Abstract
Study Design
SPARC-null mice were examined for behavioural signs of chronic low back and/or radicular pain.
Objective
To assess SPARC-null mice as a rodent model of chronic low back and/or radicular pain due to degenerative disc disease.
Summary of Background Data
Degeneration of intervertebral discs is a major cause of chronic low back and radicular pain in humans. Inactivation of the SPARC (Secreted Protein, Acidic and Rich in Cysteine, also known as osteonectin and BM-40) gene in mice results in premature intervertebral disc degeneration. The impact of disc degeneration on behavioural measures of chronic pain has not been evaluated in this model.
Methods
Cohorts of young and old (3 and 6-12 months, respectively) SPARC-null and wild-type control mice were screened for behavioural indices of low back and/or radiating pain. Sensitivity to mechanical, cold and heat stimuli, locomotor impairment, and movement-evoked hypersensitivity were determined. Animals were challenged with three analgesic agents with different mechanisms: morphine, dexamethasone, and gabapentin.
Results
SPARC-null mice showed signs of movement-evoked discomfort as early as 3 months of age. Hypersensitivity to cold stimuli on both the lower back and hindpaws developed with increasing age. SPARC-null mice had normal sensitivity to tactile and heat stimuli, and locomotor skills were not impaired. The hypersensitivity to cold was reversed by morphine, but not by dexamethasone or gabapentin.
Conclusion
SPARC-null mice display behavioural signs consistent with chronic low back and radicular pain that we attribute to intervertebral disc degeneration. We predict that the SPARC-null mouse is a useful model of chronic back pain due to degenerative disc disease.
Keywords: back pain, animal model, osteonectin, degenerative disc disease, BM-40, matricellular
Introduction
The American Pain Society estimates that 45% of the U.S. population seeks medical help for chronic pain at some point in their lives.1 Those affected include the 15% of North Americans with persistent back pain.2,3 A common cause of chronic low back pain (LBP) is degenerative disc disease (DDD). Whereas age-related disc degeneration is common in asymptomatic individuals, disc degeneration is also associated with low back and sciatic pain.4-6 Despite the significant impairment associated with this disease, there is currently no animal model that incorporates both the anatomical and functional consequences that characterize human DDD.7
SPARC (Secreted Protein, Acidic and Rich in Cysteine, also known as osteonectin and BM-40) is a matricellular protein important in tissue remodeling and response to injury.8 SPARC is detected in cells within both the annulus fibrosus and nucleus pulposus in human intervertebral discs (IVDs), and its expression is decreased as a function of aging and disc degeneration.9
Targeted deletion of the SPARC gene results in accelerated disc degeneration in the aging mouse.10 SPARC-null mice demonstrate signs of DDD as early as 2 months of age and, by the 2nd year of life, signs of extensive disc degeneration are observed. These signs include decreased proteoglycan content, cell loss, and irregular collagen fibrils. As a consequence, the discs cannot meet the structural demands placed upon them and disc herniation and spinal compression are observed.10 Given the severe disc degeneration and herniation observed in this model, the probability of associated sensory changes is high.6
The aim of this study was to assess the utility of the SPARC-null mouse as a model of LBP due to DDD. We hypothesized that SPARC-null mice would present with behavioural signs of hypersensitivity indicative of chronic back pain and that this phenotype would become increasingly severe with advancing age and degeneration.
Materials and Methods
Animals
The SPARC-null mice were developed on a mixed C57BL/6 × 129SVJ background.11 Because inbred mouse strains can have different pain behavioral responses, these SPARC-null mice have been backcrossed onto a standard C57BL/6 background for >12 generations and are considered to be fully congenic. We therefore used commercially available C57BL/6 mice (Charles River, QC) as wild-type (WT) controls as done previously.12
Two cohorts of male mice were used in this study. The young cohort was composed of 3-month old SPARC-null mice (n = 10) and age-matched WT mice (n = 9), both bred in-house. The old cohort was composed of a group of 9-month old SPARC-null animals bred at the Benaroya Research Institute and transported to McGill University (n = 5), and two groups of WT mice (6-month old, n = 9 and 12-month old retired breeders, n=9; Charles River, Québec). The use of the two WT control groups for the 9-month old SPARC-null mice was due to the lack of availability of age-matched animals. The SPARC-null mice (3-month=23.7± 0.4g; 9-month=27.4±0.7g) were slightly smaller than WT mice (3-month=26.0±0.7g; 6-month=33.6±0.6g; 12-month=34.4±1.0g).
All experiments were performed blind to genotype and treatment, were approved by the Animal Care Committee at McGill University, and conformed to ethical guidelines of the Canadian Council on Animal Care.
Behavioural screening for hypersensitivity to cutaneous mechanical, cold, and heat stimuli
Animals were placed individually in the test chamber for 60 min prior to testing. Animals were tested for only one modality per day to avoid interference between assays. All testing was conducted between 9:00 AM and 3:00 PM. For each modality tested, three body sites were assessed whenever possible: hindpaw, tail, and low back. The hindpaw and tail measures are commonly used in other animal models of chronic pain13, but to our knowledge we are the first to apply them to a model of disc degeneration. The measurement of cutaneous hypersensitivity on the skin of the low back was adapted from studies of referred visceral pain.14
Mechanical sensitivity
Hindpaw and Back
Calibrated von Frey Filaments (Stoelting Co., Wood Dale, IL) were applied for 4 sec or until withdrawal, and the 50% threshold to withdraw (grams) was calculated.15 The stimulus intensity ranged from 0.6-4.0g, corresponding to filament numbers (3.84, 4.08, 4.17, 4.31, 4.56). For each animal, the actual filaments used within the aforementioned series were determined based on the lowest filament to evoke a positive response followed by 5 consecutive stimulations using the up-down method. The filament range and average interval were then incorporated with the response pattern into each individual threshold calculation.15,16 Mechanical sensitivity was assessed on the plantar surface of the left hindpaw (response = flexion reflex) and on the bony structures of the L6-S1 lumbar spine (response = lordosis). The low back region was shaved one day prior to testing.
Cold sensitivity
Hindpaw and Back
Cold sensitivity was assessed by measurement of the total time spent in acetone-evoked behaviours over 1 minute after a drop (25 μl) of acetone was applied gently to the plantar surface of the left hindpaw (behaviours = paw elevation, flinching, biting, licking, and scratching time) or the low back region (behaviours = biting, licking, scratching, and checking time).
Tail
Cold sensitivity was assessed by the cold water (2°C) tail immersion assay. Half of the length of the tail was dipped into the cold water, and the latency to tail withdrawal was measured. A maximum cut-off of 30 sec was set to avoid tissue damage.
Heat sensitivity
Hindpaw
Heat sensitivity was assessed by the latency to withdrawal of the right hind paw from a thermal stimulus.17 Briefly, mice were placed in Plexiglas cages on top of a glass sheet. A thermal stimulus (IITC Life Science Inc., Woodland Hills, CA) was focused on the centre of the plantar surface of the hindpaw. Withdrawal latencies were measured 3 times at 10-minute intervals and the average was calculated. A cutoff of 17 sec was set to prevent tissue damage.
Tail
Heat sensitivity was assessed by recording the withdrawal latency to withdraw the tail in response to noxious heating. Briefly, tails were exposed to a focused beam of light (IITC Life Science Inc.), the withdrawal latency was measured was measured twice at 10-minute intervals and the average was calculated. A cutoff latency of 17 sec was set to prevent tissue damage.
Locomotor Capacity
Locomotor capacity was measured by the use of an accelerating rotarod (IITC Life Science Inc.) with the mouse adapter (rod diameter = 3.2 cm). The task includes a speed ramp from 0 to 30 rotations per minute over 60 sec, followed by an additional 240 sec at the maximal speed. Latency and rotation speed at fall were determined.
Movement-evoked Hypersensitivity with Stretching
Grip Force Assay
Mice grip a metal bar attached to a Grip Strength Meter (Stoelting Co., Wood Dale, IL) and are gently pulled back by the tail to exert a stretching force.18 The peak force in grams at the point of release was recorded twice at a 10-minute interval, and the average measurement was calculated. Although this assay has not been used previously to measure back pain, it has been validated in models of deep muscle inflammation and cancer pain.19,20
Tail Suspension Assay
Mice were individually suspended by the tail underneath a platform. Adhesive tape was used to attach the tail (0.5-1 cm from the base) to the platform and were videotaped for 180 sec. The duration of time spent in a) immobility (not moving but stretched out), b) rearing (trying to reach the underside of the platform), c) full extension (actively reaching for the floor), and d) self-supported (holding either the base of its tail or the tape), was analyzed by a blinded observer using digital software (Labspy®, Montreal, QC) over the entire testing period (Figure 3C). This assay is commonly used as a measure of depression in mice.21 To date, it has only been applied to chronic pain studies to assess depression in neuropathic mice and no differences were reported.22
Figure 3.
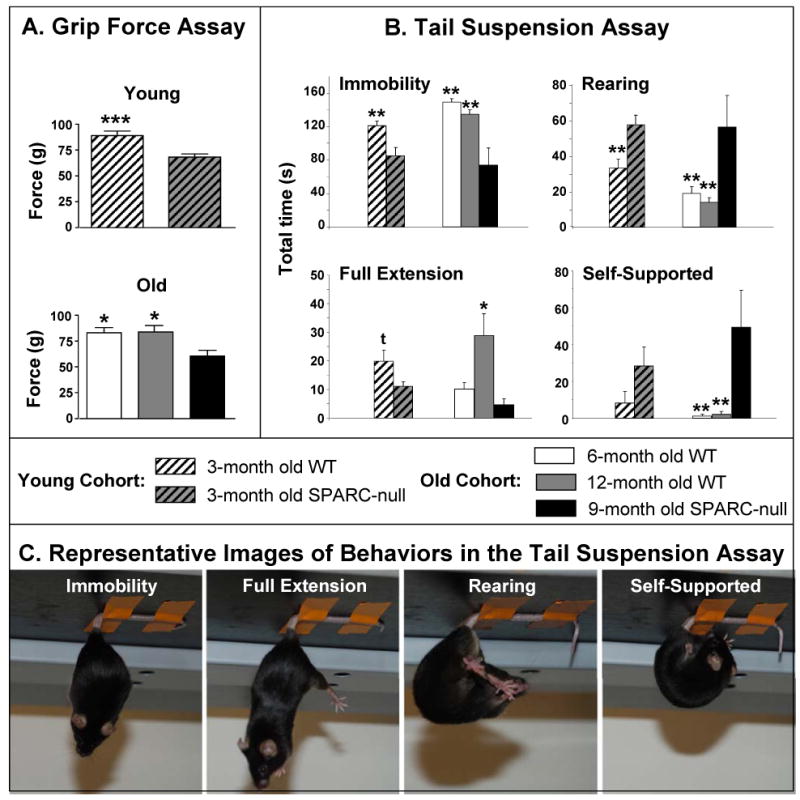
Movement-evoked discomfort in young and old SPARC-null and WT mice. (A) Grip force: SPARC-null animals display a lower resistive force than WT. (B) Tail suspension: SPARC-null mice spend less time immobile (gravity-induced stretching) and avoid this position by increased rearing and self-supported time. These differences increase with age. (C) Representative images of the postures observed in the tail suspension assay. t=p<0.1; *=p<0.05; **=p<0.01, ***=p<0.0001.
Pharmacological manipulation of cold sensitivity
Baseline measurements of cold sensitivity were determined as described above in the old cohort of SPARC-null mice (n=5) 1 month following completion of the behavioural screening. Animals were subsequently treated with drug or vehicle, and cold sensitivity was assessed 45, 90, 135, and 180 min post-treatment. After a wash-out period of at least 48h, the procedure was repeated with alternative treatments.
Drugs
Morphine (6 mg/kg in 2 ml/kg, intra-peritoneal (i.p.), Medisca Inc., Montreal, Quebec); Dexamethasone (3 mg/kg in 2 ml/kg, i.p., Sigma-Aldrich Canada Ltd., Oakville, Ontario); Gabapentin (300 mg/kg in 5 ml/kg, per os (p.o.), MUHC Pharmacy, Montreal, Quebec).
Vehicle controls
Saline solution (2 ml/kg, i.p. for morphine and dexamethasone; 5 ml/kg, p.o. for gabapentin).
Statistics
All data are plotted as mean ±S.E.M. For behavioural assays, measurements were analyzed by one-way ANOVA followed by a Dunnett test (old cohort), or by an unpaired t-test (young cohort). For the pharmacological treatments, each post-drug measure was normalized to the average pre-drug baseline and analyzed by paired t-test (one-tailed).
Results
Behavioural Indices of Low Back Pain
Cutaneous Stimulus-Evoked Hypersensitivity (Figure 1)
Figure 1.
Behavioural screening for sensitivity to cutaneous mechanical, cold, and heat stimuli in (A) young (3-month old WT (n=10) and SPARC-null (n=9)) and (B) old (6- and 12-month old WT (n=9/group) and 9-month old SPARC-null (n=5)) mice. * = p<0.05; ** = p<0.01
Mechanical, cold, and heat sensitivity were assessed in the young and old cohorts on the hindpaw, lower back, and/or tail. In the young cohort, no significant differences were observed between SPARC-null and WT animals in any assays (Figure 1A). In the old cohort, no differences were observed between SPARC-null and WT mice in sensitivity to mechanical (Figure 1B, top row) or heat stimuli (Figure 1B, bottom row) or in the cold-water tail immersion assay (Figure 1B, middle row).
In contrast, old SPARC-null mice exhibited a significantly longer duration of evoked behaviours in comparison to WT control mice in response to a drop of acetone applied to the hindpaw (F(79,23)=15.56, p<0.001) or lower back (F(25, 2)=6.087, p<0.01), a result indicative of hypersensitivity to cold.
Locomotor Capacity (Figure 2)
Figure 2.
Behavioural screening for motor impairment in the rotarod test in young (3-month old WT (n=10) and SPARC-null (n=9)) and old (6- and 12-month old WT (n=9/group) and 9-month old SPARC-null (n=5)) mice. No significant differences were observed.
Both young and old SPARC-null mice performed as well in the rotarod assay as their respective WT controls (Figure 2). Locomotor capacity is therefore intact, and locomotion does not induce discomfort in either young or aging SPARC-null animals during normal ambulation.
Movement-evoked Hypersensitivity with Stretching (Figure 3)
Grip Force Assay
Both young and old SPARC-null animals displayed significantly less resistive force to stretching than their WT controls (Figure 3A). In the young cohort, WT mice tolerated stretching with a greater average resistance (89.2±4.3 grams) upon release of the grid than the SPARC-null mice (68.3±3.1 grams; p<0.0001, unpaired t-test). Similarly, the 6- and 12-month old WT mice displayed resistive forces of 82.9±5.1 grams and 83.7±6.2 grams, respectively, whereas the 9-month old SPARC-null mice released at an average of 60.5±5.4 grams (F(999, 2)=4.027, p<0.05).
Tail Suspension Assay
Young and old SPARC-null mice presented different patterns of behaviour in comparison to their respective WT controls (Figure 3B). Both 3- and 9-month old SPARC-null animals remained immobile in a natural gravity-induced stretch for significantly less time (85.0±9.8 sec and 73.8±20.5 sec, respectively) than did 3-, 6- and 12-month old WT mice (121.2±5.8 sec, 149.6±3.9 sec, and 134.9±5.2 sec., respectively). In parallel, both 3- and 9-month old SPARC-null animals spent more time rearing (57.6±5.5 sec and 56.4±18.0 sec, respectively) compared to 3-, 6- and 12-month old WT mice (33.2±5.1 sec, 19.0±4.0 sec, 14.1±2.6 sec, respectively). Rearing is an attempt to catch the base of the tail to alleviate the weight-induced stretching of the spine. As a consequence, the time spent in the supported position (when the animal grabs the base of its tail or the adhesive tape) was negligible in 6- and 12- month old WT mice (1.2±1 sec, and 2.1±1.5 sec, respectively) but was atypically elevated in 9-month old SPARC-null mice (56.4±20.2 sec).
Pharmacological Manipulation of Cold Sensitivity (Figure 4)
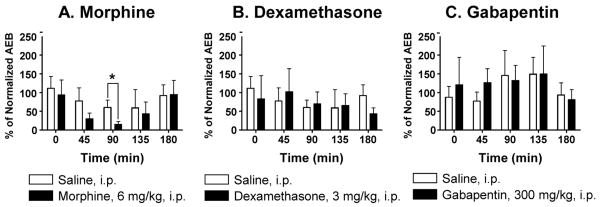
Figure 4.
SPARC-null mice were treated with saline or (A) morphine (6 mg/kg, i.p.), (B) dexamethasone (3 mg/kg, i.p.), or (C) gabapentin (300 mg/kg, p.o.), and the duration of acetone-evoked behaviour was assessed 45, 90, 135, and 180 min post-treatment. Data were normalized to the average pre-treatment baseline.
During the behavioural screening described above, SPARC-null mice displayed stretch-induced discomfort at both 3- and 9-months of age. In contrast, hypersensitivity to cold developed as a function of age. We therefore decided to use cold sensitivity in the hindpaw as our behavioural measure to test pharmacological sensitivity in aging SPARC-null mice one month after the behavioural screening.
First, baseline responses to acetone administered to the plantar surface of the hindpaw were re-assessed in 7- and 13- month old WT and 10-month old SPARC-null animals; total time spent in acetone-evoked behaviours were 1.35±0.25 sec, 1.62±0.38 sec, 5.194±1.07 sec, respectively. Since WT mice did not display a sufficient behavioural response to allow detection of pharmacological inhibition, only 10-month old SPARC-null animals were included in the study.
Ninety minutes after an intra-peritoneal injection of morphine (6 mg/kg, Figure 4A), acetone-evoked behaviours were significantly attenuated relative to saline-treated controls (morphine: = 16.2±6.2% of baseline; saline: = 61.2±16.9% of baseline; p=0.0346, paired t-test, one-tail). After systemic treatment with dexamethasone (3mg/kg, i.p., Figure 4B) or gabapentin (300 mg/kg, p.o., Figure 4C), acetone-evoked behaviours were not different from saline at any of the time-points tested.
Discussion
One of the primary causes of chronic low back and/or radicular pain is Degenerative Disc Disease. There are numerous animal models of DDD. In these models the degeneration is naturally occurring (e.g., the Desert Sand Rat),23 induced by injury to the disc (e.g., stabbing),24,25 initiated by chemical mediators that produce inflammation,26,27 or due to genetic inactivation of a protein important to disc integrity (e.g., the SPARC-null mouse).10,28 Despite the availability of these animal models, there is a lack of data relating disc degeneration to behavioural signs of pain and disability. An animal model that incorporates both anatomical and functional components of the disease will allow us to relate disc pathology directly to chronic low back and/or radicular pain and disability. We have confirmed the previously reported incidence of disc degeneration in SPARC-null mice by histological and x-ray image analysis. For example, signs of degeneration including wedging and loss of negatively charged proteoglycans are observed in lumbar IVDs by 6-months of age in SPARC-null but not WT mice (data not shown).10 The objective of the current study was to assess the utility of SPARC-null mice as a rodent model of chronic low back and/or radicular pain due to DDD.
Behavioural Phenotype of Aging SPARC-null Mice
Whereas young SPARC-null animals have normal cutaneous mechanical, cold, and heat sensitivity, significant cold hypersensitivity develops with increasing age in both the hindpaw and the lower back region (Figure 1). The plantar cold hypersensitivity could be reversed by systemic treatment with morphine, but not with dexamethasone or gabapentin (Figure 4). That motor ability is not impaired in SPARC-null animals supports the absence of generalized nervous system dysfunction (Figure 2). Finally, SPARC-null animals are reluctant to stretch in two different behavioural assays: they tolerate less stretch-induced force in the grip force assay and present with an atypical behavioural strategy in the tail suspension assay (Figure 3). Specifically, they actively avoid the natural gravity-induced stretching of the spine by increasing the time spent rearing and/or holding the base of the tail.
Cold Allodynia in SPARC-null Mice
In the present study, SPARC-null mice developed cold allodynia on the plantar surface of the hindpaw and on the lumbar skin as a function of age, indicative of referred hypersensitivity.29 The presence of cold but not mechanical or heat hypersensitivity in the current study differs from models of radicular pain following i) nerve compression,30-32 ii) exposure of nerve to nucleus pulposus33 and iii) nerve root inflammation,34,35 in which mechanical hypersensitivity is typically observed. Our model differs from the aforementioned in that it is not initiated by acute injury or inflammation to the nerve. It was recently shown that intrathecal administration of an inflammatory mediator, complement C5, evoked cold allodynia in the absence of mechanical hypersensitivity.36 Inflammatory mediators released as a consequence of disc degeneration could therefore theoretically produce cold allodynia in the absence of tactile changes.
The allodynia observed in SPARC-null mice is consistent with the human condition in which individuals experience coldness, radiating pain, and cold allodynia down one or both legs.37-39
Stretch-Induced Discomfort in SPARC-null Mice
The tail suspension assay is typically used in models of depression.21 In the current study, SPARC-null mice decreased the time spent in immobility and increased the time spent rearing and/or holding the base of the tail. This difference is not likely to be related to the slightly greater body mass of the WT mice; the increased tension on the spine of heavier animals would result in enhanced escape behaviors in WT which was not observed. Rather, we interpret this altered behavioural pattern as the avoidance of gravity-induced stretching of the spine in SPARC-null mice. To our knowledge, this study is the first to apply the tail suspension assay in the context of nociception in mice.
SPARC-null mice were impaired in the grip force assay, a phenotype observed during deep tissue pain in mice.18 It is unlikely that this deficit is due to motor impairment because SPARC-null animals exhibited i) normal reflexes in response to mechanical and heat stimuli (Figure 1), ii) increased activity during the tail suspension task (Figure 3), and iii) intact locomotor capacity (Figure 2).
The results from the grip test assay and the tail suspension task indicate that SPARC-null mice experience significant stretch-induced discomfort suggestive of axial low back pain as early as 3 months of age. At this age, SPARC-null animals show signs of IVD degeneration but not herniation.10 We propose that disc abnormalities drive the stretching-induced discomfort and hypothesize that the increased difference between aging SPARC-null and WT mice reflects the degree of degeneration. Furthermore, we propose that sensitivity to stretching will have predictive value for future drug screening, because patients affected by DDD also complain of lumbar stiffness.40 Validation of these hypotheses will require further studies, for example, extended behavioural characterization of animals (1-24 months of age), systematic assessment of the severity of DDD and its correlation to stretch-induced discomfort, and pharmacological studies.
Pharmacological Manipulation of Cold Allodynia in SPARC-null Mice
In individual patients suffering from low back and/or radicular pain due to DDD, morphine and gabapentin exhibit some analgesic efficacy.41 In contrast, systemic glucocorticoids such as dexamethasone are not more effective than placebo for the treatment of sciatica.42
In our study, morphine was the only treatment that reversed referred cold allodynia in 10-month old SPARC-null mice. This reversal was not due to sedation because higher doses are typically required to induce sedation43 and the current dose did not impair locomotor capacity in SPARC-null mice (data not shown). The ineffectiveness of gabapentin was surprising. Either the nerve injury might have been too severe at 10 months for the cold allodynia to be reversed by gabapentin, or the dose was insufficient. Future studies investigating these possibilities are required. The failure of dexamethasone to reverse referred nerve-injury induced pain is consistent with its lack of efficacy in patients42 and in animal studies of neuropathic pain.44
Conclusion
This study reports a behavioural phenotype in SPARC-null mice suggestive of chronic low back and radicular pain that is attenuated by treatment with morphine. These behavioural changes are likely a consequence of the premature disc degeneration characteristic of these mice. The lack of mechanical hypersensitivity and motor impairment supports the absence of generalized sensory nervous system dysfunction. Furthermore, none of the other characteristics described in the extensive literature on these mice explains the currently reported symptoms.
The existence of a model of disc degeneration in rodents that incorporates both disc pathology and behavioural indices of chronic pain and disability will provide a platform for studies addressing the relationships between anatomical abnormalities and functional changes in vivo. Furthermore, this model will enable us to test potential therapeutic interventions in the context of both disc degeneration and chronic pain. We propose that the SPARC-null mouse is an important animal model of chronic pain due to DDD.
Key Points.
Degeneration of intervertebral discs is a major cause of chronic low back and radicular pain in humans.
Inactivation of the SPARC gene in mice results in premature intervertebral disc degeneration.
Screening of SPARC-null mice in a battery of nociceptive assays revealed behavioural signs suggestive of low back pain including movement-evoked discomfort and hypersensitivity to cold.
The hypersensitivity to cold was reversed by morphine.
The SPARC-null mouse is a useful animal model of chronic pain due to degenerative disc disease.
Acknowledgments
The authors thank the Alan Edwards Centre for Research on Pain for access to facilities and equipment and Ms. Leigh MacIntyre and Ms. Lina Naso for technical support. This work was funded by a New Faculty Award to LSS from McGill University, CIHR/CPS/AstraZeneca Young Investigator Grant (MOP-86691) to LSS, FRSQ Bourse de chercheur-boursier to LSS, an American Pain Society Future Leaders in Pain Research Award to MM, and National Institutes of Health grant GM-40711 to EHS. All experiments were approved by the Animal Care Committee at McGill University and conformed to ethical guidelines of the Canadian Council on Animal Care.
Contributor Information
Magali Millecamps, McGill University, Alan Edwards Centre for Research on Pain, Faculty of Dentistry, Montreal, QC Canada.
Maral Tajerian, McGill University, Alan Edwards Centre for Research on Pain, Department of Neurological Sciences, Montreal, QC Canada.
E. Helene Sage, Hope Heart Program, Benaroya Research Institute at Virginia Mason, Seattle, WA, Department of Biological Structure, University of Washington School of Medicine, Seattle, WA, Department of Genitourinary Oncology, M.D. Anderson Cancer Center, Univ. of Texas, Houston, TX.
Laura S Stone, McGill University, Alan Edwards Centre for Research on Pain, Department of Biomedical Sciences, Faculty of Dentistry, Department of Pharmacology and Toxicology, Faculty of Medicine, Montreal, QC Canada.
References
- 1.APS. Chronic Pain in America: Roadblocks to Relief. 1999 [Google Scholar]
- 2.Rapoport J, Jacobs P, Bell NR, et al. Refining the measurement of the economic burden of chronic diseases in Canada. Chronic Diseases in Canada. 2004;25 [PubMed] [Google Scholar]
- 3.Conrad DA, Holland J, Liu J. Cost of low back pain problems: an economic analysis. In: Weinstein JN, Gordon SL, editors. Low Back Pain: A Scientific Overview. Rosemont, IL: American Academy of Orthopedic Surgeons; 1996. [Google Scholar]
- 4.Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 5.Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–8. [PubMed] [Google Scholar]
- 6.Luoma K, Riihimaki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–92. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 7.Singh K, Masuda K, An HS. Animal models for human disc degeneration. Spine J. 2005;5:267S–79S. doi: 10.1016/j.spinee.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–54. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruber HE, Ingram JA, Leslie K, et al. Cellular, but not matrix, immunolocalization of SPARC in the human intervertebral disc: decreasing localization with aging and disc degeneration. Spine. 2004;29:2223–8. doi: 10.1097/01.brs.0000142225.07927.29. [DOI] [PubMed] [Google Scholar]
- 10.Gruber HE, Sage EH, Norton HJ, et al. Targeted deletion of the SPARC gene accelerates disc degeneration in the aging mouse. J Histochem Cytochem. 2005;53:1131–8. doi: 10.1369/jhc.5A6687.2005. [DOI] [PubMed] [Google Scholar]
- 11.Norose K, Clark JI, Syed NA, et al. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci. 1998;39:2674–80. [PubMed] [Google Scholar]
- 12.Brekken RA, Puolakkainen P, Graves DC, et al. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest. 2003;111:487–95. doi: 10.1172/JCI16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong Y, Holden JE. Commonly used preclinical models of pain. West J Nurs Res. 2008;30:350–64. doi: 10.1177/0193945907304439. [DOI] [PubMed] [Google Scholar]
- 14.Millecamps M, Etienne M, Jourdan D, et al. Decrease in non-selective, non-sustained attention induced by a chronic visceral inflammatory state as a new pain evaluation in rats. Pain. 2004;109:214–24. doi: 10.1016/j.pain.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 16.Millecamps M, Centeno MV, Berra HH, et al. D-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain. 2007;132:108–23. doi: 10.1016/j.pain.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 18.Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–43. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- 19.Wacnik PW, Kehl LJ, Trempe TM, et al. Tumor implantation in mouse humerus evokes movement-related hyperalgesia exceeding that evoked by intramuscular carrageenan. Pain. 2003;101:175–86. doi: 10.1016/s0304-3959(02)00312-3. [DOI] [PubMed] [Google Scholar]
- 20.Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–43. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- 21.Steru L, Chermat R, Thierry B, et al. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 22.Hasnie FS, Wallace VC, Hefner K, et al. Mechanical and cold hypersensitivity in nerve-injured C57BL/6J mice is not associated with fear-avoidance- and depression-related behaviour. Br J Anaesth. 2007;98:816–22. doi: 10.1093/bja/aem087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruber HE, Johnson T, Norton HJ, et al. The sand rat model for disc degeneration: radiologic characterization of age-related changes: cross-sectional and prospective analyses. Spine. 2002;27:230–4. doi: 10.1097/00007632-200202010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Rousseau MA, Ulrich JA, Bass EC, et al. Stab incision for inducing intervertebral disc degeneration in the rat. Spine. 2007;32:17–24. doi: 10.1097/01.brs.0000251013.07656.45. [DOI] [PubMed] [Google Scholar]
- 25.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 26.Aoki Y, Ohtori S, Ino H, et al. Disc inflammation potentially promotes axonal regeneration of dorsal root ganglion neurons innervating lumbar intervertebral disc in rats. Spine. 2004;29:2621–6. doi: 10.1097/01.brs.0000146051.11574.b4. [DOI] [PubMed] [Google Scholar]
- 27.Aoki Y, Ohtori S, Takahashi K, et al. Innervation of the lumbar intervertebral disc by nerve growth factor-dependent neurons related to inflammatory pain. Spine. 2004;29:1077–81. doi: 10.1097/00007632-200405150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Boyd LM, Richardson WJ, Allen KD, et al. Early-onset degeneration of the intervertebral disc and vertebral end plate in mice deficient in type IX collagen. Arthritis Rheum. 2008;58:164–71. doi: 10.1002/art.23231. [DOI] [PubMed] [Google Scholar]
- 29.Walczak JS, Beaulieu P. Comparison of three models of neuropathic pain in mice using a new method to assess cold allodynia: the double plate technique. Neurosci Lett. 2006;399:240–4. doi: 10.1016/j.neulet.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 30.Bennett GJ, Xie YK. A peripheral neuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Fink DJ, Mata M. Vector-mediated gene transfer to express inhibitory neurotransmitters in dorsal root ganglion reduces pain in a rodent model of lumbar radiculopathy. Spine. 2006;31:1555–8. doi: 10.1097/01.brs.0000222060.88919.58. [DOI] [PubMed] [Google Scholar]
- 32.LaCroix-Fralish ML, Rutkowski MD, Weinstein JN, et al. The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. Spine. 2005;30:1821–7. doi: 10.1097/01.brs.0000174122.63291.38. [DOI] [PubMed] [Google Scholar]
- 33.Cuellar JM, Montesano PX, Carstens E. Role of TNF-alpha in sensitization of nociceptive dorsal horn neurons induced by application of nucleus pulposus to L5 dorsal root ganglion in rats. Pain. 2004;110:578–87. doi: 10.1016/j.pain.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Amaya F, Samad TA, Barrett L, et al. Periganglionic inflammation elicits a distally radiating pain hypersensitivity by promoting COX-2 induction in the dorsal root ganglion. Pain. 2009;142:59–67. doi: 10.1016/j.pain.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eliav E, Herzberg U, Ruda MA, et al. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. Pain. 1999;83:169–82. doi: 10.1016/s0304-3959(99)00102-5. [DOI] [PubMed] [Google Scholar]
- 36.Griffin RS, Costigan M, Brenner GJ, et al. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci. 2007;27:8699–708. doi: 10.1523/JNEUROSCI.2018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 38.Lindholm RV, Myllyla T, Sarvaranta J. The cold foot symptom in sciatica. A clinical and thermographic study. Ann Chir Gynaecol. 1981;70:176–81. [PubMed] [Google Scholar]
- 39.Nygaard OP, Mellgren SI. The function of sensory nerve fibers in lumbar radiculopathy. Use of quantitative sensory testing in the exploration of different populations of nerve fibers and dermatomes. Spine. 1998;23:348–52. doi: 10.1097/00007632-199802010-00012. discussion 53. [DOI] [PubMed] [Google Scholar]
- 40.Borenstein DG. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheumatol. 2000;12:143–9. doi: 10.1097/00002281-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Gilron I, Bailey JM, Tu D, et al. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:1324–34. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 42.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478–91. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 43.Sukhotina IA, Bespalov AY. Effects of the NMDA receptor channel blockers memantine and MRZ 2/579 on morphine withdrawal-facilitated aggression in mice. Psychopharmacology (Berl) 2000;149:345–50. doi: 10.1007/s002130000386. [DOI] [PubMed] [Google Scholar]
- 44.Rodrigues-Filho R, Campos MM, Ferreira J, et al. Pharmacological characterisation of the rat brachial plexus avulsion model of neuropathic pain. Brain Res. 2004;1018:159–70. doi: 10.1016/j.brainres.2004.05.058. [DOI] [PubMed] [Google Scholar]