Abstract
In the title compound, C15H17NO3S, the two aromatic rings make a dihedral angle of 69.42 (9)° with each other and the bridging C—N—S—C torsion angle is 65.76 (16)°. Weak intramolecular C—H⋯O interactions may affect the molecular conformation. Two neighbouring molecules generate a hydrogen-bonded dimer about a center of inversion through a pair of intermolecular N—H⋯O interactions, forming an R 2 2(8) ring motif. Furthermore, two intermolecular C—H⋯π interactions contribute to the stability of the crystal packing.
Related literature
For the biological activity of sulfonamides, see: Berredjem et al. (2000 ▶); Lee & Lee (2002 ▶); Soledade et al. (2006 ▶); Xiao & Timberlake (2000 ▶).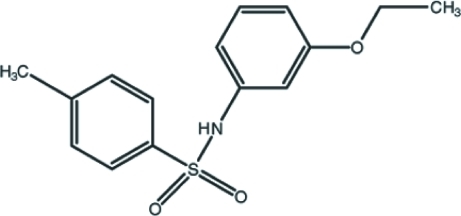
Experimental
Crystal data
C15H17NO3S
M r = 291.37
Monoclinic,
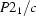
a = 8.4612 (3) Å
b = 13.1862 (5) Å
c = 13.4237 (4) Å
β = 99.326 (2)°
V = 1477.90 (9) Å3
Z = 4
Mo Kα radiation
μ = 0.23 mm−1
T = 296 K
0.34 × 0.18 × 0.16 mm
Data collection
Bruker APEXII CCD diffractometer
13221 measured reflections
3608 independent reflections
2532 reflections with I > 2σ(I)
R int = 0.036
Refinement
R[F 2 > 2σ(F 2)] = 0.043
wR(F 2) = 0.118
S = 1.00
3608 reflections
186 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.30 e Å−3
Δρmin = −0.31 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶) and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810022427/sj5019sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810022427/sj5019Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 and Cg2 are the centroids of the C2–C7 and C8–C13 benzene rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯O2i | 0.821 (16) | 2.140 (17) | 2.9476 (19) | 167.9 (16) |
| C4—H4⋯O2 | 0.93 | 2.54 | 2.914 (2) | 104 |
| C13—H13⋯O1 | 0.93 | 2.42 | 3.019 (2) | 122 |
| C14—H14A⋯Cg1ii | 0.97 | 2.90 | 3.752 (3) | 147 |
| C15—H15C⋯Cg2iii | 0.96 | 2.96 | 3.763 (3) | 147 |
Symmetry codes: (i) 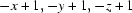 ; (ii)
; (ii) 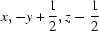 ; (iii)
; (iii) 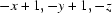 .
.
Acknowledgments
The authors are grateful to the Higher Education Commission for financial support to purchase the diffractometer.
supplementary crystallographic information
Comment
Sulfonamide is an important functionality found in a number of synthetic as well as natural compounds possessing versatile type of biological activities e.g. herbicidal, anti-malarial, anti-convulsant and anti-hypertensive (Soledade et al., 2006; Xiao & Timberlake, 2000; Berredjem et al., 2000; Lee & Lee, 2002) activities. In the present paper, the structure of N-(3-ethoxyphenyl)-4-methylbenzene sulfonamide has been determined as part of a research program involving the synthesis and biological evaluation of sulfur containing compounds.
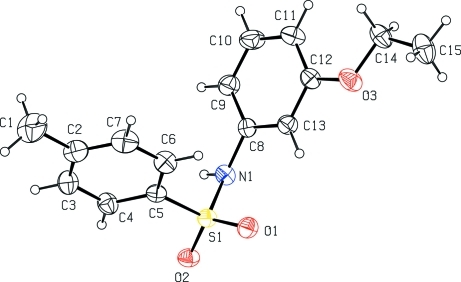
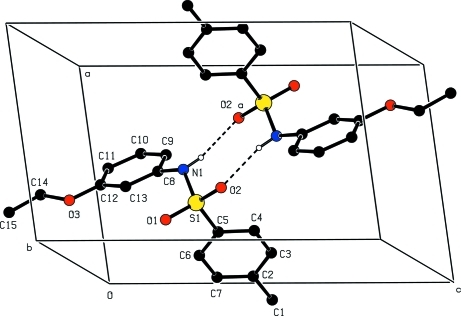
In the title compound (I), Fig. 1), the dihedral angle between the two aromatic rings (C2–C7 and C8–C13) is 69.42 (9)° and the bridging C5—S1—N1—C8 torsion angle is 65.76 (16)°. In the crystal structure, two neighbouring molecules generate a hydrogen-bonded dimer about a center of inversion through a pair of intermolecular N—H···O interactions, forming an R22(8) ring motif (Table 1, Fig. 2).
In the structure, two intermolecular C—H···π interactions contribute to the stability of crystal packing (Table 1).
Experimental
A mixture of 4-methyl benzene sulfonyl chloride (10.0 mmoles; 1.90 g), 3-ethoxy aniline (meta-phenetidine) (10.0 mmoles; 1.25 g), aqueous sodium carbonate (10%; 10.0 ml) and water (25 ml) was stirred for half an hour at room temperature. The crude mixture was washed with water and dried. Product was dissolved in methanol and crystallized by slow evaporation of the solvent. Yield 72%. 4-Methyl benzene sulfonyl chloride and meta-phenetidine were purchased from Sigma Aldrich while all other chemicals involved were obtained from Merk, Germany.
Refinement
H atoms bonded to C atoms were positioned geometrically and refined using a riding model, with C—H = 0.93–0.97Å and Uiso(H) = 1.2–1.5Ueq (C). The amino H-atom was found in a difference Fourier map, and refined with a distance restraint of N–H 0.86 (2) Å. The H-atom Uiso parameter was fixed at 1.2Ueq(N) for the N—H group.
Figures
Fig. 1.
The title molecule of (I), with displacement ellipsoids drawn at the 30% probability level.
Fig. 2.
View of the N—H···O dimer in the unit cell of (I). H-atoms not involved in hydrogen bonds have been omitted for clarity.
Crystal data
| C15H17NO3S | F(000) = 616 |
| Mr = 291.37 | Dx = 1.309 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 4223 reflections |
| a = 8.4612 (3) Å | θ = 2.9–26.1° |
| b = 13.1862 (5) Å | µ = 0.23 mm−1 |
| c = 13.4237 (4) Å | T = 296 K |
| β = 99.326 (2)° | Needle, colourless |
| V = 1477.90 (9) Å3 | 0.34 × 0.18 × 0.16 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD diffractometer | 2532 reflections with I > 2σ(I) |
| Radiation source: sealed tube | Rint = 0.036 |
| graphite | θmax = 28.3°, θmin = 3.4° |
| φ and ω scans | h = −11→11 |
| 13221 measured reflections | k = −17→15 |
| 3608 independent reflections | l = −17→17 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.043 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.118 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0564P)2 + 0.2791P] where P = (Fo2 + 2Fc2)/3 |
| 3608 reflections | (Δ/σ)max < 0.001 |
| 186 parameters | Δρmax = 0.30 e Å−3 |
| 1 restraint | Δρmin = −0.31 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.26616 (5) | 0.52544 (3) | 0.38686 (3) | 0.0408 (1) | |
| O1 | 0.17864 (15) | 0.57574 (10) | 0.30139 (9) | 0.0518 (4) | |
| O2 | 0.32376 (14) | 0.58201 (9) | 0.47639 (9) | 0.0495 (4) | |
| O3 | 0.30011 (17) | 0.42300 (12) | −0.00035 (9) | 0.0653 (5) | |
| N1 | 0.42808 (16) | 0.47848 (12) | 0.35383 (11) | 0.0439 (5) | |
| C1 | −0.1104 (3) | 0.1674 (2) | 0.4986 (2) | 0.1033 (13) | |
| C2 | −0.0197 (2) | 0.25798 (16) | 0.47044 (18) | 0.0623 (7) | |
| C3 | 0.0767 (2) | 0.31285 (17) | 0.54323 (16) | 0.0639 (8) | |
| C4 | 0.1627 (2) | 0.39529 (15) | 0.51876 (14) | 0.0532 (6) | |
| C5 | 0.15151 (18) | 0.42365 (13) | 0.41926 (13) | 0.0414 (5) | |
| C6 | 0.0547 (2) | 0.37042 (16) | 0.34460 (15) | 0.0562 (7) | |
| C7 | −0.0295 (2) | 0.28784 (18) | 0.37117 (18) | 0.0673 (8) | |
| C8 | 0.43243 (18) | 0.41644 (13) | 0.26721 (12) | 0.0402 (5) | |
| C9 | 0.5253 (2) | 0.32988 (15) | 0.28030 (15) | 0.0548 (6) | |
| C10 | 0.5428 (3) | 0.27331 (17) | 0.19682 (16) | 0.0668 (8) | |
| C11 | 0.4705 (2) | 0.30085 (16) | 0.10134 (15) | 0.0591 (7) | |
| C12 | 0.3773 (2) | 0.38714 (15) | 0.08949 (13) | 0.0484 (6) | |
| C13 | 0.3581 (2) | 0.44493 (14) | 0.17269 (13) | 0.0466 (5) | |
| C14 | 0.3347 (3) | 0.37772 (18) | −0.09048 (13) | 0.0624 (7) | |
| C15 | 0.2431 (3) | 0.4353 (2) | −0.17758 (16) | 0.0836 (9) | |
| H1A | −0.20100 | 0.15520 | 0.44690 | 0.1550* | |
| H1B | −0.14680 | 0.18010 | 0.56160 | 0.1550* | |
| H1C | −0.04160 | 0.10910 | 0.50520 | 0.1550* | |
| H1N | 0.486 (2) | 0.4612 (14) | 0.4062 (12) | 0.0530* | |
| H3 | 0.08400 | 0.29390 | 0.61060 | 0.0770* | |
| H4 | 0.22780 | 0.43140 | 0.56900 | 0.0640* | |
| H6 | 0.04630 | 0.38990 | 0.27740 | 0.0670* | |
| H7 | −0.09440 | 0.25150 | 0.32100 | 0.0810* | |
| H9 | 0.57500 | 0.31030 | 0.34430 | 0.0660* | |
| H10 | 0.60510 | 0.21490 | 0.20500 | 0.0800* | |
| H11 | 0.48420 | 0.26180 | 0.04570 | 0.0710* | |
| H13 | 0.29490 | 0.50300 | 0.16470 | 0.0560* | |
| H14A | 0.30240 | 0.30710 | −0.09370 | 0.0750* | |
| H14B | 0.44870 | 0.38110 | −0.09240 | 0.0750* | |
| H15A | 0.13040 | 0.42930 | −0.17630 | 0.1250* | |
| H15B | 0.26700 | 0.40800 | −0.23980 | 0.1250* | |
| H15C | 0.27350 | 0.50550 | −0.17240 | 0.1250* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0391 (2) | 0.0417 (2) | 0.0397 (2) | 0.0033 (2) | 0.0004 (2) | −0.0018 (2) |
| O1 | 0.0518 (7) | 0.0531 (8) | 0.0479 (7) | 0.0098 (6) | 0.0001 (6) | 0.0050 (6) |
| O2 | 0.0518 (7) | 0.0455 (7) | 0.0489 (7) | 0.0015 (6) | 0.0017 (5) | −0.0086 (5) |
| O3 | 0.0699 (9) | 0.0826 (11) | 0.0410 (7) | 0.0166 (8) | 0.0018 (6) | −0.0055 (7) |
| N1 | 0.0358 (7) | 0.0540 (9) | 0.0399 (8) | 0.0022 (7) | 0.0003 (6) | −0.0013 (7) |
| C1 | 0.0842 (17) | 0.085 (2) | 0.149 (3) | −0.0305 (15) | 0.0439 (18) | −0.0015 (18) |
| C2 | 0.0435 (10) | 0.0584 (13) | 0.0886 (15) | −0.0062 (9) | 0.0214 (10) | −0.0050 (11) |
| C3 | 0.0656 (12) | 0.0657 (14) | 0.0642 (13) | −0.0056 (11) | 0.0218 (10) | 0.0055 (10) |
| C4 | 0.0529 (10) | 0.0608 (12) | 0.0452 (10) | −0.0080 (9) | 0.0060 (8) | −0.0050 (8) |
| C5 | 0.0345 (8) | 0.0430 (10) | 0.0457 (9) | 0.0040 (7) | 0.0037 (7) | −0.0041 (7) |
| C6 | 0.0475 (10) | 0.0644 (13) | 0.0532 (11) | −0.0058 (9) | −0.0022 (8) | −0.0056 (9) |
| C7 | 0.0462 (11) | 0.0712 (15) | 0.0816 (15) | −0.0128 (10) | 0.0013 (10) | −0.0184 (12) |
| C8 | 0.0346 (8) | 0.0438 (10) | 0.0425 (9) | −0.0024 (7) | 0.0069 (7) | 0.0006 (7) |
| C9 | 0.0572 (11) | 0.0536 (12) | 0.0514 (10) | 0.0122 (9) | 0.0018 (9) | 0.0024 (9) |
| C10 | 0.0728 (13) | 0.0579 (13) | 0.0669 (13) | 0.0228 (11) | 0.0030 (11) | −0.0065 (10) |
| C11 | 0.0590 (11) | 0.0627 (13) | 0.0549 (11) | 0.0076 (10) | 0.0076 (9) | −0.0155 (9) |
| C12 | 0.0431 (9) | 0.0583 (11) | 0.0428 (9) | 0.0007 (8) | 0.0044 (7) | −0.0032 (8) |
| C13 | 0.0441 (9) | 0.0489 (10) | 0.0459 (9) | 0.0067 (8) | 0.0049 (7) | 0.0001 (8) |
| C14 | 0.0626 (12) | 0.0828 (15) | 0.0420 (10) | −0.0107 (11) | 0.0092 (9) | −0.0129 (10) |
| C15 | 0.0858 (16) | 0.116 (2) | 0.0468 (12) | −0.0155 (16) | 0.0038 (11) | 0.0010 (13) |
Geometric parameters (Å, °)
| S1—O1 | 1.4245 (13) | C11—C12 | 1.379 (3) |
| S1—O2 | 1.4313 (13) | C12—C13 | 1.383 (3) |
| S1—N1 | 1.6291 (15) | C14—C15 | 1.500 (3) |
| S1—C5 | 1.7517 (17) | C1—H1A | 0.9600 |
| O3—C12 | 1.360 (2) | C1—H1B | 0.9600 |
| O3—C14 | 1.422 (2) | C1—H1C | 0.9600 |
| N1—C8 | 1.427 (2) | C3—H3 | 0.9300 |
| N1—H1N | 0.821 (16) | C4—H4 | 0.9300 |
| C1—C2 | 1.501 (3) | C6—H6 | 0.9300 |
| C2—C7 | 1.379 (3) | C7—H7 | 0.9300 |
| C2—C3 | 1.373 (3) | C9—H9 | 0.9300 |
| C3—C4 | 1.377 (3) | C10—H10 | 0.9300 |
| C4—C5 | 1.375 (3) | C11—H11 | 0.9300 |
| C5—C6 | 1.379 (3) | C13—H13 | 0.9300 |
| C6—C7 | 1.379 (3) | C14—H14A | 0.9700 |
| C8—C13 | 1.374 (2) | C14—H14B | 0.9700 |
| C8—C9 | 1.381 (3) | C15—H15A | 0.9600 |
| C9—C10 | 1.374 (3) | C15—H15B | 0.9600 |
| C10—C11 | 1.377 (3) | C15—H15C | 0.9600 |
| S1···H13 | 3.0500 | H1N···O2ii | 2.140 (17) |
| S1···H10i | 3.0600 | H3···H1B | 2.4700 |
| O1···C13 | 3.019 (2) | H4···O2 | 2.5400 |
| O2···N1ii | 2.9476 (19) | H4···H15Bvii | 2.5500 |
| O1···H13 | 2.4200 | H6···O1 | 2.6900 |
| O1···H7iii | 2.8600 | H7···H1A | 2.4000 |
| O1···H10i | 2.6000 | H7···O1vi | 2.8600 |
| O1···H15Aiv | 2.8700 | H9···H1N | 2.3300 |
| O1···H6 | 2.6900 | H9···O2ii | 2.8100 |
| O2···H4 | 2.5400 | H10···S1viii | 3.0600 |
| O2···H11i | 2.9200 | H10···O1viii | 2.6000 |
| O2···H1Nii | 2.140 (17) | H11···C14 | 2.5600 |
| O2···H9ii | 2.8100 | H11···H14A | 2.3000 |
| N1···O2ii | 2.9476 (19) | H11···H14B | 2.4100 |
| C6···C8 | 3.569 (2) | H11···O2viii | 2.9200 |
| C8···C6 | 3.569 (2) | H13···S1 | 3.0500 |
| C13···O1 | 3.019 (2) | H13···O1 | 2.4200 |
| C7···H14Av | 3.0400 | H13···H1Aiii | 2.5500 |
| C11···H14A | 2.7700 | H14A···C11 | 2.7700 |
| C11···H14B | 2.7900 | H14A···H11 | 2.3000 |
| C14···H11 | 2.5600 | H14A···C7ix | 3.0400 |
| H1A···H7 | 2.4000 | H14B···C11 | 2.7900 |
| H1A···H13vi | 2.5500 | H14B···H11 | 2.4100 |
| H1B···H3 | 2.4700 | H15A···O1iv | 2.8700 |
| H1N···H9 | 2.3300 | H15B···H4x | 2.5500 |
| O1—S1—O2 | 119.70 (8) | C2—C1—H1B | 109.00 |
| O1—S1—N1 | 107.95 (8) | C2—C1—H1C | 109.00 |
| O1—S1—C5 | 108.68 (8) | H1A—C1—H1B | 109.00 |
| O2—S1—N1 | 103.87 (7) | H1A—C1—H1C | 109.00 |
| O2—S1—C5 | 108.53 (8) | H1B—C1—H1C | 110.00 |
| N1—S1—C5 | 107.48 (8) | C2—C3—H3 | 119.00 |
| C12—O3—C14 | 118.24 (16) | C4—C3—H3 | 119.00 |
| S1—N1—C8 | 125.00 (11) | C3—C4—H4 | 120.00 |
| C8—N1—H1N | 116.6 (12) | C5—C4—H4 | 120.00 |
| S1—N1—H1N | 106.5 (12) | C5—C6—H6 | 121.00 |
| C1—C2—C7 | 121.2 (2) | C7—C6—H6 | 121.00 |
| C3—C2—C7 | 118.23 (19) | C2—C7—H7 | 119.00 |
| C1—C2—C3 | 120.6 (2) | C6—C7—H7 | 119.00 |
| C2—C3—C4 | 121.5 (2) | C8—C9—H9 | 121.00 |
| C3—C4—C5 | 119.36 (17) | C10—C9—H9 | 121.00 |
| S1—C5—C4 | 119.72 (13) | C9—C10—H10 | 119.00 |
| C4—C5—C6 | 120.44 (17) | C11—C10—H10 | 119.00 |
| S1—C5—C6 | 119.81 (14) | C10—C11—H11 | 120.00 |
| C5—C6—C7 | 118.99 (19) | C12—C11—H11 | 121.00 |
| C2—C7—C6 | 121.5 (2) | C8—C13—H13 | 120.00 |
| N1—C8—C9 | 117.35 (15) | C12—C13—H13 | 120.00 |
| N1—C8—C13 | 121.82 (15) | O3—C14—H14A | 110.00 |
| C9—C8—C13 | 120.63 (16) | O3—C14—H14B | 110.00 |
| C8—C9—C10 | 118.64 (18) | C15—C14—H14A | 110.00 |
| C9—C10—C11 | 121.7 (2) | C15—C14—H14B | 110.00 |
| C10—C11—C12 | 118.99 (19) | H14A—C14—H14B | 108.00 |
| O3—C12—C13 | 114.97 (17) | C14—C15—H15A | 109.00 |
| O3—C12—C11 | 124.94 (17) | C14—C15—H15B | 109.00 |
| C11—C12—C13 | 120.09 (17) | C14—C15—H15C | 109.00 |
| C8—C13—C12 | 119.93 (17) | H15A—C15—H15B | 110.00 |
| O3—C14—C15 | 107.41 (19) | H15A—C15—H15C | 109.00 |
| C2—C1—H1A | 109.00 | H15B—C15—H15C | 109.00 |
| O1—S1—N1—C8 | −51.29 (16) | C1—C2—C3—C4 | 179.04 (19) |
| O2—S1—N1—C8 | −179.35 (14) | C2—C3—C4—C5 | 0.2 (3) |
| C5—S1—N1—C8 | 65.76 (16) | C3—C4—C5—C6 | 0.3 (3) |
| O1—S1—C5—C4 | −147.64 (14) | C3—C4—C5—S1 | −177.61 (14) |
| O2—S1—C5—C4 | −15.96 (16) | C4—C5—C6—C7 | −0.6 (3) |
| N1—S1—C5—C4 | 95.79 (15) | S1—C5—C6—C7 | 177.28 (14) |
| O1—S1—C5—C6 | 34.49 (16) | C5—C6—C7—C2 | 0.4 (3) |
| O2—S1—C5—C6 | 166.16 (14) | N1—C8—C9—C10 | −174.51 (18) |
| N1—S1—C5—C6 | −82.09 (15) | C9—C8—C13—C12 | −0.6 (3) |
| C14—O3—C12—C13 | 169.88 (18) | N1—C8—C13—C12 | 174.16 (16) |
| C12—O3—C14—C15 | −176.27 (18) | C13—C8—C9—C10 | 0.5 (3) |
| C14—O3—C12—C11 | −9.5 (3) | C8—C9—C10—C11 | 0.0 (3) |
| S1—N1—C8—C9 | −133.67 (15) | C9—C10—C11—C12 | −0.4 (3) |
| S1—N1—C8—C13 | 51.4 (2) | C10—C11—C12—C13 | 0.3 (3) |
| C3—C2—C7—C6 | 0.1 (3) | C10—C11—C12—O3 | 179.66 (19) |
| C1—C2—C7—C6 | −179.4 (2) | O3—C12—C13—C8 | −179.21 (16) |
| C7—C2—C3—C4 | −0.4 (3) | C11—C12—C13—C8 | 0.2 (3) |
Symmetry codes: (i) −x+1, y+1/2, −z+1/2; (ii) −x+1, −y+1, −z+1; (iii) −x, y+1/2, −z+1/2; (iv) −x, −y+1, −z; (v) x, −y+1/2, z+1/2; (vi) −x, y−1/2, −z+1/2; (vii) x, y, z+1; (viii) −x+1, y−1/2, −z+1/2; (ix) x, −y+1/2, z−1/2; (x) x, y, z−1.
Hydrogen-bond geometry (Å, °)
| Cg1 and Cg2 are the centroids of the C2–C7 and C8–C13 benzene rings, respectively. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O2ii | 0.821 (16) | 2.140 (17) | 2.9476 (19) | 167.9 (16) |
| C4—H4···O2 | 0.93 | 2.54 | 2.914 (2) | 104 |
| C13—H13···O1 | 0.93 | 2.42 | 3.019 (2) | 122 |
| C14—H14A···Cg1ix | 0.97 | 2.90 | 3.752 (3) | 147 |
| C15—H15C···Cg2xi | 0.96 | 2.96 | 3.763 (3) | 147 |
Symmetry codes: (ii) −x+1, −y+1, −z+1; (ix) x, −y+1/2, z−1/2; (xi) −x+1, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ5019).
References
- Berredjem, M., Re’ gainia, Z., Djahoudi, A., Aouf, N. E., Dewinter, G. & Montero, J. L. (2000). Phosphorus Sulfur Silicon Relat. Elem.165, 249–264.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Lee, J. S. & Lee, C. H. (2002). Bull. Korean Chem. Soc.23, 167–169.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Soledade, M., Pedras, C. & Jha, M. (2006). Bioorg. Med. Chem.14, 4958–4979. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Xiao, Z. & Timberlake, J. W. (2000). J. Heterocycl. Chem.37, 773–777.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810022427/sj5019sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810022427/sj5019Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report