Abstract
The title compound, C18H23N3O3, crystallized with two independent molecules (A and B) in the asymmetric unit. The phenyl ring and the 1,2,4-oxadiazole ring are inclined to one another by 19.9 (3)° in molecule A and 7.3 (3)° in molecule B. The absolute structure of the title compound was referred to the transfered chiral center (S) of one of the starting reactants. In the crystal, A molecules are linked by C—H⋯N interactions involving the two oxadiazole N atoms.
Related literature
For the oxadiazole nucleus as a core structural unit of various muscarinic agonists, see: Orlek & Blaney (1991 ▶). For benzodiazepine receptor partial agonists, see: Watjen & Baker (1989 ▶). For dopamine transporters, see: Gray & Abrahm (1993 ▶). For 5-HT agonists, see: Swain & Baker (1991 ▶). For inhibitors of HIV, see: Matthew et al. (2007 ▶). For GABAA receptor agonists, see: Michaela & Holger (2008 ▶). For bond-length data, see: Allen et al. (1987 ▶).
Experimental
Crystal data
C18H23N3O3
M r = 329.39
Monoclinic,
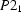
a = 6.464 (3) Å
b = 15.515 (8) Å
c = 17.847 (9) Å
β = 99.880 (7)°
V = 1763.2 (15) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 293 K
0.24 × 0.15 × 0.12 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2008 ▶) T min = 0.980, T max = 0.990
7378 measured reflections
3258 independent reflections
2520 reflections with I > 2σ(I)
R int = 0.073
Refinement
R[F 2 > 2σ(F 2)] = 0.073
wR(F 2) = 0.198
S = 1.01
3258 reflections
440 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.29 e Å−3
Δρmin = −0.44 e Å−3
Data collection: SMART (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97 and PLATON.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810018714/su2175sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810018714/su2175Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C13—H13A⋯N2i | 0.97 | 2.61 | 3.352 (7) | 133 |
| C18—H18A⋯N1i | 0.96 | 2.61 | 3.490 (9) | 153 |
Symmetry code: (i) 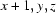 .
.
supplementary crystallographic information
Comment
The oxadiazole nucleus is a well studied pharmacophoric scaffold that has emerged as a core structural unit of various muscarinic agonists (Orlek & Blaney, 1991), benzodiazepine receptor partial agonists (Watjen & Baker, 1989), dopamine transporters (Gray & Abrahm, 1993), 5-HT agonists (Swain & Baker, 1991), inhibitors of HIV (Matthew, 2007), and GABAA receptor agonists (Michaela & Holger,2008). Among the oxadiazoles, 1,2,4-oxadiazole derivatives have gained importance in medicinal chemistry. The interest in five-membered systems containing one oxygen and two nitrogen atoms (positions 1, 2, and 4) is due to the occurrence of 1,2,4-oxadiazoles in biological activite compounds and natural products. In spite of extensive investigations, there are few studies on the crystal structures of oxadiazol-piperidines. Herein, we report on the crystal structure of the title compound, a new oxadiazol-piperidine. It can be reacted with acid, sulfonlchloride and chloride, followed by deprotection of the protective group, to give many usefull compounds.
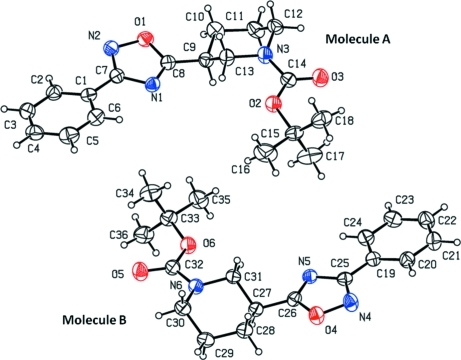
The title compound crystallized in the chiral monoclinic space group P21, with two independent molecules (A and B) in the asymmetric unit (Fig. 1). It was obtained from a chiral source, hence its absolute structure, (S), was confirmed by the transfered chiral center; atom C9 in molecule A and atom C27 in molecule B (Fig. 1). The bond distances in the two molecules are very similar and close to normal values (Allen et al., 1987). The two molecules differ in the orientation of the phenyl ring with respect to the oxadiazole mean plane. In molecule A this dihedral angle is 19.9 (3)°, while in molecule B it is only 7.3 (3)°. In both molecules the piperidine ring has a chair conformation.
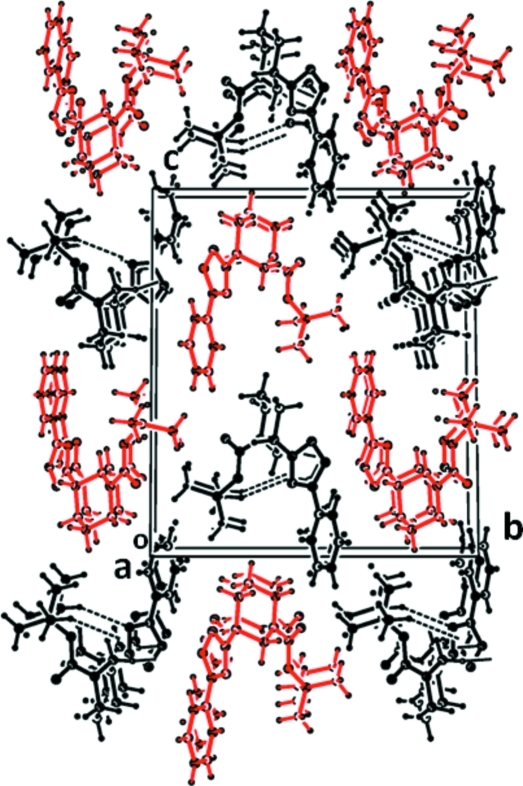
In the crystal symmetry related A molecules are linked via C-H···N interactions (see Table 1 and Fig. 2 for details).
Experimental
A suspension of hydroxylamine hydrochloride (4.09 g), potassium carbonate (2.76 g), benzonitrile (1.03 g) in absolute ethanol (200 mL) was heated at reflux for 10 h. After the reaction was completed, monitored by TLC, the mixture was cooled, filtered to remove inorganic salts, and concentrated under vacuum. The residue was purified by column chromatography, by use of a gradient elution of dichloromethane to 40% acetone in dichloromethane, to give (E)-N-hydroxybenzamidine (1.36g). 1H NMR (300 MHz, DMSO-d6): 9.59 (s, 1H), 7.62-7.67 (m, 2H), 7.32-7.37 (m, 3H), 5.77 (s, 2H); EI (M+) 136 A mixture of (S)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid (1.15 g) in absolute THF (50 mL), isobutyl carbonochloridate (5ml) and trimethylamine (2ml) were mixted together and stirred for 30min at rt, followed by slow dropwise addition of (E)-N-Hydroxy-benzamidine (1.36g) in THF (15mL). After the reaction was completed, monitored by TLC, the mixture was injected into n-Bu4NF (1 M in THF, 3 mL), warmed to reflux and was stirred for 24 h. After this reaction was completed, monitored by TLC, the mixture was poured into EtOAc and washed with water and brine. The organic layer was dried (MgSO4) and concentrated in vacuo. The residue was purified by column chromatography by use of a gradient elution of EtOAc/hexanes. The material was crystallized from EtOH to give the title compound as a white solid. Colourless-rod-like crystals, suitable for X-ray analysis, were obtained by recrystallization from EtOH. 1H NMR (300MHz, CDCl3): 8.05,-8.08 (m, 2H), 7.43-7.48 (m, 3H), 3.95 (d, 1H, J=8.4 Hz), 3.12-3.17 (m, 1H), 3.98 (t, 1H, J=12.6 Hz), 2.42 (d, 1H, J=12.6 Hz), 1.86 (t, 2H, J=12.6 Hz), 1.55-1.65 (m, 1H), 1.45 (s, 9H), 0.83-0.92 (m, 2H); ESI (M++23) 352.
Refinement
In the final cycles of refinement, in the absence of significant anomalous scattering effects, 4120 Friedel pairs were merged and Δf " set to zero. The H-atoms could all be located in difference Fourier maps. In the final cycles of refinment they were placed in calculated positions and treated as riding atoms: C—H 0.93, 0.96, 0.97 and 0.98 Å, for H-methine, H-methyl, H-methylene and H-aromtic, respectively, with Uiso(H) = k × Ueq(C), where k = 1.5 for H-methyl and = 1.2 for all other H-atoms.
Figures
Fig. 1.
The molecular structure of the two independent molecules (A and B) of the title compound, with atom labels and 30% probability displacement ellipsoids.
Fig. 2.
A view along the a-axis of the crystal packing of the title compound (Molecule A is black; Molecule B is red; C-H···N interactions are shown as dotted lines; see Table 1 for details).
Crystal data
| C18H23N3O3 | F(000) = 704 |
| Mr = 329.39 | Dx = 1.241 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2yb | Cell parameters from 818 reflections |
| a = 6.464 (3) Å | θ = 2.7–23.3° |
| b = 15.515 (8) Å | µ = 0.09 mm−1 |
| c = 17.847 (9) Å | T = 293 K |
| β = 99.880 (7)° | Rod, colourless |
| V = 1763.2 (15) Å3 | 0.24 × 0.15 × 0.12 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 3258 independent reflections |
| Radiation source: fine-focus sealed tube | 2520 reflections with I > 2σ(I) |
| graphite | Rint = 0.073 |
| phi and ω scans | θmax = 25.1°, θmin = 1.8° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2008) | h = −7→7 |
| Tmin = 0.980, Tmax = 0.990 | k = −18→16 |
| 7378 measured reflections | l = −21→14 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.073 | H-atom parameters constrained |
| wR(F2) = 0.198 | w = 1/[σ2(Fo2) + (0.1416P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max < 0.001 |
| 3258 reflections | Δρmax = 0.29 e Å−3 |
| 440 parameters | Δρmin = −0.44 e Å−3 |
| 1 restraint | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.013 (4) |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell esds are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.4355 (6) | 0.9886 (3) | 0.68689 (18) | 0.0605 (12) | |
| O2 | 1.1738 (6) | 0.7663 (3) | 0.79331 (18) | 0.0644 (15) | |
| O3 | 1.2986 (7) | 0.7271 (3) | 0.6870 (2) | 0.0747 (16) | |
| N1 | 0.5574 (6) | 0.9308 (3) | 0.7976 (2) | 0.0536 (14) | |
| N2 | 0.3265 (7) | 1.0282 (3) | 0.7402 (2) | 0.0603 (17) | |
| N3 | 1.0660 (7) | 0.8361 (3) | 0.6855 (2) | 0.0626 (16) | |
| C1 | 0.3287 (8) | 1.0093 (3) | 0.8751 (3) | 0.0524 (19) | |
| C2 | 0.1341 (9) | 1.0467 (4) | 0.8740 (3) | 0.062 (2) | |
| C3 | 0.0626 (11) | 1.0639 (4) | 0.9411 (3) | 0.070 (2) | |
| C4 | 0.1837 (11) | 1.0425 (4) | 1.0097 (3) | 0.072 (2) | |
| C5 | 0.3769 (12) | 1.0042 (4) | 1.0113 (3) | 0.079 (2) | |
| C6 | 0.4488 (9) | 0.9868 (4) | 0.9447 (3) | 0.067 (2) | |
| C7 | 0.4026 (7) | 0.9904 (3) | 0.8048 (3) | 0.0496 (17) | |
| C8 | 0.5670 (7) | 0.9310 (3) | 0.7267 (3) | 0.0496 (17) | |
| C9 | 0.6996 (8) | 0.8775 (4) | 0.6851 (3) | 0.0529 (17) | |
| C10 | 0.6740 (9) | 0.8942 (5) | 0.6013 (3) | 0.068 (2) | |
| C11 | 0.8260 (9) | 0.8384 (5) | 0.5657 (3) | 0.067 (2) | |
| C12 | 1.0481 (9) | 0.8513 (4) | 0.6046 (3) | 0.061 (2) | |
| C13 | 0.9262 (8) | 0.8882 (4) | 0.7234 (2) | 0.0584 (18) | |
| C14 | 1.1864 (8) | 0.7715 (4) | 0.7210 (3) | 0.0542 (17) | |
| C15 | 1.2965 (10) | 0.7014 (4) | 0.8439 (3) | 0.067 (2) | |
| C16 | 1.2285 (14) | 0.7220 (6) | 0.9186 (3) | 0.106 (3) | |
| C17 | 1.2262 (15) | 0.6118 (5) | 0.8167 (4) | 0.102 (3) | |
| C18 | 1.5243 (11) | 0.7145 (5) | 0.8480 (4) | 0.086 (3) | |
| O4 | 0.6093 (6) | 0.1946 (3) | 0.82130 (18) | 0.0652 (13) | |
| O5 | −0.2882 (7) | 0.4596 (3) | 0.8012 (2) | 0.0755 (16) | |
| O6 | −0.1493 (6) | 0.4210 (3) | 0.69793 (18) | 0.0678 (15) | |
| N4 | 0.7414 (7) | 0.1666 (4) | 0.7705 (2) | 0.0672 (19) | |
| N5 | 0.4557 (7) | 0.2334 (3) | 0.7072 (2) | 0.0514 (14) | |
| N6 | 0.0024 (7) | 0.3759 (3) | 0.8124 (2) | 0.0583 (15) | |
| C19 | 0.7234 (8) | 0.1768 (3) | 0.6348 (3) | 0.0502 (17) | |
| C20 | 0.9211 (9) | 0.1437 (4) | 0.6355 (3) | 0.0614 (17) | |
| C21 | 0.9925 (10) | 0.1274 (4) | 0.5684 (3) | 0.072 (2) | |
| C22 | 0.8669 (11) | 0.1471 (4) | 0.4995 (3) | 0.075 (3) | |
| C23 | 0.6724 (10) | 0.1798 (4) | 0.4977 (3) | 0.070 (2) | |
| C24 | 0.5980 (9) | 0.1963 (4) | 0.5641 (3) | 0.0617 (19) | |
| C25 | 0.6435 (8) | 0.1934 (3) | 0.7043 (2) | 0.0489 (17) | |
| C26 | 0.4423 (8) | 0.2318 (3) | 0.7778 (3) | 0.0500 (16) | |
| C27 | 0.2658 (9) | 0.2633 (3) | 0.8154 (3) | 0.0514 (16) | |
| C28 | 0.3205 (9) | 0.2705 (4) | 0.9012 (3) | 0.0626 (19) | |
| C29 | 0.1270 (10) | 0.2999 (4) | 0.9314 (3) | 0.069 (2) | |
| C30 | 0.0361 (10) | 0.3818 (4) | 0.8946 (3) | 0.0642 (19) | |
| C31 | 0.1823 (9) | 0.3471 (4) | 0.7790 (3) | 0.0569 (19) | |
| C32 | −0.1542 (9) | 0.4222 (4) | 0.7715 (3) | 0.0570 (19) | |
| C33 | −0.2846 (9) | 0.4763 (4) | 0.6425 (3) | 0.0621 (19) | |
| C34 | −0.2377 (13) | 0.5693 (5) | 0.6619 (4) | 0.088 (3) | |
| C35 | −0.2098 (11) | 0.4529 (5) | 0.5692 (3) | 0.083 (3) | |
| C36 | −0.5115 (10) | 0.4536 (5) | 0.6379 (4) | 0.081 (3) | |
| H2A | 0.05080 | 1.06050 | 0.82770 | 0.0740* | |
| H3A | −0.06750 | 1.08990 | 0.93990 | 0.0850* | |
| H4A | 0.13540 | 1.05380 | 1.05490 | 0.0870* | |
| H5A | 0.45900 | 0.99000 | 1.05770 | 0.0950* | |
| H6A | 0.57800 | 0.95980 | 0.94620 | 0.0800* | |
| H9A | 0.66130 | 0.81720 | 0.69160 | 0.0640* | |
| H10A | 0.70080 | 0.95460 | 0.59260 | 0.0820* | |
| H10B | 0.53080 | 0.88150 | 0.57760 | 0.0820* | |
| H11A | 0.78820 | 0.77820 | 0.56920 | 0.0800* | |
| H11B | 0.81450 | 0.85280 | 0.51230 | 0.0800* | |
| H12A | 1.13950 | 0.81190 | 0.58350 | 0.0730* | |
| H12B | 1.09210 | 0.90970 | 0.59590 | 0.0730* | |
| H13A | 0.96590 | 0.94840 | 0.72190 | 0.0700* | |
| H13B | 0.94070 | 0.87100 | 0.77630 | 0.0700* | |
| H16A | 1.26510 | 0.78050 | 0.93250 | 0.1590* | |
| H16B | 1.29790 | 0.68390 | 0.95720 | 0.1590* | |
| H16C | 1.07920 | 0.71480 | 0.91360 | 0.1590* | |
| H17A | 1.26240 | 0.60190 | 0.76740 | 0.1530* | |
| H17B | 1.07690 | 0.60700 | 0.81350 | 0.1530* | |
| H17C | 1.29500 | 0.56980 | 0.85190 | 0.1530* | |
| H18A | 1.55570 | 0.77500 | 0.85240 | 0.1300* | |
| H18B | 1.56650 | 0.69220 | 0.80270 | 0.1300* | |
| H18C | 1.59900 | 0.68480 | 0.89160 | 0.1300* | |
| H20A | 1.00750 | 0.13220 | 0.68170 | 0.0740* | |
| H21A | 1.12460 | 0.10330 | 0.56930 | 0.0860* | |
| H22A | 0.91670 | 0.13770 | 0.45430 | 0.0900* | |
| H23A | 0.58780 | 0.19140 | 0.45120 | 0.0840* | |
| H24A | 0.46540 | 0.22030 | 0.56240 | 0.0740* | |
| H27A | 0.15230 | 0.22090 | 0.80430 | 0.0620* | |
| H28A | 0.36690 | 0.21510 | 0.92300 | 0.0750* | |
| H28B | 0.43330 | 0.31180 | 0.91510 | 0.0750* | |
| H29A | 0.02150 | 0.25480 | 0.92260 | 0.0830* | |
| H29B | 0.16320 | 0.30870 | 0.98580 | 0.0830* | |
| H30A | −0.09650 | 0.39390 | 0.91100 | 0.0770* | |
| H30B | 0.13090 | 0.42930 | 0.91090 | 0.0770* | |
| H31A | 0.29170 | 0.39050 | 0.78690 | 0.0680* | |
| H31B | 0.13950 | 0.33890 | 0.72460 | 0.0680* | |
| H34A | −0.29080 | 0.58380 | 0.70740 | 0.1320* | |
| H34B | −0.30370 | 0.60510 | 0.62080 | 0.1320* | |
| H34C | −0.08860 | 0.57840 | 0.67010 | 0.1320* | |
| H35A | −0.06140 | 0.46290 | 0.57470 | 0.1240* | |
| H35B | −0.28190 | 0.48780 | 0.52850 | 0.1240* | |
| H35C | −0.23880 | 0.39320 | 0.55790 | 0.1240* | |
| H36A | −0.55900 | 0.47250 | 0.68330 | 0.1220* | |
| H36B | −0.52850 | 0.39230 | 0.63280 | 0.1220* | |
| H36C | −0.59270 | 0.48150 | 0.59450 | 0.1220* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.058 (2) | 0.070 (2) | 0.052 (2) | 0.0050 (18) | 0.0049 (16) | 0.0100 (17) |
| O2 | 0.071 (3) | 0.081 (3) | 0.0403 (19) | 0.015 (2) | 0.0071 (16) | 0.0061 (17) |
| O3 | 0.083 (3) | 0.089 (3) | 0.053 (2) | 0.020 (2) | 0.0144 (18) | 0.0008 (19) |
| N1 | 0.052 (3) | 0.056 (2) | 0.051 (2) | 0.006 (2) | 0.0039 (17) | 0.0042 (19) |
| N2 | 0.052 (3) | 0.069 (3) | 0.060 (3) | 0.008 (2) | 0.0096 (19) | 0.011 (2) |
| N3 | 0.066 (3) | 0.085 (3) | 0.036 (2) | 0.019 (2) | 0.0064 (17) | 0.002 (2) |
| C1 | 0.060 (4) | 0.046 (3) | 0.050 (3) | −0.004 (2) | 0.006 (2) | 0.003 (2) |
| C2 | 0.055 (4) | 0.055 (3) | 0.073 (4) | −0.002 (3) | 0.007 (2) | 0.005 (3) |
| C3 | 0.084 (5) | 0.056 (3) | 0.076 (4) | 0.002 (3) | 0.028 (3) | −0.005 (3) |
| C4 | 0.106 (5) | 0.057 (3) | 0.059 (3) | −0.010 (3) | 0.029 (3) | −0.006 (3) |
| C5 | 0.097 (5) | 0.078 (4) | 0.058 (3) | −0.006 (4) | 0.002 (3) | 0.000 (3) |
| C6 | 0.066 (4) | 0.069 (4) | 0.062 (3) | 0.000 (3) | 0.002 (3) | −0.001 (3) |
| C7 | 0.035 (3) | 0.052 (3) | 0.060 (3) | −0.006 (2) | 0.003 (2) | 0.002 (2) |
| C8 | 0.037 (3) | 0.055 (3) | 0.054 (3) | −0.003 (2) | −0.0004 (19) | 0.004 (2) |
| C9 | 0.044 (3) | 0.058 (3) | 0.056 (3) | −0.003 (2) | 0.007 (2) | 0.001 (2) |
| C10 | 0.048 (3) | 0.103 (5) | 0.048 (3) | −0.003 (3) | −0.007 (2) | 0.002 (3) |
| C11 | 0.066 (4) | 0.097 (4) | 0.036 (3) | −0.003 (3) | 0.005 (2) | 0.002 (3) |
| C12 | 0.062 (4) | 0.078 (4) | 0.044 (3) | 0.005 (3) | 0.012 (2) | 0.008 (2) |
| C13 | 0.058 (3) | 0.078 (4) | 0.037 (2) | 0.013 (3) | 0.002 (2) | −0.002 (2) |
| C14 | 0.057 (3) | 0.062 (3) | 0.042 (3) | −0.002 (3) | 0.004 (2) | −0.001 (2) |
| C15 | 0.074 (4) | 0.069 (4) | 0.053 (3) | 0.006 (3) | −0.002 (2) | 0.019 (3) |
| C16 | 0.128 (7) | 0.137 (7) | 0.048 (3) | 0.004 (6) | 0.004 (4) | 0.024 (4) |
| C17 | 0.127 (7) | 0.081 (5) | 0.087 (5) | −0.020 (5) | −0.011 (4) | 0.022 (4) |
| C18 | 0.074 (5) | 0.083 (5) | 0.093 (4) | 0.003 (3) | −0.012 (3) | 0.022 (4) |
| O4 | 0.052 (2) | 0.094 (3) | 0.0467 (18) | 0.017 (2) | 0.0005 (14) | 0.0030 (18) |
| O5 | 0.079 (3) | 0.100 (3) | 0.049 (2) | 0.026 (2) | 0.0150 (19) | 0.003 (2) |
| O6 | 0.072 (3) | 0.090 (3) | 0.0415 (18) | 0.026 (2) | 0.0104 (16) | 0.0123 (18) |
| N4 | 0.045 (3) | 0.099 (4) | 0.056 (3) | 0.016 (2) | 0.0039 (18) | 0.004 (2) |
| N5 | 0.054 (3) | 0.057 (2) | 0.042 (2) | 0.008 (2) | 0.0049 (16) | 0.0065 (17) |
| N6 | 0.073 (3) | 0.067 (3) | 0.0359 (19) | 0.017 (2) | 0.0124 (18) | 0.0042 (19) |
| C19 | 0.042 (3) | 0.053 (3) | 0.053 (3) | −0.004 (2) | 0.001 (2) | 0.002 (2) |
| C20 | 0.055 (3) | 0.067 (3) | 0.061 (3) | 0.006 (3) | 0.007 (2) | 0.003 (3) |
| C21 | 0.060 (4) | 0.081 (4) | 0.075 (4) | 0.010 (3) | 0.015 (3) | −0.005 (3) |
| C22 | 0.083 (5) | 0.081 (4) | 0.066 (4) | 0.002 (3) | 0.027 (3) | −0.007 (3) |
| C23 | 0.073 (4) | 0.080 (4) | 0.055 (3) | −0.008 (3) | 0.006 (3) | 0.001 (3) |
| C24 | 0.059 (3) | 0.076 (4) | 0.048 (3) | 0.004 (3) | 0.003 (2) | 0.004 (3) |
| C25 | 0.048 (3) | 0.050 (3) | 0.046 (3) | −0.003 (2) | 0.0002 (19) | 0.005 (2) |
| C26 | 0.050 (3) | 0.051 (3) | 0.046 (2) | 0.006 (2) | −0.0001 (19) | 0.001 (2) |
| C27 | 0.060 (3) | 0.055 (3) | 0.038 (2) | 0.007 (2) | 0.005 (2) | 0.001 (2) |
| C28 | 0.071 (4) | 0.071 (3) | 0.044 (3) | 0.010 (3) | 0.005 (2) | 0.006 (2) |
| C29 | 0.089 (4) | 0.083 (4) | 0.037 (3) | 0.014 (3) | 0.014 (3) | 0.014 (2) |
| C30 | 0.084 (4) | 0.070 (3) | 0.040 (3) | 0.007 (3) | 0.015 (2) | 0.002 (2) |
| C31 | 0.064 (4) | 0.065 (3) | 0.043 (3) | 0.011 (2) | 0.013 (2) | 0.009 (2) |
| C32 | 0.070 (4) | 0.059 (3) | 0.042 (3) | 0.009 (3) | 0.010 (2) | 0.000 (2) |
| C33 | 0.069 (4) | 0.071 (3) | 0.042 (3) | 0.006 (3) | −0.003 (2) | 0.006 (2) |
| C34 | 0.108 (6) | 0.076 (4) | 0.075 (4) | −0.009 (4) | 0.000 (4) | 0.008 (3) |
| C35 | 0.086 (5) | 0.111 (6) | 0.048 (3) | 0.005 (4) | 0.004 (3) | 0.007 (3) |
| C36 | 0.072 (5) | 0.092 (5) | 0.073 (4) | −0.002 (3) | −0.006 (3) | 0.017 (3) |
Geometric parameters (Å, °)
| O1—N2 | 1.417 (6) | C13—H13A | 0.9700 |
| O1—C8 | 1.349 (6) | C13—H13B | 0.9700 |
| O2—C14 | 1.309 (6) | C16—H16A | 0.9600 |
| O2—C15 | 1.487 (7) | C16—H16B | 0.9600 |
| O3—C14 | 1.233 (7) | C16—H16C | 0.9600 |
| O4—C26 | 1.347 (6) | C17—H17C | 0.9600 |
| O4—N4 | 1.416 (6) | C17—H17A | 0.9600 |
| O5—C32 | 1.235 (7) | C17—H17B | 0.9600 |
| O6—C33 | 1.477 (7) | C18—H18B | 0.9600 |
| O6—C32 | 1.319 (6) | C18—H18C | 0.9600 |
| N1—C7 | 1.385 (6) | C18—H18A | 0.9600 |
| N1—C8 | 1.278 (6) | C19—C20 | 1.375 (8) |
| N2—C7 | 1.312 (6) | C19—C24 | 1.411 (8) |
| N3—C13 | 1.462 (7) | C19—C25 | 1.446 (7) |
| N3—C14 | 1.357 (7) | C20—C21 | 1.379 (8) |
| N3—C12 | 1.448 (6) | C21—C22 | 1.386 (8) |
| N4—C25 | 1.309 (6) | C22—C23 | 1.351 (10) |
| N5—C25 | 1.372 (7) | C23—C24 | 1.377 (8) |
| N5—C26 | 1.278 (6) | C26—C27 | 1.501 (8) |
| N6—C31 | 1.465 (7) | C27—C28 | 1.515 (8) |
| N6—C32 | 1.349 (7) | C27—C31 | 1.511 (8) |
| N6—C30 | 1.449 (6) | C28—C29 | 1.515 (9) |
| C1—C7 | 1.447 (7) | C29—C30 | 1.503 (9) |
| C1—C2 | 1.382 (8) | C33—C34 | 1.503 (10) |
| C1—C6 | 1.392 (8) | C33—C35 | 1.515 (8) |
| C2—C3 | 1.382 (8) | C33—C36 | 1.497 (9) |
| C3—C4 | 1.376 (8) | C20—H20A | 0.9300 |
| C4—C5 | 1.379 (10) | C21—H21A | 0.9300 |
| C5—C6 | 1.375 (8) | C22—H22A | 0.9300 |
| C8—C9 | 1.481 (7) | C23—H23A | 0.9300 |
| C9—C13 | 1.516 (7) | C24—H24A | 0.9300 |
| C9—C10 | 1.499 (8) | C27—H27A | 0.9800 |
| C10—C11 | 1.527 (9) | C28—H28A | 0.9700 |
| C11—C12 | 1.497 (8) | C28—H28B | 0.9700 |
| C15—C17 | 1.516 (10) | C29—H29A | 0.9700 |
| C15—C16 | 1.508 (9) | C29—H29B | 0.9700 |
| C15—C18 | 1.476 (10) | C30—H30A | 0.9700 |
| C2—H2A | 0.9300 | C30—H30B | 0.9700 |
| C3—H3A | 0.9300 | C31—H31A | 0.9700 |
| C4—H4A | 0.9300 | C31—H31B | 0.9700 |
| C5—H5A | 0.9300 | C34—H34A | 0.9600 |
| C6—H6A | 0.9300 | C34—H34B | 0.9600 |
| C9—H9A | 0.9800 | C34—H34C | 0.9600 |
| C10—H10A | 0.9700 | C35—H35A | 0.9600 |
| C10—H10B | 0.9700 | C35—H35B | 0.9600 |
| C11—H11A | 0.9700 | C35—H35C | 0.9600 |
| C11—H11B | 0.9700 | C36—H36A | 0.9600 |
| C12—H12B | 0.9700 | C36—H36B | 0.9600 |
| C12—H12A | 0.9700 | C36—H36C | 0.9600 |
| N2—O1—C8 | 105.9 (3) | H17B—C17—H17C | 110.00 |
| C14—O2—C15 | 121.5 (5) | C15—C18—H18A | 109.00 |
| N4—O4—C26 | 105.9 (4) | C15—C18—H18B | 109.00 |
| C32—O6—C33 | 123.1 (5) | C15—C18—H18C | 109.00 |
| C7—N1—C8 | 104.4 (4) | H18A—C18—H18B | 110.00 |
| O1—N2—C7 | 104.0 (4) | H18A—C18—H18C | 109.00 |
| C12—N3—C14 | 121.8 (5) | H18B—C18—H18C | 109.00 |
| C13—N3—C14 | 122.9 (4) | C20—C19—C24 | 118.6 (5) |
| C12—N3—C13 | 114.9 (4) | C20—C19—C25 | 121.8 (5) |
| O4—N4—C25 | 103.3 (4) | C24—C19—C25 | 119.6 (5) |
| C25—N5—C26 | 103.8 (4) | C19—C20—C21 | 120.6 (5) |
| C30—N6—C32 | 118.9 (5) | C20—C21—C22 | 119.8 (6) |
| C31—N6—C32 | 121.1 (4) | C21—C22—C23 | 120.3 (5) |
| C30—N6—C31 | 116.1 (4) | C22—C23—C24 | 120.7 (5) |
| C2—C1—C6 | 119.0 (5) | C19—C24—C23 | 119.9 (5) |
| C2—C1—C7 | 120.5 (5) | N4—C25—N5 | 113.8 (4) |
| C6—C1—C7 | 120.4 (5) | N4—C25—C19 | 122.0 (5) |
| C1—C2—C3 | 120.5 (5) | N5—C25—C19 | 124.2 (4) |
| C2—C3—C4 | 120.1 (6) | O4—C26—N5 | 113.2 (5) |
| C3—C4—C5 | 119.8 (5) | O4—C26—C27 | 118.5 (4) |
| C4—C5—C6 | 120.4 (5) | N5—C26—C27 | 128.3 (5) |
| C1—C6—C5 | 120.2 (6) | C26—C27—C28 | 114.4 (5) |
| N2—C7—C1 | 122.8 (4) | C26—C27—C31 | 109.3 (4) |
| N1—C7—N2 | 112.7 (4) | C28—C27—C31 | 112.0 (4) |
| N1—C7—C1 | 124.6 (4) | C27—C28—C29 | 108.5 (5) |
| O1—C8—N1 | 113.0 (4) | C28—C29—C30 | 112.6 (5) |
| O1—C8—C9 | 118.3 (4) | N6—C30—C29 | 111.6 (5) |
| N1—C8—C9 | 128.8 (5) | N6—C31—C27 | 109.7 (4) |
| C8—C9—C10 | 115.4 (5) | O5—C32—O6 | 124.9 (5) |
| C8—C9—C13 | 108.1 (4) | O5—C32—N6 | 122.3 (5) |
| C10—C9—C13 | 111.3 (4) | O6—C32—N6 | 112.8 (5) |
| C9—C10—C11 | 110.4 (5) | O6—C33—C34 | 109.3 (5) |
| C10—C11—C12 | 111.4 (5) | O6—C33—C35 | 101.5 (5) |
| N3—C12—C11 | 110.3 (5) | O6—C33—C36 | 111.2 (5) |
| N3—C13—C9 | 110.9 (4) | C34—C33—C35 | 110.3 (5) |
| O3—C14—N3 | 121.4 (5) | C34—C33—C36 | 113.3 (6) |
| O2—C14—N3 | 112.2 (5) | C35—C33—C36 | 110.6 (5) |
| O2—C14—O3 | 126.3 (5) | C19—C20—H20A | 120.00 |
| C16—C15—C17 | 111.1 (6) | C21—C20—H20A | 120.00 |
| O2—C15—C18 | 111.1 (5) | C20—C21—H21A | 120.00 |
| O2—C15—C17 | 109.1 (5) | C22—C21—H21A | 120.00 |
| O2—C15—C16 | 100.8 (5) | C21—C22—H22A | 120.00 |
| C16—C15—C18 | 111.5 (6) | C23—C22—H22A | 120.00 |
| C17—C15—C18 | 112.6 (6) | C22—C23—H23A | 120.00 |
| C1—C2—H2A | 120.00 | C24—C23—H23A | 120.00 |
| C3—C2—H2A | 120.00 | C19—C24—H24A | 120.00 |
| C2—C3—H3A | 120.00 | C23—C24—H24A | 120.00 |
| C4—C3—H3A | 120.00 | C26—C27—H27A | 107.00 |
| C3—C4—H4A | 120.00 | C28—C27—H27A | 107.00 |
| C5—C4—H4A | 120.00 | C31—C27—H27A | 107.00 |
| C6—C5—H5A | 120.00 | C27—C28—H28A | 110.00 |
| C4—C5—H5A | 120.00 | C27—C28—H28B | 110.00 |
| C1—C6—H6A | 120.00 | C29—C28—H28A | 110.00 |
| C5—C6—H6A | 120.00 | C29—C28—H28B | 110.00 |
| C13—C9—H9A | 107.00 | H28A—C28—H28B | 108.00 |
| C8—C9—H9A | 107.00 | C28—C29—H29A | 109.00 |
| C10—C9—H9A | 107.00 | C28—C29—H29B | 109.00 |
| C9—C10—H10A | 110.00 | C30—C29—H29A | 109.00 |
| C9—C10—H10B | 110.00 | C30—C29—H29B | 109.00 |
| C11—C10—H10A | 110.00 | H29A—C29—H29B | 108.00 |
| C11—C10—H10B | 110.00 | N6—C30—H30A | 109.00 |
| H10A—C10—H10B | 108.00 | N6—C30—H30B | 109.00 |
| C10—C11—H11A | 109.00 | C29—C30—H30A | 109.00 |
| C10—C11—H11B | 109.00 | C29—C30—H30B | 109.00 |
| C12—C11—H11B | 109.00 | H30A—C30—H30B | 108.00 |
| H11A—C11—H11B | 108.00 | N6—C31—H31A | 110.00 |
| C12—C11—H11A | 109.00 | N6—C31—H31B | 110.00 |
| H12A—C12—H12B | 108.00 | C27—C31—H31A | 110.00 |
| C11—C12—H12B | 110.00 | C27—C31—H31B | 110.00 |
| N3—C12—H12A | 110.00 | H31A—C31—H31B | 108.00 |
| N3—C12—H12B | 110.00 | C33—C34—H34A | 109.00 |
| C11—C12—H12A | 110.00 | C33—C34—H34B | 110.00 |
| N3—C13—H13A | 109.00 | C33—C34—H34C | 110.00 |
| N3—C13—H13B | 110.00 | H34A—C34—H34B | 110.00 |
| C9—C13—H13A | 109.00 | H34A—C34—H34C | 109.00 |
| C9—C13—H13B | 109.00 | H34B—C34—H34C | 110.00 |
| H13A—C13—H13B | 108.00 | C33—C35—H35A | 110.00 |
| C15—C16—H16A | 109.00 | C33—C35—H35B | 109.00 |
| C15—C16—H16B | 109.00 | C33—C35—H35C | 109.00 |
| C15—C16—H16C | 110.00 | H35A—C35—H35B | 109.00 |
| H16B—C16—H16C | 110.00 | H35A—C35—H35C | 109.00 |
| H16A—C16—H16B | 109.00 | H35B—C35—H35C | 109.00 |
| H16A—C16—H16C | 109.00 | C33—C36—H36A | 110.00 |
| H17A—C17—H17C | 109.00 | C33—C36—H36B | 109.00 |
| C15—C17—H17A | 109.00 | C33—C36—H36C | 109.00 |
| C15—C17—H17B | 109.00 | H36A—C36—H36B | 110.00 |
| C15—C17—H17C | 109.00 | H36A—C36—H36C | 109.00 |
| H17A—C17—H17B | 109.00 | H36B—C36—H36C | 109.00 |
| N2—O1—C8—N1 | −1.4 (6) | C2—C1—C7—N2 | 20.5 (8) |
| N2—O1—C8—C9 | 178.5 (4) | C6—C1—C2—C3 | 2.0 (9) |
| C8—O1—N2—C7 | −0.3 (5) | C7—C1—C2—C3 | 179.3 (5) |
| C15—O2—C14—O3 | −1.6 (9) | C2—C1—C6—C5 | −2.0 (9) |
| C15—O2—C14—N3 | −178.6 (5) | C7—C1—C6—C5 | −179.4 (5) |
| C14—O2—C15—C17 | −63.1 (7) | C2—C1—C7—N1 | −158.8 (5) |
| C14—O2—C15—C18 | 61.6 (7) | C6—C1—C7—N2 | −162.2 (5) |
| C14—O2—C15—C16 | 179.9 (6) | C6—C1—C7—N1 | 18.5 (8) |
| N4—O4—C26—C27 | 176.2 (4) | C1—C2—C3—C4 | −1.1 (9) |
| N4—O4—C26—N5 | −2.2 (6) | C2—C3—C4—C5 | 0.3 (10) |
| C26—O4—N4—C25 | 2.7 (6) | C3—C4—C5—C6 | −0.4 (10) |
| C33—O6—C32—N6 | −171.1 (5) | C4—C5—C6—C1 | 1.3 (9) |
| C32—O6—C33—C36 | −65.0 (7) | N1—C8—C9—C10 | −179.6 (5) |
| C32—O6—C33—C34 | 60.9 (7) | O1—C8—C9—C13 | 125.9 (5) |
| C33—O6—C32—O5 | 10.3 (9) | O1—C8—C9—C10 | 0.6 (7) |
| C32—O6—C33—C35 | 177.4 (5) | N1—C8—C9—C13 | −54.3 (7) |
| C7—N1—C8—O1 | 2.3 (6) | C8—C9—C10—C11 | 177.3 (5) |
| C8—N1—C7—N2 | −2.5 (6) | C13—C9—C10—C11 | 53.6 (7) |
| C8—N1—C7—C1 | 176.8 (5) | C8—C9—C13—N3 | 179.4 (4) |
| C7—N1—C8—C9 | −177.6 (5) | C10—C9—C13—N3 | −52.9 (7) |
| O1—N2—C7—N1 | 1.7 (5) | C9—C10—C11—C12 | −55.1 (7) |
| O1—N2—C7—C1 | −177.7 (4) | C10—C11—C12—N3 | 54.9 (7) |
| C13—N3—C14—O3 | 178.0 (5) | C24—C19—C20—C21 | −2.0 (8) |
| C13—N3—C12—C11 | −55.9 (7) | C25—C19—C20—C21 | 179.1 (5) |
| C14—N3—C12—C11 | 117.0 (6) | C20—C19—C24—C23 | 1.8 (8) |
| C12—N3—C13—C9 | 54.8 (6) | C25—C19—C24—C23 | −179.3 (5) |
| C14—N3—C13—C9 | −118.0 (5) | C20—C19—C25—N4 | −10.0 (8) |
| C12—N3—C14—O2 | −177.1 (5) | C20—C19—C25—N5 | 173.9 (5) |
| C12—N3—C14—O3 | 5.7 (8) | C24—C19—C25—N4 | 171.1 (5) |
| C13—N3—C14—O2 | −4.8 (7) | C24—C19—C25—N5 | −4.9 (8) |
| O4—N4—C25—N5 | −2.4 (6) | C19—C20—C21—C22 | 2.1 (9) |
| O4—N4—C25—C19 | −178.8 (4) | C20—C21—C22—C23 | −2.0 (10) |
| C26—N5—C25—C19 | 177.4 (5) | C21—C22—C23—C24 | 1.8 (10) |
| C25—N5—C26—O4 | 0.8 (6) | C22—C23—C24—C19 | −1.7 (9) |
| C26—N5—C25—N4 | 1.1 (6) | O4—C26—C27—C28 | 15.2 (7) |
| C25—N5—C26—C27 | −177.4 (5) | O4—C26—C27—C31 | 141.7 (5) |
| C32—N6—C30—C29 | 150.6 (5) | N5—C26—C27—C28 | −166.7 (5) |
| C31—N6—C30—C29 | −51.5 (7) | N5—C26—C27—C31 | −40.2 (7) |
| C32—N6—C31—C27 | −149.7 (5) | C26—C27—C28—C29 | −178.1 (4) |
| C30—N6—C32—O5 | −10.6 (9) | C31—C27—C28—C29 | 56.8 (6) |
| C30—N6—C32—O6 | 170.8 (5) | C26—C27—C31—N6 | 176.9 (4) |
| C31—N6—C32—O5 | −167.4 (6) | C28—C27—C31—N6 | −55.3 (6) |
| C31—N6—C32—O6 | 14.0 (8) | C27—C28—C29—C30 | −54.6 (6) |
| C30—N6—C31—C27 | 52.9 (6) | C28—C29—C30—N6 | 51.8 (7) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C13—H13A···N2i | 0.97 | 2.61 | 3.352 (7) | 133 |
| C18—H18A···N1i | 0.96 | 2.61 | 3.490 (9) | 153 |
Symmetry codes: (i) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SU2175).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bruker (2007). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Gray, L. & Abrahm, P. (1993). J. Med. Chem.36, 2886–2890. [DOI] [PubMed]
- Matthew, D. C., Deng, B.-L., Hartmann, T. L., Watson, K. M., Buckheit, R. W. Jr, Pannecouque, C., De Clercq, E. & Cushman, M. (2007). J. Med. Chem.50, 4854–4867. [DOI] [PubMed]
- Michaela, J. & Holger, R. (2008). J. Med. Chem.51, 4430–4448.
- Orlek, B. S. & Blaney, F. E. (1991). J. Med. Chem.34, 2726–2735. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Swain, C. & Baker, R. (1991). J. Med. Chem.34, 140–151. [DOI] [PubMed]
- Watjen, F. & Baker, R. (1989). J. Med. Chem.32, 2282–2291. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810018714/su2175sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810018714/su2175Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report