Abstract
In the title compound, C6H14N2 2+·2C6H2N3O7 −, the cation possesses crystallographically imposed twofold rotation symmetry. In the crystal structure, the cation and anions are linked into a trimeric aggregate by intermolecular N—H⋯O hydrogen bonds. The trimeric units are further connected by π–π interactions [centroid–centroid distances = 3.507 (2)–3.660 (3) Å], forming layers parallel to the bc plane.
Related literature
For a discussion on hydrogen bonding in in the title crystal, see: Kumai et al. (2007 ▶); Horiuchi et al. (2005 ▶). For related structures, see: Dabros et al. (2007 ▶); Jin et al. (2004 ▶); Glidewell et al. (1999 ▶); Chen et al. (2009 ▶).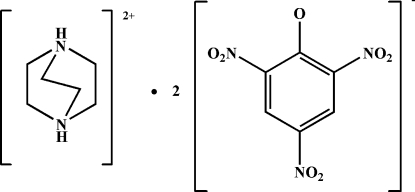
Experimental
Crystal data
C6H14N2 2+·2C6H2N3O7 −
M r = 570.40
Monoclinic,

a = 15.3808 (11) Å
b = 7.1520 (5) Å
c = 25.3527 (14) Å
β = 125.496 (2)°
V = 2270.6 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.15 mm−1
T = 93 K
0.1 × 0.1 × 0.1 mm
Data collection
Rigaku SCXmini diffractometer
Absorption correction: multi-scan (CrystalClear; Rigaku, 2005 ▶) T min = 0.857, T max = 1.000
10700 measured reflections
2590 independent reflections
2218 reflections with I > 2σ(I)
R int = 0.028
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.098
S = 1.07
2590 reflections
181 parameters
H-atom parameters constrained
Δρmax = 0.54 e Å−3
Δρmin = −0.59 e Å−3
Data collection: CrystalClear (Rigaku, 2005 ▶); cell refinement: CrystalClear (Rigaku, 2005 ▶); data reduction: CrystalClear (Rigaku, 2005 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: PRPKAPPA (Ferguson, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810021021/rz2442sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810021021/rz2442Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4—H4A⋯O1 | 0.93 | 1.69 | 2.589 (2) | 161 |
| N4—H4A⋯O2 | 0.93 | 2.42 | 2.954 (2) | 117 |
Acknowledgments
The author is grateful to the starter fund of Southeast University for financial support to buy the X-ray diffractometer.
supplementary crystallographic information
Comment
The co-crystals of 1,4-diazabicyclo[2.2.2]octane (DABCO) and phenols are typically characterized by the presence of N—H···O or O—H···N hydrogen-bonded adducts (Kumai et al. (2007); Horiuchi et al., 2005). Many of this type of co-crystals have been designed by employing crystal-engineering strategies, and their structures have been studied extensively (Dabros et al., 2007; Jin et al., 2004; Glidewell et al., 1999). As a continuation of a study of phase transitions in hydrogen-bonded co-crystalline compounds between phenols and tertiary amines as N–H···O-type systems (Chen et al., 2009), the crystal structure of the 1:2 co-crystal of DABCO and 2,4,6-trinitrophenol obtained by a single-crystal X-ray analysis is reported herein. The compound shows no dielectric irregularity in the temperature range of 93–373K.
The title compound (Fig. 1) was obtained from the reaction of 1,4-diazabicyclo[2.2.2]octane and 2,4,6-trinitrophenol. The cation has crystallographically imposed twofold rotation symmetry. The two protonated N atoms in the cation are almost equivalent with very close C–N bond lengths [1.4930 (19) to 1.4952 (18) Å] and C–N–C angles [109.79 (11)° to 110.72 (11)°]. Within the benzene ring of the 2,4,6-trinitrophenol anion, the C–C–C bond angles of the three nitro-connected C atoms are in the range 121.92 (13)–126.76 (13)°, and are a little larger than the remaining three C–C–C bond angles. In the crystal structure (Fig. 2), cation and anions are linked into a trimeric aggregate by intermolecular N—H···O hydrogen bonds (Table 1). The trimeric units are further connected by π–π interactions (centroid-to-centroid distance = 3.507 (2)–3.660 (3) Å) to form layers parallel to the bc plane.
Experimental
1,4-Diazabicyclo[2.2.2]octane (DABCO) (2.5 mmol) was dissolved in ethanol (10 ml).The clear solution obtained was added to a solution of 2,4,6-trinitrophenol(5 mmol) in ethanol (20 ml). The formed precipitate was then filtered and the obtained yellow solid was redissolved in DMF (15 ml). Yellow co-crystals of the title compound suitable for X-ray diffraction analysis were obtained by slow evaporation of the mixture at room temperature after 7 days.
Refinement
All the H atoms were calculated geometrically and were allowed to ride, with C—H = 0.95-0.99 Å, N—H = 0.93 Å, and with Uiso(H) = 1.2Ueq(C, N).
Figures
Fig. 1.
The molecular structure of the title compound, with the atomic numbering scheme. Displacement ellipsoids are drawn at the 30% probability level. Atoms labelled with suffix A are generated by the symmetry operation (-x, y, 0.5-z).
Fig. 2.
Crystal packing of the title compound viewed along the c axis. Dashed lines indicate hydrogen bonds.
Crystal data
| C6H14N22+·2C6H2N3O7− | F(000) = 1176 |
| Mr = 570.40 | Dx = 1.669 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71075 Å |
| Hall symbol: -C 2yc | Cell parameters from 4042 reflections |
| a = 15.3808 (11) Å | θ = 3.5–27.6° |
| b = 7.1520 (5) Å | µ = 0.15 mm−1 |
| c = 25.3527 (14) Å | T = 93 K |
| β = 125.496 (2)° | Prism, yellow |
| V = 2270.6 (3) Å3 | 0.1 × 0.1 × 0.1 mm |
| Z = 4 |
Data collection
| Rigaku SCXmini diffractometer | 2590 independent reflections |
| Radiation source: fine-focus sealed tube | 2218 reflections with I > 2σ(I) |
| graphite | Rint = 0.028 |
| Detector resolution: 13.6612 pixels mm-1 | θmax = 27.5°, θmin = 3.1° |
| ω scans | h = −19→18 |
| Absorption correction: multi-scan (CrystalClear; Rigaku, 2005) | k = −9→9 |
| Tmin = 0.857, Tmax = 1.000 | l = −32→32 |
| 10700 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.039 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.098 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0406P)2 + 3.4753P] where P = (Fo2 + 2Fc2)/3 |
| 2590 reflections | (Δ/σ)max < 0.001 |
| 181 parameters | Δρmax = 0.54 e Å−3 |
| 0 restraints | Δρmin = −0.59 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.06908 (8) | 0.27695 (17) | 0.12769 (5) | 0.0196 (2) | |
| O2 | −0.12313 (9) | 0.44433 (16) | 0.07242 (5) | 0.0189 (2) | |
| O3 | −0.23908 (8) | 0.41893 (16) | −0.03110 (5) | 0.0192 (2) | |
| O4 | −0.13536 (9) | 0.27577 (18) | −0.16941 (5) | 0.0248 (3) | |
| O5 | 0.01746 (9) | 0.14572 (17) | −0.13108 (5) | 0.0241 (3) | |
| O6 | 0.28037 (11) | 0.2978 (2) | 0.13599 (8) | 0.0563 (5) | |
| O7 | 0.23717 (10) | 0.01540 (18) | 0.13396 (7) | 0.0404 (4) | |
| N1 | −0.14821 (10) | 0.39809 (17) | 0.01833 (6) | 0.0135 (3) | |
| N2 | −0.04785 (10) | 0.21799 (18) | −0.12408 (6) | 0.0167 (3) | |
| N3 | 0.21729 (10) | 0.17069 (18) | 0.11151 (6) | 0.0156 (3) | |
| N4 | 0.00848 (9) | 0.28214 (18) | 0.20398 (6) | 0.0136 (3) | |
| H4A | 0.0147 | 0.2825 | 0.1696 | 0.016* | |
| C1 | 0.03700 (11) | 0.2720 (2) | 0.06954 (7) | 0.0135 (3) | |
| C2 | −0.06664 (11) | 0.3209 (2) | 0.01256 (7) | 0.0129 (3) | |
| C3 | −0.09268 (11) | 0.30461 (19) | −0.04961 (7) | 0.0133 (3) | |
| H3A | −0.1616 | 0.3403 | −0.0859 | 0.016* | |
| C4 | −0.01856 (12) | 0.2367 (2) | −0.05864 (7) | 0.0143 (3) | |
| C5 | 0.08530 (12) | 0.1875 (2) | −0.00629 (7) | 0.0141 (3) | |
| H5A | 0.1364 | 0.1409 | −0.0126 | 0.017* | |
| C6 | 0.10876 (11) | 0.2103 (2) | 0.05401 (7) | 0.0137 (3) | |
| C7 | 0.01049 (12) | 0.4793 (2) | 0.22402 (7) | 0.0163 (3) | |
| H7A | −0.0450 | 0.5536 | 0.1862 | 0.020* | |
| H7B | 0.0809 | 0.5363 | 0.2414 | 0.020* | |
| C8 | 0.10044 (12) | 0.1762 (2) | 0.25921 (7) | 0.0182 (3) | |
| H8A | 0.1687 | 0.2302 | 0.2707 | 0.022* | |
| H8B | 0.0973 | 0.0438 | 0.2468 | 0.022* | |
| C9 | −0.09404 (11) | 0.1889 (2) | 0.18263 (7) | 0.0149 (3) | |
| H9A | −0.0970 | 0.0621 | 0.1660 | 0.018* | |
| H9B | −0.1552 | 0.2620 | 0.1474 | 0.018* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0162 (5) | 0.0311 (6) | 0.0120 (5) | 0.0027 (5) | 0.0085 (4) | 0.0029 (4) |
| O2 | 0.0207 (5) | 0.0237 (6) | 0.0143 (5) | 0.0014 (4) | 0.0113 (5) | −0.0021 (4) |
| O3 | 0.0138 (5) | 0.0258 (6) | 0.0153 (5) | 0.0029 (4) | 0.0069 (4) | 0.0023 (4) |
| O4 | 0.0198 (6) | 0.0389 (7) | 0.0128 (5) | −0.0009 (5) | 0.0078 (5) | 0.0008 (5) |
| O5 | 0.0306 (6) | 0.0267 (6) | 0.0222 (6) | 0.0043 (5) | 0.0194 (5) | −0.0029 (5) |
| O6 | 0.0256 (7) | 0.0389 (9) | 0.0517 (9) | −0.0181 (6) | −0.0076 (7) | 0.0214 (7) |
| O7 | 0.0271 (7) | 0.0164 (6) | 0.0401 (8) | 0.0023 (5) | −0.0020 (6) | 0.0064 (5) |
| N1 | 0.0144 (6) | 0.0126 (6) | 0.0138 (6) | −0.0010 (5) | 0.0083 (5) | 0.0008 (4) |
| N2 | 0.0201 (6) | 0.0165 (6) | 0.0153 (6) | −0.0040 (5) | 0.0114 (5) | −0.0029 (5) |
| N3 | 0.0138 (6) | 0.0181 (6) | 0.0164 (6) | 0.0000 (5) | 0.0096 (5) | 0.0013 (5) |
| N4 | 0.0142 (6) | 0.0167 (6) | 0.0113 (5) | 0.0000 (5) | 0.0082 (5) | 0.0000 (4) |
| C1 | 0.0150 (7) | 0.0125 (7) | 0.0137 (6) | −0.0023 (5) | 0.0087 (6) | 0.0003 (5) |
| C2 | 0.0138 (7) | 0.0118 (6) | 0.0147 (6) | −0.0013 (5) | 0.0092 (6) | −0.0003 (5) |
| C3 | 0.0140 (6) | 0.0108 (6) | 0.0137 (6) | −0.0028 (5) | 0.0073 (6) | −0.0002 (5) |
| C4 | 0.0192 (7) | 0.0118 (7) | 0.0133 (6) | −0.0034 (5) | 0.0103 (6) | −0.0018 (5) |
| C5 | 0.0168 (7) | 0.0105 (7) | 0.0182 (7) | −0.0008 (5) | 0.0120 (6) | −0.0006 (5) |
| C6 | 0.0129 (6) | 0.0118 (6) | 0.0156 (7) | −0.0006 (5) | 0.0078 (6) | 0.0024 (5) |
| C7 | 0.0199 (7) | 0.0155 (7) | 0.0142 (7) | −0.0031 (6) | 0.0102 (6) | −0.0015 (5) |
| C8 | 0.0142 (7) | 0.0256 (8) | 0.0141 (7) | 0.0063 (6) | 0.0077 (6) | 0.0023 (6) |
| C9 | 0.0137 (6) | 0.0167 (7) | 0.0127 (6) | −0.0030 (5) | 0.0067 (6) | −0.0027 (5) |
Geometric parameters (Å, °)
| O1—C1 | 1.2543 (17) | C2—C3 | 1.3876 (19) |
| O2—N1 | 1.2348 (16) | C3—C4 | 1.375 (2) |
| O3—N1 | 1.2297 (16) | C3—H3A | 0.9500 |
| O4—N2 | 1.2274 (17) | C4—C5 | 1.405 (2) |
| O5—N2 | 1.2307 (17) | C5—C6 | 1.361 (2) |
| O6—N3 | 1.2054 (19) | C5—H5A | 0.9500 |
| O7—N3 | 1.2037 (18) | C7—C7i | 1.528 (3) |
| N1—C2 | 1.4535 (18) | C7—H7A | 0.9900 |
| N2—C4 | 1.4510 (18) | C7—H7B | 0.9900 |
| N3—C6 | 1.4718 (18) | C8—C9i | 1.536 (2) |
| N4—C7 | 1.4930 (19) | C8—H8A | 0.9900 |
| N4—C9 | 1.4942 (18) | C8—H8B | 0.9900 |
| N4—C8 | 1.4952 (18) | C9—C8i | 1.536 (2) |
| N4—H4A | 0.9300 | C9—H9A | 0.9900 |
| C1—C6 | 1.440 (2) | C9—H9B | 0.9900 |
| C1—C2 | 1.4409 (19) | ||
| O3—N1—O2 | 122.45 (12) | C5—C4—N2 | 119.00 (13) |
| O3—N1—C2 | 118.70 (12) | C6—C5—C4 | 116.45 (13) |
| O2—N1—C2 | 118.83 (12) | C6—C5—H5A | 121.8 |
| O4—N2—O5 | 123.34 (12) | C4—C5—H5A | 121.8 |
| O4—N2—C4 | 118.88 (12) | C5—C6—C1 | 126.76 (13) |
| O5—N2—C4 | 117.78 (12) | C5—C6—N3 | 119.86 (13) |
| O7—N3—O6 | 123.01 (14) | C1—C6—N3 | 113.38 (12) |
| O7—N3—C6 | 118.46 (12) | N4—C7—C7i | 108.68 (7) |
| O6—N3—C6 | 118.41 (13) | N4—C7—H7A | 110.0 |
| C7—N4—C9 | 110.72 (11) | C7i—C7—H7A | 110.0 |
| C7—N4—C8 | 109.79 (11) | N4—C7—H7B | 110.0 |
| C9—N4—C8 | 109.80 (12) | C7i—C7—H7B | 110.0 |
| C7—N4—H4A | 108.8 | H7A—C7—H7B | 108.3 |
| C9—N4—H4A | 108.8 | N4—C8—C9i | 108.41 (11) |
| C8—N4—H4A | 108.8 | N4—C8—H8A | 110.0 |
| O1—C1—C6 | 119.29 (13) | C9i—C8—H8A | 110.0 |
| O1—C1—C2 | 128.51 (14) | N4—C8—H8B | 110.0 |
| C6—C1—C2 | 112.20 (12) | C9i—C8—H8B | 110.0 |
| C3—C2—C1 | 122.68 (13) | H8A—C8—H8B | 108.4 |
| C3—C2—N1 | 116.72 (12) | N4—C9—C8i | 108.72 (11) |
| C1—C2—N1 | 120.56 (12) | N4—C9—H9A | 109.9 |
| C4—C3—C2 | 119.91 (13) | C8i—C9—H9A | 109.9 |
| C4—C3—H3A | 120.0 | N4—C9—H9B | 109.9 |
| C2—C3—H3A | 120.0 | C8i—C9—H9B | 109.9 |
| C3—C4—C5 | 121.92 (13) | H9A—C9—H9B | 108.3 |
| C3—C4—N2 | 119.08 (13) | ||
| O1—C1—C2—C3 | −178.37 (14) | N2—C4—C5—C6 | 179.14 (13) |
| C6—C1—C2—C3 | 1.4 (2) | C4—C5—C6—C1 | 2.8 (2) |
| O1—C1—C2—N1 | 4.2 (2) | C4—C5—C6—N3 | −177.06 (12) |
| C6—C1—C2—N1 | −175.98 (12) | O1—C1—C6—C5 | 176.35 (14) |
| O3—N1—C2—C3 | 10.33 (19) | C2—C1—C6—C5 | −3.5 (2) |
| O2—N1—C2—C3 | −167.99 (13) | O1—C1—C6—N3 | −3.80 (19) |
| O3—N1—C2—C1 | −172.10 (13) | C2—C1—C6—N3 | 176.37 (12) |
| O2—N1—C2—C1 | 9.59 (19) | O7—N3—C6—C5 | −90.91 (18) |
| C1—C2—C3—C4 | 1.0 (2) | O6—N3—C6—C5 | 93.1 (2) |
| N1—C2—C3—C4 | 178.54 (12) | O7—N3—C6—C1 | 89.23 (18) |
| C2—C3—C4—C5 | −1.9 (2) | O6—N3—C6—C1 | −86.80 (19) |
| C2—C3—C4—N2 | 179.07 (13) | C9—N4—C7—C7i | 55.03 (18) |
| O4—N2—C4—C3 | 5.2 (2) | C8—N4—C7—C7i | −66.36 (18) |
| O5—N2—C4—C3 | −175.42 (13) | C7—N4—C8—C9i | 56.78 (15) |
| O4—N2—C4—C5 | −173.87 (13) | C9—N4—C8—C9i | −65.17 (13) |
| O5—N2—C4—C5 | 5.5 (2) | C7—N4—C9—C8i | −64.33 (15) |
| C3—C4—C5—C6 | 0.1 (2) | C8—N4—C9—C8i | 57.06 (14) |
Symmetry codes: (i) −x, y, −z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N4—H4A···O1 | 0.93 | 1.69 | 2.589 (2) | 161 |
| N4—H4A···O2 | 0.93 | 2.42 | 2.954 (2) | 117 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RZ2442).
References
- Chen, L.-Z., Zhao, H., Ge, J.-Z., Xiong, R.-G. & Hu, H.-W. (2009). Cryst. Growth Des.9, 3828–3831.
- Dabros, M., Emery, P.-R. & Thalladi, V.-R. (2007). Angew. Chem. Int. Ed.46, 4132–4135. [DOI] [PubMed]
- Ferguson, G. (1999). PRPKAPPA University of Guelph, Canada.
- Glidewell, C., Ferguson, G., Gregson, R. M. & Lough, A. J. (1999). Acta Cryst. C55, 2133–2136.
- Horiuchi, S., Ishii, F., Kumai, R., Okimoto, Y., Tachibana, H., Nagaosa, N. & Tokura, Y. (2005). Nat. Mater.4, 163–166. [DOI] [PubMed]
- Jin, Z.-M., Lin, C.-S., Wang, H.-B., Hu, M.-L., Shen, L. & Huang, L.-R. (2004). Acta Cryst. C60, o765–o767. [DOI] [PubMed]
- Kumai, R., Horiuchi, S., Sagayama, H., Arima, T.-H., Watanabe, M., Noda, Y. & Tokura, Y. (2007). J. Am. Chem. Soc.129, 12920–12921. [DOI] [PubMed]
- Rigaku (2005). CrystalClear Version 1.4.0. Rigaku Corporation, Tokyo,Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810021021/rz2442sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810021021/rz2442Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




