After hepatic transplantation, liver allografts continue to produce donor-phenotype proteins and other synthetic products, allowing this operation to be used to correct numerous liver-based inborn errors of metabolism (1). Because the same retention of donor specificity is expected after successful hepatic xenotransplantation, the consequence of successfully engrafting a liver xenograft could be the imposition on the recipient of an interspecies metabolic incompatibility. To examine this question, we have used clotting factors known to be synthesized in the liver as metabolic markers after orthotopic hepatic xenotransplantation from male LVG hamsters (100–150 g) to Lewis rats (240–280 g) with a previously described cuff technique (2). Revascularization was with portal venous inflow, omitting hepatic artery reconstruction. No blood transfusions were given. Immunosuppression with FK 506 was started several hours after the transplantation was completed and continued in doses of 1 mg/kg/day until sacrifice. Particular attention was paid to the clotting factors (in italics) that require vitamin K: I, II, V, VII, X, VIII, IX, XI, XII, XIII, Fletcher, Fitzgerald, Protein C, Protein S, plasminogen, and the inhibitors antithrombin III and antiplasmin. For these studies, about 5 ml of blood were obtained from unanesthetized hamsters by cardiac puncture. Rats were anesthetized followed by the immediate opening of the abdomen and collection of blood (about 10 ml) from the exposed vena cava by puncture with a #20 butterfly. Syringes for both species were rinsed with 0.1 M sodium citrate and the blood was transferred to plastic tubes containing sufficient additional citrate to bring the citrate volume to 1/10 that of the blood. Methods for coagulation tests have been detailed elsewhere (3, 4). The Stachrom Protein C assay was used, following the manufacturer’s (Diagnostica Stageo, Asnieres-sur-seine, France) directions.
Although hamsters and rats are both rodents, the phylogenetic distance by paleontologic and genetic evidence has been estimated at 15–40 million years (5). The consequent diversity between the hamster and rat is a significant one, requiring the surmounting of a moderately difficult immunologic barrier including preformed lymphocytotoxic antibodies (6). The 3 clotting tests with the greatest disparity between normal hamsters and rats are shown in Table 1; the most specific is protein C, which was always present in hamsters, but was undetectable in normal rats. In rats that had been transplanted with hamster livers 8, 40, and >100 days previously (n=2 at each time), the profile was changed to that of the hamster. There was no crossover within 2 SD of any of the 3 tests. The transition of the rat recipient into the hamster range of values was complete by 8 days, with the exception of the protein C values, which were not in metabolic equilibrium until the next analysis at 40 days. In these and other hamster to rat hepatic xenotransplantation experiments, neither bleeding nor clotting has been observed clinically more than after rat liver allotransplantation. By 40 days (n=5) and >100 days (n=6), bleeding time in the rat recipients of hamster xenografts had shortened toward the normal range for hamsters (Table 1).
Table 1.
Coagulation tests in controls and hamster liver transplanted rats
| Tests | Hamster |
Rat |
Transplanted rats (days) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | 8 | 40 | >100 | ||||
| Prothrombin time (sec) | 22 | 9.0 | 0.8 | 15 | 15.3 | 2.1 | 9.8 | 9.0 | 8.0 | 8.3 | 9.5 | 10.3 |
| Factor X (U/ml) | 22 | 3.1 | 1.2 | 15 | 0.30 | 0.07 | 0.63 | 1.2 | 5.1 | 7.1 | 2.1 | 2.1 |
| Protein C (U/ml) | 14 | 0.51 | 0.06 | 15 | <0.01 | 0 | 0.11 | 0.17 | 0.68 | 0.56 | 0.62 | 0.57 |
| Bleeding time (sec) | 5 | 36.2 | 6.3 | 5 | 51.8 | 5.5 | ND | 42.6 | 3.9 | 40.5 | 2.8 | |
| (n = 5) | (n = 6) | |||||||||||
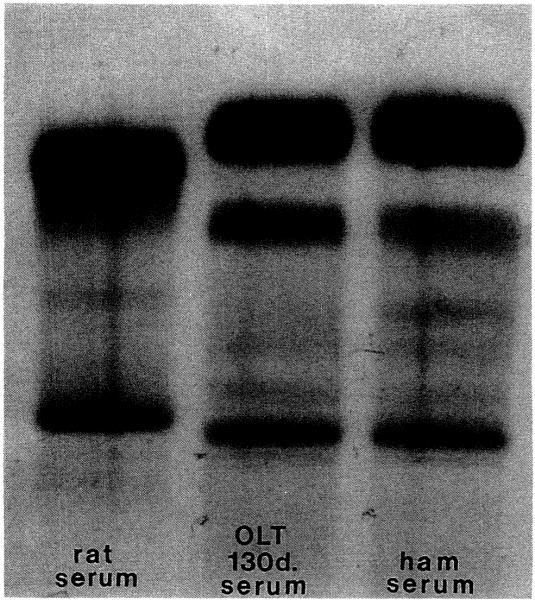
The results indicate that the donor-specific products of hepatic synthesis in some species combinations, such as hamster to rat, do not present an insurmountable metabolic barrier to liver xenotransplantation. That the metabolic changes are sweeping was shown with serial serum protein electrophoresis. During the first 3 weeks, there was a transition from rat to a rat/hamster and then an exclusively hamster albumin. This was unchanged up to 130 days (Fig. 1).
Figure 1.
Serum protein electrophoresis. Albumin: dark upper band (SPE kit, Beckman Instruments, Inc., Brea, CA.
Footnotes
This work was supported by Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Starzl TE, Demetris AJ, Van Thiel DH. Medical progress: Liver Transplantation. N Engl J Med (Part I) 1989;321:1014. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valdivia LA, Monden M, Gotoh M, et al. Prolonged survival of hamster-to-rat liver xenografts using splenectomy and cyclosporine administration. Transplantation. 1987;44:759. doi: 10.1097/00007890-198712000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JH. Hemostasis and hemorrhage. Sci Clin. 1971;1:1. [Google Scholar]
- 4.Lewis JH, Spero JA, Hasiba U. Diagnostic methods: laboratory tests. In: Lewis JH, editor. Bleeding disorders. Medical Exam; Garden City, NY: 1978. p. 22. [Google Scholar]
- 5.Hartenberger JL. The order Rodentia: major questions on their evolutionary origin, relationships and suprafamilial systematics. In: Luckett WP, Hartenberger JL, editors. Evolutionary relationships among rodents. A multidisciplinary analysis. Plenum Press; New York: 1985. p. 92. [Google Scholar]
- 6.Valdivia LA, Fung JJ, Demetris AJ, Starzl TE. Differential surival of hamster-to-rat liver and cardiac xenografts under FK 506 immunosuppression. Transplant Proc. 1991;23:3269. [PMC free article] [PubMed] [Google Scholar]