Abstract
Several mucosotropic human papillomaviruses (HPV), including HPV type 16 (HPV-16), are etiologic agents of a subset of anogenital cancers and head and neck squamous cell carcinomas. In mice, HPV-16 E7 is the most potent of the papillomaviral oncogenes in the development of cervical disease. Furthermore, interfering specifically with the expression of E7 in HPV-positive cell lines derived from human cervical cancers inhibits their ability to proliferate, indicating that the expression of E7 is important in maintaining the transformed phenotype in vitro. To assess the temporal role of E7 in maintaining HPV-associated tumors and precancerous lesions in vivo, we generated Bi-L E7 transgenic mice that harbor a tetracycline-inducible transgene that expresses both HPV-16 E7 and firefly luciferase. When we crossed Bi-L E7 mice to a K5-tTA transgene-inducing line of mice, which expresses a tetracycline-responsive transactivator selectively in the stratified squamous epithelia, the resulting Bi-L E7/K5-tTA bitransgenic mice expressed E7 and luciferase in the skin and cervical epithelium, and doxycycline repressed this expression. Bitransgenic mice displayed several overt and acute epithelial phenotypes previously shown to be associated with the expression of E7, and these phenotypes were reversed on treatment with doxycycline. Repressing the expression of E7 caused the regression of high-grade cervical dysplasia and established cervical tumors, indicating that they depend on the continuous expression of E7 for their persistence. These results suggest that E7 is a relevant target not only for anticancer therapy but also for the treatment of HPV-positive dysplastic cervical lesions.
Introduction
Human papillomaviruses (HPV) are small, epitheliotropic, dsDNA viruses that cause proliferative lesions, including warts. A subset of mucosotropic HPVs—the “high-risk” HPVs, including HPV type 16 (HPV-16) and HPV-18—is associated etiologically with the vast majority of cervical cancers, other types of anogenital cancers, and approximately a quarter of head and neck squamous cell carcinomas (1). HPV-16, which is a causative agent of over half of cervical cancers (2), encodes three oncogenes: E5, E6, and E7 (3–5). E7 is best known for its ability to bind to and degrade the tumor suppressor pRb (6), although its ability to drive carcinogenesis is not due exclusively to this interaction (7).
To characterize the oncogenic properties of E7 in vivo, our laboratory previously generated transgenic mice that express E7 under the control of the keratin 14 (K14) promoter (8). These K14E7 mice display a variety of acute epithelial phenotypes indicative of the ability of E7 to disrupt the cell cycle (9). Furthermore, K14E7 mice develop high-grade dysplasia and invasive cervical cancer when treated chronically with estrogen for as little as 6 months, whereas nontransgenic mice and K14E53 and K14E6 (10) transgenic mice do not. These data, coupled with previous demonstrations of the ability of E7 to transform cells in culture (11–13), indicate the importance of E7 in the genesis of cervical cancer.
The observation that cancerous cells depend on the continuous expression of oncogenes for their survival has been described in many murine systems for several oncogenes, including BCR-ABL (14), MYC (15–17), H-Ras (18), and K-Ras (19). Whether HPV-associated cancers require the continuous expression of viral oncogenes has been examined in tissue culture by using cell lines derived from HPV-positive human cervical cancers. Repressing the expression of E6 in the HPV-18–positive HeLa cell line by using the papillomaviral E2 transcription factor results in sporadic senescence and widespread apoptosis, whereas silencing the expression of E7 causes senescence only (20). Interfering with the expression of E6 and E7 in HeLa cells using short hairpin RNA (shRNA) induces senescence at low doses of a lentiviral vector containing the shRNA, but at high doses, apoptosis occurs (21). Conflicts in apoptotic versus senescent responses between these and similar studies (22–24) are difficult to reconcile and may reflect differences in the experimental systems being used. It remains unclear whether the requirements for high-risk E6 and E7 observed in such studies are predictive of the in vivo dependence of cervical cancers on their presence or specifically reflect their importance in maintaining the growth competence of cells in tissue culture.
To assess the temporal role of E7 in the maintenance of HPV-associated cervical cancers in vivo, we generated Bi-L E7 transgenic mice that harbor a construct containing HPV-16 E7 and firefly luciferase, the expression of which is driven by bidirectional promoters under the control of a tetracycline response element (TRE). When we produced bitransgenic mice by crossing Bi-L E7 mice to a line of mice that expresses the tetracycline transactivator (tTA) under the control of the keratin 5 promoter (K5-tTA mice; ref. 25), they expressed both luciferase and E7 in the skin and in the epithelium of the female reproductive tract. By administering doxycycline, an analogue of tetracycline, to Bi-L E7/K5-tTA bitransgenic mice, the expression of E7 and luciferase was eliminated. Bitransgenic mice displayed an array of epithelial phenotypes previously shown to be associated with the expression of E7, and these phenotypes were reversed on treatment with doxycycline. More importantly, we found that repressing the expression of E7 caused the regression of high-grade cervical dysplasia and established cervical tumors, indicating that both depend on the continuous expression of E7 for their persistence. Our findings have important implications for the treatment of HPV-associated cervical disease.
Materials and Methods
For a full description of the Materials and Methods, see Supplementary Information.
Transgenic mice
K14E7 (8) and K5-tTA (25) transgenic mice have been described previously. Bi-L E7 mice were genotyped by PCR using the primers LucF (5′-GAAATGTCCGTTCGGTTGGCAGAAGC-3′) and LucR (5′-CCAAAACCGTGATGGAATGGAACAACA-3′).
Treatment with estrogen
To induce persistent estrus and to eliminate cycling through estrus, continuous release pellets delivering 0.05 mg of 17β-estradiol (estrogen) per 60-d period (Innovative Research of America) were implanted s.c. into the fat pads of the shoulders of adult female virgin mice.
Treatment with doxycycline
Doxycycline-containing chow (2 g/kg; Bio-Serv) was used to repress the expression of the Bi-L E7 transgene.
Procurement of female reproductive tracts and histologic analysis
One hour before sacrifice, mice were injected with 5-bromo-2′-deoxyuridine (BrdUrd; 12.5 mg/mL in PBS) at 10 μL/g body weight. Procured female reproductive tracts were fixed overnight at 4°C in 4% paraformaldehyde (w/v), embedded in paraffin, sectioned, and stained with H&E for histologic analysis of neoplastic and dysplastic disease.
Analysis of luciferase
Tissue lysates were analyzed for the activity of luciferase using the Luciferase Assay System (Promega Corp.) according to the manufacturer’s instructions.
Analysis of E7
To examine the expression of E7 by Western blot, a mixture of mouse anti-HPV-16 E7 primary antibodies was used (Invitrogen Corp.; Santa Cruz Biotechnology).
Irradiation
Adult mice were mock treated or exposed to 5 Gy of ionizing radiation from a 137Cs source and sacrificed 24 h later. One hour before sacrifice, mice were injected with BrdUrd (12.5 mg/mL in PBS) at 10 μL/g body weight.
Immunohistochemistry
Mouse anti-BrdUrd (Calbiochem Immuno-chemicals) and mouse anti–minichromosome maintenance protein 7 (MCM7; NeoMarkers Corp.) primary antibodies were used for immuno-histochemical analysis.
Quantification of BrdUrd
Nuclei of keratinocytes in 10 (dorsal skin) or 8 (endocervices) visual fields at ×40 magnification were scored as either positive (brown) or negative (blue) for BrdUrd.
Results
The expression of luciferase and E7 in the skin and epithelium of the female reproductive tracts of Bi-L E7/K5-tTA bitransgenic mice is controlled by doxycycline
To study the temporal requirement for the expression of E7 in cervical cancers, we generated transgenic mice using a Bi-L E7 construct. Briefly, this transgene contains a TRE, composed of seven copies of the tetracycline operator (tetO) sequence, flanked by two minimal cytomegalovirus promoters in opposite orientations, which drive the expression of firefly luciferase in one direction and HPV-16 E7 in the other (Supplementary Fig. S1A). We initially screened nine lines of Bi-L E7 mice to determine which lines expressed luciferase under the control of the tTA protein by crossing them to an inducing line of K5-tTA mice that expresses tTA specifically in the stratified squamous epithelia (Supplementary Fig. S1B). In eight of the nine lines examined, there was no significant increase in the expression of luciferase in the dorsal skin of singly transgenic Bi-L E7 mice over background levels observed in nontransgenic mice (P > 0.05, two-sided Wilcoxon rank-sum test), indicating that there was no leaky expression of luciferase in the absence of the tTA protein (Supplementary Fig. S2). When we examined lysates from the dorsal skin of Bi-L E7/K5-tTA bitransgenic mice, we observed a significant induction of luciferase in seven of the nine lines of Bi-L E7 mice examined (P < 0.05 versus nontransgenic, two-sided Wilcoxon rank-sum test; Supplementary Fig. S2). Based on our results, we chose one line of Bi-L E7 mice, line 406, which reproducibly expressed luciferase in a manner dependent on the presence of tTA. In all data presented hereafter, we used Bi-L E7 mice exclusively from line 406.
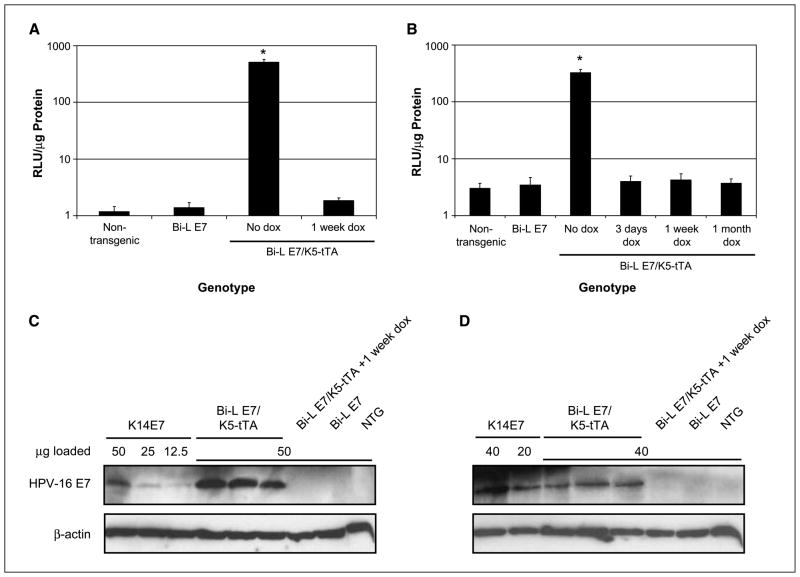
Administering chow containing 2 g/kg doxycycline for 1 week to Bi-L E7/K5-tTA bitransgenic mice eliminated the expression of luciferase in the skin, indicating that, when the transcriptional activity of the tTA protein was inactivated by doxycycline, the expression of luciferase was abolished (Fig. 1A). We also examined the expression of luciferase in lysates from the lower female reproductive tract, where we observed an induction of luciferase exclusively in bitransgenic mice (P < 0.05 versus nontransgenic mice, two-sided Wilcoxon rank-sum test) that was eliminated in mice given as little as 3 days of doxycycline (Fig. 1B). These data indicate that luciferase is induced and can be repressed with doxycycline in the stratified squamous epithelia of Bi-L E7/K5-tTA bitransgenic mice.
Figure 1.
The expression of luciferase and E7 in the dorsal skin and female reproductive tracts of Bi-L E7/K5-tTA bitransgenic mice is eliminated on treatment with doxycycline (dox). A and B, luciferase assays done on lysates from dorsal skin (A) or female reproductive tracts (B) from mice of the indicated genotypes. Columns, mean; bars, SD. *, P < 0.05 versus nontransgenic mice by a two-sided Wilcoxon rank-sum test, and n ≥ 3 for all groups of mice. C and D, Western blots to examine the expression of E7 in lysates from dorsal skin (C) or female reproductive tracts (D). To compare the level of expression of E7 in line 406 Bi-L E7/K5-tTA bitransgenic mice to that seen in K14E7 mice, equivalent amounts of lysates from three K14E7 mice were pooled, and the indicated amounts of this pool were analyzed in parallel with lysates from mice of the indicated genotypes. Detection of β-actin was used as a loading control.
We next examined the expression of E7 in Bi-L E7/K5-tTA bitransgenic mice by performing Western blots on lysates from the dorsal skin and lower female reproductive tract. By using loading controls consisting of known amounts of protein from lysates from K14E7 mice, a line of transgenic mice that we generated previously to characterize the oncogenic properties of E7 in vivo (8), we determined that the level of expression of E7 in the dorsal skin of Bi-L E7/K5-tTA mice was slightly higher than that seen in K14E7 mice (Fig. 1C). In the lower female reproductive tract, the level of expression of E7 in bitransgenic mice was approximately half that observed in K14E7 mice (Fig. 1D). As expected, we did not detect E7 in lysates from bitransgenic mice given doxycycline for 1 week or in singly transgenic Bi-L E7 mice.
These data indicate that the expression of luciferase and E7 is induced in the dorsal skin and lower female reproductive tracts of Bi-L E7/K5-tTA bitransgenic mice and that this expression is repressed by administering doxycycline.
Bi-L E7/K5-tTA bitransgenic mice display striking overt phenotypes that are reversible on treatment with doxycycline
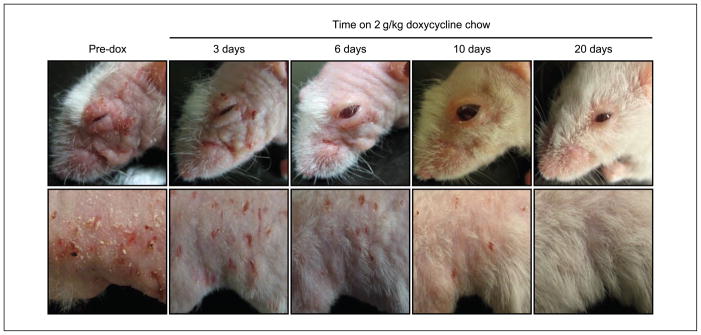
We noted that Bi-L E7/K5-tTA bitransgenic mice presented numerous, fully penetrant overt phenotypes, including alopecia, runting, excessive scratching, and dry and wrinkled hyperplastic skin (Fig. 2). These phenotypes were apparent by 4 weeks of age and invariably worsened over time. By 6 to 7 months of age, bitransgenic mice often were nearly devoid of hair, and the excessive scratching led to the appearance of flaky, raw, and scabbed skin on and around the shoulders of the animals. To determine whether we could reverse these overt phenotypes by silencing the expression of E7, we administered doxycycline to bitransgenic mice and monitored the amelioration of these phenotypes over the course of 20 days (Fig. 2). After as little as 3 days on doxycycline, the skin of bitransgenic mice was noticeably less dry and hyperplastic when compared with mice that never received doxycycline, and by 6 days on the drug, there was obvious regrowth of hair over the entire body. The overt phenotypes continued to regress as the mice remained on doxycycline, and by 20 days, bitransgenic mice continuously fed doxycycline were nearly indistinguishable from nontransgenic mice. These observations show that the overt phenotypes displayed by Bi-L E7/K5-tTA bitransgenic mice are reversed on treatment with doxycycline, regardless of their severity.
Figure 2.
Bi-L E7/K5-tTA bitransgenic mice display striking overt phenotypes that are reversible on treatment with doxycycline. Shown are photographs of the head (top) and flank (bottom) of the same mouse, either before treatment with doxycycline (Pre-dox) or after the indicated times on doxycycline. Although images from only one mouse are shown, similar results were obtained with all 10 mice treated and followed for 20 d.
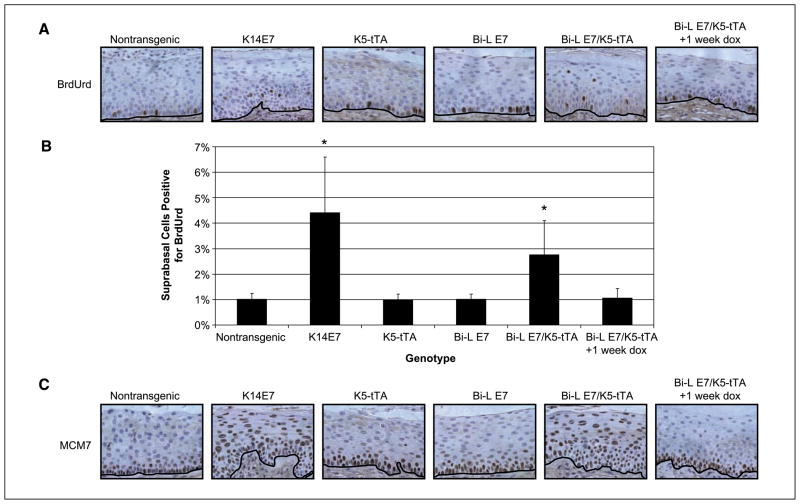
Bi-L E7/K5-tTA bitransgenic mice display several acute, microscopic phenotypes in the stratified squamous epithelia that can be reversed with doxycycline
We have shown previously that E7 can induce DNA synthesis in the normally quiescent suprabasal compartment of the endocervical epithelium of K14E7 mice (7). To investigate whether this induction occurs in the endocervical epithelium of Bi-L E7/K5-tTA mice, we performed immunohistochemical staining for BrdUrd (Fig. 3A) in sections from mice injected with the nucleotide analogue 1 hour before sacrifice and quantified the results (Fig. 3B). In Bi-L E7/K5-tTA bitransgenic mice, we observed a significant induction of DNA synthesis in the suprabasal compartment of the epithelium (P < 0.005 versus nontransgenic, two-sided Wilcoxon rank-sum test); this induction was statistically indistinguishable from the level observed in K14E7 mice (P = 0.28, two-sided Wilcoxon rank-sum test) and absent in singly transgenic mice. Administering doxycycline for 1 week to bitransgenic mice eliminated this induction of suprabasal DNA synthesis. We also examined the expression of MCM7, which we previously have shown is up-regulated in the endocervical epithelium of K14E7 mice and which is a marker in humans for high-grade cervical dysplasia and HPV-associated cancers (26). In nontransgenic mice, cells staining positively for MCM7 were restricted to the basal and parabasal layers of the epithelium; in contrast, we observed the expression of MCM7 throughout the full thickness of the endocervical epithelium of Bi-L E7/K5-tTA bitransgenic mice (Fig. 3C). The pattern of expression of MCM7 in singly transgenic Bi-L E7 mice and in bitransgenic mice given doxycycline for 1 week resembled that seen in nontransgenic mice.
Figure 3.
The endocervical epithelium of Bi-L E7/K5-tTA bitransgenic mice displays aberrant proliferation. A, immunohistochemical analysis of the incorporation of BrdUrd in sections of the endocervical epithelium from mice of the indicated genotypes. Brown nuclei represent cells positive for BrdUrd, and the black line delineates the basement membrane. B, quantification of nuclei positive for BrdUrd in sections shown in A. Eight visual fields at ×40 magnification were scored for nuclei positive (brown) or negative (blue) for BrdUrd. Data are presented as the mean ± SD. *, P < 0.005 versus nontransgenic mice by a two-sided Wilcoxon rank-sum test, and n ≥ 3 for all groups of mice. C, immunohistochemical analysis of the expression of MCM7 in sections of the endocervical epithelium from mice of the indicated genotypes. Brown nuclei represent cells positive for MCM7, and the black line delineates the basement membrane.
We also investigated whether Bi-L E7/K5-tTA bitransgenic mice displayed acute phenotypes in the dorsal skin, where their overt phenotypes had been most obvious. As described fully in Supplementary Information, in the dorsal skin of Bi-L E7/K5-tTA mice, we observed an induction of DNA synthesis in the suprabasal compartment of the epidermis (Supplementary Fig. S3A, quantified in Supplementary Fig. S3C), a disruption of epithelial differentiation (Supplementary Fig. S3B), and an abrogation of the response to DNA damage induced by ionizing radiation (Supplementary Fig. S3D), all of which we have observed previously in the epidermis of K14E7 mice (9, 27, 28). In addition, all of these phenotypes in bitransgenic mice regressed once we administered doxycycline for 1 week (Supplementary Fig. S3).
Taken together, these data indicate that Bi-L E7/K5-tTA bitransgenic mice display an array of acute epithelial phenotypes associated with the expression of E7 and that these phenotypes regress on treatment with doxycycline.
Bi-L E7/K5-tTA bitransgenic mice develop high-grade cervical dysplasia and cervical cancer when treated chronically with estrogen
We have shown previously that estrogen is a necessary cofactor for the development (10) and maintenance (29) of cervical cancers in K14E7 mice. Thus, to induce cervical cancers in female Bi-L E7/K5-tTA bitransgenic mice, we treated them for 6 to 7 months with exogenous estrogen. At the end points, we sacrificed the mice, harvested and fixed the reproductive tracts, embedded them in paraffin, sectioned them, and stained the sections with H&E to allow us to score for the presence of dysplastic disease and cancer. To do this, we categorized each mouse based on the most severe state of disease observed. The categories were nondysplastic hyperplasia, low-grade dysplasia [cervical intraepithelial neoplasia 1 (CIN1)], mid-grade dysplasia (CIN2), high-grade dysplasia/carcinoma in situ (CIN3/CIS), and cervical cancer. The results are summarized in Table 1. As a positive control, we treated K14E7 mice with estrogen for 7 months and observed a 40% incidence of cervical cancer. In contrast, mice that did not express E7—singly transgenic Bi-L E7 mice, K5-tTA transgenic mice, and nontransgenic mice—developed nothing worse than sporadic CIN1 lesions. When we examined tracts from Bi-L E7/K5-tTA bitransgenic mice, we found that 33% of mice treated for 6 months with estrogen developed cervical cancer, and this proportion increased to 53% after 7 months. Both results were statistically significant compared with nontransgenic mice (P < 0.05, two-sided Fisher’s exact test). In all bitransgenic mice, regardless of the presence of cancer, mid- to high-grade dysplasia was ubiquitous throughout the female reproductive tract; statistically, this led to an increase in the severity of disease in bitransgenic mice over what was observed in nontransgenic mice (P < 1 × 10−5, two-sided Wilcoxon rank-sum test). Consistent with our prior findings with K14E7 mice (10, 26, 29), these results indicate that the expression of E7 in the female reproductive tracts of Bi-L E7/K5-tTA bitransgenic mice, in combination with estrogen, leads to the development of high-grade dysplasia and cervical cancer.
Table 1.
Incidence of cervical disease in mice treated chronically with estrogen
| Genotype | Estrogen (mo) | Doxycycline (mo) | Grade of cervical disease |
||||
|---|---|---|---|---|---|---|---|
| Hyperplasia | CIN1 | CIN2 | CIN3/CIS | Cervical cancer (%) | |||
| Nontransgenic | 6 | — | 5 | 8 | |||
| K14E7 | 7 | — | 6 | 4 (40)*† | |||
| K14E7 | 7 | 1 | 4 | 6 (60)*† | |||
| K5-tTA | 6 | — | 7 | 8 | |||
| Bi-L E7 | 6 | — | 8 | 8 | |||
| Bi-L E7/K5-tTA | 6 | — | 5 | 5 | 5 (33)*† | ||
| Bi-L E7/K5-tTA | 7 | — | 3 | 6 | 10 (53)*† | ||
| Bi-L E7/K5-tTA | 7 | 1 | 8 | 9 | |||
NOTE: Mice of the indicated genotypes were treated with estrogen for the duration listed and either treated or not treated (—) with doxycycline for 1 mo. Based on the most severe state of disease observed in each animal, mice were placed into the following categories: nondysplastic hyperplasia, CIN1, CIN2, CIN3/CIS, and cervical cancer. The percentage of animals with cervical cancer is listed in parentheses.
P < 1 × 10−5, when comparing the average severity of disease with that of nontransgenic mice by a two-sided Wilcoxon rank-sum test.
P < 0.05, when comparing the incidence of cancers with that of nontransgenic mice by a two-sided Fisher’s exact test.
Continuous expression of E7 is required for the maintenance of high-grade dysplasia and cervical cancer in Bi-L E7/K5-tTA bitransgenic mice
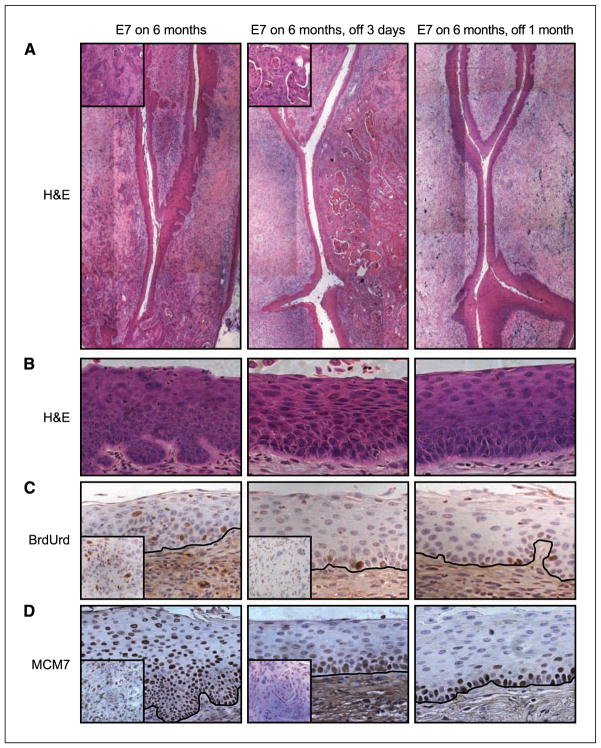
To evaluate the dependence of cervical cancers on the continuous presence of E7, we treated a subset of bitransgenic mice with doxycycline for the final month of the 7-month treatment with estrogen. By comparing the incidence of cancers in this group with the two groups of bitransgenic mice that did not receive doxycycline, we were able to determine the effect on cervical cancers of turning off the expression of E7. As shown in Table 1 and Fig. 4A, bitransgenic mice receiving 1 month of treatment with doxycycline had no cervical cancers, indicating that cervical cancers in these mice regressed after we administered doxycycline. Furthermore, we observed a complete regression of high-grade dysplasia in bitransgenic mice given doxycycline for 1 month (Fig. 4B), although in a subset of these mice we still observed isolated CIN1 lesions. This was in stark contrast to what we observed in bitransgenic mice not given doxycycline, where we saw dysplasia throughout the female reproductive tract. To ensure that these results were not due to an effect of the doxycycline other than the silencing of the Bi-L E7 transgene, we treated a subset of K14E7 mice with doxycycline for the final month of a 7-month treatment with estrogen and observed no difference in the severity of dysplastic disease or the incidence of cervical cancer between this group and a group never given doxycycline (Table 1).
Figure 4.
The expression of E7 is required for the maintenance of high-grade cervical dysplasia and cervical cancer in Bi-L E7/K5-tTA bitransgenic mice. Shown in all panels are images of sections of the endocervices from bitransgenic mice expressing E7 for the indicated duration. A, composite images at ×10 magnification of sections of the endocervix stained with H&E. Insets, ×40 magnification of cancers in mice receiving no doxycycline (left) or 3 d of doxycycline (middle). B, images at ×40 magnification of sections from the endocervical epithelium stained with H&E, illustrating a region of high-grade dysplasia observed in a bitransgenic mouse receiving no doxycycline (left) and the regression of high-grade dysplasia on treatment of bitransgenic mice with doxycycline for 3 d (middle) or 1 mo (right). C and D, immunohistochemical analysis of BrdUrd (C) and MCM7 (D) in the endocervical epithelium of mice receiving no doxycycline (left), 3 d of doxycycline (middle), or 1 mo of doxycycline (right). The black line delineates the basement membrane. Insets, results from tumors from the same animals.
To determine how quickly the dysplasia in bitransgenic mice regressed once we administered doxycycline, we treated mice given 6 months of estrogen with doxycycline for 3 days. Even after this brief treatment with doxycycline, we observed a complete regression of all high-grade dysplasia in the reproductive tracts of female Bi-L E7/K5-tTA mice (Fig. 4B), although cancers still remained (Fig. 4A). We also performed immunohistochemical staining for BrdUrd (Fig. 4C) and MCM7 (Fig. 4D) to verify that the expression of the Bi-L E7 transgene was silenced within 3 days of administering doxycycline, and these results, coupled with the data from luciferase assays (Fig. 1B), indicated that this was the case. In summary, these findings show that both high-grade cervical dysplasia and cervical cancer depend on the continued presence of E7 for their maintenance.
Mechanism of regression
Studies in tissue culture have argued that suppressing the transcription of HPV oncogenes in cell lines derived from human cervical cancers leads to the rapid induction of senescence or apoptosis and halts cellular growth (20–24). In addition, apoptosis and senescence have been observed within 1 to 8 days of inducing the regression of cancers in many other animal models in which the expression of oncogenes or tumor suppressors can be regulated temporally (14, 15, 18, 19, 30). In our model, however, we were unable to detect an increase in apoptosis (Supplementary Fig. S4; data not shown) or an induction of senescence-associated β-galactosidase (SA-β-gal) activity (Supplementary Fig. S5) in either cervical cancers or the endocervical epithelium after administering doxycycline to Bi-L E7/K5-tTA bitransgenic mice for 1, 3, or 6 days following prolonged treatment with estrogen. The explanation for the observed regression of cervical disease simply may be that silencing E7 leads to the restoration of the normal cellular program for proliferation and differentiation (Fig. 4B–D), resulting in the elimination of high-grade cervical dysplasia and cervical cancers; however, it remains possible that senescence is occurring in our samples but is not manifesting as an induction of SA-β-gal in this tissue.4
Discussion
The hypothesis that interfering with the expression of HPV oncogenes might trigger the regression of HPV-associated tumors has been bolstered by several lines of evidence obtained from studying HPV-positive cell lines derived from human cervical cancers (20–24). Although it has been shown in vivo that an array of cancers requires the continuous expression of their respective oncogenes for their maintenance (14–19), our results (Table 1; Fig. 4A and B) are the first demonstration in the context of an animal model that the expression of HPV E7 is required for the maintenance of high-grade cervical dysplasia and cervical cancer.
Oncogenic addiction, oncogenic amnesia, and HPV-associated cervical cancer
The hypothesis of oncogenic addiction (31) asserts that the intracellular circuitry in cancerous cells is altered significantly when compared with that of normal cells. In the context of this altered circuitry, an oncogene may fulfill a different and more important role than it does in normal cells; therefore, cancerous cells become “addicted” to the presence of this oncogene for their survival. Recently, the hypothesis of oncogenic amnesia (32) was proposed to explain differently the reason that tumors depend on the continuous presence of an oncogene. It states that no single oncogenic lesion can inactivate all of the checkpoints that regulate cellular proliferation and genomic integrity; instead, a given oncogene will inactivate only some of these checkpoints. Further genomic changes will continue to inactivate additional pathways and eventually will lead to carcinogenesis. As long as oncogenes are expressed, a cell remains “ignorant” of the further accumulation of genomic damage. Once an oncogene is silenced, however, the pathways with which it interfered become activated, recognize the genomic damage that has occurred, and initiate a response—senescence, apoptosis, or differentiation, depending on the reactivated pathways—to deal with the accumulated genomic insults. Unless the pathways with which the oncogene interfered become redundantly inactivated genetically or epigenetically while the oncogene is expressed, they will be reactivated on silencing the oncogene and will drive the regression of tumors.
Although the hypotheses of oncogenic addiction and oncogenic amnesia are not mutually exclusive and both have their caveats (31, 32), the assertions of oncogenic amnesia seem especially relevant in the context of HPV-associated cervical carcinogenesis. There is an average latency of 15 years between infection with HPV and the appearance of cervical carcinoma (33), suggesting that cervical carcinogenesis results from the cooperation of HPV oncoproteins with other genetic and epigenetic alterations. Whereas most human tumors contain mutations in the p53 (34) or pRb (35) pathways, these networks are unaltered in HPV-positive cell lines (36); presumably, mutations in the p53 or pRb pathway do not confer any advantage in growth to a cell in which these pathways already are dysregulated by E6 and E7 and do not enhance tumorigenesis. In fact, despite their aneuploidy (37), HeLa cells retain functional p53 and pRb pathways, and on silencing E6 and E7, both pathways are induced and can trigger an arrest in cellular growth (38). Our immunohistochemical data show that the expression of MCM7, an E2F-responsive gene negatively regulated by pRb (39), is lost in suprabasal endocervical cells and in tumors once E7 is silenced (Fig. 4D), suggesting that the pRb pathway remains functional in the cancers and high-grade dysplasia arising in Bi-L E7/K5-tTA bitransgenic mice. Thus, oncogenic mutations that cooperate with HPV to drive tumorigenesis spare the cellular pRb and p53 pathways, and the reactivation of these pathways likely plays an important role in mediating the regression of cancers on the silencing of HPV oncogenes.
Regression of high-grade dysplasia on silencing E7
The results presented here are the first to show in any context that high-grade cervical dysplasia depends on the presence of E7 for its persistence. The elimination of high-grade dysplasia was complete after just 3 days of treatment with doxycycline (Fig. 4B), although cancers still remained (Fig. 4A); thus, even brief interference with the expression of E7 can cause the rapid regression of high-grade cervical dysplasia, the development of which generally requires several years (33). However, even after the treatment of Bi-L E7/K5-tTA bitransgenic mice with doxycycline for 1 month, sporadic CIN1 lesions remained in about half of the mice (Table 1). Currently, it is unclear whether these low-grade lesions represent remnants of high-grade lesions that still are in the process of regressing, lesions whose regression has stalled, or emerging E7-independent lesions that potentially could develop into high-grade lesions and even cancer. Determining the nature of these residual CIN1 lesions will have important therapeutic implications.
Implications for the treatment of HPV-positive cervical disease
Results from our (Table 1; Fig. 4A) and previous (20–24) studies reinforce the notion that anti-HPV therapy that targets the expression or function of E7 holds great promise for the treatment of invasive cervical disease. Currently, the treatment of cervical cancer often involves radiotherapy, chemotherapy, or a partial or total hysterectomy, which can be especially devastating for women of childbearing age. The benefits of potential antiviral therapies over these current methods of treatment, provided these therapies have minimal side effects, are obvious.
Currently, the most common treatment for mid- to high-grade cervical dysplasia—CIN2, CIN3, and CIS—is loop electrosurgical excision procedure (LEEP; reviewed in ref. 40). Although LEEP does not have deleterious effects on fertility, it does carry an overall rate of complication of 9.7%. Although LEEP is considered very effective for the treatment of CIN, the effectiveness is reduced for patients who test positively for HPV DNA at follow-up and varies inversely with the severity of dysplasia at the time of treatment. Our results suggest that the development of antiviral therapy targeting the expression or function of E7 may be a rapid and effective treatment for HPV-associated high-grade cervical dysplasia (Table 1; Fig. 4B). The benefits over LEEP of targeting HPV E7 potentially include eliminating the need for surgery and its associated complications to remove CIN lesions, increasing the effectiveness of treating the highest-grade dysplastic lesions, and reducing the recurrence of lesions if HPV DNA persists.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute grants CA022443 and CA098428.
We thank Amy Liem for technical assistance, Dr. Henry Pitot for consultations about pathology, Drs. Bill Sugden and Shannon Kenney for critical reading of this manuscript, Drs. Jonathan Ewald and David Jarrard for positive control samples for our analysis of senescence, and Dr. Ewald for helpful discussions about this analysis.
Footnotes
Maufort et al., unpublished data.
J. Ewald, personal communication.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Leechanachai P, Banks L, Moreau F, Matlashewski G. The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus. Oncogene. 1992;7:19–25. [PubMed] [Google Scholar]
- 4.Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–10. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–21. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblasto-ma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4. [PubMed] [Google Scholar]
- 7.Balsitis S, Dick F, Dyson N, Lambert PF. Critical roles for non-pRb targets of human papillomavirus type 16 E7 in cervical carcinogenesis. Cancer Res. 2006;66:9393–400. doi: 10.1158/0008-5472.CAN-06-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herber R, Liem A, Pitot H, Lambert PF. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–81. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balsitis SJ, Sage J, Duensing S, Munger K, Jacks T, Lambert PF. Recapitulation of the effects of the human papillomavirus type 16 E7 oncogene on mouse epithelium by somatic Rb deletion and detection of pRb-independent effects of E7 in vivo. Mol Cell Biol. 2003;23:9094–103. doi: 10.1128/MCB.23.24.9094-9103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–71. [PubMed] [Google Scholar]
- 11.Vousden KH, Doniger J, DiPaolo JA, Lowy DR. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncogene Res. 1988;3:167–75. [PubMed] [Google Scholar]
- 12.Phelps WC, Yee CL, Munger K, Howley PM. The human papillomavirus type 16 E7 gene encodes trans-activation and transformation functions similar to those of adenovirus E1A. Cell. 1988;53:539–47. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- 13.Matlashewski G, Osborn K, Banks L, Stanley M, Crawford L. Transformation of primary human fibro-blast cells with human papillomavirus type 16 DNA and EJ-ras. Int J Cancer. 1988;42:232–8. doi: 10.1002/ijc.2910420215. [DOI] [PubMed] [Google Scholar]
- 14.Huettner CS, Zhang P, Van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nat Genet. 2000;24:57–60. doi: 10.1038/71691. [DOI] [PubMed] [Google Scholar]
- 15.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 16.D’Cruz CM, Gunther EJ, Boxer RB, et al. c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat Med. 2001;7:235–9. doi: 10.1038/84691. [DOI] [PubMed] [Google Scholar]
- 17.Jain M, Arvanitis C, Chu K, et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–4. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 18.Chin L, Tam A, Pomerantz J, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–72. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 19.Fisher GH, Wellen SL, Klimstra D, et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–62. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol. 2003;77:1551–63. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu W, Putral L, Hengst K, et al. Inhibition of cervical cancer cell growth in vitro and in vivo with lentiviral-vector delivered short hairpin RNA targeting human papillomavirus E6 and E7 oncogenes. Cancer Gene Ther. 2006;13:1023–32. doi: 10.1038/sj.cgt.7700971. [DOI] [PubMed] [Google Scholar]
- 22.Hall AH, Alexander KA. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J Virol. 2003;77:6066–9. doi: 10.1128/JVI.77.10.6066-6069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura A, Nakahara T, Ueno T, et al. Requirement of E7 oncoprotein for viability of HeLa cells. Microbes Infect. 2006;8:984–93. doi: 10.1016/j.micinf.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin EC, Yang E, Lee CJ, Lee HW, DiMaio D, Hwang ES. Rapid induction of senescence in human cervical carcinoma cells. Proc Natl Acad Sci U S A. 2000;97:10978–83. doi: 10.1073/pnas.97.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated trans-activators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–94. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 26.Brake T, Connor JP, Petereit DG, Lambert PF. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 2003;63:8173–80. [PubMed] [Google Scholar]
- 27.Gulliver GA, Herber RL, Liem A, Lambert PF. Both conserved region 1 (CR1) and CR2 of the human papillomavirus type 16 E7 oncogene are required for induction of epidermal hyperplasia and tumor formation in transgenic mice. J Virol. 1997;71:5905–14. doi: 10.1128/jvi.71.8.5905-5914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song S, Gulliver GA, Lambert PF. Human papilloma-virus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci U S A. 1998;95:2290–5. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brake T, Lambert PF. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Natl Acad Sci U S A. 2005;102:2490–5. doi: 10.1073/pnas.0409883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–80. doi: 10.1158/0008-5472.CAN-07-3293. discussion 80. [DOI] [PubMed] [Google Scholar]
- 32.Felsher DW. Oncogene addiction versus oncogene amnesia: perhaps more than just a bad habit? Cancer Res. 2008;68:3081–6. doi: 10.1158/0008-5472.CAN-07-5832. discussion 6. [DOI] [PubMed] [Google Scholar]
- 33.Meijer CJ, Snijders PJ, van den Brule AJ. Screening for cervical cancer: should we test for infection with high-risk HPV? CMAJ. 2000;163:535–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 36.Scheffner M, Munger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991;88:5523–7. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macville M, Schrock E, Padilla-Nash H, et al. Comprehensive and definitive molecular cytogenetic characterization of HeLa cells by spectral karyotyping. Cancer Res. 1999;59:141–50. [PubMed] [Google Scholar]
- 38.Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Natl Acad Sci U S A. 2000;97:12513–8. doi: 10.1073/pnas.97.23.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki S, Adachi A, Hiraiwa A, Ohashi M, Ishibashi M, Kiyono T. Cloning and characterization of human MCM7 promoter. Gene. 1998;216:85–91. doi: 10.1016/s0378-1119(98)00323-0. [DOI] [PubMed] [Google Scholar]
- 40.Lindeque BG. Management of cervical premalignant lesions. Best Pract Res Clin Obstet Gynaecol. 2005;19:545–61. doi: 10.1016/j.bpobgyn.2005.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.