Abstract
There are two independent molecules in the asymmetric unit of the title compound, C11H9Cl2N, both of which are essentially planar [maximum deviations of 0.072 (5) and 0.072 (7) Å]. In the crystal structure, weak π–π stacking interactions [centroid-centroid distances = 3.791 (3) Å and 3.855 (3) Å] link pairs of molecules.
Related literature
For the properties and applications of related compounds, see: Biavatti et al. (2002 ▶); Fournet et al. (1981 ▶); McCormick et al. (1996 ▶); Towers et al. (1981 ▶); Ziegler & Gelfert (1959 ▶). For similar crystal structures, see: Subashini et al. (2009 ▶); Somvanshi et al. (2008 ▶).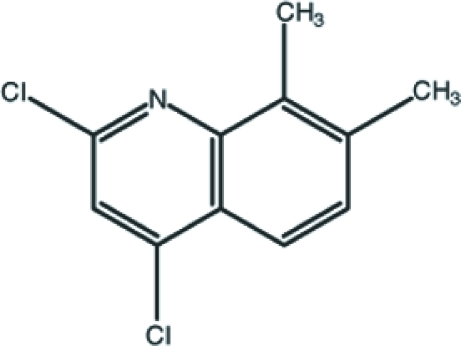
Experimental
Crystal data
C11H9Cl2N
M r = 226.09
Orthorhombic,

a = 20.3054 (9) Å
b = 3.9992 (2) Å
c = 25.5743 (11) Å
V = 2076.77 (17) Å3
Z = 8
Mo Kα radiation
μ = 0.58 mm−1
T = 295 K
0.30 × 0.24 × 0.15 mm
Data collection
Oxford Xcalibur Eos (Nova) CCD detector diffractometer
Absorption correction: multi-scan (CrysAlis PRO RED; Oxford Diffraction, 2009 ▶) T min = 0.845, T max = 0.918
19807 measured reflections
4009 independent reflections
2599 reflections with I > 2σ(I)
R int = 0.048
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.119
S = 0.94
4009 reflections
257 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.32 e Å−3
Δρmin = −0.19 e Å−3
Absolute structure: Flack (1983 ▶), 1943 Friedel pairs
Flack parameter: 0.15 (10)
Data collection: CrysAlis PRO CCD (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis PRO CCD; data reduction: CrysAlis PRO RED (Oxford Diffraction, 2009 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810020386/vm2029sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810020386/vm2029Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank the Department of Science and Technology, India, for use of the CCD facility set up under the FIST–DST program at SSCU, IISc. We also thank Professor T. N. Guru Row, IISc, Bangalore, for his help with the data collection. FNK thanks the DST for Fast Track Proposal funding.
supplementary crystallographic information
Comment
A wide range of medicinal properties have already been identified for compounds containing the quinoline ring system including antiprotozoal (Fournet et al., 1981), antibacterial (Towers et al., 1981), antifungal (Biavatti et al., 2002) and antiviral activities (McCormick et al., 1996). Reaction of aniline with malonic acid in an excess of phosphorus oxychloride at reflux to give 2,4-dichloroquinoline was first reported by Ziegler & Gelfert (1959). A similar derivative of quinoline was synthesized from the mixture of p-toluidine and malonic acid in a one-pot reaction from an aryl amine, malonic acid and phosphorous oxychloride and its cytotoxicity has been reported (Somvanshi et al., 2008). Another derivative of quinoline prepared from p-anisidine and phosphorous oxychloride has been reported (Subashini et al., 2009). In continuous of our work, the crystal structure of another derivative is reported in this paper.
The molecules A (Cl1/Cl2/N1/C1–C11) and B (Cl3/Cl4/N2/C12–C22) in the asymmetric unit of the title compound (I) are shown in Fig. 1. In both molecules A and B, the bond lengths and angles are comparable with those of similar structures (Somvanshi et al., 2008; Subashini et al., 2009). The molecules A and B are essentially planar, except the H atoms of their methyl groups, with maximum deviations of 0.072 (5)Å for C10 and 0.072 (7)Å for C21, respectively. Fitting of the non-H atoms of molecules A and B results in an r.m.s. fit of 0.063 Å). The least-squares plane through molecule A makes a dihedral angle of 56.72 (14)° with that of molecule B.
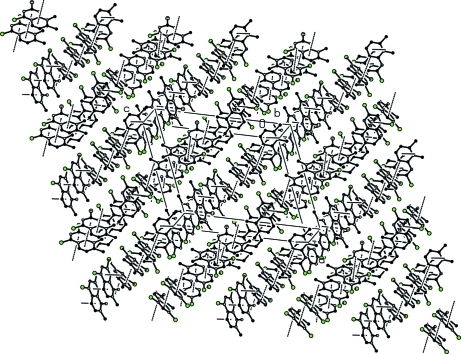
Weak intramolecular C—H···Cl and C—H···N interactions contribute to the stabilization of the molecular conformation of (I) (Table 1). In the crystal structure, weak π-π stacking interactions [Cg1···Cg2(x, 1 + y, z) = 3.791 (3) Å and Cg4···Cg5 (x, 1 + y, z) = 3.855 (3) Å; where Cg1, Cg2, Cg4 and Cg5 are centroids of the N1/C1–C4/C9, C4–C9, N2/C12–C15/C20 and C15–C20 rings, respectively] link pairs of molecules. In the structure, no classical hydrogen bonds are observed. Fig. 2 shows the crystal packing down the b axis.
Experimental
2,3-Dimethylaniline (10 mmol) and malonic acid (10 mmol) were heated under reflux in phosphorus oxychloride (30 ml), with stirring, for 5 h. The mixture was cooled, poured into crushed ice with vigorous stirring and then made alkaline with 5 M sodium hydroxide. Filtration gave the crude product as a brown solid. Column chromatography (95:5 hexane–EtOAc) yielded the pure 2,4-dichloro-7,8-dimethylquinoline. White needles of the synthesized compound have been grown from DMSO.
Refinement
H atoms were positioned geometrically with C—H = 0.93 and 0.96 Å, for aromatic and methyl H and refined as a riding method, with Uiso(H) = 1.2 or 1.5Ueq(C).
Figures
Fig. 1.
View of the two molecules in the same asymmetric unit of (I), showing the atom-numbering scheme and displacement ellipsoids drawn at the 50% probability level.
Fig. 2.
The molecular packing of (I) showing π-π stacking interactions (dashed lines) between the adjacent molecules down b axis. H atoms are omitted for clarity.
Crystal data
| C11H9Cl2N | F(000) = 928 |
| Mr = 226.09 | Dx = 1.446 Mg m−3 |
| Orthorhombic, Pca21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2c -2ac | Cell parameters from 895 reflections |
| a = 20.3054 (9) Å | θ = 1.8–24.7° |
| b = 3.9992 (2) Å | µ = 0.58 mm−1 |
| c = 25.5743 (11) Å | T = 295 K |
| V = 2076.77 (17) Å3 | Needle, colourless |
| Z = 8 | 0.30 × 0.24 × 0.15 mm |
Data collection
| Oxford Xcalibur Eos (Nova) CCD detector diffractometer | 4009 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2599 reflections with I > 2σ(I) |
| graphite | Rint = 0.048 |
| ω scans | θmax = 26.0°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis PRO RED; Oxford Diffraction, 2009) | h = −25→25 |
| Tmin = 0.845, Tmax = 0.918 | k = −4→4 |
| 19807 measured reflections | l = −31→31 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.049 | H-atom parameters constrained |
| wR(F2) = 0.119 | w = 1/[σ2(Fo2) + (0.0652P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.94 | (Δ/σ)max < 0.001 |
| 4009 reflections | Δρmax = 0.32 e Å−3 |
| 257 parameters | Δρmin = −0.19 e Å−3 |
| 1 restraint | Absolute structure: Flack (1983), 1943 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.15 (10) |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.68230 (6) | 0.6442 (5) | 0.97181 (6) | 0.0916 (6) | |
| Cl2 | 0.50757 (6) | 0.7463 (3) | 0.81738 (5) | 0.0709 (4) | |
| N1 | 0.56446 (17) | 0.4177 (12) | 0.97981 (17) | 0.0530 (12) | |
| C1 | 0.6040 (3) | 0.5681 (15) | 0.9491 (2) | 0.0630 (19) | |
| C2 | 0.5891 (2) | 0.6763 (11) | 0.89752 (18) | 0.0550 (16) | |
| C3 | 0.5272 (2) | 0.6141 (12) | 0.88079 (17) | 0.0490 (14) | |
| C4 | 0.4813 (3) | 0.4506 (11) | 0.9111 (3) | 0.0510 (19) | |
| C5 | 0.4158 (2) | 0.3859 (13) | 0.8958 (2) | 0.0593 (17) | |
| C6 | 0.3744 (2) | 0.2192 (11) | 0.9295 (2) | 0.0647 (17) | |
| C7 | 0.3943 (2) | 0.1131 (13) | 0.9795 (2) | 0.0623 (19) | |
| C8 | 0.4577 (2) | 0.1780 (10) | 0.99705 (18) | 0.0553 (17) | |
| C9 | 0.50171 (19) | 0.3483 (10) | 0.96177 (17) | 0.0477 (14) | |
| C10 | 0.3437 (3) | −0.0425 (13) | 1.0129 (2) | 0.0640 (19) | |
| C11 | 0.4804 (3) | 0.0815 (14) | 1.0504 (2) | 0.074 (2) | |
| Cl3 | 0.56576 (6) | 1.1324 (5) | 0.69164 (6) | 0.0964 (6) | |
| Cl4 | 0.73974 (6) | 1.2634 (3) | 0.84687 (5) | 0.0694 (4) | |
| N2 | 0.6857 (2) | 0.9027 (12) | 0.68457 (18) | 0.0627 (17) | |
| C12 | 0.6456 (3) | 1.0586 (13) | 0.7160 (2) | 0.0540 (17) | |
| C13 | 0.6582 (2) | 1.1805 (11) | 0.76636 (19) | 0.0553 (16) | |
| C14 | 0.7208 (2) | 1.1269 (12) | 0.78476 (17) | 0.0500 (16) | |
| C15 | 0.7689 (2) | 0.9656 (10) | 0.7534 (3) | 0.0420 (18) | |
| C16 | 0.8335 (3) | 0.8998 (14) | 0.7678 (2) | 0.0620 (17) | |
| C17 | 0.8766 (2) | 0.7485 (11) | 0.73560 (19) | 0.0590 (17) | |
| C18 | 0.8568 (2) | 0.6444 (12) | 0.6858 (2) | 0.0610 (19) | |
| C19 | 0.7928 (2) | 0.6889 (10) | 0.66826 (18) | 0.0567 (17) | |
| C20 | 0.74761 (19) | 0.8565 (11) | 0.70266 (17) | 0.0507 (16) | |
| C21 | 0.9046 (4) | 0.4700 (17) | 0.6447 (4) | 0.112 (4) | |
| C22 | 0.7674 (3) | 0.5740 (13) | 0.6163 (2) | 0.0650 (19) | |
| H2 | 0.62010 | 0.78320 | 0.87660 | 0.0660* | |
| H5 | 0.40070 | 0.45540 | 0.86320 | 0.0710* | |
| H6 | 0.33150 | 0.17480 | 0.91870 | 0.0780* | |
| H10A | 0.32250 | 0.12660 | 1.03360 | 0.0960* | |
| H10B | 0.36400 | −0.20380 | 1.03550 | 0.0960* | |
| H10C | 0.31150 | −0.15180 | 0.99130 | 0.0960* | |
| H11A | 0.46530 | 0.24420 | 1.07530 | 0.1120* | |
| H11B | 0.52760 | 0.07210 | 1.05100 | 0.1120* | |
| H11C | 0.46280 | −0.13390 | 1.05930 | 0.1120* | |
| H13 | 0.62630 | 1.29030 | 0.78600 | 0.0660* | |
| H16 | 0.84760 | 0.96260 | 0.80100 | 0.0740* | |
| H17 | 0.91970 | 0.71310 | 0.74650 | 0.0700* | |
| H21A | 0.90020 | 0.57790 | 0.61130 | 0.1670* | |
| H21B | 0.89310 | 0.23810 | 0.64140 | 0.1670* | |
| H21C | 0.94930 | 0.48880 | 0.65650 | 0.1670* | |
| H22A | 0.79460 | 0.66330 | 0.58900 | 0.0970* | |
| H22B | 0.72290 | 0.65090 | 0.61180 | 0.0970* | |
| H22C | 0.76820 | 0.33420 | 0.61490 | 0.0970* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0611 (8) | 0.1339 (13) | 0.0798 (10) | −0.0212 (8) | −0.0161 (7) | 0.0013 (12) |
| Cl2 | 0.0827 (8) | 0.0806 (7) | 0.0495 (6) | 0.0103 (6) | −0.0061 (6) | 0.0020 (6) |
| N1 | 0.047 (2) | 0.068 (2) | 0.044 (2) | 0.004 (2) | −0.0064 (19) | −0.008 (2) |
| C1 | 0.048 (3) | 0.069 (3) | 0.072 (4) | −0.003 (3) | −0.001 (3) | −0.013 (3) |
| C2 | 0.062 (3) | 0.056 (3) | 0.047 (2) | −0.002 (2) | 0.005 (2) | −0.007 (2) |
| C3 | 0.060 (3) | 0.048 (2) | 0.039 (2) | 0.006 (2) | 0.000 (2) | −0.005 (2) |
| C4 | 0.052 (3) | 0.041 (3) | 0.060 (4) | 0.009 (2) | −0.008 (3) | −0.020 (2) |
| C5 | 0.045 (3) | 0.075 (3) | 0.058 (3) | 0.010 (3) | −0.003 (2) | −0.019 (3) |
| C6 | 0.046 (3) | 0.067 (3) | 0.081 (3) | 0.007 (2) | −0.004 (2) | −0.016 (3) |
| C7 | 0.054 (3) | 0.054 (3) | 0.079 (4) | 0.005 (3) | 0.012 (3) | −0.016 (3) |
| C8 | 0.066 (3) | 0.048 (3) | 0.052 (3) | 0.004 (2) | 0.008 (2) | −0.012 (2) |
| C9 | 0.055 (2) | 0.038 (2) | 0.050 (3) | 0.001 (2) | 0.011 (2) | −0.012 (2) |
| C10 | 0.062 (4) | 0.062 (3) | 0.068 (3) | −0.004 (2) | 0.018 (3) | −0.018 (3) |
| C11 | 0.088 (4) | 0.067 (3) | 0.068 (4) | 0.002 (3) | −0.001 (3) | −0.004 (3) |
| Cl3 | 0.0610 (8) | 0.1433 (13) | 0.0848 (10) | 0.0298 (9) | −0.0193 (7) | −0.0122 (13) |
| Cl4 | 0.0783 (7) | 0.0777 (7) | 0.0523 (6) | −0.0045 (6) | −0.0028 (6) | −0.0053 (6) |
| N2 | 0.067 (3) | 0.067 (3) | 0.054 (3) | 0.006 (2) | −0.002 (2) | −0.002 (3) |
| C12 | 0.046 (3) | 0.073 (3) | 0.043 (3) | 0.005 (2) | −0.002 (2) | −0.006 (3) |
| C13 | 0.042 (2) | 0.061 (3) | 0.063 (3) | 0.008 (2) | 0.005 (2) | 0.002 (2) |
| C14 | 0.058 (3) | 0.047 (3) | 0.045 (2) | −0.003 (2) | 0.006 (2) | 0.002 (2) |
| C15 | 0.034 (3) | 0.039 (2) | 0.053 (4) | 0.0013 (16) | 0.004 (3) | 0.0138 (19) |
| C16 | 0.070 (3) | 0.057 (3) | 0.059 (3) | −0.014 (3) | −0.009 (3) | 0.004 (3) |
| C17 | 0.050 (3) | 0.060 (3) | 0.067 (3) | −0.001 (2) | −0.004 (2) | 0.006 (3) |
| C18 | 0.060 (3) | 0.043 (3) | 0.080 (4) | −0.001 (2) | 0.016 (3) | 0.018 (3) |
| C19 | 0.063 (3) | 0.049 (3) | 0.058 (3) | −0.008 (2) | 0.007 (2) | 0.011 (2) |
| C20 | 0.047 (2) | 0.054 (3) | 0.051 (3) | 0.000 (2) | 0.009 (2) | 0.007 (2) |
| C21 | 0.084 (5) | 0.086 (5) | 0.166 (8) | 0.018 (3) | 0.056 (5) | 0.007 (4) |
| C22 | 0.084 (4) | 0.072 (3) | 0.039 (3) | 0.016 (3) | 0.007 (3) | −0.002 (3) |
Geometric parameters (Å, °)
| Cl1—C1 | 1.720 (6) | C10—H10C | 0.9600 |
| Cl2—C3 | 1.752 (5) | C11—H11B | 0.9600 |
| Cl3—C12 | 1.762 (6) | C11—H11C | 0.9600 |
| Cl4—C14 | 1.723 (5) | C11—H11A | 0.9600 |
| N1—C9 | 1.383 (5) | C12—C13 | 1.401 (7) |
| N1—C1 | 1.274 (7) | C13—C14 | 1.372 (6) |
| N2—C20 | 1.352 (6) | C14—C15 | 1.419 (7) |
| N2—C12 | 1.303 (7) | C15—C16 | 1.388 (7) |
| C1—C2 | 1.421 (7) | C15—C20 | 1.436 (8) |
| C2—C3 | 1.351 (6) | C16—C17 | 1.345 (7) |
| C3—C4 | 1.377 (8) | C17—C18 | 1.399 (7) |
| C4—C5 | 1.410 (7) | C18—C19 | 1.386 (6) |
| C4—C9 | 1.421 (8) | C18—C21 | 1.592 (10) |
| C5—C6 | 1.376 (7) | C19—C20 | 1.437 (6) |
| C6—C7 | 1.407 (7) | C19—C22 | 1.498 (7) |
| C7—C10 | 1.474 (7) | C13—H13 | 0.9300 |
| C7—C8 | 1.388 (6) | C16—H16 | 0.9300 |
| C8—C11 | 1.491 (7) | C17—H17 | 0.9300 |
| C8—C9 | 1.441 (6) | C21—H21A | 0.9600 |
| C2—H2 | 0.9300 | C21—H21B | 0.9600 |
| C5—H5 | 0.9300 | C21—H21C | 0.9600 |
| C6—H6 | 0.9300 | C22—H22A | 0.9600 |
| C10—H10A | 0.9600 | C22—H22B | 0.9600 |
| C10—H10B | 0.9600 | C22—H22C | 0.9600 |
| Cl1···H22Ai | 3.0300 | H5···H16iii | 2.5500 |
| Cl2···H5 | 2.7300 | H6···H10C | 2.3100 |
| Cl2···H13ii | 3.1300 | H6···Cl4iii | 3.1500 |
| Cl2···H17iii | 3.1400 | H10A···C22viii | 3.0400 |
| Cl3···H21Civ | 2.9500 | H10A···H22Bviii | 2.3800 |
| Cl4···H6v | 3.1500 | H10B···C11 | 2.6500 |
| Cl4···H16 | 2.7600 | H10B···H11C | 2.1200 |
| N1···H11B | 2.4100 | H10C···H6 | 2.3100 |
| N2···H22B | 2.2500 | H11A···H11Cvi | 2.5200 |
| C3···C4vi | 3.558 (7) | H11B···N1 | 2.4100 |
| C4···C3ii | 3.558 (7) | H11C···H11Aii | 2.5200 |
| C5···C6vi | 3.543 (7) | H11C···C10 | 2.7200 |
| C6···C5ii | 3.543 (7) | H11C···H10B | 2.1200 |
| C8···C9ii | 3.553 (6) | H13···Cl2vi | 3.1300 |
| C9···C8vi | 3.553 (6) | H16···Cl4 | 2.7600 |
| C14···C15vi | 3.584 (6) | H16···H5v | 2.5500 |
| C15···C14ii | 3.584 (6) | H17···H21C | 2.5400 |
| C18···C21vi | 3.598 (9) | H17···Cl2v | 3.1400 |
| C19···C20ii | 3.563 (6) | H21A···C22 | 2.7000 |
| C20···C19vi | 3.563 (6) | H21A···H22A | 2.2400 |
| C21···C18ii | 3.598 (9) | H21B···C18ii | 2.7300 |
| C10···H11C | 2.7200 | H21B···C19ii | 3.0700 |
| C11···H10B | 2.6500 | H21B···C21ii | 3.0800 |
| C18···H21Bvi | 2.7300 | H21B···C22 | 2.9500 |
| C19···H21Bvi | 3.0700 | H21C···H17 | 2.5400 |
| C19···H22Cvi | 2.9600 | H21C···Cl3ix | 2.9500 |
| C20···H22Cvi | 2.9800 | H22A···C21 | 2.7600 |
| C21···H21Bvi | 3.0800 | H22A···H21A | 2.2400 |
| C21···H22C | 2.9200 | H22A···Cl1x | 3.0300 |
| C21···H22A | 2.7600 | H22B···N2 | 2.2500 |
| C22···H22Cvi | 3.0400 | H22B···H10Avii | 2.3800 |
| C22···H10Avii | 3.0400 | H22C···C19ii | 2.9600 |
| C22···H21B | 2.9500 | H22C···C20ii | 2.9800 |
| C22···H21A | 2.7000 | H22C···C21 | 2.9200 |
| H5···Cl2 | 2.7300 | H22C···C22ii | 3.0400 |
| C1—N1—C9 | 118.0 (4) | H11A—C11—H11B | 110.00 |
| C12—N2—C20 | 115.8 (5) | Cl3—C12—N2 | 115.9 (4) |
| Cl1—C1—N1 | 117.3 (4) | Cl3—C12—C13 | 115.8 (4) |
| N1—C1—C2 | 125.6 (5) | N2—C12—C13 | 128.3 (5) |
| Cl1—C1—C2 | 117.2 (4) | C12—C13—C14 | 115.5 (4) |
| C1—C2—C3 | 115.9 (4) | Cl4—C14—C13 | 118.3 (3) |
| Cl2—C3—C2 | 116.7 (3) | Cl4—C14—C15 | 120.8 (4) |
| C2—C3—C4 | 122.6 (5) | C13—C14—C15 | 121.0 (4) |
| Cl2—C3—C4 | 120.7 (4) | C14—C15—C16 | 125.9 (6) |
| C3—C4—C5 | 124.7 (6) | C14—C15—C20 | 116.2 (4) |
| C5—C4—C9 | 118.4 (5) | C16—C15—C20 | 117.8 (5) |
| C3—C4—C9 | 116.9 (5) | C15—C16—C17 | 122.5 (5) |
| C4—C5—C6 | 119.4 (5) | C16—C17—C18 | 120.3 (4) |
| C5—C6—C7 | 122.7 (4) | C17—C18—C19 | 121.7 (4) |
| C6—C7—C8 | 120.3 (4) | C17—C18—C21 | 123.8 (5) |
| C6—C7—C10 | 117.0 (4) | C19—C18—C21 | 114.5 (5) |
| C8—C7—C10 | 122.6 (5) | C18—C19—C20 | 117.4 (4) |
| C7—C8—C9 | 117.5 (4) | C18—C19—C22 | 124.8 (4) |
| C7—C8—C11 | 122.3 (4) | C20—C19—C22 | 117.8 (4) |
| C9—C8—C11 | 120.2 (4) | N2—C20—C15 | 123.2 (4) |
| N1—C9—C8 | 117.2 (4) | N2—C20—C19 | 116.6 (4) |
| C4—C9—C8 | 121.8 (4) | C15—C20—C19 | 120.2 (4) |
| N1—C9—C4 | 121.0 (4) | C12—C13—H13 | 122.00 |
| C3—C2—H2 | 122.00 | C14—C13—H13 | 122.00 |
| C1—C2—H2 | 122.00 | C15—C16—H16 | 119.00 |
| C4—C5—H5 | 120.00 | C17—C16—H16 | 119.00 |
| C6—C5—H5 | 120.00 | C16—C17—H17 | 120.00 |
| C7—C6—H6 | 119.00 | C18—C17—H17 | 120.00 |
| C5—C6—H6 | 119.00 | C18—C21—H21A | 109.00 |
| C7—C10—H10A | 110.00 | C18—C21—H21B | 109.00 |
| C7—C10—H10B | 109.00 | C18—C21—H21C | 110.00 |
| H10A—C10—H10B | 110.00 | H21A—C21—H21B | 109.00 |
| H10A—C10—H10C | 109.00 | H21A—C21—H21C | 109.00 |
| C7—C10—H10C | 109.00 | H21B—C21—H21C | 109.00 |
| H10B—C10—H10C | 109.00 | C19—C22—H22A | 109.00 |
| C8—C11—H11B | 109.00 | C19—C22—H22B | 109.00 |
| C8—C11—H11C | 110.00 | C19—C22—H22C | 110.00 |
| C8—C11—H11A | 109.00 | H22A—C22—H22B | 110.00 |
| H11A—C11—H11C | 109.00 | H22A—C22—H22C | 110.00 |
| H11B—C11—H11C | 109.00 | H22B—C22—H22C | 109.00 |
| C9—N1—C1—Cl1 | −178.7 (4) | C7—C8—C9—C4 | 0.7 (6) |
| C9—N1—C1—C2 | 0.8 (8) | C11—C8—C9—N1 | −0.4 (6) |
| C1—N1—C9—C4 | −1.9 (7) | C11—C8—C9—C4 | −178.6 (4) |
| C1—N1—C9—C8 | 180.0 (5) | C7—C8—C9—N1 | 178.9 (4) |
| C20—N2—C12—Cl3 | 177.8 (4) | Cl3—C12—C13—C14 | −179.0 (4) |
| C20—N2—C12—C13 | −0.9 (8) | N2—C12—C13—C14 | −0.3 (8) |
| C12—N2—C20—C15 | 0.8 (7) | C12—C13—C14—Cl4 | −178.9 (4) |
| C12—N2—C20—C19 | −179.2 (4) | C12—C13—C14—C15 | 1.5 (7) |
| N1—C1—C2—C3 | 0.7 (8) | Cl4—C14—C15—C16 | 0.2 (7) |
| Cl1—C1—C2—C3 | −179.8 (4) | Cl4—C14—C15—C20 | 178.9 (3) |
| C1—C2—C3—Cl2 | 179.8 (4) | C13—C14—C15—C16 | 179.7 (5) |
| C1—C2—C3—C4 | −1.2 (7) | C13—C14—C15—C20 | −1.6 (7) |
| Cl2—C3—C4—C5 | −2.4 (7) | C14—C15—C16—C17 | −179.3 (5) |
| Cl2—C3—C4—C9 | 179.2 (3) | C20—C15—C16—C17 | 2.0 (8) |
| C2—C3—C4—C9 | 0.2 (7) | C14—C15—C20—N2 | 0.3 (7) |
| C2—C3—C4—C5 | 178.7 (5) | C14—C15—C20—C19 | −179.6 (4) |
| C3—C4—C5—C6 | 179.9 (5) | C16—C15—C20—N2 | 179.2 (5) |
| C9—C4—C5—C6 | −1.7 (7) | C16—C15—C20—C19 | −0.8 (7) |
| C5—C4—C9—C8 | 0.9 (7) | C15—C16—C17—C18 | −1.3 (8) |
| C3—C4—C9—N1 | 1.4 (7) | C16—C17—C18—C19 | −0.8 (7) |
| C3—C4—C9—C8 | 179.4 (4) | C16—C17—C18—C21 | 178.8 (5) |
| C5—C4—C9—N1 | −177.2 (4) | C17—C18—C19—C20 | 1.9 (7) |
| C4—C5—C6—C7 | 1.0 (7) | C17—C18—C19—C22 | −177.8 (4) |
| C5—C6—C7—C10 | 176.0 (5) | C21—C18—C19—C20 | −177.7 (4) |
| C5—C6—C7—C8 | 0.7 (7) | C21—C18—C19—C22 | 2.6 (7) |
| C6—C7—C8—C9 | −1.5 (7) | C18—C19—C20—N2 | 178.9 (4) |
| C6—C7—C8—C11 | 177.8 (4) | C18—C19—C20—C15 | −1.1 (6) |
| C10—C7—C8—C9 | −176.5 (4) | C22—C19—C20—N2 | −1.3 (6) |
| C10—C7—C8—C11 | 2.8 (7) | C22—C19—C20—C15 | 178.7 (4) |
Symmetry codes: (i) −x+3/2, y, z+1/2; (ii) x, y−1, z; (iii) x−1/2, −y+1, z; (iv) x−1/2, −y+2, z; (v) x+1/2, −y+1, z; (vi) x, y+1, z; (vii) −x+1, −y+1, z−1/2; (viii) −x+1, −y+1, z+1/2; (ix) x+1/2, −y+2, z; (x) −x+3/2, y, z−1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C5—H5···Cl2 | 0.93 | 2.73 | 3.094 (5) | 104 |
| C11—H11B···N1 | 0.96 | 2.41 | 2.825 (7) | 106 |
| C16—H16···Cl4 | 0.93 | 2.76 | 3.135 (6) | 105 |
| C22—H22B···N2 | 0.96 | 2.25 | 2.744 (7) | 111 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: VM2029).
References
- Biavatti, M. W., Vieira, P. C., da Silva, M. F. G. F., Fernandes, J. B., Victor, S. R., Pagnocca, F. C., Albuquerque, S., Caracelli, I. & Zukerman-Schpector, J. (2002). J. Braz. Chem. Soc.13, 66–70.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Fournet, A., Barrios, A. A., Munioz, V., Hocquemiller, R., Cave, A. & Bruneton, J. (1981). J. Antimicrob. Agents Chemother.37, 859–863. [DOI] [PMC free article] [PubMed]
- McCormick, J. L., McKee, T. C., Cardellina, J. H. & Boyd, M. R. (1996). J. Nat. Prod.59, 469–471. [DOI] [PubMed]
- Oxford Diffraction (2009). CrysAlis PRO CCD and CrysAlis PRO RED Oxford Diffraction Ltd, Yarnton, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Somvanshi, R. K., Subashini, R., Dhanasekaran, V., Arulprakash, G., Das, S. N. & Dey, S. (2008). J. Chem. Crystallogr.38, 381–386.
- Subashini, R., Hathwar, V. R., Manivel, P., Prabakaran, K. & Khan, F. N. (2009). Acta Cryst. E65, o370. [DOI] [PMC free article] [PubMed]
- Towers, G. H. N., Grahanm, E. A., Spenser, I. D. & Abramowski, Z. (1981). Planta Med.41, 136–142. [DOI] [PubMed]
- Ziegler, E. & Gelfert, K. (1959). Monatsh. Chem.90, 822–826.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810020386/vm2029sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810020386/vm2029Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report