Abstract
Recent studies have shown that blue light-specific stomatal opening is reversed by green light and that far-red light can be used to probe phytochrome-dependent stomatal movements. Here, blue-green reversibility and far-red light were used to probe the stomatal responses of the npq1 mutant and the phot1 phot2 double mutant of Arabidopsis. In plants grown at 50 μmol m-2 s-1, red light (photosynthetic)-mediated opening in isolated stomata from wild type (WT) and both mutants saturated at 100 μmol m-2 s-1. Higher fluence rates caused stomatal closing, most likely due to photo-inhibition. Blue light-specific opening, probed by adding blue light (10 μmol m-2 s-1) to a 100 μmol m-2 s-1 red background, was found in WT, but not in npq1 or phot1 phot2 double mutant stomata. Under 50 μmol m-2 s-1 red light, 10 μmol m-2 s-1 blue light opened stomata in both WT and npq1 mutant stomata but not in the phot1 phot2 double mutant. In npq1, blue light-stimulated opening was reversed by far-red but not green light, indicating that npq1 has a phytochrome-mediated response and lacks a blue light-specific response. Stomata of the phot1 phot2 double mutant opened in response to 20 to 50 μmol m-2 s-1 blue light. This opening was green light reversible and far-red light insensitive, indicating that stomata of the phot1 phot2 double mutant have a detectable blue light-specific response.
Many steps in the sensory-transducing cascade mediating blue light-specific stomatal movements are well characterized (Assmann, 1993). Guard cells are turgor valves that control the dimensions of the stomatal pore by changes in water content caused by changes in their osmotic potential. Blue light-specific stomatal opening is mediated by potassium and chloride uptake and malate biosynthesis. Ion uptake is driven by an electrochemical gradient generated by a proton-pumping ATPase activated by blue light (Tallman, 1992). The activation of the ATPase is mediated by the phosphorylation of its C terminus by a Ser/Thr kinase, facilitated by the binding of a 14-3-3 protein (Kinoshita and Shimazaki, 1999). Blue light-specific stomatal opening has an action spectrum, showing a maximum at 450 nm and two minor peaks at 420 and 470 nm (Karlsson, 1986).
Genetic, biochemical, and physiological studies have identified the chloroplastic carotenoid, zeaxanthin, as a blue light photoreceptor in guard cells (Frechilla et al., 1999). Two recent studies, however, have reported that stomata from the zeaxanthin-less Arabidopsis mutant, npq1, open in response to blue light (Eckert and Kaldenhoff, 2000; Kinoshita et al., 2001) and questioned the validity of the zeaxanthin hypothesis. One of these studies further reported that stomata from the phot1 phot2 double mutant of Arabidopsis lack a blue light response and hypothesized that phototropin is the main photoreceptor mediating blue light responses in guard cells (Kinoshita et al., 2001).
The photobiological properties of guard cells complicate the analysis of stomatal opening in response to blue light (Lasceve et al., 1999). A separate, chlorophyll-based, sensory-transducing cascade mediates stomatal movements in response to photosynthetic active radiation (Zeiger et al., 2002). This photosynthesis-dependent pathway shows maximal sensitivity in the blue and red wavebands of the visible spectrum. These different photoreceptors can be separated using specific light treatments as probes. Because blue light activates both the blue light-specific and the photosynthesis-dependent pathways, blue light-specific responses are usually tested under a saturating red light background (Ogawa, 1981).
Responses mediated by the blue light-specific photoreceptor also can be probed by green light (Frechilla et al., 2000). The reversibility of specific blue light-stimulated stomatal opening by green light has been documented in Arabidopsis, Vicia faba, and several other species (Talbott et al., 2002a). Green light (maximum at 540 nm) completely inhibits stomatal opening induced by continuous blue light when given together with blue light in a 2:1 ratio. The green reversal has also been detected in pulse experiments: The opening caused by a pulse of blue light is not observed if the blue light pulse is followed by a green light pulse. The opening is restored if the green light pulse is followed by second blue light pulse (Frechilla et al., 2000).
In addition, the absorption spectrum of phytochrome extends into the blue region of the spectrum, thus making phytochrome a potential mediator of blue light opening. Phytochrome, although not usually active in stomatal responses to light, appears to mediate opening in stomata of the orchid genus Paphiopedilum (Talbott et al., 2002b), which have only minute amounts of chlorophyll and lack photosynthesis-dependent opening. Low fluence rates of red light stimulated opening in orchid stomata, and this opening was reversed by far-red light. A phytochrome-mediated blue light response should also be reversible by far-red light.
The goal of the present study was to identify as clearly as possible the blue light responses of the two Arabidopsis mutants, npq1 and the phot1 phot2 double mutant. Obtained results showed that the reported blue light response of npq1 stomata was reversed by far-red but not by green light. Stomata from the phot1 phot2 double mutant opened in response to 20 to 50 μmol m-2 s-1 blue light and that response was green light reversible and far-red light insensitive.
RESULTS
Dose Response Curves for Red Light-Stimulated Opening in Wild Type (WT), npq1 Mutant, and phot1 phot2 Double Mutant Stomata
Interpretation of blue light responses obtained in dual-beam experiments using background red light is facilitated by detailed information of the saturation levels of the red light (photosynthetic) response. Dose response curves of red light-stimulated opening were determined in isolated stomata from WT and the npq1 mutant and phot1 phot2 double mutant of Arabidopsis.
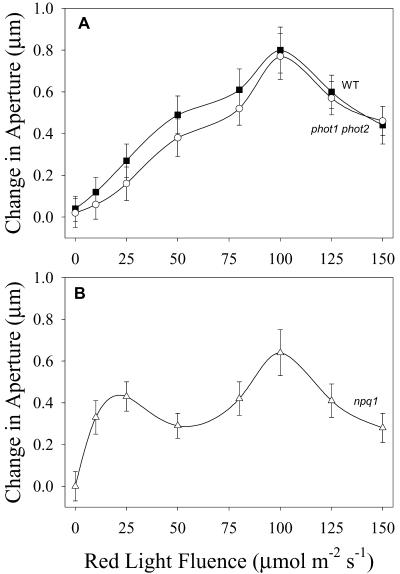
The dose response curves of the stomatal response to red light from WT and the phot1 phot2 double mutant were indistinguishable (Fig. 1A). Both curves showed a steady increase in aperture with fluence, with maximum response obtained at 100 μmol m-2 s-1 red light. Apertures declined at fluence rates higher than 100 μmol m-2 s-1, most likely due to photo-inhibition.
Figure 1.
Red light-stimulated opening in WT, npq1, and phot1 phot2 double mutant of Arabidopsis. Stomata in epidermal strips were dark adapted for 30 min and then exposed to red light for 1.5 h. Data plotted are the difference between initial and final average aperture values from three to four experiments for each fluence rate (30–40 stomata per experiment) ± se of the mean. A, Fluence response curves for WT (▪) and the phot1 phot2 double mutant (○). B, Fluence response curve for the npq1 mutant. Average initial aperture values: WT, 1.3 μm; npq1, 1.2 μm; and phot1 phot2, 1.2 μm.
The red light fluence response curve of npq1 stomata also showed a maximum at 100 μmol m-2 s-1. This response curve however, differed from WT in that it was biphasic, with a minor peak around 25 μmol m-2 s-1 red light (Fig. 1B). Opening in response to 10 μmol m-2 s-1 red light was substantially larger in npq1 than in WT stomata. Because photosynthetic rate should increase linearly with fluence in this portion of the dose response curve, the secondary peak in the 10 to 30 μmol m-2 s-1 region of the npq1 response curve suggests the operation of an additional opening mechanism activated by red light and operating at low fluence rates.
Blue Light Responses of npq1
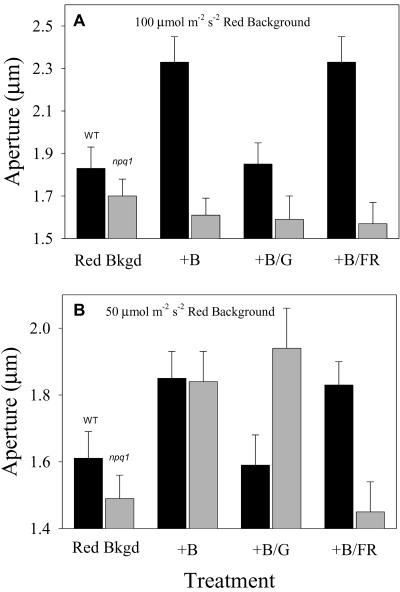
Addition of 10 μmol m-2 s-1 blue light to a 100 μmol m-2 s-1 saturating background red light resulted in additional opening in WT stomata (Fig. 2A). This blue light response obtained under a saturating red light background has been shown to have an action spectrum typical of specific blue light responses (Karlsson, 1986). As previously found in green light reversibility studies, this specific blue light response was reversed by simultaneous application of 20 μmol m-2 s-1 green light in conjunction with blue light (Fig. 2A; Talbott et al., 2002a). Far-red light did not reverse the blue light-stimulated opening in WT stomata, indicating that there was no phytochrome-mediated component in the specific blue light response of WT stomata of Arabidopsis (Fig. 2A).
Figure 2.
Blue light-stimulated opening in WT and npq1 stomata. Apertures after 1.5 h of 100 μmol m-2 s-1 (A) or 50 μmol m-2 s-1 (B) red light are shown in the first column (Red Bkgd). Subsequent columns show aperture value after an additional 1 h of exposure to 10 μmol m-2 s-1 blue light (+B), 10 μmol m-2 s-1 blue and 20 μmol m-2 s-1 green light (+B/G), or 10 μmol m-2 s-1 blue and 100 μmol m-2 s-1 far-red light (+B/FR) to the red light background. Values represent averages from four experiments.
As reported previously (Frechilla et al., 1999), no blue light-specific opening was observed in npq1 stomata upon the addition of 10 μmol m-2 s-1 blue light to a 100 μmol m-2 s-1 saturating red light background (Fig. 2A). Addition of green or far-red light to the applied blue light had no detectable effect on aperture.
The two published reports on blue light responses of npq1 stomata used non-saturating levels of background red light (Eckert and Kaldenhoff, 2000; Kinoshita et al., 2001). We confirmed the results of Kinoshita et al. (2001) in experiments in which 10 μmol m-2 s-1 blue light was added to subsaturating, 50 μmol m-2 s-1 background red light. In such conditions, the aperture increase of npq1 stomata was comparable with that observed in the WT (Fig. 2B).
A Phytochrome-Mediated Stomatal Response in npq1
Probing of the blue light response of npq1 stomata with green and far-red light showed that the blue light-stimulated opening observed in npq1 under a 50 μmol m-2 s-1 red light background was not reversible by green light but was reversible by far-red light. This is in contrast with the WT response, which showed green light reversibility and was insensitive to far-red light (Fig. 2B).
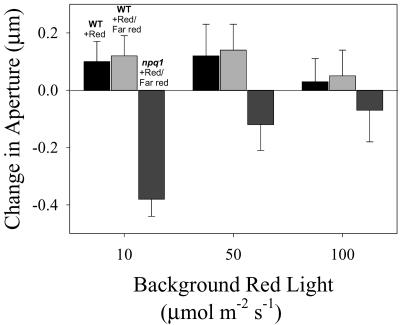
The far-red reversibility of the blue light-stimulated opening seen in npq1 stomata under subsaturating red background light suggests that phytochrome is mediating this opening response. The possible involvement of phytochrome in the regulation of stomatal apertures in npq1 was probed further by measuring aperture changes in response to 100 μmol m-2 s-1 far-red light and 10 μmol m-2 s-1 red light (used to control for a possible photosynthetic effect elicited by the previously used 10 μmol m-2 s-1 blue light). Far red had no detectable effect on WT stomata (Fig. 3). In contrast, far-red light effectively closed npq1 stomata. Strikingly, the phytochrome-mediated component of stomatal opening under red light, probed by the far-red light treatment, decreased as a function of fluence rates of red light (Fig. 3). Thus, under low fluence rates, corresponding to the initial peak of opening seen in the red light response curve for npq1 stomata (Fig. 1B), red or blue light-stimulated opening is far-red reversible. At higher fluence rates, corresponding to the major peak of red light opening, most of the red light-stimulated opening in npq1 is far-red insensitive. It is noteworthy that the phytochrome response of orchid stomata showed the same inverse relation with red light dose (Talbott et al., 2002b).
Figure 3.
Far-red light reversibility of red light-stimulated opening in WT and npq1 stomata. Stomata were exposed to 10, 50, or 100 μmol m-2 s-1 background red light for 1.5 h. Ten micromoles per meter squared per second red and 100 μmol m-2 s-1 far-red (+Red/Far red) light were then added to the red background light for an additional 1 h. Data plotted are the difference between average aperture values before and after addition of light to the red background and are the results from three experiments (30–40 stomata per experiment) ± se of the mean. Change in aperture in WT stomata caused by an additional 1 h of 10 μmol m-2 s-1 red is shown for comparison (wt + Red).
Green light (20 μmol m-2 s-1) applied under 50 μmol m-2 s-1 background red light opened npq1 stomata from an initial aperture of 1.7 ± 0.1 to 1.9 ± 0.1 μm. This green light-stimulated opening was farred reversible, as found with orchid stomata (Talbott et al., 2002b). No aperture changes were elicited by the same green light treatment in WT stomata. A positive effect of green light on phytochrome-mediated phototropism in oat (Avena sativa) and Arabidopsis has been reported (Mandoli and Briggs, 1981; Steinitz et al., 1985).
PSI Does Not Mediate the Response of npq1 Stomata to Far-Red Light
PSI responds maximally at 700 nm and absorbs poorly at 720 nm. In contrast, the Pr and Pfr forms of phytochrome absorbs nearly equally at 700 nm, whereas Pfr has a large absorption at 720 nm (Vierstra and Quail, 1983). Isolated stomata from npq1 were treated with 20 μmol m-2 s-1, 700 or 720 nm of light, obtained with interference filters, applied under a background of 10 μmol m-2 s-1 red light. Addition of 720 nm of light, which should effectively shift the phytochrome photo-equilibrium toward Pr, closed the stomata from 1.5 ± 0.1 to 1.3 ± 0.1 μm. In contrast, addition of 20 μmol m-2 s-1 700 nm of light to a 10 μmol m-2 s-1 background red light illumination failed to reverse stomatal opening, and, in fact, opened them to 1.7 ± 0.1 μm. Thus, far-red reversibility of blue and red light-stimulated stomatal opening in npq1 appears unrelated to guard cell photosynthesis and seems to be a phytochrome-mediated response.
Far-Red Light Alters the Dose Response Curve for White Light in npq1 Stomata
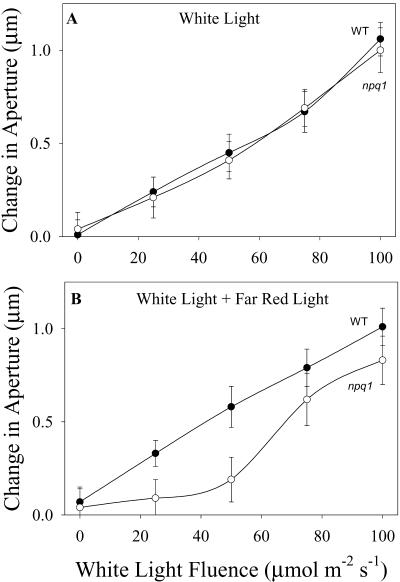
Previous studies have shown that npq1 stomata appear to have a normal response to white light (Frechilla et al., 1999), and data shown in Figure 4 confirm those observations. Given the fact that npq1 stomata are devoid of a specific blue light response (Frechilla et al., 1999; Figs. 2 and 3), a normal response to white light suggests the operation of compensating mechanisms. The possibility that phytochrome-mediated responses provide a compensating mechanism for the absence of a specific blue light response was tested in experiments in which the response of npq1 stomata to white light was tested in the presence of far-red light. Results showed that in the presence of far-red light, the opening response of npq1 stomata is impaired, primarily at low fluence rates. Thus, the operation of a phytochrome-dependent sensory transduction cascade in npq1 stomata appears to compensate for the lack of a normal blue light-specific response in this mutant and results in a WT-like dose response curve for white light.
Figure 4.
The effect of far-red light on white light-stimulated opening in WT and npq1 stomata. Isolated stomata in epidermal strips were dark adapted for 30 min and then exposed to white light for 1.5 h. Data plotted are the difference between initial and final average aperture values for WT (•) and npq1 (○) stomata and are the average of three to four experiments for each fluence rate ± se of the mean. A, White light response. B, White light response in the presence of a background of 100 μmol m-2 s-1 far-red light. Average dark aperture values: WT, 1.2 μm; and npq1, 1.2 μm.
Blue Light- and Phytochrome-Mediated Responses in Stomata from the phot1 phot2 Double Mutant
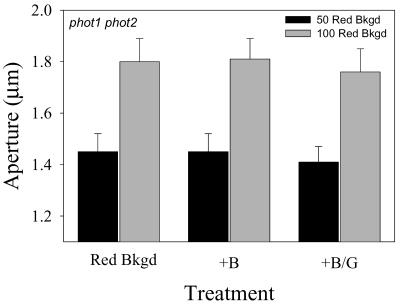
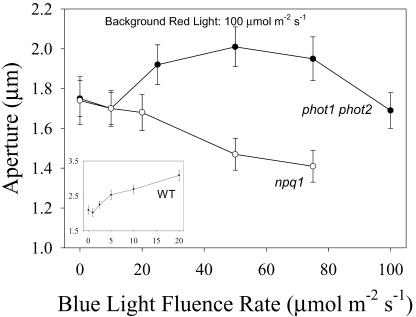
It has been reported recently that the stomata of the phot1 and phot2 mutants of Arabidopsis have a reduced blue light response, whereas the phot1 phot2 double mutant is completely devoid of a specific blue light response (Kinoshita et al., 2001). A detailed analysis of the blue light response of phot1 phot2 double mutant stomata showed no opening in response to 10 μmol m-2 s-1 blue light applied under 50 or 100 μmol m-2 s-1 red light background (Fig. 5). However, higher fluence rates of blue light applied under 100 μmol m-2 s-1 background red light stimulated a blue light response that saturated at around 50 μmol m-2 s-1 (Fig. 6). In contrast, npq1 stomata treated under the same conditions showed a progressive closing as a function of increasing fluence rates of blue light. In the absence of a specific blue light response, the closing observed with npq1 stomata mirrors that observed under high fluence rates of red light (Fig. 1B), suggesting that the applied fluence rates of blue light under the 100 μmol m-2 s-1 background red light were causing stomatal closing due to photo-inhibition. This indicates that the observed opening of stomata from the phot1 phot2 double mutant is an underestimate of the actual blue light response because of the simultaneous closing component of photosynthesis-dependent opening. The actual opening response to 50 μmol m-2 s-1 blue light can be estimated by adding the value of the expected decline in aperture from increasing photosynthetically active radiation from 100 to 150 μmol m-2 s-1 (Fig. 1) to the measured increase in aperture. Using this corrected value, the aperture increase of stomata of the phot1 phot2 double mutant in response to 50 μmol m-2 s-1 blue light is approximately equal to the aperture increase of WT stomata in response to 10 μmol m-2 s-1 blue light (Fig. 6).
Figure 5.
Apertures in stomata from the phot1 phot2 double mutant in response to 10 μmol m-2 s-1 blue light. Stomata in epidermal peels were treated with either 50 or 100 μmol m-2 s-1 background red light for 1.5 h (Red Bkgd). Ten micromoles per meter squared per second blue light (+B) or 10 μmol m-2 s-1 blue and 20 μmol m-2 s-1 green light (+B/G) were then added to the background red illumination for an additional 1 h. Aperture values are the average of four experiments.
Figure 6.
Blue light-stimulated opening in stomata from the phot1 phot2 double mutant and the npq1 mutant of Arabidopsis. Stomata in epidermal strips were treated with saturating background red light (100 μmol m-2 s-1) for 1.5 h. Data shown are final apertures after addition of the indicated fluence rate of blue light to the saturating red background for an additional 1 h. Values are the average of three experiments. The inset shows the blue light response of WT stomata.
Blue Light-Stimulated Opening in the phot1 phot2 Double Mutant Is Green Light Reversible
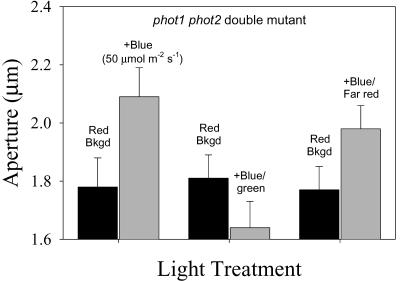
The important question of whether the blue light response found in stomata from the phot1 phot2 double mutant is a specific blue light response was addressed by testing its green light reversibility (Fig. 7). The aperture changes elicited by blue light (50 μmol m-2 s-1) applied under a 100 μmol m-2 s-1 red light background was fully reversed by simultaneous irradiation of 100 μmol m-2 s-1 green light, the typical 2:1 green/blue ratio established in previous studies. Parallel experiments measuring aperture changes in response to addition of 50 μmol m-2 s-1 red light plus 100 μmol m-2 s-1 green light showed that there was no significant closing caused by the addition of green light alone, ruling out a photo-inhibitory effect of green light (data not shown). In contrast, the blue light-stimulated opening was not altered by simultaneous irradiation with 100 μmol m-2 s-1 far-red light (Fig. 7). The green reversibility of the blue light-stimulated opening in stomata from the phot1 phot2 double mutant and its insensitivity to far-red light indicate that the blue light-opening is a specific blue light response.
Figure 7.
Green and far-red reversibility of blue light-stimulated opening in stomata of the phot1 phot2 double mutant. Stomata in epidermal strips were treated with saturating red light for 1.5 h (Red Bkgd). Fifty micromoles per meter squared per second blue (+Blue), 50 μmol m-2 s-1 blue and 100 μmol m-2 s-1 green (+Blue/green), or 50 μmol m-2 s-1 blue and 100 μmol m-2 s-1 far-red (+Blue/Far red) light was then added to the saturating red background for an additional 1 h. Aperture values are the average of four experiments.
DISCUSSION
Unambiguous identification of the photoreceptor mediating blue light-stimulated stomatal movements would significantly enhance our understanding of the sensory-transducing cascade mediating that important blue light response. The chloroplastic carotenoid, zeaxanthin, has been identified as a putative blue light photoreceptor in guard cells (Srivastava and Zeiger, 1995; Quiñones et al., 1996; Frechilla et al., 1999). Results showing that stomata from npq1, an Arabidopsis mutant that fails to accumulate zeaxanthin due to a defective violaxanthin de-epoxidase (Niyogi et al., 1998), lacks a specific blue light response have provided strong support for the zeaxanthin hypothesis (Frechilla et al., 1999).
That conclusion was challenged by two subsequent studies showing that npq1stomata opened when irradiated with blue light applied under a subsaturating red light background. However, it is not possible to clearly interpret those results because of the interactions between the specific blue light response of guard cells and guard cell photosynthesis, which is also stimulated by blue light. One of the goals of the present study was to obtain a detailed characterization of the light responses of npq1 stomata under blue and red light irradiation, which would allow an unambiguous interpretation of the photobiological properties of this mutant.
Responses of npq1 Stomata to Red Light
The dose response curve of red light-stimulated opening in npq1 saturated at about 100 μmol m-2 s-1 (Fig. 1). Stomata from WT and the phot1 phot2 double mutant showed identical saturation ranges. All three genotypes were grown at 50 μmol m-2 s-1 incandescent light, growth conditions that were designed to match those used in the Kinoshita et al. (2001) study. The red light dose required for saturation varies with growth conditions: Arabidopsis stomata from plants grown under 350 μmol m-2 s-1 white light saturated at 250 μmol m-2 s-1 (L.D. Talbott and E. Zeiger, unpublished data). The precise determination of fluence rates of red light that saturate the photosynthetic response in guard cells made it possible to measure the blue light responses of the mutants without interference from blue light-stimulated photosynthesis.
Responses of npq1 Stomata to Blue Light
WT stomata exhibited a clear opening response when irradiated with 10 μmol m-2 s-1 blue light under a saturating red light background (Fig. 2). As reported previously (Frechilla et al., 1999), npq1 stomata did not respond to blue light in the same conditions, indicating that they lack a specific blue light response.
Both npq1 and WT stomata opened in response to 10 μmol m-2 s-1 blue light applied under a 50 μmol m-2 s-1 red light background, as recently reported (Kinoshita et al., 2001). However, the green light reversal test showed that the blue light-stimulated opening observed with WT stomata was fully reversible by green light, whereas the response of the npq1 stomata was not (Fig. 2). This lack of green light reversibility of npq1 stomata provided further evidence that they lack a specific response to blue light.
Phytochrome-Mediated Stomatal Opening in npq1 Stomata
Data presented in this study clearly implicated phytochrome-mediated regulation of stomatal movements in the npq1 mutant. The blue light-stimulated opening measured at 50 μmol m-2 s-1 background red light was fully reversed by far-red light. Far-red light also reversed red light-stimulated opening, and the magnitude of the far-red light effect is inversely proportional to the fluence rate of background red light (Fig. 3). Green light stimulated a far-red light-reversible opening in a response similar to the reported green light-stimulated phototropism in oat and Arabidopsis (Mandoli and Briggs, 1981; Steinitz et al., 1985). Finally, a dose response curve for white light-stimulated opening was inhibited by far-red light in the low fluence rate portion of the curve (Fig. 4), indicating that phytochrome-mediated opening compensates for the lack of a specific blue light response in npq1 stomata. Neither WT stomata nor those of the phot1 phot2 double mutant showed any evidence of phytochrome action in stomatal regulation under any of these experimental conditions.
Despite many attempts toward its documentation, conclusive evidence for phytochrome involvement in stomatal movements has been elusive (Karlsson, 1988). Recent work with the achlorophyllous stomata from Paphiopedilum showed that phytochrome is involved in the stomatal regulation of this orchid genus (Talbott et al., 2002b). The phytochrome responses of orchid and npq1 stomata share several common features. In both cases, a far-red reversal of red light-stimulated opening at low fluence rates is inversely proportional to fluence rate of red light and cannot be observed under saturating red light fluences. Green light stimulates a far-red light-reversible opening in both cases. On the other hand, stomata of the two species differ in that npq1 stomata lack a specific blue light response and show a far-red-reversible, blue light-stimulated opening, whereas orchid stomata have a typical, green light-reversible blue light response that cannot be reversed by far-red light.
It is remarkable that phytochrome responses can be characterized readily in orchid and npq1 stomata and not in the WT. The fact that phytochrome is found in two anomalous stomatal phenotypes might suggest the presence of compensation mechanisms that deserve further investigation.
The Blue Light Response of phot1 phot2 Double Mutant Stomata
Reports indicating that the phot1 and phot2 single mutants show a reduced blue light response, combined with the severely impaired response in the double mutant (Kinoshita et al., 2001), suggested the possibility that stomata from the phot1 phot2 double mutant would exhibit a blue light response at higher fluence rates of blue light, as observed (Fig. 6). A similar but smaller opening can be observed in Figure 2C of Kinoshita et al. (2001), although the significance of the opening is obscured by the large range of apertures bracketed on the y axis. The larger magnitude of opening seen in Figure 6 is likely caused by a red light enhancement effect (Frechilla et al., 1999) stemming from the higher background red light used in the present study. The blue light response of the phot1 phot2 double mutant is distinctly different from that of npq1 stomata. The increasing fluence rates of blue light applied in a background of saturating red light caused stomatal closing in the npq1 stomata (Fig. 6) in a response matching that observed under high fluence rates of red light alone (Fig. 1). The responses of phot1 phot2 double mutant and npq1 stomata overlap in the 0 to 10 μmol m-2 s-1 range. A blue light response is activated in stomata from the phot1 phot2 double mutant at higher fluence rates, and the response saturates at around 50 μmol m-2 s-1 blue light. At higher fluence rates of blue light, the closing seen may be due to the inhibitory effects of high fluences of photosynthetically active radiation (Fig. 1).
The green light reversibility and far-red insensitivity of the stomatal response from the phot1 phot2 double mutant (Fig. 7) strongly indicate that the phot1 phot2 double mutant stomata exhibit a specific blue light response that requires higher fluence rates than WT stomata for its expression. These results indicate that phototropin has a regulatory role in the blue light response of guard cells, perhaps associated with the regulation of the Ser/Thr kinase activity (Kinoshita and Shimazaki, 1999). On the other hand, the absence of a blue light-specific response and the farred reversal of the opening observed under low light fluences (Figs. 2 and 6) clearly indicate that the zeaxanthin-less npq1 mutant is a true null function mutant for the specific blue light response of stomata. These data indicate that zeaxanthin plays a significant role in blue light photoreception in guard cells.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All experiments with WT plants used Arabidopsis ecotype Columbia. Seeds of the npq1-2 (Niyogi et al., 1998) mutant were a gift of Dr. Krishna Niyogi (University of California, Berkeley). Seeds of the phot1-5,phot2-1 double mutant were a gift of Dr. Winslow Briggs (Carnegie Institution of Washington, Stanford, CA). Seeds were given a 24-h cold treatment and a 1-h red light treatment, then planted in pots with commercial potting mix (Sunshine Mix No. 1, American Horticultural Supply, Camarillo, CA). Plants were grown in a Conviron E8 growth chamber (Conviron Inc., Asheville, NC) at 75% relative humidity in 16 h of light/8 h of dark at 22°C. Fluence rates of white light (fluorescent, GTE Sylvania F48T12/CW/VHO, Sylvania, Danvers, MA) in the growth chamber were 50 μmol m-2 s-1. Light levels were measured with a quantum sensor (LI-COR Inc., Lincoln, NE). Plants were fertilized once a week (20-10-20 mix, Grow-More Research and Manufacturing Co., Gardena, CA).
Measurement of Stomatal Aperture
Epidermal strips from dark-adapted plants were carefully detached by hand and placed in a solution of 20 mm KCl, 0.1 mm CaCl2, and 1 mm MES-NaOH (pH 6.0). The strips were briefly sonicated for 3 s at 50% power on a Branson Sonifier (model 250, Branson Ultrasonics Corporation, Danbury, CT) to remove air bubbles from the stomatal pores. The epidermal strips were then incubated in the dark for 30 min, after which sample strips were used to measure dark aperture levels and the remainder used for light treatment experiments.
Stomatal aperture was determined by selecting at least three epidermal strips for each treatment condition. Average aperture was determined from measurements of 30 to 40 digitized video images of abaxial stomata in the three epidermal peels using an Olympus BH-2 microscope (40× objective, 10× ocular, Olympus, Melville, NY) connected to a Javelin JE2362A digital imaging camera (Javelin Systems, Torrence, CA). Image processing was handled with an IBM PC-based MV-1 image analysis board (Metrabyte Corp., Taunton, MA) and JAVA image analysis software (Jandel Scientific, Corte Madera, CA). All experiments were repeated three to four times using plants from multiple plantings. Data presented are average aperture values over all replicates (a total of 120–160 aperture measurements per treatment). In light curve experiments (Figs. 1 and 4) involving large number of points collected over a period of several weeks, plotting change in aperture (average aperture after treatment minus average initial aperture) normalizes for natural variation in initial aperture.
Light Treatments
For fluence response curves, the epidermal strips were placed in a solution of 20 mm KCl, 0.1 mm CaCl2, and 1 mm MES-NaOH (pH 6.0) in small treatment dishes held in a circulating water bath at 23°C. Strips were illuminated with red or white light of the specified fluence rate for 1.5 h by means of a fiber optic, after which aperture was measured and compared with the value obtain for dark aperture.
For multibeam experiments, background red light was applied to the treatment dishes for 1.5 h, after which apertures were measured to determine values for red light-stimulated opening. Multibranched fiber optic cables were then used to deliver one or more additional beams of blue, red, green, or far-red light to the background red illumination. The additional light beam(s) were applied for 1 h, after which final aperture values were measured.
White light was provided by Dolan-Jenner fiber optic illuminators (Edmund Scientific, Barrington, NJ) using a halogen projector lamp (EKE, Ushio Inc., Tokyo). Red light was provided by a red filter (1A safelight filter, broadband, 50% cutoff, 620 nm, Eastman Kodak Co., Rochester, NY) using the fiber optic illuminator as the light source. Blue and green light were provided by a blue Plexiglas filter (No. 2424 Plexiglas, 470-nm maximum, half-bandwidth 100 nm, Rohm and Haas, Hayward, CA) and a green broad-band filter (50% cutoffs at 505 and 560 nm), respectively. Light of specific wavelengths (720 and 700 nm) was provided by interference filters (10- ± 2.5-nm bandwidth, Oriel Instruments, Stratford, CT). Far-red light was supplied by light emitting diodes having an emission maximum at 730 nm (gift of Dr. John Sager, Kennedy Space Center, FL). Light fluence rates were measured with a LI-COR quantum sensor (LI-COR Inc.) or in the case of far-red light, by a SKR110 far-red quantum sensor (Skye Instruments LTD, Llandrindad Wells, UK).
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.029587.
This work was supported by the National Science Foundation (grant no. DCB 8904254).
References
- Assmann SM (1993) Signal transduction in guard cells. Annu Rev Cell Biol 9: 345-375 [DOI] [PubMed] [Google Scholar]
- Eckert M, Kaldenhoff R (2000) Light-induced stomatal movement of selected Arabidopsis thaliana mutants. J Exp Bot 51: 1435-1442 [PubMed] [Google Scholar]
- Frechilla S, Talbott LD, Bogomolni RA, Zeiger E (2000) Reversal of blue light-stimulated stomatal opening by green light. Plant Cell Physiol 41: 171-176 [DOI] [PubMed] [Google Scholar]
- Frechilla S, Zhu J, Talbott LD, Zeiger E (1999) Stomata from npq1, a zeaxanthin-less Arabidopsis mutant, lack a specific response to blue light. Plant Cell Physiol 40: 949-954 [DOI] [PubMed] [Google Scholar]
- Karlsson PE (1986) Blue light regulation of stomata in wheat (Triticum aestivum) seedlings: II. Action spectrum and search for action dichroism. Physiol Plant 66: 207-210 [Google Scholar]
- Karlsson PE (1988) Phytochrome is not involved in the red-light-enhancement of the stomatal blue-light-response in wheat seedlings. Physiol Plant 74: 544-548 [Google Scholar]
- Kinoshita T, Doi M, Suetsuga N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656-660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548-5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasceve G, Leymarie J, Olney MA, Liscum E, Christie JM, Vavassuer A, Briggs WR (1999) Arabidopsis contains at least four independent blue-light-activated signal transduction pathways. Plant Physiol 120: 605-614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli DF, Briggs WR (1981) Phytochrome control of two low-irradiance responses in etiolated oat seedlings. Plant Physiol 67: 733-739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Bjorkman O (1998) Arabidopsis mutants define a central role for the zeaxanthin cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T (1981) Blue light response of stomata with starch-containing (Vicia faba) and starch-deficient (Allium cepa) guard cells under background illumination with red light. Plant Sci Lett 22: 103-108 [Google Scholar]
- Quiñones MA, Lu Z, Zeiger E (1996) Close correspondence between the action spectra for the blue light responses of the guard cell and coleoptile chloroplasts, and the spectra for blue light-dependent stomatal opening and coleoptile phototropism. Proc Nat Acad Sci USA 93: 2224-2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Zeiger E (1995) The inhibitor of zeaxanthin formation, dithiothreitol, inhibits blue light-stimulated stomatal opening in Vicia faba. Planta 196: 445-449 [Google Scholar]
- Steinitz B, Ren Z, Poff KL (1985) Blue and green light-induced phototropism in Arabidopsis thaliana and Lactuca sativa L. seedlings. Plant Physiol 77: 248-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Nikolova G, Ortiz A, Shmayevitch I, Zeiger E (2002a) Green light reversal of blue light-stimulated stomatal opening is found in a wide range of plant species. Am J Bot 89: 366-368 [DOI] [PubMed] [Google Scholar]
- Talbott LD, Zhu J, Han SW, Zeiger E (2002b) Phytochrome and blue light-mediated stomatal opening in the orchid, Paphiopedilum. Plant Cell Physiol 43: 639-646 [DOI] [PubMed] [Google Scholar]
- Tallman G (1992) The chemiosmotic model of stomatal opening revisited. Crit Rev Plant Sci 11: 35-57 [Google Scholar]
- Vierstra RD, Quail PH (1983) Purification and initial characterization of 124-kilodalton phytochrome from Avena. Biochemistry 22: 2498-2505 [Google Scholar]
- Zeiger E, Talbott, LD Frechilla, Srivastava A, Zhu J (2002) The guard cell chloroplast: a perspective for the twenty-first century. New Phytol 153: 415-424 [DOI] [PubMed] [Google Scholar]