Abstract
The phototropin photoreceptors transduce blue-light signals into several physiological and developmental responses in plants. A transient rise in cytoplasmic calcium (Ca2+) that begins within seconds of phototropin 1 (phot1) excitation is believed to be an important element in the transduction pathways leading to one or more of the phot1-dependent responses. The goal of the present work was to determine whether the Ca2+ response was necessary for (a) the inhibition of hypocotyl elongation that develops within minutes of the irradiation, and (b) hypocotyl phototropism (curved growth of the stem in response to asymmetric illumination). After determining that pulses of light delivering photon fluences of between 1 and 1,000 μmol m-2 induced growth inhibition mediated by phot1 without significant interference from other photosensory pathways, the effect of blocking the Ca2+ rise was assessed. Treatment of seedlings with a Ca2+ chelator prevented the rise in cytoplasmic Ca2+ and prevented phot1-mediated growth inhibition. However, the same chelator treatment did not impair phot1-mediated phototropism. Thus, it appears that the early, transient rise in cytoplasmic Ca2+ is an important intermediary process in at least one but not all phot1-signaling pathways.
The two phototropins of Arabidopsis (phototropin 1 [phot1] and phototropin 2 [phot2]) are light-activated kinases that initiate phototropic responses of stems (Briggs and Huala, 1999; Briggs and Christie, 2002), opening of stomata (Kinoshita et al., 2001), irradiance-dependent movements of chloroplasts (Sakai et al., 2001; Kagawa and Wada, 2002), the onset of hypocotyl growth inhibition (Folta and Spalding, 2001a), leaf expansion (Sakamoto and Briggs, 2002), and regulation of mRNAs encoding a chlorophyll-binding protein in greening cotyledons (Folta and Kaufman, 2003). There are undoubtedly additional ways in which phot1 and phot2 adapt a plant to the prevailing light environment to be discovered through further morphological, cellular, and biochemical studies of phot1 and phot2 single and double mutants.
Structural studies at the molecular level have characterized the initial flavin-based photochemistry and conformational changes induced by blue light in the phot1 protein (Crosson and Moffat, 2001; Corchnoy et al., 2003). Genetic, biochemical, and physiological studies have begun to delineate the signaling pathways initiated after these earliest effects of excitation (Christie and Briggs, 2001; Motchoulski and Liscum, 1999). For example, the NPH3 scaffolding protein interacts physically with phot1 and is required for phototropism (Motchoulski and Liscum, 1999). However, the phot1-initiated inhibition of hypocotyl growth, which begins within 30 s of irradiation and persists for approximately 30 min (Parks et al., 1998; Folta and Spalding, 2001a) does not depend on NPH3. Such branches in the phototropin-signaling pathways, in this case a very early bifurcation, may be the rule rather than the exception given that the two photoreceptors appear to influence a variety of responses.
Many photosensory transduction pathways across biological kingdoms include a rise in cytoplasmic [Ca+2] as a critical step following excitation of the photoreceptor (for review, see Spalding, 2000). Plants are no exception. Evidence has been obtained in studies of red-light responses that phytochrome-signaling pathways use Ca2+ as a second messenger (Wayne and Hepler, 1985; Ermolayeva et al., 1997; Neuhaus et al., 1997). Also, the effects of blue light and UV radiation on chalcone synthase expression are mediated by Ca2+ (Christie and Jenkins, 1996; Long and Jenkins, 1998). SUB1 is Ca2+-binding protein that participates in the regulation of stem elongation in blue and far-red light, as evidenced by the conspicuous phenotype of sub1 seedlings (Guo et al., 2001). Although phot1 was discovered relatively recently, strong evidence has already been accumulated indicating that cytoplasmic [Ca2+] plays an important role in its signal transduction pathway. First, experiments using the aequorin Ca2+-reporter system showed that blue light acting through phot1, but not cryptochromes, induces a transient increase in cytosolic calcium ion concentration ([Ca2+]cvt) that rises and falls over the time course of approximately 1 h (Baum et al., 1999). Second, measurements with extracellular vibrating microelectrodes demonstrated that phot1 triggers an influx of Ca2+ across the plasma membrane in Arabidopsis seedling hypocotyls (Babourina et al., 2002). Third, patch clamp studies showed that blue light acting through phot1 activates voltage-gated Ca2+ channels at the plasma membrane of mesophyll cells (Stoelzle et al., 2003). Although not all of these data were obtained with the same cells or organs, the body of evidence indicates that excitation of phot1 rapidly activates Ca2+ channels at the plasma membrane, resulting in a transient increase in [Ca2+]cyt. Presumably, the rise in [Ca2+]cyt is a signal-transducing step in a phot1 pathway, but to which downstream response(s) is the rise in [Ca2+]cyt mechanistically connected? This question is addressed here, and the results further define two phot1-signaling pathways important to seedling photomorphogenesis.
RESULTS
Low Photon Fluences Initiate Growth Inhibition through phot1
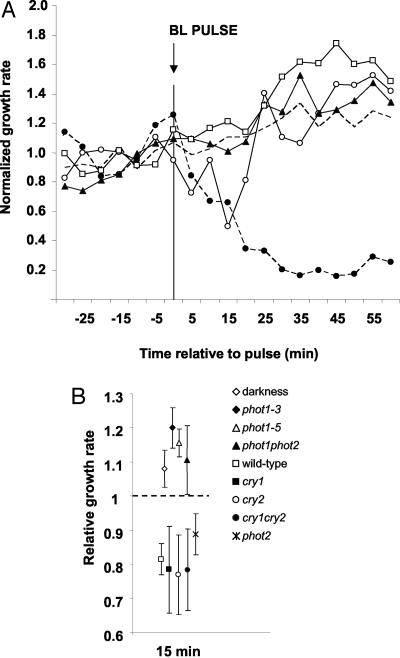
Growth of wild-type seedlings is inhibited rapidly and persistently by continuous 100 μmol m-2 s-1 blue light as shown in previous publications and here in Figure 1A. A 10-s pulse delivering 103 μmol m-2 photons induced an inhibition similar in magnitude and kinetics to the first 15 min of the response to continuous blue light (Fig. 1A). The response to the pulse was interpreted as reflecting phototropin action because phot1 was previously shown to initiate growth inhibition and maintain it during the 30 min before the cryptochromes and phytochromes developed an influence (Folta and Spalding, 2001b). Furthermore, phot1 (nph1-3) and phot1phot2 seedlings did not respond to the pulse, growing instead at an increasing rate similar to untreated dark control seedlings (Fig. 1A). A second allele of phot1 (nph1-5) produced similar results. The inhibition induced by a pulse of blue light (50 μmol m-2) was shown to result specifically from phot1 signaling, as mutations in the cryptochromes or in phot2 approximate wild-type maximal inhibition at 15 min (Fig. 1B). Conversely, the growth rates of two phot1 alleles, phot1phot2 mutants, and dark-grown seedlings cluster together at this time point. These data indicate that growth inhibition induced by a pulse of blue light can be used as an assay of early phot1 function.
Figure 1.
The phot1 receptor mediated primary growth inhibition. A, The growth rates in response to a single pulse of blue light (103 μmol m-2 delivered in 10 s) in etiolated wild-type (white circles), phot1 (white squares), and phot1phot2 (black triangles) mutant seedlings are presented. The dotted line represents the growth rate of seedlings in continuous darkness. The growth kinetics of wild-type seedlings treated with continuous irradiation (102 μmol m-2 s-1) are shown for comparison (black circles). All growth rates are normalized to the dark growth rate (set to “1”). Each data point represents the average growth rate of many (>15) independent seedlings. Error bars have been omitted for clarity and do not exceed 0.06. B, The average normalized growth rate 15 min after a single 50 μmol m-2 blue-light pulse in cryptochrome and phot mutants is shown. The dark growth rate for all genotypes is set to “1” (dashed line). Data points represent the mean growth rate of at least 10 independent seedlings, and error bars represent se.
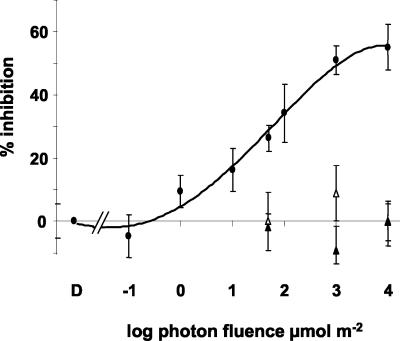
The dependence on fluence of this early phot1 action was investigated by delivering pulses ranging from 10-1 to 104 μmol m-2 to wild-type seedlings. To quantify the relationship between specific fluences of blue-light and phot1-mediated inhibition, the percent inhibition was derived from comparison of maximum inhibition of blue-light-treated seedlings with mock-treated seedlings at the 15-min time point (Fig. 2). The sigmoidal fluence-response curve indicates a response threshold between 10-1 and 100 μmol m-2 that is fully saturated by fluences on the order of 103 μmol m-2. In wild-type seedlings, saturating fluences inhibited growth to approximately 50%, but produced no inhibition in phot1 or phot1 phot2 seedlings. These data describe the range of fluences over which inhibition of growth induced by a pulse of blue light may be attributed specifically to the action of phot1.
Figure 2.
The fluence-response characteristics of phot1-mediated growth inhibition. Dark-grown wild-type seedlings (black circles) were irradiated with a single pulse of blue light ranging in fluence from 10-1 to 104 μmol m-2 or a mock pulse (D). Treatments were delivered in 10 s or less, except of the 104 treatment which was delivered in 100 s. The inhibition measured in phot1 (white triangles) and phot1phot2 (black triangles) seedlings after treatment with 50, 103, and 104 μmol m-2 blue light is presented for comparison. The results are reported as percent inhibition, calculated from the equation (1 - [growth rate at 15 min after blue-light treatment/growth rate at 15 min after a mock pulse] × 100). At least 10 seedlings were measured per fluence per genotype. Error bars represent se.
A treatment near the midpoint of the fluence-response curve was used to test whether phot1-mediated growth inhibition depended solely on the number of photons delivered or whether the duration or irradiance of the blue-light treatment were of any consequence. A fluence of 50 μmol m-2 induced an inhibition of 27.9% ± 9% when delivered as a 1-s pulse and 32% ± 8% when delivered as a 100-s pulse, so reciprocity was shown to hold for this response. Therefore, growth inhibition 15 min after a pulse of blue light in the fluence range circumscribed by the curve in Figure 2 behaves like the product of first-order reactions initiated by excited phot1 and only phot1.
Ca2+ Influx Required for phot1-Mediated Growth Inhibition
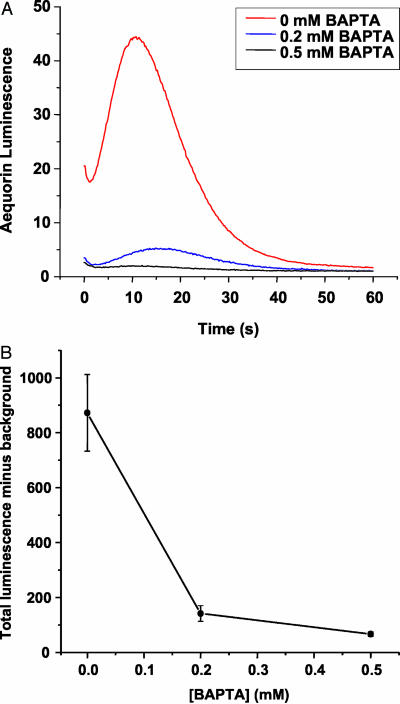
After determining the parameters that enabled the primary action of phot1 to be studied with minimal contribution of other blue-light-absorbing photoreceptors, experiments were conducted to test whether the blue-light-induced change in [Ca2+]cyt that had been previously attributed to phot1 was essential to the signal transduction process. The approach taken was to reduce the availability of Ca2+ for influx from the apoplast through the use of chelators and then assess the impact of the treatment on phot1-dependent processes. Seedlings expressing the Ca2+-dependent chemiluminescent protein aequorin were treated with or without 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,M′-tetraacetic acid (BAPTA) before assessing whether blue light induced a transient increase in [Ca2+]cyt. As shown in Figure 3, the control seedlings displayed a transient increase in [Ca2+]cyt very similar to that reported by Baum et al. (1999). The Ca2+ response was suppressed by BAPTA treatment in a concentration-dependent manner, further evidence that phot1 triggers an influx of Ca2+ across the plasma membrane from the apoplast. More importantly, these data demonstrate that BAPTA could be used to examine the role of the Ca2+ transient in phot1 signaling. To be useful as a tool for studying the link between the Ca2+ transient and ensuing effects of blue light on growth, BAPTA should not have a general effect on growth. BAPTA-treated seedlings exhibited normal absolute growth rates during growth rate assays (not shown).
Figure 3.
BAPTA suppresses blue-light-induced Ca2+ influx. A pulse of blue light induced a transient rise in aequorin luminescence ([Ca2+]cyt) that was suppressed by treating seedlings externally with the Ca2+ chelator BAPTA. A, The traces shown represent the averages of between 9 and 11 independent trials for each concentration. Error bars have been omitted for clarity. B, The average integrated luminescence (area under the curve in A, minus background) plotted against concentration. Error bars represent se.
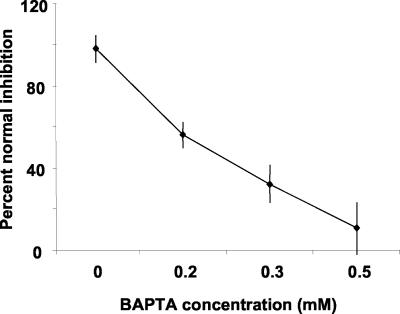
The effects of BAPTA treatments on growth inhibition induced by a 5-s pulse of blue light (50 μmol m-2) are shown in Figure 4A. The magnitude of phot1-mediated inhibition measured 15 min after the pulse was inhibited in a concentration-dependent fashion by BAPTA. The concentrations of BAPTA that almost completely inhibited the Ca2+ transient almost completely inhibited the growth inhibition. However, the growth rate of BAPTA-treated seedlings was the approximately the same as control seedlings after peak inhibition (data not shown), indicating that the treatment affected only the phot1-dependent growth response and did not speed or slow growth in general. These data indicate that Ca2+ influx is an essential step in the transduction process linking phot1 to a growth inhibition mechanism.
Figure 4.
BAPTA specifically impairs phot1-mediated growth inhibition. The magnitude of blue-light-induced, phot1-mediated growth inhibition was assessed in the presence of different concentrations of BAPTA. The results are presented as “percent of normal inhibition,” which represents the magnitude of inhibition in BAPTA-treated seedlings relative to the inhibition measured in control (no BAPTA) seedlings 15 min after a 50 μmol m-2 blue-light pulse. At least eight seedlings were measured for each BAPTA concentration. Error bars represent se.
Light/BAPTA Treatments that Affect Primary Growth Inhibition Do Not Affect Phototropism
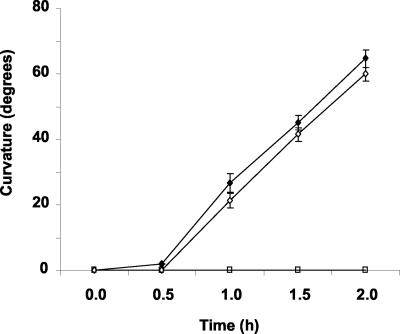
A large amount of evidence has established phot1 as the photoreceptor responsible for phototropism induced by low to moderate fluence rates of unilateral blue light (Liscum and Briggs, 1995; Huala et al., 1997; Christie and Briggs, 2001). If Ca2+ influx is a signal-transducing event on the phototropism pathway, as it is on the growth-inhibition pathway, then BAPTA should impair phototropism. The data in Figure 5 show this not to be the case. The kinetics of phototropic curvature and the magnitude of curvature induced by continuous blue light (7 × 10-3 μmol m-2 s-1) were not affected by the same BAPTA treatment that blocked phot1-dependent Ca2+ influx. Thus, it appears that the phot1-mediated transient increase in [Ca2+]cyt is a signal-transducing event on the growth inhibition pathway but not the phototropism pathway.
Figure 5.
Growth on BAPTA does not affect phototropism. Seedlings were grown vertically on agar plates containing 0 (black circles) or 300 μm BAPTA (white circles) and then were irradiated with continuous unilateral blue light at a fluence rate of 2.3 × 10-4 μmol m-2 s-1. Phototropic curvature was measured as a change in hypocotyl angle as determined from analysis of stacked images captured every 30 min for 120 min. Error bars represent se.
DISCUSSION
An early cellular response to light, such as the transient change in [Ca2+]cyt mediated by phot1 (Baum et al., 1999; Babourina et al., 2002; Harada et al., 2003; Stoelzle et al., 2003), is not necessarily a signal transduction event. It may be considered one if manipulations of the intermediary response affect the output of the transduction process. Experiments designed with this reasoning in mind were performed to determine whether the phot1-mediated change in [Ca2+]cyt can be considered a signal transduction step in either of two photobiological responses known to be mediated by phot1. After circumscribing the conditions in which the primary action of phot1 on growth could be studied as separately as possible from other blue-light systems, the effect of preventing Ca2+ influx was measured. The same was done for phot1-mediated phototropism. BAPTA treatment prevented phot1-mediated growth inhibition but the same treatment did not affect phototropism. Thus, it would appear that Ca2+ influx is an important event in the growth inhibition pathway, but not the phototropism pathway. However, it may be more accurate to say that the growth inhibition pathway depends on the change in [Ca2+]cyt to a much larger extent than does the phototropism pathway. The second statement incorporates the notion that signaling pathways are not necessarily discrete sequences of processes but instead may be groups of events that differ from each other in matters of degree. It remains to be seen how many of other phot1-controlled processes have a strong dependence on the transient change in [Ca2+]cyt.
A similar approach demonstrated that Ca2+ is required for inhibition of stem elongation by blue light in Cucumis sp. seedlings (Shinkle and Jones, 1988). Growth inhibition developed in both stem segments and intact seedlings within minutes but was not evident in segments or seedlings treated with EGTA or BAPTA. Addition of Ca+2 restored normal inhibition. The time course of inhibition and sensitivity to chelators is almost identical to what is observed in here in Arabidopsis (Figs. 1 and 3).
The fluence required to induce a measurable phot1-mediated growth inhibition (Fig. 2) is higher by 2 to 3 orders of magnitude than that required to produce measurable phototropism (Steinitz and Poff, 1986). This may be related to the fact that the signal transduction chains mediating the two are here shown to differ significantly. The phototropism pathway, the more sensitive of the two, does not include a change in [Ca2+]cyt as a signal-transducing step, but it does require the phot1-interacting protein, NPH3 (Motchoulski and Liscum, 1999). Perhaps a subset of phot1 molecules is bound to NPH3, and the complex efficiently couples the action of excited phot1 to a phototropism mechanism. The phot1 molecules not bound to NPH3 may couple less efficiently to a mechanism that leads to an increase in [Ca2+]cyt and, consequently, to growth inhibition. The fluence-response relationship of phot1 autophosphorylation (Palmer et al., 1993; Christie et al., 1998) correlates better with the latter, growth-inhibiting pathway than the phototropism pathway. This may also reflect the different degrees of NPH3 participation in the two pathways.
The time course of maximal phot1-dependent growth inhibition, which peaks between 15 and 20 min after a blue-light pulse, compares favorably with previous reports of phot1 activation and response. After peak inhibition, the growth rate trends toward dark rates. This suggests that activation and decay of the photoproduct are complete by this time point. These data agree well with observations by Steinitz and Poff (1986) in their analysis of blue-light-induced, first-positive curvature in Arabidopsis. Using multiple pulses, the authors determined that the decay of the initial photoproduct was complete after approximately 20 min. The results in Figure 1 closely parallel those obtained with cucumber (Cucumis sativus) seedlings by Gaba and Black (1983). They found that growth inhibition induced by a pulse of blue light was followed by a resumption of rapid growth after 20 min. Also, phot1 derived from plant membranes or insect cells is maximally autophosphorylated within 20 min of a blue-light pulse in vitro (Christie et al., 1998). The similar time course in these various instances probably reflects the time course of activation and decay of the phot1 photoproduct in response to a pulse of blue light. The time course of phot1 action may also be influenced by the intracellular redistribution of phot1 (release from the plasma membrane), which follows blue-light treatment (Sakamoto and Briggs, 2002).
The phot2 receptor has no detectable influence on the onset of growth inhibition. All of the data obtained to date indicate that phot2 does not compensate for the loss of phot1 to any detectable degree, and that phot1 is the primary receptor mediating the onset of hypocotyl growth inhibition. These findings are consistent with the expression patterns of phot2. Transcripts of phot2 are not abundant in dark-grown tissue, but increase in abundance after treatment with UV-A, blue, or white light (Jarillo et al., 2001).
Are the processes of phototropism and growth inhibition related beyond the simple fact that they are initiated by the same photoreceptor? The approximate coincidence of maximum phot1-mediated growth inhibition and the onset of phototropism may indicate a mechanistic link between the two phenomena. A reasonable hypothesis was that a larger growth rate differential across a hypocotyl could be established if growth of the back side could be accelerated as well as the front side slowed. This would be more easily achieved if the stem were not growing at its maximum rate, as it appears to do in darkness. Thus, it may be possible to establish a larger growth differential in a stem that has undergone some degree of growth inhibition. However, the results presented here argue against this attractive model. BAPTA treatment suppressed phot1-mediated growth inhibition without affecting phototropism. In this way, early blue-light-induced, phot1-mediated Ca+2 events may be important in early positioning of plant organs in preparation for autotrophic growth and development.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The genetic lines used in these experiments include Arabidopsis wild type (Col-0), phot1-5 (nph1-5; Huala et al., 1997), phot1-3 (nph1-3; Huala et al., 1997), phot2 (cav1), and phot1phot2. All phot lines were furnished by Dr. Winslow Briggs (Carnegie Institute of Washington, Palo Alto, CA). For each experiment, seeds were surface sterilized in 1 mL of 70% (v/v) ethanol containing 0.1% (v/v) Triton X-100 for 30 s. Seeds were dried on filter paper discs and then planted individually onto 1% (v/v) agar (Difco, Becton Lakes, NJ) plates containing 1 mm KCl and 1 mm CaCl2. The seeds were stratified for 48 h, and then were given a single 30-min treatment of white light (20 μmol m-2 s-1) to ensure even germination. Plates were transferred to absolute darkness for 30 to 36 h at 24°C. Seedlings were selected for growth experiments based on size and developmental state (approximately 2-3 mm tall and possessing a tightly closed hook) under a dim green safelight.
Hypocotyl Growth Rate Measurement
Individual seedlings with hypocotyls measuring approximately 2 to 3 mm (the stage exhibiting the most rapid rate of hypocotyl elongation) were transferred to a separate 1% (w/v) agar plate oriented vertically and perpendicular to the lens of a CCD camera (EDC1000N, Electrim, Princeton, NJ) using a close-focus lens (K52-274, Edmund Industrial Optics, Barrington, NJ). A non-photomorphogenic infrared light source was placed behind the seedlings to allow visualization of seedlings during the dark period as described (Parks and Spalding, 1999). Digital images were obtained at 5-min intervals. To test growth rates in response to constant blue light, images were captured at 5-min intervals for 1 h in darkness then for 2 h after initiation of blue-light illumination. Blue light was supplied by a blue LED array (Quantum Devices, Barneveld, WI) with a fluence rate of 100 μmol m-2 s-1. For pulse experiments, seedling growth was monitored at 5-min intervals for 1 h, a single pulse of varying total fluence was applied, and the response was monitored for 1 h. Blue light was supplied by the aforementioned LED array using blotting paper as a neutral density filter to attenuate fluence rate. Pulses were typically delivered between 1 and 100 s, except in reciprocity experiments where pulses were delivered between 1 and 1,000 s. Between 10 and 25 seedlings were measured per light treatment, per genotype. Growth rates were calculated from the series of digital images using a custom software application, written in the Lab View environment (National Instruments, Austin, TX).
Aequorin Luminescence
Seeds containing a construct encoding aequorin (Lewis et al., 1997) were sown on 1% (w/v) Difco agar containing 1.4 mm MES, pH 5.8, with or without the chelator BAPTA as specified. After stratification for 2 d at 4°C, the seeds were irradiated for 30 min with white light (20 μmol m-2 s-1) and then grown at 23°C for 3 d in a darkroom where all subsequent experimental manipulations took place. The etiolated seedlings were transferred under a green safelight to a solution identical to the growth media (minus agar) containing 10 μm coelenterazine cp (Molecular Probes, Eugene, OR) and allowed to soak overnight. Luminometer cuvettes containing 150 μL of the coelenterazine solution were loaded with five seedlings each. The seedlings were allowed to recover from the handling in darkness at 25°C for at least 1 h before background luminescence was recorded with a luminometer (TD-20/20, Turner Designs, Sunnyvale, CA). The cuvette containing seedlings was removed from the luminometer and irradiated for 10 s with 100 μmol m-2 s-1 blue light produced by a 300-W xenon arc lamp (Thermo Oriel, Stratford, CT) coupled to the shutter, lens, 450-nm interference filter (50-nm bandpass), and liquid light guide described previously (Spalding, 1995). The cuvette was returned to the luminometer immediately after the pulse ended, and luminescence from the sample was recorded continuously at 5 Hz by computer. Mean-fold luminescence was calculated by dividing each point in the recording by the average background value obtained for that cuvette and then averaging the separate trials. Total relative luminescence was calculated by integrating each of the separate recordings and then averaging the integrals.
Experiments with Chelators
Wild-type Arabidopsis (Col-0) seedlings were grown in conditions identical to those used for the luminescence experiments above. Seedlings grown under these conditions respond normally to constant, pulse, and long-term (4-d) light treatments (data not shown). Seeds were sown on plates containing 0, 200, 300, or 500 μm BAPTA (Acros Organics, Belgium) and then were stratified at 4°C for 48 h. A single 30-min pulse of white light (20 μmol m-2 s-1) was given to ensure even germination. Growth rate assessment was performed as described above, except seedlings were grown in complete darkness for 30 to 36 h (until 2-3 mm tall) and then were transferred to fresh media of identical BAPTA concentration. To test the effect of BAPTA on phototropism, seedlings were grown as described for growth rate assays and then were transferred to media containing an identical BAPTA concentration when the hypocotyl was 4 to 7 mm long (approximately 36-40 h after germination-inducing pulse). Seedlings were allowed to acclimate for a variable time between 0 and 30 min. Phototropism was induced by 450 nm broad-band light supplied by a LED light array delivered to a point source through a liquid light guide at a fluence rate of 7.0 × 10-3 μmol m-2 s-1 parallel to the plane of the agar surface for the duration of the 120-min experiment. A total fluence was approximately 50 μmol m-2, identical to the fluence that demonstrates BAPTA sensitivity during the phot1-mediated growth response (Fig. 4A). Seedlings were imaged every 30 min. Photocurvature was assessed by stacking successive images against the initial time zero (dark-grown) image and then measuring the angle in the hypocotyl using Image Tool software. The mean curvature of at least 20 independent seedlings is presented.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.024372.
This work was supported by the National Science Foundation (grant no. IBN-9974585).
References
- Babourina O, Newman I, Shabala S (2002) Blue light-induced kinetics of H+ and Ca2+ fluxes in etiolated wild-type and phototropin mutant Arabidopsis seedlings. Proc Natl Acad Sci USA 99: 2433-2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum G, Long JC, Jenkins GI, Trewavas AJ (1999) Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc Natl Acad Sci USA 96: 13554-13559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Christie JM (2002) Phototropins 1 and 2: versatile plant bluelight receptors. Trends Plant Sci 7: 204-210 [DOI] [PubMed] [Google Scholar]
- Briggs WR, Huala E (1999) Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol 15: 33-62 [DOI] [PubMed] [Google Scholar]
- Christie JM, Briggs WR (2001) Blue light sensing in higher plants. J Biol Chem 276: 11457-11460 [DOI] [PubMed] [Google Scholar]
- Christie JM, Jenkins GI (1996) Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 8: 1555-1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698-1701 [DOI] [PubMed] [Google Scholar]
- Corchnoy SB, Swartz TE, Lewis JW, Szundi I, Briggs WR, Bogomolni RA (2003) Intramolecular proton transfers and structural changes during the photocycle of the LOV2 domain of phototropin 1. J Biol Chem 278: 724-731 [DOI] [PubMed] [Google Scholar]
- Crosson S, Moffat K (2001) Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction. Proc Natl Acad Sci USA 98: 2995-3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolayeva E, Sanders D, Johannes E (1997) Ionic mechanism and role of phytochrome-mediated membrane depolarisation in caulonemal side branch initial formation in the moss Physcomitrella patens. Planta 201: 109-118 [Google Scholar]
- Folta KM, Kaufman LS (2003) Phototropin 1 is required for high-fluence blue-light-mediated mRNA destabilization. Plant Mol Biol 51: 609-618 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001a) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471-478 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001b) Opposing roles of phytochrome A and phytochrome B in early cryptochrome-mediated growth inhibition. Plant J 28: 333-340 [DOI] [PubMed] [Google Scholar]
- Gaba V, Black M (1983) Photocontrol of hypocotyl elongation in deetiolated Cucumis sativus L.: a blue-light-induced post-illumination burst of growth. Photochem Photobiol 38: 473-476 [Google Scholar]
- Guo H, Mockler T, Duong H, Lin C (2001) SUB1, an Arabidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science 291: 487-490 [DOI] [PubMed] [Google Scholar]
- Harada A, Sakai T, Okada K (2003) phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci USA 100: 8583-8588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120-2123 [DOI] [PubMed] [Google Scholar]
- Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410: 952-954 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Wada M (2002) Blue light-induced chloroplast relocation. Plant Cell Physiol 43: 367-371 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656-660 [DOI] [PubMed] [Google Scholar]
- Lewis BD, Karlin-Neumann G, Davis RW, Spalding EP (1997) Ca2+-activated anion channels and membrane depolarizations induced by blue light and cold in Arabidopsis seedlings. Plant Physiol 114: 1327-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JC, Jenkins GI (1998) Involvement of plasma membrane redox activity and calcium homeostasis in the UV-B and UV-A/blue light induction of gene expression in Arabidopsis. Plant Cell 10: 2077-2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E (1999) Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961-964 [DOI] [PubMed] [Google Scholar]
- Neuhaus G, Bowler C, Hiratsuka K, Yamagata H, Chua NH (1997) Phytochrome-regulated repression of gene expression requires calcium and cGMP. EMBO J 16: 2554-2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, Short TW, Gallagher S, Briggs WR (1993) Blue light-induced phosphorylation of a plasma membrane-associated protein in Zea mays L. Plant Physiol 102: 1211-1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Cho MH, Spalding EP (1998) Two genetically separable phases of growth inhibition induced by blue light in Arabidopsis seedlings. Plant Physiol 118: 609-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Spalding EP (1999) Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc Natl Acad Sci USA 96: 14142-14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969-6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Briggs WR (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723-1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle JR, Jones RL (1988) Inhibition of stem elongation in Cucumis seedlings by blue light requires calcium. Plant Physiol 86: 960-966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP (1995) An apparatus for studying rapid electrophysiological responses to light demonstrated on Arabidopsis leaves. Photochem Photobiol 62: 934-939 [DOI] [PubMed] [Google Scholar]
- Spalding EP (2000) Ion channels and the transduction of light signals. Plant Cell Environ 23: 665-674 [DOI] [PubMed] [Google Scholar]
- Steinitz B, Poff KL (1986) A single positive phototropic response induced with pulsed-light in hypocotyls of Arabidopsis thaliana seedlings. Planta 168: 305-315 [DOI] [PubMed] [Google Scholar]
- Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P (2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100: 1456-1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R, Hepler PK (1985) Red light stimulates an increase in intracellular calcium in the spores of Onoclea sensibilis. Plant Physiol 77: 8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]