Abstract
Hepatocyte growth factor (HGF) and its receptor Met are responsible for a wide variety of cellular responses, both physiologically during embryo development and tissue homeostasis, and pathologically, particularly during tumor growth and dissemination. In cancer, Met can act as an oncogene on tumor cells, as well as a pro-angiogenic factor activating endothelial cells and inducing new vessel formation. Molecules interfering with Met activity could be valuable therapeutic agents. Here we have investigated the antiangiogenic properties of a synthetic peptide mimicking the docking site of the Met carboxyl-terminal tail, which was delivered into the cells by fusion with the internalization sequences from Antennapedia or HIV-Tat. We showed that these peptides inhibit ligand-dependent endothelial cell proliferation, motility, invasiveness and morphogenesis in vitro to an even greater extent and with much less toxicity than the Met inhibitor PHA-665752, which correlated with interference of HGF-dependent downstream signaling. In vivo, the peptides inhibited HGF-induced angiogenesis in the matrigel sponge assay and impaired xenograft tumor growth and vascularization in Kaposi's sarcoma. These data show that interference with the Met receptor intracellular sequence impairs HGF-induced angiogenesis, suggesting the use of antidocking site compounds as a therapeutic strategy to counteract angiogenesis in cancer as well as in other diseases.
Keywords: Met, cell-penetrating peptides, Antennapedia, Tat, angiogenesis, cancer
Introduction
Angiogenesis contributes to cancer initiation and progression and to several other pathologies, such as chronic inflammation, retinopathies and arthritis (Carmeliet and Jain, 2000). The formation of a tumor-associated vasculature, a process referred to as tumor angiogenesis, is essential for cancer progression and metastasis and has become a clinical target for anticancer therapy. Current clinical approaches all focus on blocking a common hypoxia-associated angiogenic factor, vascular endothelial growth factor (VEGF), by either sequestering the factor itself or inhibiting its receptors. Although effective in slowing tumor progression, tumor escape through a variety of mechanisms, including up-regulation of other angiogenic growth factors, is frequently encountered (Loges et al., 2009).
The receptor for the human hepatocyte growth factor (HGF), encoded by MET oncogene, has an important physiological role in a complex morphogenetic program known as invasive growth, occurring both during embryonic development and during adult life. In pathological conditions, unregulated HGF-Met signaling can trigger tumor growth and metastasis (Boccaccio and Comoglio, 2006). Met is predominantly expressed by epithelial cells (Di Renzo et al., 1991; Prat et al., 1991), although it is also expressed in other cell types, including endothelial (Bussolino et al., 1992), myoblasts (Anastasi et al., 1997), hematopoietic (Nishino et al., 1995) and neuronal cells (Ebens et al., 1996). HGF binding triggers receptor activation through dimerization and auto/trans-phosphorylation of tyrosine residues. Two tyrosine residues within the catalytic loop (Y1234, Y1235) are first to be phosphorylated, leading to full kinase activation (Longati et al., 1994). Subsequently, two other tyrosine residues (Y1349, Y1356) in the carboxyl-terminal tail are phosphorylated. These are embedded in a multifunctional consensus sequence (Ponzetto et al., 1994), acting as a docking site for different SH2-containing transducers and adaptors involved in several pathways, including the GRB2–SOS–RAS–RAF–MEK–ERK (extracellular signal-regulated protein kinase) pathway, the phosphoinositide 3-kinase–AKT cascade, SRC, STAT3, SHC, PLC-γ, cbl, Gab1 and Rho-like GTPases such as RAC1 (Birchmeier et al., 2003; Lock et al., 2003).
Different strategies to target Met have the aim of inhibiting tumor growth and metastatic dissemination (Comoglio et al., 2008). Met can be blocked by small-molecule inhibitors, early examples for which are K252a (Morotti et al., 2002), SU11274 (Berthou et al., 2004) and PHA-665752 (Christensen et al., 2003), all of which compete for ATP binding to block Met catalytic activity. Several of these molecules (PF-2341066, XL880, ARQ197, MK2461, MP470, SGX523 and JNJ38877605) are in phase I/II clinical trials (Zou et al., 2007; Comoglio et al., 2008). Owing to the way in which they were selected and the inhibitory mechanism with which they operate, none of these drugs shows absolute specificity for Met, but also target other kinases, mostly receptor tyrosine kinases (Comoglio et al., 2008). Higher levels of specificity in Met inhibition can be obtained using biological molecules, either natural or designed expressly on the basis of the structure of HGF or Met. They act as antagonists that interfere in the ligand/receptor interaction, which normally leads to receptor dimerization and subsequent activation. HGF can be antagonized by its shorter forms: NK2—a natural fragment of HGF, or NK4—a truncated form of HGF, or by its uncleavable form, all of which bind the receptor but cannot fully activate it (Chan et al., 1991; Matsumoto and Nakamura, 2003; Mazzone et al., 2004). Similarly, anti-HGF-neutralizing antibodies act by subtracting functional HGF in the microenvironment (Cao et al., 2001), some of which are in pre-clinical or phase II clinical trials (Kim et al., 2006; Jun et al., 2007), whereas decoy Met, a soluble truncated Met extracellular receptor domain, both sequesters the ligand and impairs receptor dimerization (Michieli et al., 2004). The main limit of using HGF inhibitors is that they only inhibit HGF-dependent receptor activation. Few antagonist antireceptor monoclonal antibodies are available; these antibodies tend to have agonistic rather than antagonistic properties due to their bivalent structure (Prat et al., 1998). One promising monoclonal antibody, DN-30, is able to downregulate Met and inhibit tumor growth in experimental models (Vigna et al., 2008). Gene silencing based on antisense, ribozyme and RNA interference techniques have been used experimentally to silence Met and HGF expression (Paranjpe et al., 2007; Corso et al., 2008; Chu et al., 2009).
Finally, peptides interfering with the association of signal transducers to the Met receptor docking site could block the propagation of Met signaling. Previous studies have shown that a peptide derived from the carboxyl-terminal tail of Met specifically inhibited kinase activity and HGF-induced invasive growth in normal and transformed epithelial cells in vitro (Bardelli et al., 1999).
On this basis, we investigated whether synthetic Met peptides could interfere with angiogenesis in vitro and in vivo. The peptide was delivered into cells by fusion with the internalization sequences from either the Antennapedia homeodomain (Antp) or HIV-Tat previously shown to translocate peptides efficiently across the plasma membrane (Deshayes et al., 2005; Gupta et al., 2005; Foged and Nielsen, 2008; Morris et al., 2008; Stewart et al., 2008).
We show that micromolar concentrations of these peptides specifically inhibited HGF-induced in vitro migration, invasion and morphogenesis of endothelial cells, interfering with downstream signaling by HGF. Comparison with the inhibitor PHA-665752 showed that the peptides exerted greater inhibitory effects, yet no toxicity, whereas PHA-665752 showed substantial toxicity. In vivo, the peptides impaired HGF-dependent angiogenesis and significantly inhibited the growth of Kaposi's sarcoma xenograft, as well as vascularization. These data suggest a possible pharmacological mechanism to interfere with Met-mediated angiogenesis.
Results
Cell internalization of the Met docking site peptide
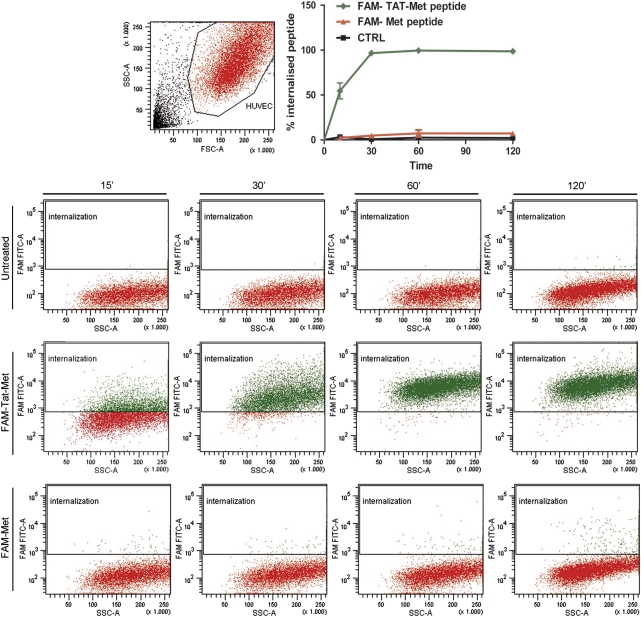
The plasma membrane, which limits cell internalization of most proteins and peptides, can be crossed by cell-penetrating peptides, such as Tat and Antp, which can be used to deliver peptides into living cells. We verified efficient peptide internalization by incubating 5-(and-6)-carboxyfluorescein (FAM)-conjugated Tat-Met peptide (10 μ) with human umbilical vein endothelial cells (HUVEC) and monitoring fluorescence with flow cytofluorimetry. Peptide uptake was time dependent, with more than 50% of the cells being fluorescent after as little as 10 min and nearly 100% after 30 min (Figure 1). An FAM-conjugated Met peptide lacking the Tat cell-penetrating peptide motif did not cross the cell membrane even after extensive incubation, showing levels of fluorescence similar to those of control-untreated HUVEC.
Figure 1.
The Tat-Met peptide enters into HUVECs. Tat-Met was conjugated at the N-terminus with FAM. HUVECs (1 × 105) were incubated for different periods of time with 10 μ peptide, washed twice and analyzed by fluorescence-activated cell sorting. Labeled Met peptide devoid of the cell-penetrating peptide motif and untreated cells (CTRL) were used as controls. In the lower panels, representative dot plots of peptide internalization percentage are shown.
Met docking site peptides inhibit HUVEC proliferation
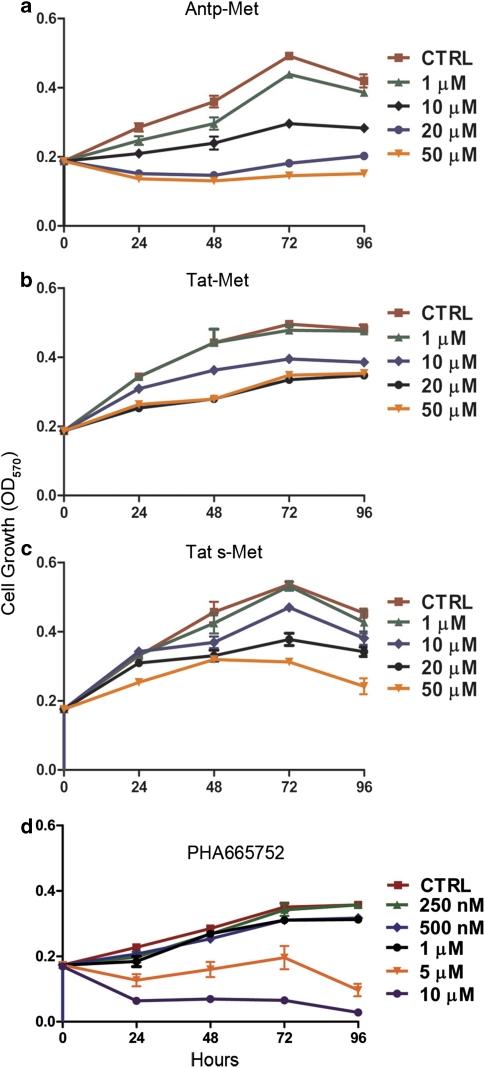
The cell-penetrating Antp-Met and Tat-Met peptides were tested for their ability to interfere with HGF-induced proliferation of HUVEC and compared with PHA-665752. Significant inhibition of cell growth in MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays was observed at doses of 10 μ Antp-Met already after 24 h of treatment (Figure 2a). Higher concentrations of the Antp-Met completely abolished cell proliferation. Tat-Met and Tat s-Met (containing a shorter Tat peptide), used at 10 μ concentrations, showed a significant reduction of cell proliferation only after 48 h (Figures 2b and c). At higher concentrations (20 and 50 μ) they displayed a cytostatic activity starting from 24 h of incubation. Lower concentrations of peptides (1 μ) did not have any effect even in longer treatments.
Figure 2.
The Met docking site peptide inhibits HUVEC proliferation (MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)assay). HUVECs were plated at low density, and then starved in low serum. Cell proliferation was evaluated at fixed times after the addition of HGF (50 ng/ml) +/− Antp-Met (a), Tat-Met (b), Tat s-Met (c) or PHA-665752 (d) at different concentrations in 2% FBS-containing medium. The inhibitory effect of Antp-Met (10 μ) was already significant after 24 h, whereas the inhibitory effect of Tat-Met or Tat s-Met peptides were significant only after 48 h and when 20 μ concentrations were used. PHA-665752 showed a marked reduction in cell numbers starting from 24 h at micromolar concentrations (5 and 10 μ), whereas lower concentrations did not exert a significant reduction of proliferation with respect to the control. Means±s.e.m. are shown (P<0.05).
In contrast, we observed a marked inhibitory effect starting from the 24-h time point when we exposed endothelial cells to micromolar concentrations (5 and 10 μ) of PHA-665752 (Figure 2d). Lower PHA-665752 concentrations did not exert any significant reduction in proliferation with respect to the control.
These data show that the internalized Met peptides inhibited cell proliferation; the Antp-coupled Met peptide appears to have a stronger inhibitory activity, whereas PHA-665752 required relatively high concentrations to attain comparable effects.
Met docking site peptides do not exert toxicity or induce apoptosis in endothelial cells
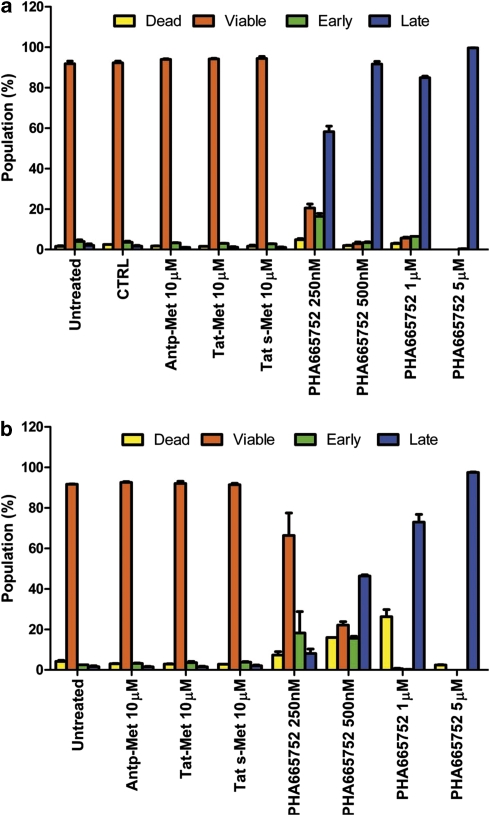
The possibility that the Met inhibitors used could exert toxicity or induce endothelial cell apoptosis was evaluated by cytofluorimetric analysis. After only 2 h of treatment of endothelial cells in the presence of HGF with 250 n PHA-665752, approximately 60% of the cells (Figure 3a) were already undergoing apoptosis (both Annexin V and 7-amino-actinomycin D positive). In contrast, cells treated with the peptides, even at highest concentrations, showed a high percentage (about 95%) of viable endothelial cells and no apoptosis (Figure 3a). The toxicity of PHA-665752 was observed even in the presence of serum (fetal bovine serum (FBS)) with clear dose-dependent effects (Figure 3b). These data suggest that the growth inhibition exerted by PHA-665752 may be due to cytotoxicity and apoptosis.
Figure 3.
Met docking site peptides do not induce toxicity or apoptosis in endothelial cells. In the apoptosis assay, HUVECs were plated onto six-well plates and were treated with either the Antp-Met peptide (10 μ), Tat-Met peptide (10 μ) or Tat s-Met peptide (10 μ) or with increasing concentrations of PHA-665752 (250 and 500 n, and 1 and 5 μ). Vehicle (dimethyl sulfoxide) and growth medium alone were used as controls, and HGF 50 ng/ml (panel a) or 10% FBS (panel b) were added to the growth medium. After 2 h, cells were recovered, washed and stained with two markers, Annexin V and 7-amino-actinomycin D, used to detect viable, dead or cells undergoing apoptosis. After only 2 h, 250 n PHA-665752 induced late apoptosis in about 60% of endothelial cells in HGF-containing media (a); on the contrary, 10 μ peptides showed an high percentage of viable endothelial cells (about 95% of the population; a). The toxicity of PHA-665752 was marked both in the presence of HGF (a) or FBS (b). The experiment was performed three times and each condition was in triplicate.
The Met docking site peptide specifically inhibits HGF-dependent HUVEC migration and invasion
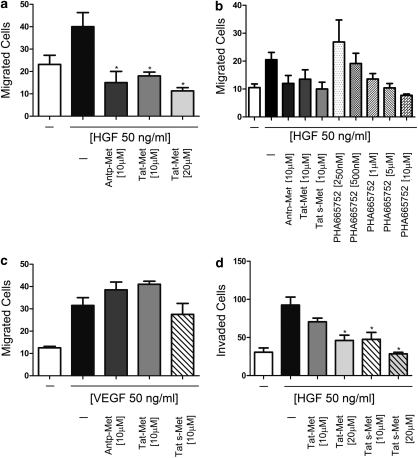
Endothelial cells must cross basement membranes to form new blood vessels during angiogenesis; we investigated whether the peptides could affect HGF-dependent HUVEC motility and invasion. Under the effect of HGF (50 ng/ml), the number of migrated cells in a Boyden chamber assay doubled; a non-cytotoxic dose of 10 μ peptides abrogated this migration (Figure 4a; *P<0.05). No significant differences in migration-inhibitory activity were found among the different peptides. We observed equivalent inhibitory effects with PHA-6657525 only in the presence of the highest (cytotoxic) concentrations (5 and 10 μ) (Figure 4b). The Met peptides did not inhibit VEGF-dependent cell migration (Figure 4c), thus showing clear specificity.
Figure 4.
The Met docking site peptide strongly inhibits HGF-dependent HUVEC migration and invasion. In the migration assay, HUVECs were seeded into the upper compartment of Boyden chambers in the presence or absence of Antp-, Tat- and Tat s-Met peptides or PHA-665752, whereas chemoattractants (50 ng/ml HGF (a) or 10 ng/ml VEGF (c)) were placed in the lower compartment. After 6 h incubation, cells in the upper side of the filter were removed, and cells that migrated to the lower surface were fixed, stained and counted. Serum-free medium (SFM) was used as negative control. Met mimicking cell-penetrating peptides inhibited HUVEC migration induced by HGF, but not that induced by VEGF. The inhibitor PHA-665752 showed the same inhibitory effect of peptides on HGF-dependent migration only at the highest concentrations (5 and 10 μ). The invasion assay was performed similarly, except that the filter separating the two compartments was pre-coated with matrigel and incubation was for 18 h. Tat- and Tat s-Met peptides inhibited HGF-induced matrigel invasion in dose-dependent manner (d). All experiments were performed in triplicate and repeated three times. Means±s.e.m. are shown (*P<0.05).
In an invasion assay performed in Boyden chambers with filters pre-coated with a reconstituted basement membrane (matrigel), treatment with Tat-Met or Tat s-Met significantly reduced the HGF-induced invasion (Figure 4d). Tat s-Met displayed a higher inhibitory activity than Tat-Met (*P<0.05), and in both cases a dose-dependent effect was observed (Figure 4d).
The Met docking site peptide inhibits endothelial morphogenesis in vitro
Human umbilical vein endothelial cells can organize into capillary-like structures when cultured on a thick matrigel layer in the presence of angiogenic factors. The Met peptides interfered with HGF-induced HUVEC morphogenesis with the same efficacy shown in inhibiting cell proliferation (Supplementary data). Antp-Met was effective at 10 μ (Supplementary data), whereas Tat-Met and Tat s-Met were effective at 20 μ. Antp-Met did not affect FBS-dependent network formation, showing specificity of the inhibitory effect (Supplementary data). PHA-665752 interfered with HGF-dependent tube formation to a similar extent as that of the peptides only at cytotoxic micromolar concentrations (Supplementary data).
The Met docking site peptide inhibits HGF-dependent downstream signaling
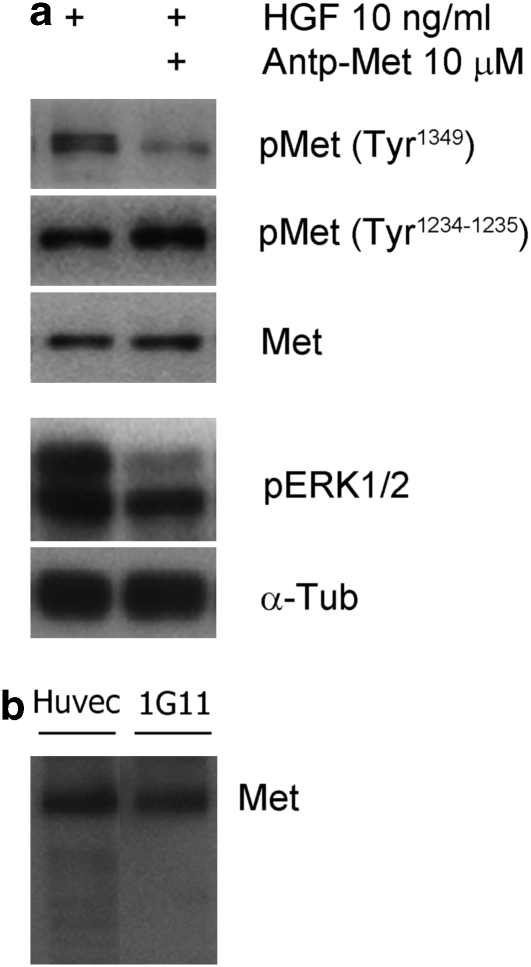
The different biological responses triggered by HGF and mediated by Met depend on efficient downstream signal transduction, which in turn depends on receptor tyrosine phosphorylation. The binding of HGF leads to the activation of the two major signaling pathways, the ERK1/2 and the phosphoinositide 3-kinase (Birchmeier et al., 2003; Boccaccio and Comoglio, 2006). We tested whether Antp-Met impaired Met receptor phosphorylation in the catalytic loop and in the docking site. Although phosphorylation on tyrosines 1234−1235, localized in catalytic loop, was not affected, phosphorylation on tyrosine 1349, localized in the carboxyl-terminal docking site, showed a 70% reduction when Antp-Met was added to cells 2 h before stimulation with 10 ng/ml HGF (Figure 5a). Phosphorylation of ERK1/2, which is mainly involved in transducing proliferation and motility responses (Prat et al., 1998; Birchmeier et al., 2003), was also impaired by the Antp-Met peptide (Figure 5a). Taken together, these data show that Met-derived peptides inhibit the phosphorylation of the tyrosines located in this motif, and block activation of the downstream signaling, but do not affect phosphorylation of the tyrosines located in the activation loop.
Figure 5.
The Met docking site peptide inhibits the HGF-dependent downstream signaling. Quiescent HUVECs were pre-treated or not for 2 h with Antp-Met before stimulation with 10 ng/ml HGF for 15 min, and then lysed and processed for Western blot analysis. Blots were probed with anti-phospho Met (tyrosine 1349), anti-phospho Met (tyrosines 1234–1235), anti-phospho ERK1/2, anti-Met and anti-α-tubulin. Antp-Met inhibited the phosphorylation of tyrosine 1349, but not of tyrosines 1234–1235. Besides, the phosphorylation of ERK1/2 was impaired by Antp-Met (a). (b) Human and murine endothelial cell lines, HUVEC and 1G11, respectively, express Met receptor protein.
The Met docking site peptide inhibits in vivo angiogenesis
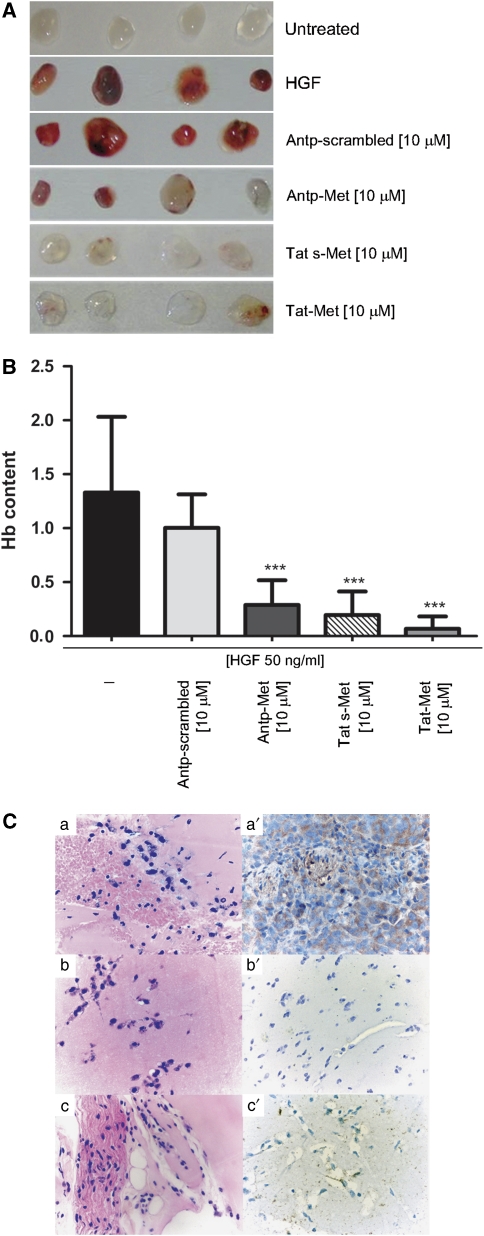
We assessed the effect of the Met peptides on in vivo angiogenesis using a rapid and quantitative matrigel sponge assay. A cocktail of HGF (50 ng/ml) and heparin (50 U/ml) promoted a hemorrhagic vascularization of the matrigel sponge, which was clearly detectable at 4 days post-implantation (Figure 6a). Addition of the different Met-derived peptides (10 μ) significantly inhibited the HGF-induced angiogenic response, as detected by visual inspection of the pellets and measured in a hemoglobin quantification assay (***P<0.001) (Figure 6b). The inhibition was specific, as shown by the lack of effect on the angiogenic response of an Antp-Met peptide whose sequence had been scrambled (Figure 6b). Hematoxylin- and eosin-stained sections of pellets showed that the extensive vascularized areas in mice treated with HGF and heparin were substantially reduced in mice treated with the Met peptides (10 μ) (Figures 6Ca, b, c).
Figure 6.
The Met docking site peptide inhibits in vivo angiogenesis. A cocktail of HGF (50 ng/ml) and heparin (50 U/ml) promoted a hemorrhagic vascularization of the matrigel sponge clearly detectable at 4 days post-implantation. HGF-induced vascularization of the subcutaneously injected matrigel implants was significantly inhibited by the addition of micromolar concentration of Met peptides, as detectable at visual inspection (A) and quantified by measuring hemoglobin (Hb) content of the pellets (B) (***P<0.001). Representative histological specimens that were stained with hematoxylin and eosin show extensive necrotic areas (C (a, b and c)). CD11b/c immunohistochemistry on adjacent sections show abrogation/impairment of cellular infiltrates in the pellets treated with the peptides (C (a′, b′ and c′)) ( × 400 magnification).
Immunohistochemistry showed that CD11b/c-positive cells were readily detected within pellets containing HGF and heparin, indicating the presence of an inflammatory response; this infiltrate was significantly reduced when Met peptides were co-injected in matrigel (Figures 6Ca′, b′, c′).
The Met docking site peptide inhibits the growth and vascularization of Kaposi's sarcomas in nude mice
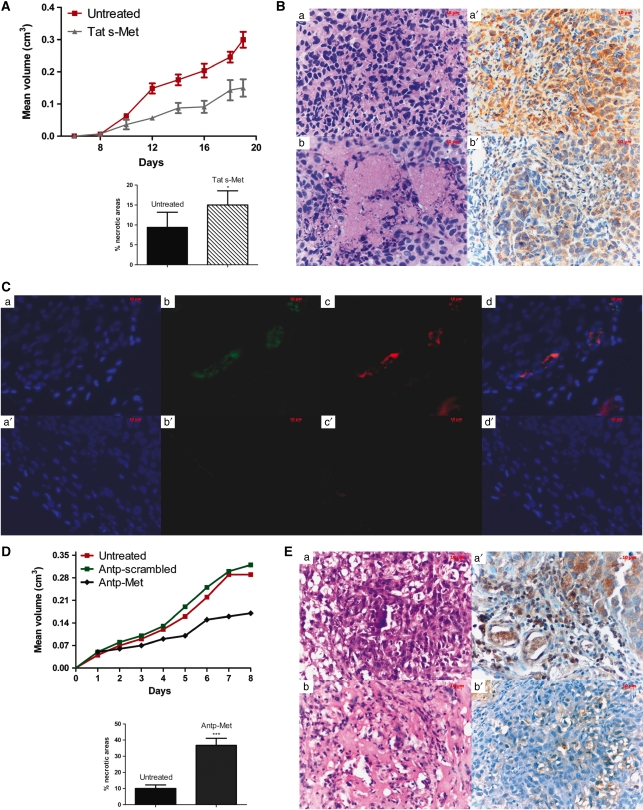
We next examined whether the peptides were able to effect growth and vascularization of tumors in vivo. KS-Imm Kaposi's sarcoma cells were injected in liquid matrigel with or without Tat s-Met (10 μ), followed by treatment with peptides subcutaneously every second day until termination of the experiment. Treatment with the Tat s-Met peptide clearly inhibited tumor growth (Figure 7a). Hematoxylin–eosin staining of sections of tumors showed extensive areas of vascularization in control tumors, whereas extensive necrotic areas and strongly reduced vascularization were found in tumors of animals receiving peptides (Figures 7Ba, b). Immunohistochemical staining indicated that in peptide-treated tumors CD11b/c-positive inflammatory cells were significantly reduced (Figures 7Ba′, b′). To confirm the reduction of vascularization in tumors treated with peptide, we performed an immunofluorescence analysis for the endothelial marker von Willebrand (Figures 7Cb, b′) and we observed a higher number of von Willebrand-positive cells (Figure 7Cb) in control tumors than in tumors treated with Tat s-Met (Figure 7Cb′). We observed colocalization of the Met receptor and the von Willibrand endothelial marker in the tumors (Figures 7Cc, c′), suggesting that murine tumor endothelial cells express Met. This was consistent with the presence of Met (Figure 5b) in the 1G11 murine lung microvascular endothelial cell line (Dong et al., 1997).
Figure 7.
The Met docking site peptide impairs the growth and vascularization of established Kaposi's sarcomas in nude mice. Mice with xenografted Kaposi's sarcoma tumors (either established (D, E) or not (A, B)) were injected peri-tumorally with equimolar concentrations of either Antp-Met, Tat s-Met or Antp-scrambled (150 μl final volume of a 10 μ solution) or vehicle alone (control) every second day, as indicated. (A) Tumor growth analysis showed a significant inhibition of tumor growth by Tat s-Met. The inset shows a quantitative analysis of the areas necrosis (as a percentage) in control and Tat s-Met-treated tumors (mean±s.e.; *P<0.001). (B) Hematoxylin and eosin staining of the Kaposi's sarcoma xenografts grown in mice treated with Tat s-Met (b) showed extensive necrotic areas, when compared with tumors from control mice receiving only vehicle (a). Immunostaining of adjacent sections with CD11b/c, a marker of inflammatory infiltrates, showed that Tat s-Met treatment strongly inhibited the inflammation associated with the tumor (b′), relative to treatment with vehicle alone (control) (a′). (C) Immunofluorescence staining of Kaposi's sarcoma xenograft sections for the endothelial marker von Willibrand (b and b′) and Met receptor (c and c′). The number of positive von Willibrand cells in control tumors (b) was much higher than that in xenografts treated with Tat-s (b′). The effect of the peptide on vascularization was Met-related, as showed by the colocalization of the Met receptor with the von Willibrand endothelial cell marker in the tumors (c and c′). (D) Antp-Met peptide was also able to significantly inhibit tumor growth in vivo. In contrast, a scrambled control peptide had no effect on tumor growth, showing the role of specific Met inhibition. The inset shows a quantitative analysis of the areas necrosis (as a percentage) in control and Antp-Met-treated tumors (mean±s.e.; ***P<0.001). (E) Hematoxylin and eosin staining of the Kaposi's sarcoma xenografts grown in mice treated with Antp-Met (b) showed extensive necrotic areas, as compared with tumors from control mice receiving only vehicle (a). CD11b/c immunostaining of adjacent sections also showed a strong Antp-Met inhibition of tumor-associated inflammation (b′) as compared with controls (a′) (× 400 magnification).
In another set of experiments, Antp-Met, or a scrambled peptide (both at 10 μ), was injected in the peri-tumor area every second day after the tumor was established. Again, the peptide significantly inhibited tumor growth, whereras the scrambled peptide had no effect on tumor growth (Figure 7D). The central necrotic areas (Figures 7Ea, b) and the strong inflammatory infiltrate (Figures 7Ea′, b′) again were strongly reduced in the presence of the Met peptide as compared with controls receiving either vehicle alone or scrambled peptide. These data indicate that the Met peptides, while not toxic for healthy endothelial cells, strongly and specifically inhibited tumor angiogenesis, inflammation and growth.
Discussion
Angiogenesis is associated with several pathologies, including chronic inflammation and arthritis, atherosclerosis, macular degeneration and cancer (Carmeliet and Jain, 2000); therapeutic strategies have envisioned angiogenesis as a promising target (Folkman, 1985; Carmeliet and Jain, 2000; Ferrara, 2004; Goh et al., 2007). HGF and its receptor Met are able to stimulate endothelial cell migration, proliferation and organization into capillary-like tubes and angiogenesis (Bussolino et al., 1992; Grant et al., 1993; Silvagno et al., 1995). HGF can induce pro-angiogenic cytokine expression, directly activating angiogenic pathways (Dong et al., 2001; Sengupta et al., 2003) or downregulating the expression of angiogenic inhibitors (Zhang et al., 2003) and promoting lymphangiogenesis (Cao et al., 2006). The HGF/Met axis has been proposed as a target for antiangiogenic therapy (You and McDonald, 2008).
Structural and functional integrity of endothelial cells is a fundamental factor for vessel homeostasis and function. Alterations in the endothelium are associated with increased risk for several diseases, including cardiovascular diseases and adverse events. Agents affecting endothelial integrity, inducing apoptosis or direct toxicity, are likely to induce vascular dysfunction. Several small-molecule drugs targeting Met and other kinases show toxicity; biological molecules could be used to obtain higher levels of specificity and lower toxicity in Met inhibition.
Here we describe the antiangiogenic properties of synthetic cell-permeable peptides containing the carboxyl-terminal tail of Met in the absence of cytotoxicity or apoptosis induction. These peptides inhibit HGF-induced proliferation, migration, invasiveness and capillary-like network formation in endothelial cells in vitro. Comparison of our peptides with the ATP-competitive inhibitor PHA-665752 showed that similar inhibitory effects were obtained only at high concentrations of PHA-665752 that were associated with apoptosis and toxicity. The ability of these peptides to interfere with pathological responses with no cytotoxic effects would be of clinical relevance to minimize the additive toxicity phenomena due to the administration of multiple inhibitor drugs.
The Met docking site peptides were active in vivo, blocking HGF-induced angiogenesis and restraining growth and vascularization of xenografted tumors; this is the first report of the in vivo inhibiting activity of a Met-specific cell-penetrating peptide. Homozygous genetic deletion of either Met or HGF is lethal in utero due to defects in placental development (Bladt et al., 1995; Schmidt et al., 1995; Uehara et al., 1995). However, the Met−/− mice did not show an endothelial phenotype (Bladt et al., 1995) and the morphological appearance of endothelium in HGF−/− embryos was not altered (Uehara et al., 1995). These data suggest that the Met/HGF axis may have a major role in pathological but not in physiological angiogenesis, as proposed for other angiogenic factors such as PlGF (Fischer et al., 2008). This would be consistent with the role of Met in endothelial cells (Figure 6b), the co-expression with the endothelial marker in vivo (Figure 7b) and the lack of toxicity in vitro and in vivo.
Biochemically, the peptides strongly impaired HGF-dependent activation of the Ras-MAPK ERK1/2 transduction pathway (Ponzetto et al., 1994; Birchmeier et al., 2003), consistent with previous in vitro studies in epithelial cells (Bardelli et al., 1999). The Met-specific peptides were delivered into cells by fusion with the internalization sequences from the Antp homeodomain 3 or the HIV-Tat basic domain, which proved to act efficiently as transporters across membranes (Deshayes et al., 2005; Gupta et al., 2005; Foged and Nielsen, 2008; Morris et al., 2008; Stewart et al., 2008). A similar peptide fused to the Tat internalizing sequence enhanced in vitro chemosensitivity of glioma cells to a cis-platinum derivative (Lou et al., 2009). The three peptides containing the Met docking site coupled to different internalizing sequences showed only small differences in their inhibitory efficiency in vitro and in vivo. The three peptides displayed clear specificity for Met receptor; they did not impair VEGF- or FBS-induced effects on endothelial cells in vitro and a scrambled peptide was ineffective in vivo.
The Met-specific peptide inhibited the phosphorylation of Y1349, which binds the p85 regulatory subunit of phosphoinositide 3-kinase (Graziani et al., 1991; Ponzetto et al., 1994; Bardelli et al., 1999; Lou et al., 2009), mainly involved in transducing HGF-dependent motogenic and antiapoptotic responses (Ponzetto et al., 1993; Khwaja et al., 1998; Giordano et al., 2000; Deregibus et al., 2003). Phosphorylation of the MAPK ERK1/2, whose activation is required for the proliferative and invasive responses (Ponzetto et al., 1994; Prat et al., 1998; Birchmeier et al., 2003; Bardelli et al., 2005), was impaired. Although strongly interfering with the activation of the tyrosines located in the docking site, the peptide had no effect on phosphorylation of Y1234, Y1235, localized in the activation loop, as it appears to compete with substrate access to the catalytic pocket (Bardelli et al., 1999).
Current clinical approaches to targeting angiogenesis have focused on blocking VEGF signaling by either anti-VEGF antibodies or multitargeted receptor tyrosine kinase inhibitors (Folkman, 1985; Carmeliet and Jain, 2000; Ferrara, 2004; Goh et al., 2007; Loges et al., 2009). Current clinical experience has revealed that VEGF-targeted therapy prolongs overall survival of cancer patients by only months, and experimental models have recently shown that anti-VEGF treatment inhibits primary tumor growth, but may promote invasiveness and metastasis (Pennacchietti et al., 2003; Ebos et al., 2009; Paez-Ribes et al., 2009). The fact that Met is both a pro-angiogenic factor and an oncogene suggested that it may be an optimal target for anticancer treatment, simultaneously inhibiting angiogenesis and oncogenesis (Michieli et al., 2004) and potentially complementing VEGF targeted antiangiogenic therapy.
Our observations provide the novel result that Met-specific peptides mimicking the tail multifunctional docking site can block angiogenesis and consequent cancer growth and related metastatic dissemination.
Materials and methods
Reagents
Murine recombinant VEGF-A, human recombinant VEGF-A165, murine recombinant tumor necrosis factor-α and human recombinant HGF in its biologically active form (Gherardi et al., 2006) were purchased from Peprotech (Offenbach, Germany); heparin was obtained from Sigma (Sigma-Aldrich Chemie, Taufkirchen, Germany). Matrigel was prepared from the Engelbreth–Holm–Swarm sarcoma as described previously (Kleinman et al., 1986).
Cell-permeable peptides containing the Met docking site sequence (ref. seq: NP_000236), or a control scrambled sequence, fused to the internalization sequences of either the homeodomain 3 of the Antennapedia protein (ref. seq: NP_996167) or the Tat transactivator domain (ref. seq: NP_057853) at their N-terminus were synthesized and purified by Primm (Milan, Italy). Two Tat sequences of different lengths were used. Peptides conjugated to FAM at their N-terminus were used to evaluate internalization. Peptide sequences are reported in Table 1.
Table 1. Peptide sequences.
| Antp-Met | RQIKIWFQNRRMKWKK IGEHYVHVNATYVNVKCVA |
| Tat-Met | GRKKRRQRRRPP IGEHYVHVNATYVNVKCVA |
| Tat s-Met | RKKRRQRRR IGEHYVHVNATYVNVKCVA |
| Antp-scrambled | RQIKIWFQNRRMKWKK IVAVNCGVHYEHTNVKVYA |
| Met | IGEHYVHVNATYVNVKCVA |
Cell-permeable peptides derived from the Met receptor docking site fused to internalization sequences from the Antennapedia and Tat proteins. Met sequence alone was used as control of cell internalization.
Cell cultures
Human umbilical vein endothelial cells were purchased from Promo Cell (Heidelberg, Germany) and grown on 1% gelatin-coated tissue culture plates in M199 endothelial growth medium (Sigma, St Louis, MO, USA), supplemented with 10% heat-inactivated FBS, 1% -glutamine, fibroblast growth factors (1 μg acid-fibroblast growth factor plus 1 μg basic-fibroblast growth factor /100 ml), epidermal growth factor (1 μg/100 ml), heparin (10 mg/100 ml) and hydrocortisone (0.1 mg/100 ml). Cells were used between the second and eighth passage in vitro. The murine lung endothelial cell line (1G11) was the kind gift from Annunciata Vecchi (Istituto Clinico Humanitas, IRCCS and University of Milan, Milan, Italy).
Cytofluorimetric analysis
Human umbilical vein endothelial cells (6 × 105) were incubated with 10 μ concentrations of FAM-labeled peptides for different periods of time (10, 30, 60 and 120 min). Cells were then recovered, washed twice with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde and analyzed by flow fluorocytometry in a FACSCanto (BD Biosciences, San Jose, CA, USA).
To compare the effect of our peptides with that of the Met-specific inhibitor PHA-665752 (the kind gift of Professor Livio Trusolino, Univ. Torino), 1 × 105 HUVECs were plated onto six-well plates and allowed to adhere overnight. The next day, the cells were treated in the growth medium (in the presence of HGF 50 ng/ml or 10% FBS) with the Antp-Met peptide (10 μ), Tat-Met peptide (10 μ) or Tat s-Met peptide (10 μ) or with increasing concentrations of PHA-665752 (250 and 500 n, and 1 and 5 μ). Dimethyl sulfoxide and growth medium alone (in the presence of HGF 50 ng/ml or 10% FBS) were used as controls. After 2 h, cells were recovered, washed with PBS and transferred to test tubes. Cells were pelleted and resuspended in Annexin V-binding buffer (0.01 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (pH 7.4); 0.14 NaCl; 2.5 m CaCl2). Fluorescein isothiocyanate Annexin V and 7-amino-actinomycin D (BD Biosciences) were added to each test tube and incubated for 15 min at room temperature in the dark. Cells were then washed in PBS, supernatants discarded and resuspended in 400 μl of binding buffer. Samples were acquired by flow fluorocytometry using a FACSCanto (BD Biosciences) and analyzed using FACSDiva Software 6.1.2. The experiment was performed three times and each condition was a triplicate.
In vitro cell viability
Human umbilical vein endothelial cell viability was evaluated in the MTT assay. Cells (1000/well) were seeded into 96-multiwell plates in complete medium and, after complete adhesion, the medium was replaced with 2% FBS fresh medium with or without 50 ng/ml HGF and/or different peptides at different concentrations (1–50 μ) or with or without PHA-665752 at increasing concentrations (250 n–10 μ). After different periods of incubation (24, 48, 72 and 96 h), plates were processed and absorbance read at 570 nm.
Migration, invasion and morphogenesis assays
These assays were performed in modified Boyden chambers, as described previously (Albini et al., 1987; Albini and Benelli, 2007). HUVECs (5 × 104) were washed with PBS, resuspended in serum-free medium and placed in the upper compartment. Chemoattractants (50 ng/ml HGF or 10 ng/ml VEGF) in serum-free M199 medium were added in the lower compartment and the different peptides or PHA-665752 at different concentrations added. Pore-size polycarbonate filters (12 μ) were pre-coated with collagen (50 μg/ml) or matrigel (1 mg/ml). After 6 h (migration) or overnight (invasion) incubation, the filters were recovered, cells on the upper surface mechanically removed and cells migrated to or invaded the lower filter surface fixed with absolute ethanol and stained with toluidine blue. Cells were counted in a double-blind manner in eight consecutive fields each with a light microscope. All experiments were performed three times in triplicate. The in vitro morphogenesis assay was performed as described by Grant et al. (1989) in the presence of 50 ng/ml HGF or 10% FBS, and with the peptides or PHA-665752 in increasing concentrations. After 6 h incubation on matrigel, the dimensional organization of the cells was examined under an inverted microscope (Zeiss, Oberkochen, Germany), equipped with CCD optics and a digital analysis system.
Western blotting
Human umbilical vein endothelial cells, pre-incubated in serum-free medium for 24 h, were pre-treated for 2 h with 10 μ Antp-Met peptide before stimulation with 10 ng/ml HGF for 15 min (Prat et al., 1998). Lysates were prepared using Cell Lysis Buffer (Cell Signaling Technology, Beverly, MA, USA) and protein concentrations evaluated by the DC Protein Assay (Bio-Rad, Hercules, CA, USA). Proteins were separated on 8% SDS–polyacrylamide gel electrophoresis under reducing conditions and transferred onto polyvinylidene fluoride membranes (Amersham, Biosciences, Otelfingen, CH, USA), which were then incubated with the antibodies directed against the following human antigens: phospho-ERK1/2, tubulin, phospho-Met (tyrosine 1349), phospho-Met (tyrosines 1234–1235) (Cell Signaling Technology) and Met (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) diluted in 5% bovine serum albumin–Tris-buffered saline–0.1% Tween. Horseradish-peroxidase-conjugated secondary antibodies against rabbit or anti-mouse immunoglobulin (Cell Signaling Technology; 1:5000) were then used and reactions were visualized with the enhanced chemiluminescence kit (ECL) from Amersham Biosciences (Pittsburg, PA, USA). Lysates, HUVEC and 1G11 cells, were incubated with antibodies directed against human Met and mouse Met (both provided by Santa Cruz Biotechnology Inc), respectively.
In vivo matrigel sponge assay
The assay was performed as described (Albini et al., 1994). HGF (50 ng/ml in the presence of heparin 50 U/ml), with or without peptides, was added to unpolymerized liquid matrigel and slowly injected subcutaneously into the flanks of 6- to 8-week-old C57/BL6 male mice (Charles River Laboratories, Calco (Lecco), Italy) in groups of 4–8 mice for each treatment. Four days after injection, the animals were killed and the pellets were removed, weighed, and either formalin-fixed and paraffin-embedded for histological examination, or minced and diluted in water for hemoglobin content measurement with a Drabkin reagent kit (Sigma). The final hemoglobin concentration was calculated from a standard calibration curve, after spectrophotometric analysis at 540 nm.
In vivo xenograft tumor growth
Xenografted tumors were obtained by subcutaneous injection of 5 × 106 human KS-Imm cells/mouse, and mixed with liquid matrigel into the flanks of seven-week-old male nude nu/nu (CD-1) BR mice (Charles River Laboratories) as described previously (Albini et al., 1997, 2001). Tat s-Met peptide was injected with cells (300 μl final volume of 10 μ peptide corresponding to 34 μg/ml) and then every second day by peri-tumor injection, whereas control animals received vehicle alone. In another experiment, the Antp-Met peptide (300 μl final volume of 10 μ peptide corresponding to 43 μg/ml) was injected after the tumors had become established. Animals were weighed and tumor growth monitored at regular intervals by measuring the two tumor diameters with a caliper. After the animal was killed, tumors were removed, weighed and processed for histological examination. All procedures were performed in adherence with the ECC Directive for Care and Italian Laws on animal experimentation (Law by Decree 116/92).
Histological analysis
Sections of 3 μ thickness were stained with hematoxylin–eosin for histological examination. For immunohistochemistry, slides were deparaffinized in xylene, rehydrated and antigens retrieved in EDTA (pH 8); thereafter, the sections were treated with 3% of hydrogen peroxide, incubated with rabbit polyclonal CD11b/c antibody (1:600) (ABR, Golden, CO, USA) and peroxidase-coupled anti-rabbit immunoglobulin secondary antibodies, followed by reaction with 3,3′-diaminobenzidine (Dako REAL EnVision system, Peroxidase/diaminobenzidine+, Dako, Glostrup, Denmark) and finally counterstained with Mayer's hematoxylin (Sigma).
For immunofluorescence microscopy, primary antibodies against von-Willibrand factor (rabbit anti-mouse, 1:100) (Millipore, Temecula, CA, USA) and Met (mouse anti-mouse, 1:100) (Santa Cruz Biotechnology Inc) were diluted in background reducing diluent (Dako) and applied for 1 h at room temperature, after the incubation with blocking serum (3% goat serum). Fluorescent secondary antibodies Alexa Fluor goat anti-rabbit 488 (1:50) (Invitrogen, Eugene, OR, USA) and goat anti-mouse 555 (1:30) (Invitrogen) were diluted in PBS buffer and applied for 1 h at room temperature. Nuclei were labeled with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA).
Statistical analyses
Statistical analyses were performed using two-tailed t-tests for comparison of two data sets, one-way analysis of variance for multiple data sets and two-way analysis of variance for growth curves, using the Graph Pad Prism statistics and graphing program.
Acknowledgments
These studies were supported by AIRC (Associazione Italiana per la Ricerca sul Cancro), ISS, Compagnia San Paolo and the MIUR. We thank Drs Monica Morini and Raffaella Dell'Eva (Istituto Nazionale per la Ricerca sul Cancro, Genova) for preliminary observations. We are indebted to Dr Paola Corradino (CBA, Genova) for literature searches and Alessandra Panvini-Rosati (Multimedica, Milan) for assistance. ARC is a PhD student at the Department of Structural and Functional Biology, Via Dunant 5, Varese, CF is a MIUR ‘Grande Progetto Strategico' fellow.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- Albini A, Benelli R. The chemoinvasion assay: a method to assess tumor and endothelial cell invasion and its modulation. Nat Protocol. 2007;2:504–511. doi: 10.1038/nprot.2006.466. [DOI] [PubMed] [Google Scholar]
- Albini A, Fontanini G, Masiello L, Tacchetti C, Bigini D, Luzzi P, et al. Angiogenic potential in vivo by Kaposi's sarcoma cell-free supernatants and HIV-1 tat product: inhibition of KS-like lesions by tissue inhibitor of metalloproteinase-2. AIDS. 1994;8:1237–1244. doi: 10.1097/00002030-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- Albini A, Morini M, D'Agostini F, Ferrari N, Campelli F, Arena G, et al. Inhibition of angiogenesis-driven Kaposi's sarcoma tumor growth in nude mice by oral N-acetylcysteine. Cancer Res. 2001;61:8171–8178. [PubMed] [Google Scholar]
- Albini A, Paglieri I, Orengo G, Carlone S, Aluigi MG, DeMarchi R, et al. The beta-core fragment of human chorionic gonadotrophin inhibits growth of Kaposi's sarcoma-derived cells and a new immortalized Kaposi's sarcoma cell line. AIDS. 1997;11:713–721. doi: 10.1097/00002030-199706000-00003. [DOI] [PubMed] [Google Scholar]
- Anastasi S, Giordano S, Sthandier O, Gambarotta G, Maione R, Comoglio P, et al. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J Cell Biol. 1997;137:1057–1068. doi: 10.1083/jcb.137.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardelli A, Longati P, Williams TA, Benvenuti S, Comoglio PM. A peptide representing the carboxyl-terminal tail of the met receptor inhibits kinase activity and invasive growth. J Biol Chem. 1999;274:29274–29281. doi: 10.1074/jbc.274.41.29274. [DOI] [PubMed] [Google Scholar]
- Bardelli C, Sala M, Cavallazzi U, Prat M. Agonist Met antibodies define the signalling threshold required for a full mitogenic and invasive program of Kaposi's sarcoma cells. Biochem Biophys Res Commun. 2005;334:1172–1179. doi: 10.1016/j.bbrc.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Berthou S, Aebersold DM, Schmidt LS, Stroka D, Heigl C, Streit B, et al. The Met kinase inhibitor SU11274 exhibits a selective inhibition pattern toward different receptor mutated variants. Oncogene. 2004;23:5387–5393. doi: 10.1038/sj.onc.1207691. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Su Y, Oskarsson M, Zhao P, Kort EJ, Fisher RJ, et al. Neutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal models. Proc Natl Acad Sci USA. 2001;98:7443–7448. doi: 10.1073/pnas.131200498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, et al. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Chan AM, Rubin JS, Bottaro DP, Hirschfield DW, Chedid M, Aaronson SA. Identification of a competitive HGF antagonist encoded by an alternative transcript. Science. 1991;254:1382–1385. doi: 10.1126/science.1720571. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- Chu SH, Feng DF, Zhang H, Chen ET, Duan ZX, Li XY, et al. c-Met-targeted RNA interference inhibits growth and metastasis of glioma U251 cells in vitro. J Neurooncol. 2009;93:183–189. doi: 10.1007/s11060-008-9772-5. [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- Corso S, Migliore C, Ghiso E, De Rosa G, Comoglio PM, Giordano S. Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene. 2008;27:684–693. doi: 10.1038/sj.onc.1210697. [DOI] [PubMed] [Google Scholar]
- Deregibus MC, Buttiglieri S, Russo S, Bussolati B, Camussi G. CD40-dependent activation of phosphatidylinositol 3-kinase/Akt pathway mediates endothelial cell survival and in vitro angiogenesis. J Biol Chem. 2003;278:18008–18014. doi: 10.1074/jbc.M300711200. [DOI] [PubMed] [Google Scholar]
- Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62:1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo MF, Narsimhan RP, Olivero M, Bretti S, Giordano S, Medico E, et al. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991;6:1997–2003. [PubMed] [Google Scholar]
- Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001;61:5911–5918. [PubMed] [Google Scholar]
- Dong QG, Bernasconi S, Lostaglio S, De Calmanovici RW, Martin-Padura I, Breviario F, et al. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol. 1997;17:1599–1604. doi: 10.1161/01.atv.17.8.1599. [DOI] [PubMed] [Google Scholar]
- Ebens A, Brose K, Leonardo ED, Hanson MG, Jr, Bladt F, Birchmeier C, et al. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy. Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- Foged C, Nielsen HM. Cell-penetrating peptides for drug delivery across membrane barriers. Expert Opin Drug Deliv. 2008;5:105–117. doi: 10.1517/17425247.5.1.105. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Gherardi E, Sandin S, Petoukhov MV, Finch J, Youles ME, Ofverstedt LG, et al. Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc Natl Acad Sci USA. 2006;103:4046–4051. doi: 10.1073/pnas.0509040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano S, Maffe A, Williams TA, Artigiani S, Gual P, Bardelli A, et al. Different point mutations in the met oncogene elicit distinct biological properties. FASEB J. 2000;14:399–406. doi: 10.1096/fasebj.14.2.399. [DOI] [PubMed] [Google Scholar]
- Goh PP, Sze DM, Roufogalis BD. Molecular and cellular regulators of cancer angiogenesis. Curr Cancer Drug Targets. 2007;7:743–758. doi: 10.2174/156800907783220462. [DOI] [PubMed] [Google Scholar]
- Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, et al. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DS, Tashiro K, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Graziani A, Gramaglia D, Cantley LC, Comoglio PM. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J Biol Chem. 1991;266:22087–22090. [PubMed] [Google Scholar]
- Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev. 2005;57:637–651. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Jun HT, Sun J, Rex K, Radinsky R, Kendall R, Coxon A, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87MG cells and xenografts. Clin Cancer Res. 2007;13:6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Lehmann K, Marte BM, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Wang L, Su YC, Gillespie GY, Salhotra A, Lal B, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12:1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Lock LS, Frigault MM, Saucier C, Park M. Grb2-independent recruitment of Gab1 requires the C-terminal lobe and structural integrity of the Met receptor kinase domain. J Biol Chem. 2003;278:30083–30090. doi: 10.1074/jbc.M302675200. [DOI] [PubMed] [Google Scholar]
- Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Longati P, Bardelli A, Ponzetto C, Naldini L, Comoglio PM. Tyrosines1234–1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor) Oncogene. 1994;9:49–57. [PubMed] [Google Scholar]
- Lou X, Zhou Q, Yin Y, Zhou C, Shen Y. Inhibition of the met receptor tyrosine kinase signaling enhances the chemosensitivity of glioma cell lines to CDDP through activation of p38 MAPK pathway. Mol Cancer Ther. 2009;8:1126–1136. doi: 10.1158/1535-7163.MCT-08-0904. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. NK4 (HGF-antagonist/angiogenesis inhibitor) in cancer biology and therapeutics. Cancer Sci. 2003;94:321–327. doi: 10.1111/j.1349-7006.2003.tb01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone M, Basilico C, Cavassa S, Pennacchietti S, Risio M, Naldini L, et al. An uncleavable form of pro-scatter factor suppresses tumor growth and dissemination in mice. J Clin Invest. 2004;114:1418–1432. doi: 10.1172/JCI22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73. doi: 10.1016/j.ccr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Morotti A, Mila S, Accornero P, Tagliabue E, Ponzetto C. K252a inhibits the oncogenic properties of Met, the HGF receptor. Oncogene. 2002;21:4885–4893. doi: 10.1038/sj.onc.1205622. [DOI] [PubMed] [Google Scholar]
- Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- Nishino T, Hisha H, Nishino N, Adachi M, Ikehara S. Hepatocyte growth factor as a hematopoietic regulator. Blood. 1995;85:3093–3100. [PubMed] [Google Scholar]
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe S, Bowen WC, Bell AW, Nejak-Bowen K, Luo JH, Michalopoulos GK. Cell cycle effects resulting from inhibition of hepatocyte growth factor and its receptor c-Met in regenerating rat livers by RNA interference. Hepatology. 2007;45:1471–1477. doi: 10.1002/hep.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Maina F, Longati P, Panayotou G, Dhand R, et al. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol Cell Biol. 1993;13:4600–4608. doi: 10.1128/mcb.13.8.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Prat M, Crepaldi T, Pennacchietti S, Bussolino F, Comoglio PM. Agonistic monoclonal antibodies against the Met receptor dissect the biological responses to HGF. J Cell Sci. 1998;111 Part 2:237–247. doi: 10.1242/jcs.111.2.237. [DOI] [PubMed] [Google Scholar]
- Prat M, Narsimhan RP, Crepaldi T, Nicotra MR, Natali PG, Comoglio PM. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991;49:323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Gherardi E, Sellers LA, Wood JM, Sasisekharan R, Fan TP. Hepatocyte growth factor/scatter factor can induce angiogenesis independently of vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2003;23:69–75. doi: 10.1161/01.atv.0000048701.86621.d0. [DOI] [PubMed] [Google Scholar]
- Silvagno F, Follenzi A, Arese M, Prat M, Giraudo E, Gaudino G, et al. In vivo activation of met tyrosine kinase by heterodimeric hepatocyte growth factor molecule promotes angiogenesis. Arterioscler Thromb Vasc Biol. 1995;15:1857–1865. doi: 10.1161/01.atv.15.11.1857. [DOI] [PubMed] [Google Scholar]
- Stewart KM, Horton KL, Kelley SO. Cell-penetrating peptides as delivery vehicles for biology and medicine. Org Biomol Chem. 2008;6:2242–2255. doi: 10.1039/b719950c. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Vigna E, Pacchiana G, Mazzone M, Chiriaco C, Fontani L, Basilico C, et al. Active' cancer immunotherapy by anti-Met antibody gene transfer. Cancer Res. 2008;68:9176–9183. doi: 10.1158/0008-5472.CAN-08-1688. [DOI] [PubMed] [Google Scholar]
- You WK, McDonald DM. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep. 2008;41:833–839. doi: 10.5483/bmbrep.2008.41.12.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci USA. 2003;100:12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.