Abstract
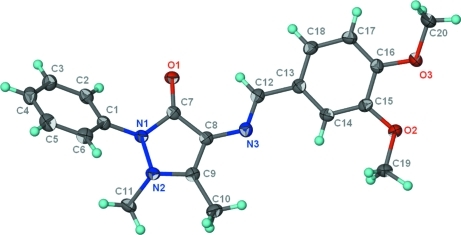
The imino–carbon double-bond in the title Schiff base, C20H21N3O3, has an E configuration; the six-membered aromatic substituent (r.m.s. deviation = 0.012 Å) is nearly coplanar with five-membered pyrazole substituent (r.m.s. deviation = 0.031 Å), the dihedral angle between the two systems being 11.4 (1)°]. The phenyl ring connected to the pyrazole ring is aligned at 45.5 (1)° with respect to this five-membered ring. The N atoms in the ring show pyramidal coordinations.
Related literature
For background literature on Schiff bases derived from 4-aminoantipyridine, see: Montalvo-González & Ariza-Castolo (2003 ▶).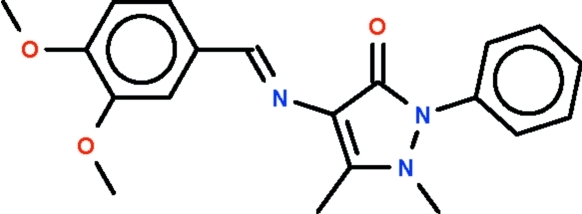
Experimental
Crystal data
C20H21N3O3
M r = 351.40
Monoclinic,
a = 12.5584 (8) Å
b = 10.4752 (7) Å
c = 14.6002 (9) Å
β = 109.039 (1)°
V = 1815.6 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 100 K
0.35 × 0.25 × 0.15 mm
Data collection
Bruker SMART APEX diffractometer
16900 measured reflections
4164 independent reflections
3442 reflections with I > 2σ(I)
R int = 0.031
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.103
S = 1.00
4164 reflections
239 parameters
H-atom parameters constrained
Δρmax = 0.23 e Å−3
Δρmin = −0.24 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810023238/cv2733sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810023238/cv2733Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank King Abdul Aziz University and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
4-Aminoantipyrine (4-amino-1,2-dihydro-1,5-dimethyl-2-phenyl-3H-pyrazol-3-one) possesses an aminopyrazolone unit, a feature that allows the compound to condense with aromatic aldehydes to yield Schiff bases. The Schiff base derived from the benzaldehyde homolog has nearly coplanar phenyl and pyrazoly rings (Montalvo-González & Ariza-Castolo, 2003). In the title benzaldehyde analog (Scheme I, Fig. 1), the 6-membered ring is nearly coplanar with 5-membered pyrazolyl ring [dihedral angle between the two systems 11.4 (1) °]. The phenyl ring connected to the pyrazolyl ring is aligned at 45.5 (1)°.
Experimental
3,4-Dimethoxybenzaldehyde (0.36 g, 2.2 mmol) and 4-aminoantipyrine (0.45 g, 2.2 mmol) here heated in methanol (15 ml) for 5 h to afford a colorless precipitate. The solid material was collected and recrystallized from methanol.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C–H 0.95 to 0.98 Å, U(H) 1.2 to 1.5Ueq(C)] and were included in the refinement in the riding model approximation.
Figures
Fig. 1.
Displacement ellipsoid plot (Barbour, 2001) of C20H21N3O3 at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C20H21N3O3 | F(000) = 744 |
| Mr = 351.40 | Dx = 1.286 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 6153 reflections |
| a = 12.5584 (8) Å | θ = 2.4–28.2° |
| b = 10.4752 (7) Å | µ = 0.09 mm−1 |
| c = 14.6002 (9) Å | T = 100 K |
| β = 109.039 (1)° | Prism, colourless |
| V = 1815.6 (2) Å3 | 0.35 × 0.25 × 0.15 mm |
| Z = 4 |
Data collection
| Bruker SMART APEX diffractometer | 3442 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.031 |
| graphite | θmax = 27.5°, θmin = 1.7° |
| ω scans | h = −16→16 |
| 16900 measured reflections | k = −13→12 |
| 4164 independent reflections | l = −18→17 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.037 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.103 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0548P)2 + 0.5659P] where P = (Fo2 + 2Fc2)/3 |
| 4164 reflections | (Δ/σ)max = 0.001 |
| 239 parameters | Δρmax = 0.23 e Å−3 |
| 0 restraints | Δρmin = −0.24 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.28425 (6) | 0.77513 (8) | 0.59527 (6) | 0.01980 (19) | |
| O2 | 0.86240 (7) | 0.62990 (8) | 0.50221 (6) | 0.02147 (19) | |
| O3 | 0.86452 (8) | 0.84966 (8) | 0.42541 (7) | 0.0292 (2) | |
| N1 | 0.26678 (8) | 0.60441 (9) | 0.69085 (7) | 0.0185 (2) | |
| N2 | 0.33485 (8) | 0.50111 (9) | 0.73701 (7) | 0.0190 (2) | |
| N3 | 0.50498 (8) | 0.62594 (9) | 0.60372 (7) | 0.0187 (2) | |
| C1 | 0.18777 (9) | 0.65675 (11) | 0.73138 (8) | 0.0189 (2) | |
| C2 | 0.09077 (9) | 0.71284 (11) | 0.66951 (9) | 0.0204 (2) | |
| H2 | 0.0778 | 0.7155 | 0.6017 | 0.025* | |
| C3 | 0.01286 (10) | 0.76508 (12) | 0.70792 (9) | 0.0235 (3) | |
| H3 | −0.0530 | 0.8052 | 0.6662 | 0.028* | |
| C4 | 0.03033 (10) | 0.75915 (12) | 0.80662 (9) | 0.0252 (3) | |
| H4 | −0.0240 | 0.7936 | 0.8323 | 0.030* | |
| C5 | 0.12751 (11) | 0.70267 (13) | 0.86781 (9) | 0.0258 (3) | |
| H5 | 0.1394 | 0.6982 | 0.9354 | 0.031* | |
| C6 | 0.20713 (10) | 0.65278 (12) | 0.83089 (9) | 0.0230 (3) | |
| H6 | 0.2745 | 0.6161 | 0.8731 | 0.028* | |
| C7 | 0.31965 (9) | 0.67280 (11) | 0.63587 (8) | 0.0171 (2) | |
| C8 | 0.41934 (9) | 0.59911 (11) | 0.64197 (8) | 0.0173 (2) | |
| C9 | 0.42229 (9) | 0.49573 (11) | 0.70009 (8) | 0.0179 (2) | |
| C10 | 0.50361 (10) | 0.38826 (12) | 0.72347 (9) | 0.0231 (3) | |
| H10A | 0.5663 | 0.4077 | 0.6996 | 0.035* | |
| H10B | 0.4657 | 0.3102 | 0.6924 | 0.035* | |
| H10C | 0.5326 | 0.3759 | 0.7938 | 0.035* | |
| C11 | 0.27490 (10) | 0.38645 (11) | 0.75111 (9) | 0.0225 (3) | |
| H11A | 0.2343 | 0.4054 | 0.7965 | 0.034* | |
| H11B | 0.3292 | 0.3177 | 0.7774 | 0.034* | |
| H11C | 0.2212 | 0.3597 | 0.6888 | 0.034* | |
| C12 | 0.49724 (9) | 0.72136 (11) | 0.54678 (8) | 0.0191 (2) | |
| H12 | 0.4304 | 0.7713 | 0.5274 | 0.023* | |
| C13 | 0.59014 (9) | 0.75419 (11) | 0.51128 (8) | 0.0187 (2) | |
| C14 | 0.68242 (9) | 0.67130 (11) | 0.52633 (8) | 0.0182 (2) | |
| H14 | 0.6838 | 0.5919 | 0.5582 | 0.022* | |
| C15 | 0.77102 (9) | 0.70504 (11) | 0.49495 (8) | 0.0186 (2) | |
| C16 | 0.77122 (10) | 0.82494 (11) | 0.45064 (9) | 0.0213 (2) | |
| C17 | 0.68007 (11) | 0.90626 (12) | 0.43516 (9) | 0.0241 (3) | |
| H17 | 0.6794 | 0.9867 | 0.4049 | 0.029* | |
| C18 | 0.58924 (10) | 0.86946 (11) | 0.46420 (9) | 0.0222 (3) | |
| H18 | 0.5257 | 0.9242 | 0.4515 | 0.027* | |
| C19 | 0.87115 (10) | 0.51317 (11) | 0.55464 (9) | 0.0229 (3) | |
| H19A | 0.9404 | 0.4687 | 0.5564 | 0.034* | |
| H19B | 0.8060 | 0.4590 | 0.5226 | 0.034* | |
| H19C | 0.8730 | 0.5316 | 0.6209 | 0.034* | |
| C20 | 0.86692 (15) | 0.96944 (14) | 0.37878 (14) | 0.0452 (4) | |
| H20A | 0.9381 | 0.9775 | 0.3652 | 0.068* | |
| H20B | 0.8605 | 1.0393 | 0.4213 | 0.068* | |
| H20C | 0.8038 | 0.9735 | 0.3179 | 0.068* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0190 (4) | 0.0167 (4) | 0.0230 (4) | 0.0009 (3) | 0.0060 (3) | 0.0034 (3) |
| O2 | 0.0217 (4) | 0.0182 (4) | 0.0269 (4) | 0.0041 (3) | 0.0112 (3) | 0.0044 (3) |
| O3 | 0.0333 (5) | 0.0184 (4) | 0.0463 (6) | 0.0023 (4) | 0.0273 (4) | 0.0066 (4) |
| N1 | 0.0171 (4) | 0.0173 (5) | 0.0208 (5) | 0.0008 (4) | 0.0060 (4) | 0.0036 (4) |
| N2 | 0.0183 (4) | 0.0165 (5) | 0.0216 (5) | 0.0006 (4) | 0.0057 (4) | 0.0036 (4) |
| N3 | 0.0178 (4) | 0.0192 (5) | 0.0192 (5) | −0.0019 (4) | 0.0062 (4) | −0.0020 (4) |
| C1 | 0.0170 (5) | 0.0173 (5) | 0.0228 (6) | −0.0038 (4) | 0.0071 (4) | −0.0013 (5) |
| C2 | 0.0188 (5) | 0.0214 (6) | 0.0208 (6) | −0.0040 (4) | 0.0060 (4) | −0.0001 (5) |
| C3 | 0.0176 (5) | 0.0232 (6) | 0.0293 (6) | −0.0018 (5) | 0.0071 (5) | −0.0010 (5) |
| C4 | 0.0241 (6) | 0.0237 (6) | 0.0319 (7) | −0.0058 (5) | 0.0147 (5) | −0.0083 (5) |
| C5 | 0.0302 (6) | 0.0271 (7) | 0.0212 (6) | −0.0061 (5) | 0.0097 (5) | −0.0051 (5) |
| C6 | 0.0220 (6) | 0.0236 (6) | 0.0211 (6) | −0.0021 (5) | 0.0039 (5) | −0.0013 (5) |
| C7 | 0.0164 (5) | 0.0169 (5) | 0.0167 (5) | −0.0032 (4) | 0.0038 (4) | −0.0016 (4) |
| C8 | 0.0163 (5) | 0.0172 (5) | 0.0174 (5) | −0.0008 (4) | 0.0042 (4) | −0.0013 (4) |
| C9 | 0.0169 (5) | 0.0179 (5) | 0.0170 (5) | −0.0012 (4) | 0.0030 (4) | −0.0019 (4) |
| C10 | 0.0232 (6) | 0.0190 (6) | 0.0258 (6) | 0.0036 (5) | 0.0064 (5) | 0.0027 (5) |
| C11 | 0.0249 (6) | 0.0185 (6) | 0.0251 (6) | −0.0033 (5) | 0.0096 (5) | 0.0031 (5) |
| C12 | 0.0184 (5) | 0.0198 (6) | 0.0189 (5) | 0.0007 (4) | 0.0060 (4) | −0.0012 (4) |
| C13 | 0.0207 (5) | 0.0190 (6) | 0.0171 (5) | −0.0008 (4) | 0.0070 (4) | −0.0022 (4) |
| C14 | 0.0219 (5) | 0.0160 (5) | 0.0170 (5) | −0.0006 (4) | 0.0069 (4) | 0.0001 (4) |
| C15 | 0.0206 (5) | 0.0161 (5) | 0.0192 (5) | 0.0017 (4) | 0.0069 (4) | −0.0019 (4) |
| C16 | 0.0250 (6) | 0.0184 (6) | 0.0253 (6) | −0.0005 (5) | 0.0149 (5) | −0.0008 (5) |
| C17 | 0.0325 (6) | 0.0166 (6) | 0.0284 (6) | 0.0030 (5) | 0.0169 (5) | 0.0034 (5) |
| C18 | 0.0256 (6) | 0.0191 (6) | 0.0241 (6) | 0.0046 (5) | 0.0113 (5) | 0.0006 (5) |
| C19 | 0.0246 (6) | 0.0176 (6) | 0.0273 (6) | 0.0028 (5) | 0.0095 (5) | 0.0041 (5) |
| C20 | 0.0552 (9) | 0.0222 (7) | 0.0797 (12) | 0.0092 (7) | 0.0513 (9) | 0.0175 (7) |
Geometric parameters (Å, °)
| O1—C7 | 1.2354 (14) | C9—C10 | 1.4830 (16) |
| O2—C15 | 1.3669 (14) | C10—H10A | 0.9800 |
| O2—C19 | 1.4277 (14) | C10—H10B | 0.9800 |
| O3—C16 | 1.3627 (14) | C10—H10C | 0.9800 |
| O3—C20 | 1.4326 (16) | C11—H11A | 0.9800 |
| N1—C7 | 1.3950 (14) | C11—H11B | 0.9800 |
| N1—N2 | 1.4072 (13) | C11—H11C | 0.9800 |
| N1—C1 | 1.4206 (15) | C12—C13 | 1.4638 (16) |
| N2—C9 | 1.3731 (15) | C12—H12 | 0.9500 |
| N2—C11 | 1.4673 (14) | C13—C18 | 1.3876 (17) |
| N3—C12 | 1.2833 (15) | C13—C14 | 1.4067 (16) |
| N3—C8 | 1.3926 (14) | C14—C15 | 1.3804 (16) |
| C1—C2 | 1.3883 (16) | C14—H14 | 0.9500 |
| C1—C6 | 1.3933 (17) | C15—C16 | 1.4132 (16) |
| C2—C3 | 1.3890 (17) | C16—C17 | 1.3851 (17) |
| C2—H2 | 0.9500 | C17—C18 | 1.3946 (17) |
| C3—C4 | 1.3865 (18) | C17—H17 | 0.9500 |
| C3—H3 | 0.9500 | C18—H18 | 0.9500 |
| C4—C5 | 1.3879 (19) | C19—H19A | 0.9800 |
| C4—H4 | 0.9500 | C19—H19B | 0.9800 |
| C5—C6 | 1.3839 (18) | C19—H19C | 0.9800 |
| C5—H5 | 0.9500 | C20—H20A | 0.9800 |
| C6—H6 | 0.9500 | C20—H20B | 0.9800 |
| C7—C8 | 1.4486 (15) | C20—H20C | 0.9800 |
| C8—C9 | 1.3688 (16) | ||
| C15—O2—C19 | 116.78 (9) | H10B—C10—H10C | 109.5 |
| C16—O3—C20 | 116.62 (10) | N2—C11—H11A | 109.5 |
| C7—N1—N2 | 109.98 (9) | N2—C11—H11B | 109.5 |
| C7—N1—C1 | 124.72 (10) | H11A—C11—H11B | 109.5 |
| N2—N1—C1 | 119.67 (9) | N2—C11—H11C | 109.5 |
| C9—N2—N1 | 106.39 (9) | H11A—C11—H11C | 109.5 |
| C9—N2—C11 | 122.28 (10) | H11B—C11—H11C | 109.5 |
| N1—N2—C11 | 115.93 (9) | N3—C12—C13 | 120.78 (10) |
| C12—N3—C8 | 120.78 (10) | N3—C12—H12 | 119.6 |
| C2—C1—C6 | 120.57 (11) | C13—C12—H12 | 119.6 |
| C2—C1—N1 | 118.44 (10) | C18—C13—C14 | 119.19 (11) |
| C6—C1—N1 | 120.99 (10) | C18—C13—C12 | 120.08 (10) |
| C1—C2—C3 | 119.23 (11) | C14—C13—C12 | 120.71 (10) |
| C1—C2—H2 | 120.4 | C15—C14—C13 | 120.15 (11) |
| C3—C2—H2 | 120.4 | C15—C14—H14 | 119.9 |
| C4—C3—C2 | 120.56 (11) | C13—C14—H14 | 119.9 |
| C4—C3—H3 | 119.7 | O2—C15—C14 | 125.03 (10) |
| C2—C3—H3 | 119.7 | O2—C15—C16 | 114.87 (10) |
| C3—C4—C5 | 119.73 (11) | C14—C15—C16 | 120.10 (10) |
| C3—C4—H4 | 120.1 | O3—C16—C17 | 125.23 (11) |
| C5—C4—H4 | 120.1 | O3—C16—C15 | 115.00 (10) |
| C6—C5—C4 | 120.37 (12) | C17—C16—C15 | 119.77 (11) |
| C6—C5—H5 | 119.8 | C16—C17—C18 | 119.66 (11) |
| C4—C5—H5 | 119.8 | C16—C17—H17 | 120.2 |
| C5—C6—C1 | 119.50 (11) | C18—C17—H17 | 120.2 |
| C5—C6—H6 | 120.2 | C13—C18—C17 | 121.05 (11) |
| C1—C6—H6 | 120.2 | C13—C18—H18 | 119.5 |
| O1—C7—N1 | 123.87 (10) | C17—C18—H18 | 119.5 |
| O1—C7—C8 | 131.33 (10) | O2—C19—H19A | 109.5 |
| N1—C7—C8 | 104.77 (9) | O2—C19—H19B | 109.5 |
| C9—C8—N3 | 122.68 (10) | H19A—C19—H19B | 109.5 |
| C9—C8—C7 | 107.92 (10) | O2—C19—H19C | 109.5 |
| N3—C8—C7 | 129.26 (10) | H19A—C19—H19C | 109.5 |
| C8—C9—N2 | 110.34 (10) | H19B—C19—H19C | 109.5 |
| C8—C9—C10 | 128.36 (11) | O3—C20—H20A | 109.5 |
| N2—C9—C10 | 121.31 (10) | O3—C20—H20B | 109.5 |
| C9—C10—H10A | 109.5 | H20A—C20—H20B | 109.5 |
| C9—C10—H10B | 109.5 | O3—C20—H20C | 109.5 |
| H10A—C10—H10B | 109.5 | H20A—C20—H20C | 109.5 |
| C9—C10—H10C | 109.5 | H20B—C20—H20C | 109.5 |
| H10A—C10—H10C | 109.5 | ||
| C7—N1—N2—C9 | −8.00 (12) | C7—C8—C9—N2 | −3.61 (13) |
| C1—N1—N2—C9 | −162.78 (10) | N3—C8—C9—C10 | −8.03 (19) |
| C7—N1—N2—C11 | −147.65 (10) | C7—C8—C9—C10 | 175.83 (11) |
| C1—N1—N2—C11 | 57.57 (13) | N1—N2—C9—C8 | 7.08 (12) |
| C7—N1—C1—C2 | 58.12 (15) | C11—N2—C9—C8 | 143.54 (11) |
| N2—N1—C1—C2 | −151.03 (10) | N1—N2—C9—C10 | −172.41 (10) |
| C7—N1—C1—C6 | −121.50 (12) | C11—N2—C9—C10 | −35.94 (16) |
| N2—N1—C1—C6 | 29.34 (16) | C8—N3—C12—C13 | 176.26 (10) |
| C6—C1—C2—C3 | −0.05 (17) | N3—C12—C13—C18 | −168.62 (11) |
| N1—C1—C2—C3 | −179.68 (10) | N3—C12—C13—C14 | 9.85 (17) |
| C1—C2—C3—C4 | −1.31 (18) | C18—C13—C14—C15 | 0.35 (17) |
| C2—C3—C4—C5 | 1.22 (18) | C12—C13—C14—C15 | −178.13 (10) |
| C3—C4—C5—C6 | 0.25 (19) | C19—O2—C15—C14 | −6.31 (16) |
| C4—C5—C6—C1 | −1.59 (19) | C19—O2—C15—C16 | 174.22 (10) |
| C2—C1—C6—C5 | 1.50 (18) | C13—C14—C15—O2 | −177.20 (10) |
| N1—C1—C6—C5 | −178.89 (11) | C13—C14—C15—C16 | 2.23 (17) |
| N2—N1—C7—O1 | −172.69 (10) | C20—O3—C16—C17 | −0.3 (2) |
| C1—N1—C7—O1 | −19.46 (17) | C20—O3—C16—C15 | 178.96 (13) |
| N2—N1—C7—C8 | 5.75 (12) | O2—C15—C16—O3 | −2.53 (15) |
| C1—N1—C7—C8 | 158.98 (10) | C14—C15—C16—O3 | 177.98 (11) |
| C12—N3—C8—C9 | 177.56 (11) | O2—C15—C16—C17 | 176.81 (11) |
| C12—N3—C8—C7 | −7.18 (18) | C14—C15—C16—C17 | −2.68 (18) |
| O1—C7—C8—C9 | 176.90 (12) | O3—C16—C17—C18 | 179.80 (12) |
| N1—C7—C8—C9 | −1.37 (12) | C15—C16—C17—C18 | 0.52 (19) |
| O1—C7—C8—N3 | 1.1 (2) | C14—C13—C18—C17 | −2.54 (18) |
| N1—C7—C8—N3 | −177.18 (11) | C12—C13—C18—C17 | 175.95 (11) |
| N3—C8—C9—N2 | 172.54 (10) | C16—C17—C18—C13 | 2.10 (19) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CV2733).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Montalvo-González, R. & Ariza-Castolo, A. (2003). J. Mol. Struct.655, 375–389.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst.43 Submitted.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810023238/cv2733sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810023238/cv2733Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report