk of PD in the elderly [Bartzokis et al., 2004; Haaxma et al., 2007]. Supporting evidence is also found among women, where compared to men, levels of Hb and brain ferritin iron are low, as is the risk of PD [Bartzokis et al., 2007; Haaxma et al., 2007]. Whether higher Hb in the elderly (when it is expected to be low) could be used to target individuals for further MRI screening of brain iron ferritin levels and preventive therapeutic interventions in neurodegeneration warrants consideration [Bartzokis et al., 2007; Kaur et al., 2009].
Supplementary Material
Acknowledgments
Supported by a contract (N01-AG-4-2149) and grant (1-R01-AG17155-01A1) from the National Institute on Aging, a contract (N01-HC-05102) from the National Heart, Lung, and Blood Institute, a grant (1-R01-NS41265-01) from the National Institute of Neurological Disorders and Stroke, a grant from the United States Department of the Army (DAMD17-98-1-8621), and by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs. The information contained in this article does not necessarily reflect the position or the policy of the United States Government, and no official endorsement should be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott RD. Logistic regression in survival analysis. Am J Epidemiol. 1985;121:465–471. doi: 10.1093/oxfordjournals.aje.a114019. [DOI] [PubMed] [Google Scholar]
- Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, Curb JD, Petrovitch H. Excessive daytime sleepiness and subsequent development of Parkinson’s disease. Neurology. 2005;65:1442–1449. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- Abbott RD, Launer LJ, Rodriguez BL, Ross GW, Wilson PWF, Masaki KH, Strozyk D, Curb JD, Yano K, Popper JS, Petrovitch H. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68:563–568. doi: 10.1212/01.wnl.0000254473.88647.ca. [DOI] [PubMed] [Google Scholar]
- Andre C, Truong TT, Robert JF, Guillaume YC. Effect of metals on herbicides-α-synuclein association: A possible factor in neurodegenerative disease studied by capillary electrophoresis. Electrophoresis. 2005;26:3256–3264. doi: 10.1002/elps.200500169. [DOI] [PubMed] [Google Scholar]
- Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, Chilcote TJ. Red blood cells are a major source of alpha-synuclein in blood. Neurodegenerative Dis. 2008;5:55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Shin IS, Lu PH, Cummings JL. Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann N Y Acad Sci. 2004;1012:224–236. doi: 10.1196/annals.1306.019. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J. Brain ferritin iron may influence age- and gender related risks of neurodegeneration. Neurobiol Aging. 2007;28:414–423. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Bollinger PB, Drewinko B, Brailas CD, Smeeten NA, Trujillo JM. The technicon H*1 – an automated hematology analyzer for today and tomorrow: Complete blood cell parameters. Am J Clin Pathol. 1987;87:71–78. doi: 10.1093/ajcp/87.1.71. [DOI] [PubMed] [Google Scholar]
- Cammack R, Shergill JK, Ananda IV, Hughes MN. Applications of electron paramagnetic resonance spectroscopy to study interactions of iron proteins in cells with nitric oxide. Spectrochim Acta A Mol Biomol Spectrosc. 1998;54A:2393–2402. doi: 10.1016/s1386-1425(98)00219-4. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Kumar MJ, Hsu M, Rajagopalan S, Kaur D, Rane A, Nichols DG, Choi J, Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons results in nigrostriatal degeneration. J Neurosci. 2007;25:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mallen P, Checkoway H, Zabeti A, Edenfield MJ, Swanson PD, Longstreth WT, Jr, Franklin GM, Smith-Weller T, Sadrzadeh SM. The functional polymorphism of the hemoglobin-binding protein haptoglobin influences susceptibility to idiopathic Parkinson’s disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:216–222. doi: 10.1002/ajmg.b.30593. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. J R Stat Soc. 1972;34(series B):187–202. [Google Scholar]
- Ferrucci L, Guralnik JM, Bandinelli S, Semba RD, Lauretani F, Corsi A, Ruggiero C, Ershler WB, Longo DL. Unexplained anaemia in older persons is characterized by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol. 2007;136:849–855. doi: 10.1111/j.1365-2141.2007.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leeanders KL, Eshuis S, Booji J, Dluzen DE, Horstink MW. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Kaur D, Andersen J. Does cellular iron dysregulation play a causative role in Parkinson’s disease? Age Res Rev. 2004;3:327–343. doi: 10.1016/j.arr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Kaur D, Rajagopalan S, Andersen JK. Chronic expression of H-ferritin in dopaminergic midbrain neurons results in an age-related expansion of the labile iron pool and subsequent neurodgeneration: Implications for Parkinson’s disease. Brain Res. 2009;1297:17–22. doi: 10.1016/j.brainres.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane PW, Nelder JA. Analysis of covariance and standardization as instances of prediction. Biometrics. 1982;38:613–621. [PubMed] [Google Scholar]
- Logroscino G, Marder K, Graziano J, Freyer G, Slavkovich V, Lojacono N, Cote L, Mayeux R. Dietary iron, animal fats, and risk of Parkinson’s disease. Mov Disord. 1998;13(suppl 1):13–16. [PubMed] [Google Scholar]
- Logroscino G, Gao X, Chen H, Wing A, Ascherio A. Dietary iron intake and risk of Parkinson’s disease. Am J Epidemiol. 2008;168:1381–1388. doi: 10.1093/aje/kwn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta CR, Patel NR. Exact logistic regression: Theory and examples. Stat Med. 1995;14:2143–2160. doi: 10.1002/sim.4780141908. [DOI] [PubMed] [Google Scholar]
- Morens DM, Davis JW, Grandinetti A, Ross GW, Popper JS, White LR. Epidemiologic observations on Parkinson’s disease: Incidence and mortality in a prospective study of middle-aged men. Neurology. 1996;46:1044–1050. doi: 10.1212/wnl.46.4.1044. [DOI] [PubMed] [Google Scholar]
- Pinero DJ, Li NQ, Connor JR, Beard JL. Variations in dietary iron alter brain iron metabolism in developing rats. J Nutr. 2000;130:254–263. doi: 10.1093/jn/130.2.254. [DOI] [PubMed] [Google Scholar]
- Powers KM, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD, Checkoway H. Parkinson’s disease risk associated with dietary iron, manganese, and other nutrient intakes. Neurology. 2003;60:1761–1766. doi: 10.1212/01.wnl.0000068021.13945.7f. [DOI] [PubMed] [Google Scholar]
- Rhodes SL, Ritz B. Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol Dis. 2008;32:183–195. doi: 10.1016/j.nbd.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol. 2004;56:532–539. doi: 10.1002/ana.20226. [DOI] [PubMed] [Google Scholar]
- Savica R, Grossardt BR, Carlin JM, Icen M, Bower JH, Ahlskog JE, Maraganore DM, Steensma DP, Rocca WA. Anemia or low hemoglobin levels preceding Parkinson disease: A case-control study. Neurology. 2009;73:1381–1387. doi: 10.1212/WNL.0b013e3181bd80c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Grass JA, Liao Z, Pepivani I, Zheng B, Eklund AC, Ney PA, Ng J, McGoldrick M, Mollenhauer B, Bresnick EH, Schlossmacher MG. GATA transcription factors directly regulate the Parkinson’s disease-linked gene α-synuclein. Proc Natl Acad Sci USA. 2008;105:10907–10912. doi: 10.1073/pnas.0802437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill JK, Cammack R, Cooper CE, Cooper M, Mann VM, Schapira AHV. Detection of nitrosyl complexes in human substantia nigra in relation to Parkinson’s disease. Biochem Biophys Res Communs. 1996;228:298–305. doi: 10.1006/bbrc.1996.1656. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- Ward CD, Gibb WR. Research diagnostic criteria for Parkinson’s disease. Adv Neurol. 1990;53:245–249. [PubMed] [Google Scholar]
- White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, Rodriguez BL, Blanchette PL, Havlik RJ, Wergowske G, Chiu D, Foley DJ, Murdaugh C, Curb JD. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
- Wolozin B, Golts N. Iron and Parkinson’s disease. Neuroscientist. 2002;8:22–32. doi: 10.1177/107385840200800107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.