Abstract
Phototropins are blue-light (BL) receptor serine (Ser)/threonine kinases, and contain two light, oxygen, and voltage (LOV) domains, and are members of the PAS domain superfamily. They mediate phototropism, chloroplast movement, leaf expansion, and stomatal opening of higher plants in response to BL. In stomatal guard cells, genetic analysis has revealed that phototropins mediate activation of the plasma membrane H+-ATPase by phosphorylation and drive stomatal opening. However, biochemical evidence for the involvement of phototropins in the BL response of stomata is lacking. Using guard cell protoplasts, we showed that broad bean (Vicia faba) phototropins (Vfphots) were phosphorylated by BL, and that this phosphorylation of Vfphots reached to the maximum level earlier than that of the H+-ATPase. Phosphorylation of both Vfphots and H+-ATPase showed similar sensitivity to BL and were similarly suppressed by protein kinase and flavoprotein inhibitors. We found that a 14-3-3 protein was bound to Vfphots upon phosphorylation, and this binding occurred earlier than the H+-ATPase phosphorylation. Vfphots (Vfphot1a and Vfphot1b) were expressed in Escherichia coli, and phosphorylation sites were determined to be Ser-358 for Vfphot1a and Ser-344 for Vfphot1b, which are localized between LOV1 and LOV2. We conclude that Vfphots act as BL receptors in guard cells and that phosphorylation of a Ser residue between LOV1 and LOV2 and subsequent 14-3-3 protein binding are likely to be key steps of BL response in stomata. The binding of a 14-3-3 protein to Vfphot was found in etiolated seedlings and leaves in response to BL, suggesting that this event was common to phototropin-mediated responses.
Stomatal pores surrounded by a pair of guard cells in the epidermis regulate gas exchange between leaves and the atmosphere and allow CO2 entry for both photosynthesis and the transpirational stream in higher plants (Zeiger, 1983; Assmann, 1993). Stomata open through the activation of a H+ pump in guard cells in response to blue light (BL; Assmann et al., 1985; Shimazaki et al., 1986). The BL-activated pump creates an inside-negative, electrical potential across the plasma membrane and drives K+ uptake through voltage-gated K+ channels (Hedrich and Schroeder, 1989; Assmann and Shimazaki, 1999; Schroeder et al., 2001). The H+ pump has been demonstrated to be the plasma membrane H+-ATPase and is activated via phosphorylation of its C terminus with concomitant binding of the 14-3-3 protein (Kinoshita and Shimazaki, 1999; Emi et al., 2001; Palmgren, 2001). Although physiological responses downstream of BL perception have been elucidated extensively in guard cells, the mechanism of BL perception itself, an initial event in the BL response of stomata, had yet to be elucidated. Recently, genetic analysis of Arabidopsis mutants strongly suggests that phototropins (Atphot1 and Atphot2) function as BL receptors in a redundant manner and mediate BL-dependent stomatal opening (Kinoshita et al., 2001), although the biochemical evidence for this is still lacking. In contrast, a carotenoid zeaxanthin localized in the thylakoid membrane of guard cell chloroplasts has been proposed to be a BL receptor on the basis of correlations between the zeaxanthin content and stomatal opening via physiological, biochemical, and genetic analysis (Zeiger and Zhu, 1998; Frechilla et al., 1999).
In recent years, molecular genetic analysis of Arabidopsis has shown that at least four different photoreceptors (cryptochrome1, cryptochrome2, phot1, and phot2) mediate plant growth and development in response to BL (390-500 nm) and UV-A light (320-390 nm; Cashmore et al., 1999; Briggs et al., 2001; Christie and Briggs, 2001; Briggs and Christie, 2002; Lin, 2002). Among these photoreceptors, phototropins represent a newly characterized class of flavoprotein photoreceptors and had been found initially as a 120-kD protein phosphorylated in response to BL in the plasma membrane of etiolated pea (Pisum sativum) epicotyls (Gallagher et al., 1988). Phototropins are suggested to regulate a wide range of responses in higher plants including phototropism (Huala et al., 1997), chloroplast movement (Jarillo et al., 2001; Kagawa et al., 2001; Sakai et al., 2001), rapid stem growth inhibition (Folta and Spalding, 2001), leaf expansion (Sakamoto and Briggs, 2002), and stomatal opening (Kinoshita et al., 2001). Phototropins contain Ser/Thr kinase domains in the C terminus and undergo BL-induced autophosphorylation (Huala et al., 1997; Christie et al., 1998, 2002; Sakai et al., 2001). The N-terminal regions of phototropins contain two repeated motifs of approximately 110 amino acids that belong to PAS domains, which are regulated by light, oxygen, and voltage (LOV), and are named LOV1 and LOV2, respectively (Huala et al., 1997). The LOV domains possess non-covalent binding sites for the chromophore FMN and Cys residues for FMN-cysteinyl adducts (Christie et al., 1998; Sakai et al., 2001; Swartz et al., 2001; Kasahara et al., 2002) and produce a covalent cysteinyl adduct with the FMN chromophore when LOVs are illuminated with BL (Salomon et al., 2000; Crosson and Moffat, 2001; Crosson et al., 2003).
The cysteinyl adduct formation by BL is the primary photochemical process associated with the LOV domains, and the adduct formation is essential for signaling that leads to phototropin autophosphorylation and subsequent physiological responses. In these processes, LOV2 rather than LOV1 in Arabidopsis phototropins serves as the principle lightsensing domain and is required for activating kinase activity and eliciting hypocotyl curvature (Christie et al., 2002). Recently, a phototropin-activated calciumpermeable channel has been identified in mesophyll cells, although activation of these channels by BL takes more than several minutes (Stoelze et al., 2003). However, the biochemical events that occur after the perception of BL by phototropins and/or the relationship between autophosphorylation and the subsequent physiological responses are still largely unknown.
Stomatal guard cells are an excellent model system for dissecting phototropin-mediated signaling cascades because guard cells seem to possess all of the signaling elements required for BL-induced stomatal opening in leaves (Zeiger and Hepler, 1977). In the present study, we provide biochemical evidence that broad bean (Vicia faba) phototropins (Vfphots) act as BL receptors and transduce light signals into activation of the plasma membrane H+-ATPase in guard cells. We also found that the 14-3-3 protein binds to Vfphots in guard cells when Vfphots are phosphorylated and that such binding occurs in both etiolated seedlings and green leaves. Furthermore, taking advantage of the fact that the 14-3-3 protein binds to specific phosphorylated motifs, we determined the site of 14-3-3 binding in Vfphots, which is located between the LOV1 and LOV2 domains. Very recently, Salomon et al. (2003) determined eight phosphorylation sites in phot1a of etiolated seedlings from oat (Avena sativa) on the N terminus upstream of LOV1 and the hinge region between LOV1 and LOV2. Sequence alignment indicated that the site of 14-3-3 binding in Vfphot was included in the hinge region of LOV1 and LOV2.
RESULTS
Tissue-Specific Expression of Phototropin mRNA in Broad Bean
We obtained two cDNAs encoding Vfphot1a and Vfphot1b (broad bean phototropin) by reverse transcriptase-PCR from GCPs of broad bean (Fig. 1A). The complete cDNA sequences of VfPHOT1a and VfPHOT1b contain open reading frames of 2,889 and 2,910 bp, respectively, and the deduced proteins consist of 963 and 970 amino acids, with predicted molecular masses of 108 and 109 kD, respectively. Vfphot1a and Vfphot1b contain typical conserved domains of LOV1 (amino acids 162-268 for Vfphot1a and 161-267 for Vfphot1b) and LOV2 (amino acids 447-553 for Vfphot1a and 443-549 for Vfphot1b), respectively. The C-terminal regions of Vfphot1a and Vfphot1b include conserved kinase domains amino acids 634 to 918 and 630 to 925, respectively. Fulllength Vfphot1a protein had an amino acid identity of 72% with Vfphot1b protein. Both Vfphot1a and Vfphot1b had an identity of 69% with Arabidopsis Atphot1 (Huala et al., 1997), but less so (60% and 57%, respectively) with Arabidopsis Atphot2 (Jarillo et al., 1998). Sequences of Vfphot1a and Vfphot1b were compared with those of phototropin homologs including Arabidopsis Atphot1 and Atphot2, rice (Oryza sativa) OsNPH1a and OsNPH1b (Kanegae et al., 2000), and pea PsNPH1 (accession no. U83281). Phylogenetic analysis by ClustalX revealed that these phototropin homolog proteins can be classified into two groups, and both Vfphot1a and Vfphot1b are placed into a group along with Atphot1, OsNPH1a, and PsNPH (Fig. 1B). We could not clone any phot2-like proteins from GCPs or etiolated seedlings of broad bean, although we used specific primers for phot2-like proteins designated from Atphot2 and OsNPH1b (data not shown).
Figure 1.
Cloning of phototropin cDNAs from guard cells of broad bean. A, Alignment of deduced amino acid sequences of Vfphot1a (AB095909) and Vfphot1b (AB095910) from broad bean and phot1 from Arabidopsis (Atphot1). Asterisks show identical amino acids. Dashes indicate gaps introduced to allow for optimal alignment of sequences. Open arrowheads indicate the conserved Cys residues in LOV domains. The solid arrowhead indicates a conserved Lys residue in the kinase domain, which is required for kinase activity (see Fig. 5). B, Phylogenetic tree showing the relationship among the plant phototropins. The relationship is based on a comparison of amino acid sequences using the ClustalX program. Amino acid sequences of Atphot1 (Arabidopsis), Atphot2 (Arabidopsis), PsNPH1 (pea), OsNPH1a (rice), and OsNPH1b (rice) were obtained from the database. The scale represents 0.05 substitutions per site. C, Northern-blot analysis of VfPHOT1a and VfPHOT1b. Each lane contained an equal amount (35 μg) of total RNA isolated from GCPs, leaves, roots, and etiolated seedlings of broad bean. Experiments repeated three times on different occasions gave similar results.
We determined the steady-state transcript levels of VfPHOT1a and VfPHOT1b in GCPs, leaves, roots, and etiolated seedlings (Fig. 1C). VfPHOT1a was expressed in GCPs and etiolated seedlings but was only found in trace amounts in leaves. VfPHOT1b was expressed in both GCPs and leaves but was only found in trace amounts in etiolated seedlings. Neither Vfphot1a nor Vfphot1b expression was detectable in roots. It is noteworthy that only GCPs expressed both transcripts of VfPHOT1a and VfPHOT1b.
Phosphorylation of Vfphots Precedes That of the Plasma Membrane H+-ATPase in Response to BL
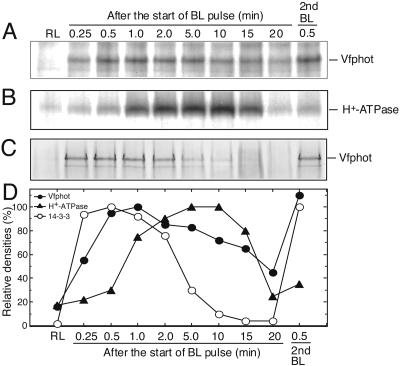
To investigate phosphorylation levels of guard cell Vfphots in response to BL, we raised polyclonal antibodies against conserved regions of Vfphots. The antibodies recognized isoforms of both Vfphot1a and Vfphot1b, but did not discriminate between individual isoforms because of their similar size. GCPs were incubated with [32P]orthophosphate for 80 min, and Vfphots from guard cell proteins were immunoprecipitated with specific antibodies at the indicated times (Fig. 2). Autoradiograms revealed that Vfphots having a molecular mass of 125 kD were phosphorylated by BL (Fig. 2A), and the phosphorylation levels reached a maximum at around 1 min after the start of BL pulse and then decreased slowly. When a second pulse of BL was applied, the Vfphots were rephosphorylated within 30 s. Western-blot analysis revealed that there was no change in the levels of Vfphots (Fig. 3B).
Figure 2.
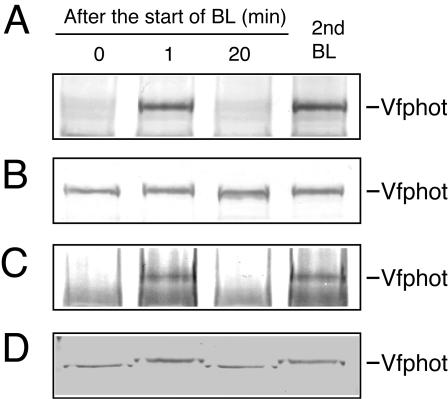
Changes in the phosphorylation levels of Vfphot and the plasma membrane H+-ATPase in GCPs in response to BL. A pulse of BL was applied to the GCP suspension for 30 s at 100 μmol m-2 s-1 superimposed on the background red light at 600 μmol m-2 s-1. All measurements were done at 24°C. A, Autoradiogram of immunoprecipitated Vfphots. The reaction was terminated by adding an equal volume of solubilizing medium to the GCP suspension at the indicated time. In each lane, the immunoprecipitated Vfphot was obtained from 200 μg of guard cell proteins. B, Autoradiogram of immunoprecipitated plasma membrane H+-ATPase. In each lane, the immunoprecipitated H+-ATPase was obtained from 50 μg of guard cell proteins. C, Far western blot of Vfphot in GCPs using glutathione S-transferase (GST)-14-3-3 fusion protein as a probe. Lower band is degradation product of Vfphot. The GST alone did not bind to Vfphots (data not shown). D, Relative density of phosphorylation levels of Vfphots (A) and H+-ATPase (B) and binding amount of the 14-3-3 protein (C). Experiments repeated three times on different occasions gave similar results.
Figure 3.
Binding of the 14-3-3 protein to Vfphot in response to BL. A, Autoradiogram of immunoprecipitated Vfphot. Other reaction conditions were the same as in Figure 2A. B, Western blotting of Vfphot in GCPs using polyclonal antibodies raised against to recombinant Vfphot. GCPs were incubated under background red light for 40 min and then illuminated with a pulse of BL. The reaction was terminated by adding an equal volume of solubilizing medium to the GCP suspension at the indicated time. Asterisk indicates a nonspecific protein recognized by Vfphot antibodies. C, Binding of the 14-3-3 protein to Vfphot. Other reaction conditions were the same as in B. Far western blotting was done using recombinant GST-14-3-3 fusion protein as a probe. Lower band is degradation product of Vfphot. The GST alone did not bind to Vfphots (data not shown). D, Inhibition of binding of the 14-3-3 protein to Vfphot by staurosporine. Staurosporine at 10 μm was added to GCPs 20 min before the BL pulse. Other reaction conditions were the same as in B. Experiments repeated three times on different occasions gave similar results.
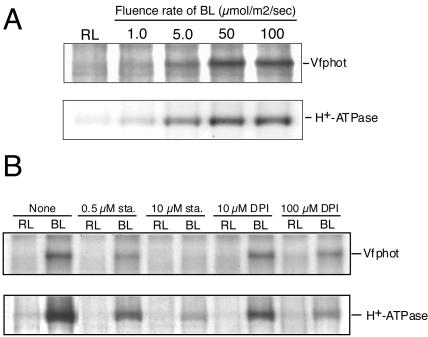
We determined phosphorylation levels of the plasma membrane H+-ATPase under the same conditions described for Vfphots according to our previous method (Kinoshita and Shimazaki, 1999). The phosphorylation levels of H+-ATPase were increased in response to BL, reached a maximum at around 5 min after the start of BL pulse, and then decreased gradually to basal levels in this experiment. Time required for reaching maximum phosphorylation levels in Vfphots was shorter than that for the plasma membrane H+-ATPase in response to BL stimulation. This supports the contention that Vfphots function as BL receptors and transduce light signals into activation of the membrane H+-ATPase, as was evidenced genetically using a phot1 phot2 double mutant of Arabidopsis (Kinoshita et al., 2001). We further determined fluence dependencies in phosphorylation of Vfphots and the plasma membrane H+-ATPase in response to BL. Because the time required for reaching the maximum phosphorylation levels after a pulse of BL is different between Vfphots (approximately 1 min) and H+-ATPase (approximately 5 min), we measured phosphorylation at these maximum levels for both proteins. The phosphorylation levels of both Vfphots and the H+-ATPase increased with the fluence rate, and both saturated at 50 μmol m-2 s-1 when the pulse duration was 30 s (Fig. 4A). The half-saturating intensity of BL was 7.5 μmol m-2 s-1 (30 s) for both Vfphots and the H+-ATPase. We further determined the effects of the protein kinase inhibitor, staurosporine (Tamaoki et al., 1986), and the flavoprotein inhibitor, DPI (O'Donnell et al., 1993), on BL-induced phosphorylation of both Vfphots and the plasma membrane H+-ATPase (Fig. 4B). Staurosporine inhibited phosphorylation of both Vfphots and the membrane H+-ATPase in a similar concentration dependency, with complete inhibition of both measured at 10 μm (Fig. 4B). It is most likely that the inhibition of phosphorylation of the H+-ATPase by staurosporine is due to the specific inhibition of Vfphot kinase activity because phosphorylation of the H+-ATPase is insensitive to both staurosporine (data not shown) and a general protein kinase inhibitor K-252a when the phosphorylation was induced by a fungal toxin fusicoccin (Kinoshita and Shimazaki, 2001). DPI inhibited phosphorylation of both by 50% at 10 μm and 80% at 100 μm. From these separate three lines of evidence, we conclude that Vfphots act as BL receptors in stomatal guard cells and that phosphorylation of Vfphots is a prerequisite for the activation of the plasma membrane H+-ATPase.
Figure 4.
A, Phosphorylation of Vfphot and H+-ATPase in GCPs as a function of BL fluence rate. Autoradiograms of immunoprecipitated Vfphot and the H+-ATPase. Reactions were terminated 1.0 min (Vfphot) and 5.0 min (H+-ATPase) after the start of the BL pulse at various fluence rates by adding solubilizing medium. B, Effects of inhibitors on phosphorylation of Vfphot and the H+-ATPase in GCPs. Autoradiograms of immunoprecipitated Vfphot and H+-ATPase at various concentrations of staurosporine (sta.), an inhibitor of protein kinases, and diphenyleneiodonium chloride (DPI), an inhibitor of flavoproteins. These inhibitors were added to the GCP suspension 20 min before the BL pulse. Other reaction conditions were the same as in A. Experiments repeated three times on different occasions gave similar results.
A 14-3-3 Protein Binds to Phosphorylated Vfphots in Guard Cells
Previously, during the course of far western-blot analysis (an overlay assay) using the recombinant GST-14-3-3 fusion protein on guard cell proteins separated by SDS-PAGE, we found that the 14-3-3 protein bound to a 125-kD protein in addition to a 94-kD plasma membrane H+-ATPase (Kinoshita and Shimazaki, 1999). We identified the 125-kD protein as Vfphots by western-blot analysis (Fig. 3B). The 14-3-3 protein bound to phosphorylated Vfphots, but it did not bind 20 min after the initial pulse of BL. When Vfphots were rephosphorylated by a second BL pulse, a 14-3-3 protein bound again (Fig. 3C). The binding was specific to a 14-3-3 protein because recombinant GST did not bind to Vfphots (data not shown). In the presence of staurosporine at 10 μm, which inhibits the phosphorylation of Vfphots completely, a 14-3-3 protein did not bind to Vfphots, indicating that the binding is phosphorylation dependent (Fig. 3D).
We predicted that the amount of 14-3-3 protein bound to Vfphots would be proportional to the phosphorylation levels of Vfphots. Unexpectedly, this turned out not to be the case (Fig. 2). The amount of the 14-3-3 protein bound to Vfphots increased sharply and reached a maximum level within 30 s of the start of the BL pulse, and decreased gradually to a basal level within 15 min. In contrast, the phosphorylation levels of Vfphots reached a maximum level after 1 min, decreased very slowly, and showed significant levels of phosphorylation 10 to 20 min after the pulse. Vfphots contain a phosphorylation site(s) that does not appear to be involved in 14-3-3 binding. A decrease in the amount of 14-3-3 protein bound to Vfphots is thus most likely to be related to an increase in phosphorylation levels of the plasma membrane H+-ATPase (Fig. 2C).
Determination of BL-Induced Phosphorylation Site in Vfphots
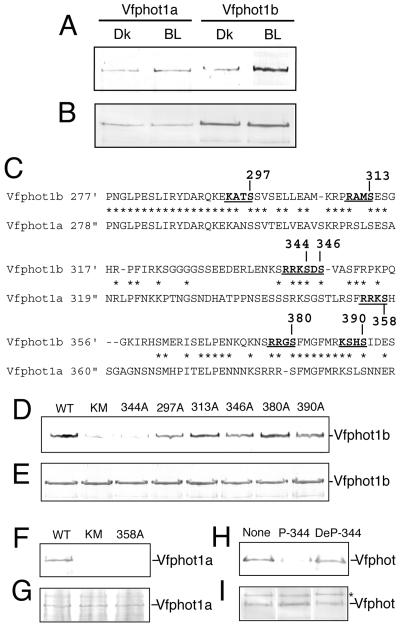
Full-length Vfphot1a and Vfphot1b proteins were expressed in Escherichia coli (Fig. 5B) with Vfphot1b being expressed at a higher level. Stimulation of E. coli cells with BL induced binding of 14-3-3 protein to both Vfphot1a and Vfphot1b, although small amounts of the 14-3-3 protein also bound to Vfphots in the dark (Fig. 5A). Amount of the binding 14-3-3 protein was increased by BL 1.9 times for Vfphot1a and 4.8 times for Vfphot1b. The 14-3-3 protein did not bind to mutant Vfphots that lack kinase activity in response to BL (Fig. 5, D and F, lane KM; Hanks et al., 1988). These results suggest that binding of 14-3-3 protein to Vfphots requires the autophosphorylation in response to BL.
Figure 5.
Identification of the binding site of the 14-3-3 protein using recombinant Vfphot. BL illumination of E. coli was for 30 s at 100 μmol m-2 s-1. E. coli cells were solubilized before (Dk) and 1 min after the start of the BL pulse (BL). Far western blotting was done using GST-14-3-3 protein as a probe. The GST alone did not bind to Vfphots (data not shown). A, Binding of the 14-3-3 protein with recombinant Vfphot in response to BL. B, Western blotting of Vfphot in E. coli cells using Vfphot antibodies. C, Amino acid sequence alignment of the junction of LOV domains of Vfphot1b (position 277-394) and Vfphot1a (position 278-399). Asterisks show identical amino acids. Dashes indicate gaps introduced to allow for optimal alignment of sequences. Underlines indicate potential phosphorylation sites, and Ser residues in these sites were substituted with Ala. D, Far western blotting of Vfphot1b in BL-illuminated E. coli cells using GST-14-3-3 protein as a probe. Ser residues in wild-type Vfphot1b (WT) were substituted with Ala at the indicated positions. KM is a kinase mutant in which a Lys residue was substituted by Asn in the kinase domain (Fig. 1A). E, western blotting of Vfphot1b in E. coli using Vfphot antibodies. F, Far western blotting of Vfphot1a in BL-illuminated E. coli cells using GST-14-3-3 protein as a probe. Ser-358 in wild-type Vfphot1a (WT) was substituted with Ala. KM is a kinase mutant (Fig. 1A). G, Western blotting of Vfphot1a in E. coli using Vfphot antibodies. H, Effect of phosphopeptides on binding of a 14-3-3 protein to Vfphot in BL-illuminated GCPs. GST-14-3-3 protein (0.05 μm) with or without 10 μm peptides was used as a probe for far western blotting. I, Western blotting of Vfphot using Vfphot antibodies. Experiments repeated three times on different occasions gave similar results.
14-3-3 proteins bind to their targets in a sequence-specific and phosphorylation-dependent manner, the phospho-Ser consensus motifs usually being R/KXXpSXP and R/KXXXpSXP (Muslin et al., 1996; Yaffe et al., 1997; Sehnke et al., 2002). Taking advantage of this aspect of 14-3-3, we can determine the phosphorylation site of Vfphot in response to BL. Unfortunately, Vfphot1a and Vfphot1b lack these typical 14-3-3 consensus motifs, but recently, several 14-3-3 binding proteins have been shown to bind to simpler motifs represented by R/KXXS/T (Aitken, 2002) in animal organisms. We found that such candidates existed also in Vfphots and localized exclusively between LOV1 and LOV2 domains, being KANS298, RPRS313, RSLS315, KSGS349, RRKS358, RRRS385, and KSKS395 in Vfphot1a; and KATS297, RAMS313, RRKS344, KSDS346, RRGS380, and KSHS390 in Vfphot1b. Because Vfphot1b was expressed at a higher level than Vfphot1a in E. coli cells (Fig. 5B), we substituted all of the candidate Ser residues with Ala residues in Vfphot1b by site-directed mutagenesis and looked for inhibition of 14-3-3 binding. The results indicated that only the RRKS344 mutant of Vfphot1b caused binding inhibition, but no other mutations had this effect. We found the same motif (RRKS358), in Vfphot1a, and a repeat experiment found that this Vfphot1a mutant also caused complete inhibition of 14-3-3 protein binding. From these results, we conclude that Ser-358 in Vfphot1a and Ser-344 in Vfphot1b are at the sites of binding for the 14-3-3 protein and that RRKpS is most likely to be the binding motif for the 14-3-3 protein in Vfphots.
We confirmed the binding site of the 14-3-3 protein using synthetic phosphopeptides containing Ser-344 and neighboring amino acid sequences in Vfphot1b (P-344; LENKSRRKphospho-SDSVASFRPQ) as shown previously (Kinoshita and Shimazaki, 2002). P-344 strongly prevented binding of the 14-3-3 protein to the Vfphots in a competitive manner (Fig. 5H), but the dephosphorylated peptide (DeP-344; LENKSRRKSDSVASFRPQ) had no effect on the binding.
Binding of the 14-3-3 Protein to Vfphots in Etiolated Seedlings and Leaves
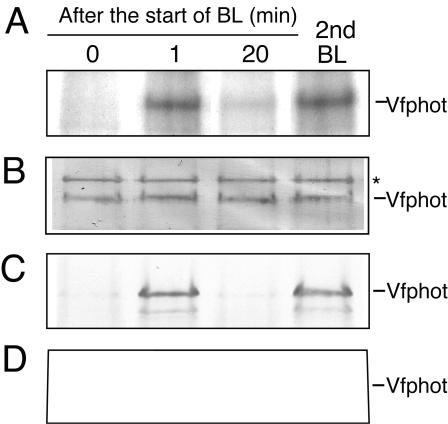
We investigated whether binding of the 14-3-3 protein to Vfphots occurred in etiolated seedlings and green leaves in response to BL by far western-blot analysis. Etiolated seedlings and leaves of broad bean were kept in the dark for 1 h and then illuminated with BL for 30 s at 100 μmol m-2 s-1. The etiolated seedlings and leaves were disrupted at the indicated times. Microsomes from the etiolated seedlings and the plasma membrane from green leaves were isolated, and they were subjected to SDS-PAGE (Fig. 6). In etiolated seedlings, the 14-3-3 protein bound to Vfphots in response to BL but did not bind 20 min after the pulse, and it bound again by the time of the second pulse (Fig. 6A). Binding of the 14-3-3 protein is likely to be dependent on phosphorylation of Vfphots because a mobility shift was induced by BL (Fig. 6B). In green leaves, the 14-3-3 protein also bound to Vfphots in response to BL, and bound again by the time of the second pulse (Fig. 6C). Western analysis revealed that a clear mobility shift of Vfphots was induced by BL, indicating that binding of the 14-3-3 protein is likely to be dependent on phosphorylation of Vfphots (Fig. 6D). These results suggest that binding of the 14-3-3 protein to the phosphorylated phototropin is common to phototropin-mediated responses, such as phototropism, chloroplast movement, and stomatal opening.
Figure 6.
Binding of the 14-3-3 protein to Vfphot in etiolated seedlings and leaves. BL illumination of intact etiolated seedlings was for 30 s at 100 μmol m-2 s-1. Etiolated seedlings and leaves were disrupted at the indicated time, and microsome protein from the etiolated seedlings (50 μg of protein) and plasma membrane protein from the green leaves (50 μg of protein) were separated by SDS-PAGE. A, Far western blotting in etiolated seedlings using GST-14-3-3 protein as a probe. The GST alone did not bind to Vfphots (data not shown). B, Western blotting of Vfphot in etiolated seedlings using Vfphot antibodies. C, Far western blotting in leaves using GST-14-3-3 protein as a probe. BL illumination of intact leaves was also for 30 s at 100 μmol m-2 s-1. The GST alone did not bind to Vfphots (data not shown). D, Western blotting of Vfphot in leaves using Vfphot antibodies. Experiments repeated three times on different occasions gave similar results.
DISCUSSION
Cloning and Expression of Vfphots
We cloned two cDNAs encoding typical phototropin proteins, Vfphot1a and Vfphot1b, from broad bean. Both Vfphot1a and Vfphot1b had a higher homology with that of Arabidopsis phot1 than with phot2 (Fig. 1B). Expression profiles of VfPHOT1a and VfPHOT1b were similar to those of PHOT1 and PHOT2 in Arabidopsis, and OsNPH1a and OsNPH1b in rice, respectively (Fig. 1C; Huala et al., 1997; Kanegae et al., 2000; Jarillo et al., 2001; Sakai et al., 2001). Interestingly, both VfPHOT1a and VfPHOT1b were expressed in guard cells. A simultaneous expression of both VfPHOT1a and VfPHOT1b in guard cells is consistent with physiological data obtained from Arabidopsis showing that phot1 and phot2 function redundantly in BL-induced stomatal opening (Kinoshita et al., 2001).
Biochemical Evidence for Vfphots as BL Receptors in Stomata
It has been demonstrated that phototropin in etiolated seedlings is phosphorylated in response to BL and that this phosphorylation is an early event in the signal transduction of hypocotyl phototropism (Briggs and Christie, 2002; Christie et al., 2002). However, there is no such biochemical evidence for the function of phototropin in stomatal guard cells, and phosphorylation of phototropin has not yet been demonstrated other than in etiolated seedlings (Kagawa, 2003). Because BL activates the plasma membrane H+-ATPase by phosphorylation in guard cells (Kinoshita and Shimazaki, 1999), we predicted that phosphorylation of phototropins would occur earlier than that of the H+-ATPase. Using antibodies against Vfphot1a, we immunoprecipitated Vfphots from GCPs and demonstrated phosphorylation of Vfphots in response to BL. As expected, Vfphots reached to the maximum level of phosphorylation earlier than the H+-ATPase; Vfphot was phosphorylated 15 s after the pulse of BL, but the H+-ATPase was not yet phosphorylated but subsequently became gradually phosphorylated (Fig. 2, A and B). We further showed that phosphorylation of Vfphots and H+-ATPase showed the same fluence dependency on BL (Fig. 4A). Furthermore, BL-induced phosphorylation of Vfphots and the plasma membrane H+-ATPase revealed a similar sensitivity to both a protein kinase inhibitor and a flavoprotein inhibitor (Fig. 4B). Because phosphorylation levels of the H+-ATPase were almost proportional to those of Vfphots in all these different experiments, it can be interpreted that phosphorylated Vfphots function at the limiting step in this BL signaling. From these results, we conclude that Vfphots function as BL receptors upstream of the H+-ATPase in stomatal guard cells and phosphorylation of Vfphots has an essential role in signal transduction. With respect to sensitivity to BL and to specific inhibitors, we noted that Vfphots in guard cells have similar properties to phototropins that have been described in etiolated seedlings of several plant species (Gallagher et al., 1988; Short and Briggs, 1990; Reymond et al., 1992; Palmer et al., 1993). This, therefore, is the first experimental evidence, to our knowledge, indicating that a phototropin is phosphorylated in response to BL in tissues other than etiolated seedlings.
Binding of the 14-3-3 Protein to Vfphots
Phosphorylation of phototropins is most likely to be both a general and an early event of phototropin-mediated responses. For example, mutant phototropin alleles that carry single amino acid substitution in the kinase domain lost phototropism (Huala et al., 1997) and chloroplast movement (Kagawa et al., 2001). However, the specific events following phosphorylation are largely unknown. We found that the 14-3-3 protein reversibly binds to Vfphots upon phosphorylation in guard cells (Figs. 3 and 5). Furthermore, binding of the 14-3-3 protein to Vfphots in response to BL seems to be a common event leading to different responses including phototropism, chloroplast movement, and stomatal opening. In accordance with this notion, 14-3-3 binding was observed both in etiolated seedlings and green leaves of broad bean (Fig. 6). Also in support of this, we have found BL-dependent binding of a 14-3-3 protein to phototropin-like proteins (having molecular masses around 125 kD) in etiolated seedlings from pea, tobacco (Nicotiana tabacum), oat, Arabidopsis, and green leaves from Arabidopsis (data not shown).
According to the model proposed by Crosson et al. (2003), light absorption by LOV domain could change in binding affinity between the LOV domain and the C-terminal kinase domain. Because the LOV domain may serve as an autoinhibitor, this may result in the autophosphorylation. If the 14-3-3 protein binds to the autophosphorylation site in Vfphot proteins, further conformational change of Vfphot will occur, and the Vfphot-14-3-3 complex may come to interact with new protein components. Therefore, the Vfphot-14-3-3 protein complex is most likely a signaling state of Vfphot that transduces light signals into downstream elements and finally activates the plasma membrane H+-ATPase in guard cells. As shown in Figure 2, B and C, binding of the 14-3-3 protein to Vfphots preceded phosphorylation of the plasma membrane H+-ATPase. The complex formation seems to be transient and release of a 14-3-3 protein from Vfphots coincides well with subsequent phosphorylation of the H+-ATPase (Fig. 2). However, the molecular mechanisms underlying Vfphot-14-3-3 protein complex transduction of light signal into downstream elements are unknown. It has been proposed that binding of the 14-3-3 protein to target proteins modifies enzyme activity, changes intracellular localization, and generates a scaffold for other protein interactions (Morrison, 1994; Ferl, 1996; Huber et al., 2002; Sehnke et al., 2002). In most cases, the activities of the target proteins do not change until binding to a 14-3-3 proteins occurs. For example, activities of nitrate reductase and Suc-phosphate synthase in plants decrease upon binding of a 14-3-3 protein (Bachmann et al., 1996; Toroster et al., 1998). The plasma membrane H+-ATPase in guard cells (Kinoshita and Shimazaki, 2002) and some protein kinases, such as Raf-1 and calcium-dependent protein kinase (Fantl et al., 1994; Freed et al., 1994; Irie et al., 1994; Camoni et al., 1998; Sehnke et al., 2002), were activated only when the 14-3-3 protein bound to these enzymes. Because Vfphots are Ser/Thr kinases, it is possible that only the Vfphot-14-3-3 complex can phosphorylate substrate proteins and/or interact with downstream elements. We note that NPH3 acts as a scaffold protein in phototropin-mediated responses (Motchoulski and Liscum, 1999), although the relationship between NPH3 and a 14-3-3 protein has yet to be elucidated.
Although binding of the 14-3-3 protein to Vfphot was phosphorylation dependent, the amount of 14-3-3 protein bound to Vfphots did not always coincide with the levels of phosphorylation. It is noteworthy that binding of the 14-3-3 protein to Vfphots was more rapid than phosphorylation of Vfphot and release of 14-3-3 protein from Vfphots was faster than dephosphorylation of Vfphots (Fig. 2). These observations suggest that there may be at least two phosphorylation sites in Vfphots: one being the binding site for the 14-3-3 protein and another that is not related to this binding. The result is consistent with previous reports (Short et al., 1994; Salomon et al., 2003) showing that phototropins from etiolated seedlings are phosphorylated on multiple Ser residues in response to BL. We can hypothesize that there are at least several different conformational states of phosphorylated Vfphots: phosphorylated but not bound by a 14-3-3 protein, phosphorylated bound by a 14-3-3 protein, and phosphorylated released by a 14-3-3 protein. The late phosphorylated Vfphot state with no bound a 14-3-3 protein had a relatively long life (approximately 40 min), and this state may be more closely related to the restoration process of responsiveness to the second pulse of BL. The restoration of complete responsiveness to the second BL took more than 30 min in BL-dependent stomatal opening in leaves and H+ pumping in GCPs (Iino et al., 1985; Shimazaki et al., 1986). Additionally, the late Vfphot phosphorylated state may have become BL desensitized until it will be dephosphorylated. Further investigation will be clearly needed to elucidate this.
Expression of Vfphots in E. coli and Determination of the 14-3-3 Binding Site
In this study, we found that a 14-3-3 protein bound to Vfphots in a phosphorylation-dependent manner. Because 14-3-3 proteins bind to target proteins that have a sequence-specific consensus motif (Aitken, 2002; Sehnke et al., 2002), we searched for such sites in Vfphots. It was found that all of the candidate sites were located between the LOV1 and LOV2 domains. To elucidate the phosphorylation site using site-directed mutagenesis, we tried to express recombinant Vfphots in insect cells as described previously (Christie et al., 1998, 2002), but unfortunately we found that endogenous protein kinases catalyzed phosphorylation of Vfphot without BL stimulation. We therefore expressed full-length Vfphots in E. coli and demonstrated that binding of a 14-3-3 protein to recombinant Vfphots in E. coli was increased by BL. Hence, this system was suitable for the determination of the 14-3-3-binding Vfphot phosphorylation sites.
Because expression of Vfphot1b in E. coli was higher than Vfphot1a, we substituted each candidate Ser with Ala residues in Vfphot1b and pinpointed the Ser mutant that caused inhibition of 14-3-3 binding. This was shown to be Ser-344 in Vfphot1b, and on the basis of the amino acid consensus sequence, we determined that the corresponding Ser in Vfphot1a is Ser-358. Both Ser residues were contained within an RRKS motif. No other Ser to Ala mutations caused inhibition of 14-3-3 binding, but this does not necessarily mean that these other Ser residues are not phosphorylated in response to BL. From these results, we conclude that Ser-358 in Vfphot1a and Ser-344 in Vfphot1b are sites of phosphorylation-dependent binding of the 14-3-3 protein and that RRKpS is most likely to be the 14-3-3-binding motif in Vfphots.
14-3-3 binding seems to be mediated by Vfphot autophosphorylation because it did not bind to mutant Vfphots that had lost their kinase activity (Fig. 6, D and F, lane KM). RRKpS is demonstrated to be a binding motif for the 14-3-3 protein in Vfphots. We must note, however, that there is no such motif in other plant phototropins except for PsNPH from pea. Phototropins in other plants species, however, have similar putative consensus motifs such as R/KXXS/T. An example includes Arabidopsis phot1, which contains the motifs RALS350, KSES364, RRMS376, RRNS387, RRNS394, RKSS410, KKKS420, KSES422, and RPES450 at the junction of its LOV domains. Arabidopsis phot2 has the motifs KPDS300, RVST328, and KLKS336. Further biochemical analysis of each plant isoform should provide more general consensus motif for 14-3-3 binding in plant phototropins. Very recently, using phot1a from rice (Asphot1a), Salomon et al. (2003) have identified eight autophosphorylation sites as Ser-27 and Ser-30 in the N terminus upstream of LOV1 and Ser-274, Ser-300, Ser-317, S-325, Ser-332, and Ser-349 in the hinge region of LOV1 and LOV2, by elegant combination of biochemistry and site-directed mutagenesis. Sequence alignment indicated that Ser-358 in Vfphot1a corresponded to Ser-325 in Asphot1a. The results support our conclusion that binding of a 14-3-3 protein to Vfphot depends on autophosphorylation in the Ser residue on a junction of two LOV domains.
In summary, we have demonstrated that a pulse of BL induces phosphorylation of Vfphot in guard cells and that the 14-3-3 protein binds to phosphorylated Vfphot. Binding of a 14-3-3 protein to Vfphots required phosphorylation of a specific Ser residue at the junction of LOV domains, and the Vfphot-14-3-3 protein complex may have a key role in activation of the plasma membrane H+-ATPase via a guard cell-specific signaling pathway. Further experiments will be needed to clarify the physiological significance and the functional consequences of 14-3-3 binding to Vfphot.
MATERIALS AND METHODS
Plant Materials and Isolation of GCPs
Broad bean (Vicia faba) was cultured hydroponically in a greenhouse as described previously (Shimazaki et al., 1992). GCPs were isolated enzymatically from the lower epidermis of 4- to 6-week-old leaves according to the previous method (Kinoshita and Shimazaki, 1999). Isolated GCPs were stored in the dark in 0.4 m mannitol and 1 mm CaCl2 on ice until use. Protein concentrations were determined by the method of Bradford as described in the instructions accompanying the protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Cloning of VfPHOT1a and VfPHOT1b in GCPs
Two degenerate oligonucleotide primers (5′-CCNGAYAAYCCNATHATHTTYGC-3′ and 5′-TAYTCYTCNGTNCCNACRAA-3′) were designed using highly conserved sequences in the LOV2 domain (PDNPIIFA) and the protein kinase domain (FVGTEEY) of plant phototropins, respectively. These oligonucleotides were used in reverse transcriptase-PCR with the first-strand cDNA as a template. The first-strand cDNA were synthesized from total RNA of GCPs by SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) using oligo(dT)12-18 as a primer. Primers (5′-CAAATGACAAGAAGAAAGGACAACATGG-3′) for VfPHOT1a and (5′-CAGCTTATACTTCCAGCTACTGAGG-3′) for VfPHOT1b were used for the 3′-RACE. Primers (5′-AAATTTTCTCCCTCCTTAGCTGTA-3′) for VfPHOT1a and (5′-AATAGTTCTCCTTCCTTTGCAGAC-3′) for VfPHOT1b were used for the 5′-RACE. 3′- and 5′-RACE were performed according to standard procedures (Invitrogen). PCR products were then cloned into a pCRII vector (Invitrogen). Sequences were determined from both strands of the cDNA (ABI PRISM 3100, PE Applied Biosystems, Foster City, CA).
Northern Hybridization
RNA was isolated from GCPs, leaves, roots, and etiolated seedlings of broad bean with ISOGENE (Nippon Gene, Tokyo). Leaves and roots were harvested from 4-week-old plants. Etiolated seedlings were grown for 4 d in darkness. Digoxigenin-labeled probes of VfPHOT1a (2,726-3,230 bp) and VfPHOT1b (2,696-3,137 bp) were obtained by PCR using a PCR DIG labeling mix (Roche Diagnostics, Tokyo). Specificities of the probes were confirmed by Southern hybridization using Vfphot1a and Vfphot1b cDNAs. Northern hybridization was performed using a digoxigenin luminescent detection kit (Roche Diagnostics) according to the manufacturer's instruction. Signals were detected using CDP-Star system (Roche Diagnostics).
Polyclonal Antibodies
Polyclonal antibodies raised against Vfphot were prepared using recombinant Vfphot1a containing the highly conserved region between the LOV2 domain and the kinase domain as an antigen. The cDNA fragment of VfPHOT1a (1,501-2,418 bp) was amplified using two primers (5′-CCGGATCCACAGAATATAGTCGTGAAG-3′ and 5′-CCGGATCCGTTAGAGACACATGTCC-3′), which contained a BamHI site, and was cloned in-frame with GST into pGEX-2T (Pharmacia Biotech, Tokyo). GSTVfphot1a fusion protein was purified with Bulk and RediPack GST purification modules (Pharmacia Biotech) and used as an antigen in rabbit. Polyclonal antibodies raised against the plasma membrane H+-ATPase and a 14-3-3 protein were described previously (Kinoshita and Shimazaki, 1999).
Determination of Phosphorylation Levels of Vfphots and the Plasma Membrane H+-ATPase
Phosphorylation levels of Vfphots and the plasma membrane H+-ATPase were determined using 32P-labeled GCPs as described previously (Kinoshita and Shimazaki, 1999) with slight modifications. In brief, the GCP suspension (0.5 mg protein mL-1) were incubated with [32P]orthophosphate (0.25 mCi mg-1 protein) under background red light for 80 min at 24°C and then illuminated with a 30 s pulse of BL. The reaction was terminated at indicated times by addition of an equal volume of the medium containing 100 mm MOPS-KOH, pH 7.5, 5 mm EDTA, 200 mm NaCl, 1 mm phenylmethylsulfonyl fluoride, 20 μm leupeptin, 4 mm dithiothreitol, 20 mm NaF, 1 mm ammonium molybdate, 100 nm calyculin A, and 2% (w/v) Triton X-100 to the GCPs, followed by centrifugation for 1 min at 10,000g. The resulting supernatant was mixed with antiserum at 0.5% (v/v). After incubation for 12 h with gentle mixing at 4°C, protein A-agarose (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the supernatant at 2.5% (w/v) and incubated for 2 h at 4°C with gentle mixing. The sample was then centrifuged for 1 min at 10,000g, and the pellet was washed three times with 1 mL of Tris-based saline (TBS; 20 mm Tris-HCl pH 7.4, 140 mm NaCl, and 0.05% [w/v] Tween 20). The pellet was resuspended in solubilizing medium (10 mm Tris-HCl, pH 8.0, 15% [w/v] Suc, 1% [w/v] SDS, 1 mm EDTA, 2.5% [w/v] 2-mercaptoethanol, and 0.02% [w/v] Coomassie Brilliant Blue) and centrifuged at 10,000g for 1 min to remove the agarose, and the supernatant was subjected to SDS-PAGE in a 9.0% (w/v) gel. In each lane, Vfphots and the H+-ATPase immunoprecipitated from 200 and 50 μg of guard cell proteins, respectively, were loaded. Separated proteins on the gel were stained once with Coomassie Brilliant Blue for 1 h to visualize the protein profiles. The destained gel was dried on a gel drier, and autoradiography was carried out by exposing Fuji RX film (Fuji Film, Tokyo) to the dried gel for 1 d for the plasma membrane H+-ATPase and for 2 to 4 d for Vfphot at room temperature.
Filters
Background red light at 600 μmol m-2 s-1 was obtained from a tungsten lamp (EXR 300W, Philips, Eindhoven, The Netherlands) by passing the light through a red glass filter (2-61, Corning, Corning, NY). BL at 100 μmol m-2 s-1 was obtained from a tungsten lamp (EXR 150W, Sylvania, Danvers, MA) by passing the light through a glass filter (Corning 5-60). Photon flux density was measured with a quantum meter (model 185A, LI-COR, Lincoln, NE).
Immunodetection
After separation of proteins by SDS-PAGE in a 9.0% (w/v) gel, individual proteins were transferred to nitrocellulose membranes at 1 mA cm-2 in transfer buffer (48 mm Tris, 39 mm Gly, and 20% [w/v] methanol) by electroblotting (Trans-blot, Bio-Rad Laboratories). The membranes were preincubated in blocking buffer for 1 h (0.05% [w/v] Tween 20, 5% [w/v] non-fat dry milk, 20 mm Tris-HCl, pH 7.4, and 140 mm NaCl) and then reacted with polyclonal antibodies at a dilution of 1:2,000 in blocking buffer at room temperature for 2 h. The membrane was then rinsed three times for 5 min each in T-TBS containing 0.05% [w/v] Tween 20, 20 mm Tris-HCl, pH 7.4, and 140 mm NaCl and reacted with a goat anti-rabbit IgG secondary antibody conjugated to alkaline phosphatase (Bio-Rad Laboratories) at a dilution of 1:3,000 in blocking buffer at room temperature for 2 h. Development of the alkaline phosphatase reaction was performed by the addition of 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazorium.
Far Western-Blot Analysis
Far western blotting was performed according to Kinoshita and Shimazaki (1999) with some modifications. In brief, GCP proteins were subjected to SDS-PAGE in a 9.0% (w/v) gel and blotted onto nitrocellulose. The membrane was incubated with blocking buffer for 1 h at room temperature and then reacted with 0.1 μm GST-14-3-3 in the blocking buffer for 16 h at 4°C. GST alone did not bind to Vfphot (data not shown). For competition experiments, a mixture of 0.05 μm GST-14-3-3 with synthetic peptides (P-344, LENKSRRKphospho-SDSVASFRPQ; DeP-344, LENKSRRKSDSVASFRPQ) at 10 μm was used for incubation, and incubations were performed for 2 h at room temperature. After three washes with T-TBS, the membrane was incubated with anti-GST antibodies (Pharmacia Biotech) at a dilution of 1:3,000 for 2 h at room temperature in blocking buffer. The membrane was then washed three times for 5 min each in T-TBS and reacted with anti-goat IgG secondary antibodies conjugated to alkaline phosphatase (Sigma-Aldrich, St. Louis) at a dilution of 1:5,000 for 2 h at room temperature in blocking buffer. Development of alkaline phosphatase reaction was performed as described above.
Expression of Full-Length Vfphots in Escherichia coli
The primers (5′-GGGTCGACCATGGAGCCATTTACAAGAGATCA-3′ and 5′-GGGTCGACTTTCAGAAGACACTCATATCCTC-3′) for VfPHOT1a and (5′-GGGTCGACCATGGAGAAGCATTTGAAGAAATCG-3′ and 5′-GGGTCGACCCATCTTTCATAATTTAAACCTTTTAG-3′) for VfPHOT1b were used for amplification of full-length Vfphots with the first-strand cDNA as a template, all of which contained a SalI site at both ends. The resulting amplified DNA fragments were cloned into the SalI site of pET30a (Novagen, Madison, WI). E. coli cells of the BL21 strain transformed with these plasmids were grown at 20°C in Luria-Bertani medium. When the culture showed a turbidity of A600 = 0.6, isopropyl-β-d-thiogalactopyranoside at 0.5 mm was added to induce recombinant Vfphot proteins. The cells were cultured further 12 h at 20°C in darkness. The harvested cells were suspended in TBS and used for far western and western blots. Amino acid substitution was performed using a QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's instruction.
Membrane Preparations from the Etiolated Seedlings and Green Leaves
Isolation of microsomes from the etiolated seedlings and plasma membrane by aqueous two-phase partition from the green leaves was according to the previous method (Kinoshita et al., 1995).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.029629.
This work was supported by the Ministry of Education, Science, Sports, and Culture of Japan (grant nos. 14704003 to T.K. and 10170224 to K.S.).
References
- Aitken A (2002) Functional specificity in 14-3-3isoform interactions through dimmer formation and phosphorylation: chromosome location of mammalian isoforms and variants. Plant Mol Biol 50: 993-1010 [DOI] [PubMed] [Google Scholar]
- Assmann SM (1993) Signal transduction in guard cells. Annu Rev Cell Biol 9: 345-375 [DOI] [PubMed] [Google Scholar]
- Assmann SM, Shimazaki K (1999) The multisensory guard cell: stomatal responses to blue light and abscisic acid. Plant Physiol 119: 809-815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Simoncini L, Schroeder JI (1985) Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba L. Nature 318: 285-287 [Google Scholar]
- Bachmann M, Huber JL, Athwal GS, Wu K, Ferl RJ, Huber SC (1996) 14-3-3 proteins associate with the regulatory phosphorylation site of spinach leaf nitrate reductase in an isoform-specific manner and reduce dephosphorylation of Ser-543 by endogenous protein phosphatases. FEBS Lett 398: 26-30 [DOI] [PubMed] [Google Scholar]
- Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A et al. (2001) The phototropin family of photoreceptors. Plant Cell 13: 993-997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Christie JM (2002) Photropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci 7: 204-210 [DOI] [PubMed] [Google Scholar]
- Camoni L, Harper JF, Palmgren MG (1998) 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK). FEBS Lett 430: 381-384 [DOI] [PubMed] [Google Scholar]
- Cashmore AR, Jarillo JA, Wu YJ, Liu DM (1999) Blue light receptors for plants and animals. Science 284: 760-765 [DOI] [PubMed] [Google Scholar]
- Christie JM, Briggs WR (2001) Blue light sensing in higher plants. J Biol Chem 276: 11457-11460 [DOI] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698-1701 [DOI] [PubMed] [Google Scholar]
- Christie JM, Swartz TE, Bogomolni RA, Briggs WR (2002) Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J 32: 205-219 [DOI] [PubMed] [Google Scholar]
- Crosson S, Moffat K (2001) Structure of flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction. Proc Natl Acad Sci USA 98: 2995-3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson S, Rajagopal S, Moffat K (2003) The LOV domain family: photo-responsive signaling modules coupled to diverse output domains. Biochemistry 42: 2-10 [DOI] [PubMed] [Google Scholar]
- Emi T, Kinoshita T, Shimazaki K (2001) Specific binding of a vf14-3-3a isoform to the plasma membrane H+-ATPase in response to blue light and fusicoccin in guard cells of broad bean. Plant Physiol 125: 1115-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl WJ, Muslin AJ, Kikuchi A, Martin JA, MacNicol AM, Gross RW, Williams LT (1994) Activation of Raf-1 by 14-3-3 proteins. Nature 371: 612-614 [DOI] [PubMed] [Google Scholar]
- Ferl RJ (1996) 14-3-3 proteins and signal transduction. Annu Rev Plant Physiol Plant Mol Biol 47: 49-73 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-stimulated hypocotyl growth inhibition. Plant J 26: 471-478 [DOI] [PubMed] [Google Scholar]
- Frechilla S, Zhu J, Talbott LD, Zeiger E (1999) Stomata from npq1, a zeaxanthin-less Arabidopsis mutant, lack a specific response to blue light. Plant Cell Physiol 40: 949-954 [DOI] [PubMed] [Google Scholar]
- Freed E, Symons M, Macdonald SG, McCormick F, Ruggieri R (1994) Binding of 14-3-3 proteins to the protein kinase Raf and effects on its activation. Science 265: 1713-1716 [DOI] [PubMed] [Google Scholar]
- Gallagher S, Short TW, Ray PM, Pratt LH, Briggs WR (1988) Light-mediated changes in two proteins found associated with the plasma membrane fractions from pea stem sections. Proc Natl Acad Sci USA 85: 8003-8007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T (1988) The protein kinase family: Conserved features and deduced phylogeny of the catalytic domains. Science 241: 42-52 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Schroeder JI (1989) The physiology of ion channels and electrogenic pumps in higher plants. Annu Rev Plant Physiol 40: 539-569 [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120-2123 [DOI] [PubMed] [Google Scholar]
- Huber ST, MacKintosh C, Kaiser WM (2002) Metabolic enzymes as targets for 14-3-3 proteins. Plant Mol Biol 50: 1053-1063 [DOI] [PubMed] [Google Scholar]
- Iino M, Ogawa T, Zeiger E (1985) Kinetic properties of the blue-light response of stomata. Proc Natl Acad Sci USA 82: 8019-8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie K, Gotoh Y, Yashar BM, Errede B, Nishida E, Matsumoto K (1994) Stimulatory effects of yeast and mammalian 14-3-3 proteins on the Raf protein kinase. Science 265: 1716-1719 [DOI] [PubMed] [Google Scholar]
- Jarillo JA, Ahmad M, Cashmore AR (1998) NPL1: a second member of the NPH1 serine/threonine protein kinase family of Arabidopsis. Plant Physiol 117: 719 [Google Scholar]
- Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410: 952-954 [DOI] [PubMed] [Google Scholar]
- Kagawa T (2003) The phototropin family as photoreceptors for the blue light-induced chloroplast relocation. J Plant Res 116: 77-82 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138-2141 [DOI] [PubMed] [Google Scholar]
- Kanegae H, Tahir M, Savazzini F, Yamamoto K, Yano M, Sasaki T, Kanegae T, Wada M, Takano M (2000) Rice NPH1 homologues, OsNPH1a and OsNPH1b, are differently photoregulated. Plant Cell Physiol 41: 415-423 [DOI] [PubMed] [Google Scholar]
- Kasahara M, Swartz TE, Olney MA, Onodera A, Mochizuki N, Fukuzawa H, Asamizu E, Tabata S, Kanegae H, Takano M et al. (2002) Photochemical properties of the FNM-binding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas. Plant Physiol 129: 762-773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656-660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura N, Shimazaki K (1995) Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell 7: 1333-1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548-5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (2001) Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant Cell Physiol 42: 424-432 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (2002) Biochemical evidence for the requirement of 14-3-3 protein binding in activation of the guard-cell plasma membrane H+-ATPase by blue light. Plant Cell Physiol 43: 1359-1365 [DOI] [PubMed] [Google Scholar]
- Lin C (2002) Blue light receptors and signal transduction. Plant Cell 14: S207-S225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D (1994) 14-3-3: modulators of signaling proteins. Science 266: 56-57 [DOI] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E (1999) Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961-964 [DOI] [PubMed] [Google Scholar]
- Muslin AJ, Tanner JM, Allen PM, Shaw AS (1996) Interaction of 14-3-3 protein with signaling proteins is mediated by the recognition of phosphoserine. Cell 84: 889-897 [DOI] [PubMed] [Google Scholar]
- O'Donnell BV, Tew DG, Jones OT, England PJ (1993) Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290: 41-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, Short TW, Gallagher S, Briggs WR (1993) Blue light-induced phosphorylation of a plasma membrane-associated protein in Zea mays L. Plant Physiol 102: 1211-1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG (2001) Plant plasma membrane H+-ATPase: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52: 817-845 [DOI] [PubMed] [Google Scholar]
- Reymond P, Short TW, Briggs WR (1992) Blue light activates a specific protein in higher plants. Plant Physiol 100: 655-661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplasts relocation. Proc Natl Acad Sci USA 98: 6969-6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Briggs WR (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723-1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M, Christie JM, Knieb E, Lempert U, Briggs WR (2000) Photo-chemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 39: 9401-9410 [DOI] [PubMed] [Google Scholar]
- Salomon M, Knieb E, von Zeppelin T, Rüdiger W (2003) Mapping of lowand high-fluence autophosphorylation sites in phototropin 1. Biochemistry 42: 4217-4225 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627-658 [DOI] [PubMed] [Google Scholar]
- Sehnke PC, DeLille JM, Ferl RJ (2002) Consummating signal transduction: the role of 14-3-3 proteins in the completion of signal-induced transitions in protein activity. Plant Cell 14: S339-S354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Iino M, Zeiger E (1986) Blue light-dependent proton extrusion by guard-cell protoplasts of Vicia faba. Nature 319: 324-326 [Google Scholar]
- Shimazaki K, Kinoshita T, Nishimura M (1992) Involvement of calmodulin and calmodulin-dependent myosin light chain kinase in blue light-dependent H+ pumping by guard cell protoplasts from Vicia faba L. Plant Physiol 99: 1416-1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short TW, Briggs WR (1990) Characterization of a rapid, blue light-mediated change in detectable phosphorylation of a plasma membrane protein from etiolated pea (Pisum sativum L.) seedlings. Plant Physiol 92: 179-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short TW, Porst M, Palmer J, Fernbach E, Briggs WR (1994) Blue light induces phosphorylation at seryl residues on a pea (Pisum sativum L.) plasma membrane protein. Plant Physiol 104: 1317-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoelze S, Kagawa T, Wada M, Hedrich R, Dietrich P (2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100: 1456-1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz TE, Corchnoy SB, Christie JM, Lewis JW, Szundi I, Briggs WR, Bogomolni RA (2001) The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J Biol Chem 276: 36493-36500 [DOI] [PubMed] [Google Scholar]
- Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F (1986) Staurosporine, a potent inhibitor of phospholipid/Ca2+ dependent protein kinase. Biochem Biophys Res Commun 135: 397-402 [DOI] [PubMed] [Google Scholar]
- Toroster D, Athwal GS, Huber SC (1998) Site-specific regulatory interaction between spinach leaf sucrose-phosphate synthase and 14-3-3 protein. FEBS Lett 435: 110-114 [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC (1997) The structural basis for 14-3-3: phosphopeptide binding specificity. Cell 91: 961-971 [DOI] [PubMed] [Google Scholar]
- Zeiger E (1983) The biology of stomatal guard cells. Annu Rev Plant Physiol 34: 441-475 [Google Scholar]
- Zeiger E, Hepler PK (1977) Light and stomatal function: blue light stimulates swelling of guard cell protoplasts. Science 196: 887-889 [DOI] [PubMed] [Google Scholar]
- Zeiger E, Zhu J (1998) Role of zeaxanthin in blue light photoreception and the modulation of light-CO2 interactions in guard cells. J Exp Bot 49: 433-442 [Google Scholar]