Abstract
The possibility that reduced photomorphogenic responses could increase field crop yield has been suggested often, but experimental support is still lacking. Here, we report that ectopic expression of the Arabidopsis PHYB (phytochrome B) gene, a photoreceptor involved in detecting red to far-red light ratio associated with plant density, can increase tuber yield in field-grown transgenic potato (Solanum tuberosum) crops. Surprisingly, this effect was larger at very high densities, despite the intense reduction in the red to far-red light ratios and the concomitant narrowed differences in active phytochrome B levels between wild type and transgenics at these densities. Increased PHYB expression not only altered the ability of plants to respond to light signals, but they also modified the light environment itself. This combination resulted in larger effects of enhanced PHYB expression on tuber number and crop photosynthesis at high planting densities. The PHYB transgenics showed higher maximum photosynthesis in leaves of all strata of the canopy, and this effect was largely due to increased leaf stomatal conductance. We propose that enhanced PHYB expression could be used in breeding programs to shift optimum planting densities to higher levels.
The world population is predicted to reach 8 billion by 2025, and this, in combination with current trends in dietary composition, will result in a substantial increase of food demand. The ability to satisfy this demand will rely heavily on the genetic improvement of cultivated plants to increase yield potentials and/or yield stability (i.e. the constancy of performance; Khush, 2001). Although yield per unit area continues to increase for many crops, maximum yield is not showing such an obvious trend, and this has been interpreted as a result of yield potential approaching a ceiling, whereas yield stability continues to grow (Mann, 1999). Although some consider that this ceiling represents the highest physically attainable yield (Sinclair, 1993), others are more confident that incorporation of the current wealth of knowledge in plant biology will overcome the decreasing rate of increase in yield potential. However, genetic modifications that appear beneficial when analyzed at the cellular or plant level do not necessarily translate into higher yields in the field, either because of negative side effects or simply because they do not operate on the limitations under field conditions.
As incident solar radiation penetrates the canopy of commercial crops, it suffers a gradual attenuation of the photosynthetic photon flux density (PPFD) and of the red (R) to far-red (FR) ratio (Holmes and Smith, 1977). Changes in the R to FR ratio perceived by phytochromes initiate a number of responses such as increased stem extension growth (Morgan and Smith, 1976), reduced branching (Deregibus et al., 1983), and accelerated leaf senescence (Rousseaux et al., 1999), which are collectively called the shade avoidance syndrome (Smith, 2000). In growing canopies, some of these changes anticipate competition for PPFD because R to FR signals caused by FR reflected on neighboring vegetation take place before mutual plant shading (Casal et al., 1986; Ballaré et al., 1987, 1990). The responses to low R to FR ratios are potentially adaptative and increase the competitive ability of plants (Smith, 1982; Ballaré et al., 1989; Schmitt, 1997). However, this ability of plants to adjust their morphology and physiology in response to crowding in natural environments could play a deleterious role under field crop conditions. In that sense, we have observed recently that sunflower (Helianthus annuus) plants cultivated at very low densities yield less grain when the growth of the stem is stimulated by low R to FR ratios (Libenson et al., 2002).
Disabling of responses to R to FR ratio by transgenic manipulation of phytochrome genes could result in crops with a higher proportion of resources incorporated into harvestable material (Smith, 1992). In accordance with this idea, mild expression of oat (Avena sativa) phytochrome A (phyA) in tobacco (Nicotiana tabacum) resulted in reduced stem growth and stem to leaf fresh biomass ratio, particularly at high planting densities (Robson et al., 1996). However, because only biomass ratio and not leaf dry weight data are available, whether reduced stem growth translates into increased crop yield in tobacco is not known. Potato (Solanum tuberosum) plants with enhanced phytochrome B (phyB) levels due to ectopic expression of the Arabidopsis PHYB transgene do show increased tuber yield in experiments performed in pots in the greenhouse (Thiele et al., 1999) but they have not been tested under the low R to FR of dense crops cultivated under field conditions (Moffat, 2000). The latter is not a trivial problem because low R to FR ratios are predicted to reduce the differences in Pfr levels between normal and high-phyB transgenic plants. Arabidopsis plants expressing high levels of oat or Arabidopsis PHYB respond to a greater or lesser extend to R to FR than the wild type (WT) depending on the range of R to FR ratios (McCormac et al., 1993). PHYB overexpression could exaggerate or desensitize the response of potato crops to canopy density depending on the actual impact of density on R to FR ratio and the slope of the responses to R to FR ratio. In contrast to phyB, phyA is more active under low than under high R to FR ratios, and its impact can be increased at high plant densities (Robson et al., 1996). An additional reason for the field test is that the phytochrome transgenic strategy to reduce the negative effects of crop light signals has the potential drawback that plants lacking normal responses to R to FR could simultaneously fail to optimize the position of light-harvesting organs (Ballaré et al., 1995, 1997). Plant responses to R to FR signals play a key role in minimizing the interference among leaves in maize (Zea mays) crops (Maddonni et al., 2002). Furthermore, phyB can alter the timing of tuber formation (Jackson et al., 1996), and this could modify the impact of PHYB overexpression on the yield of the field crops.
The aim of the work presented here was to investigate the field performance of potato plants bearing an Arabidopsis PHYB transgene to assess whether manipulation of phytochrome levels can increase crop yield.
RESULTS
Density Dependence of Stem Length in Transgenic Plants Expressing the Arabidopsis PHYB Transgene
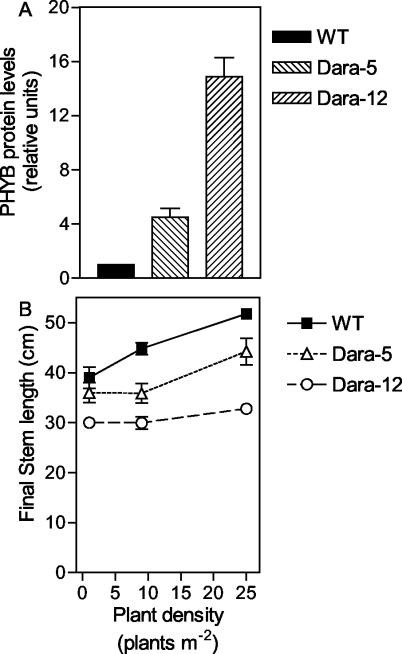
In preliminary experiments, we investigated the range of densities where manipulation of phyB levels had the greatest impact on stem growth, a typical trait under phytochrome control. WT potato cv Desireé plants or expressing the Arabidopsis PHYB transgene (Dara-5 and Dara-12; Fig. 1A) were grown outdoors in pots placed at different densities (using for each density the same distance between and within rows). In the WT, stem length increased linearly with plant density (Fig. 1B). The two transgenic lines expressing Arabidopsis PHYB failed to respond between 1 and 9 plants m-2. The line expressing higher levels of PHYB (approximately 15-fold the WT level) retained a weak response between 9 and 25 plants m-2. However, the line expressing moderate levels of PHYB (approximately 5-fold the WT level) showed a WT response between 9 and 25 plants m-2 (Fig. 1B). These observations indicate that despite the reduction in the proportion of Pfr caused by the lower R to FR ratios of higher densities, the transgenics retained higher levels of phyB in the active (Pfr) form. Because differences between the WT and the transgenics were noted both at 9 and 25 plants m-2, densities within that range were used in the field.
Figure 1.
Expression of the Arabidopsis PHYB transgene reduces stem growth responses to plant density. A, PHYB protein levels detected in leaf extracts. The ratio between PHYB-associated alkaline phosphatase band intensity and total protein Ponceau S staining is expressed relative to the WT ratio. B, Stem length plotted against plant density. Plants were grown outdoors at the indicated plant density. Data are means and se of four blocks.
Transgenic Potato Plants Expressing the Arabidopsis PHYB Transgene Show Increased Branching, Tuber Number, and Tuber Yield at High Densities
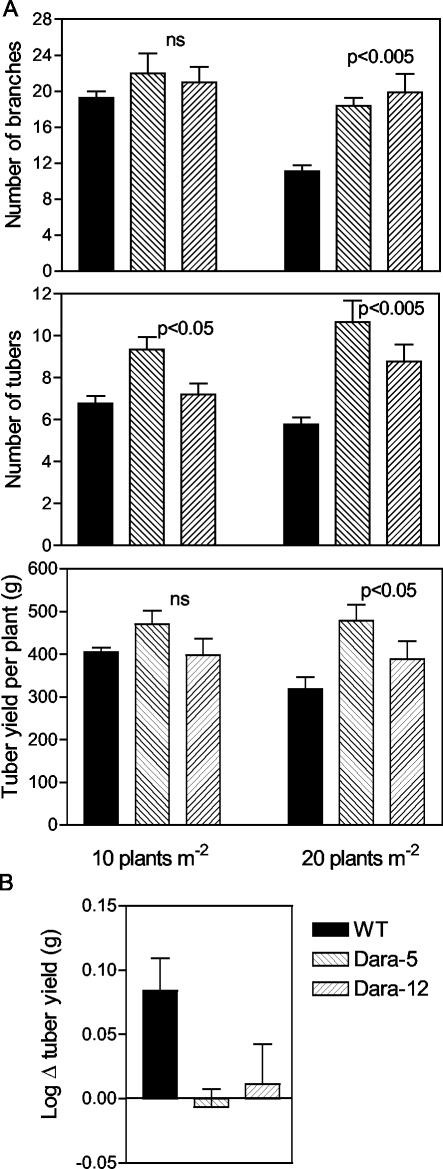
Potato plants of the WT or expressing the Arabidopsis PHYB transgene were grown in replicated plots in the field at high commercial densities of either 10 plants m-2 (planted in rows 70 cm apart) or 20 plants m-2 (planted in rows 35 cm apart). Interplant distance within the row was 15 cm in both cases. The number of branches, the number of tubers, and tuber yield per plant were enhanced by the Arabidopsis PHYB transgene particularly at 20 plants m-2 (Fig. 2A). Tuber number increased more than tuber yield in transgenic plants with the consequent reduction in weight per tuber (20 plants m-2; WT, 55.07 ± 2.55 g tuber-1; Dara-5, 47.23 ± 7.86 g tuber-1; and Dara-12, 44.87 ± 4.70 g tuber-1). In the WT, the number of tubers per plant decreased with density, but in the PHYB transgenics, the number actually increased. This suggests that the response to density could be the results of a balance between negative (e.g. lower PPFD, lower R to FR) and positive (e.g. lower temperatures; Jackson, 1999) effects on tuber number. The balance would be positive in the transgenics. Increasing plant density reduced tuber yield per plant in the WT but not in the transgenic lines (Fig. 2B). Flowering time was accelerated by the Arabidopsis PHYB transgene, particularly at high plant densities (20 plants m-2, WT, 44.1 ± 1.37 d; Dara-5, 34.7 ± 1.47 d; Dara-12, 36.2 ± 2.07 d; P < 0.01).
Figure 2.
Field performance of potato plants either of the WT (Desiree) or of lines expressing the Arabidopsis PHYB transgene (Dara-5 and Dara-12). A, Number of branches, number of tubers, and tuber yield. Plant density was 10 or 20 plants m-2 (70 or 35 cm between rows, respectively). B, Tuber yield response to plant density. Note that the se overestimates the error used in ANOVA because blocking reduced the error.
R to FR Ratio in WT and Transgenic Canopies
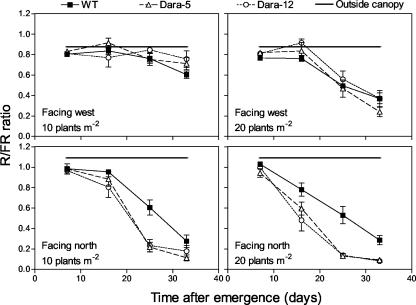
Because increased plant densities are predicted to reduce the R to FR ratio and the impact of the PHYB transgene on absolute Pfr levels, we were surprised to find higher differences on tuber yield between the WT and transgenic lines at 20 compared with 10 plants m-2. We then thought that because Dara-5 and Dara-12 plants are bushier than the WT, the transgenic canopies could undergo weaker reductions of the R to FR ratio with increasing plant densities and in this way exacerbate their impact on yield at higher densities. However, this was not the case. An R to FR sensor was placed at the base of plants facing toward the nearest neighbors within the same row (i.e. toward the north or the south in a crop with north-south-oriented rows) and in adjacent rows (i.e. toward the west or the east). When the sensor was placed facing neighbors within the row, both transgenic crops showed reduced and not higher R to FR ratios (Fig. 3). When the sensor faced toward adjacent rows, the reduced interrow distance was reflected in lower R to FR ratios, but little difference was observed between WT and transgenic crops (Fig. 3). Lower R to FR ratios in transgenic crops were observed when the sensor was placed facing upward at different heights within the canopy (data not shown).
Figure 3.
Transgenic expression of the Arabidopsis PHYB transgene modifies R to FR ratio signals. The R to FR ratio of the light propagating horizontally was measured at midday. The sensor was placed at the base of a given plant facing neighbor plants in different rows (facing west) or within the same row (facing north). Data are means and se of four independent replicate measurements.
PPFD Interception by WT and Transgenic Canopies
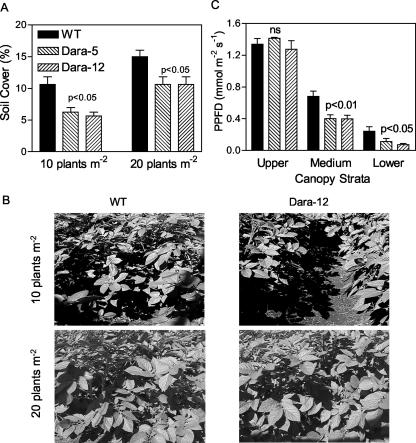
The transgenic lines expressing Arabidopsis PHYB showed reduced ability to intercept radiation at early stages of canopy growth at both plant densities (Fig. 4A). These differences narrowed down with canopy growth, reaching WT levels faster at 20 than 10 plants m-2 (Fig. 4B). The vertical distribution of the light was also affected by the Arabidopsis PHYB transgene, particularly at the highest density. The analysis of the profile of light extinction within the canopy showed reduced PPFD reaching intermediate or low strata of the canopy in both Dara-5 and Dara-12 crops compared with the WT (Fig. 4C). This indicates that enhanced phyB levels increased the proportion of radiation intercepted by higher compared with lower strata of the canopy. Therefore, we measured the photosynthetic capacity of leaves of different strata to investigate the impact of this light profile on the photosynthesis of the canopy.
Figure 4.
PPFD interception by potato crops either of the WT or of lines expressing the Arabidopsis PHYB transgene (Dara-5 and Dara-12). A, Light interception (10 d after planting). B, Transgenic crops fail to cover the soil when planted at 10 plants m-2, but this effect is diluted at 20 plants m-2 (48 d after planting) C, Irradiance reaching different strata (20 plants m-2; 48 d after planting). Data are means and se of four replicates.
Photosynthesis
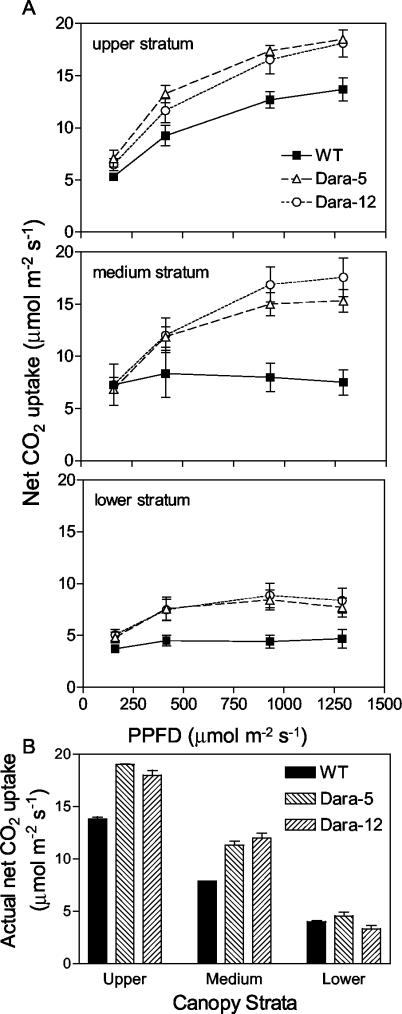
To investigate the photosynthetic capacity of potato crops expressing the Arabidopsis PHYB transgene, the net rate of CO2 uptake was recorded at different positions within the canopy and at different levels of supplementary PPFD. At a PPFD of 160 μmol m-2 s-1, the rate of CO2 uptake was not significantly different in WT and transgenic plants and showed a relatively small response to the position of the leaf within the canopy (Fig. 5A). At higher PPFD, particularly 900 or 1,300 μmol m-2 s-1, the transgenic leaves showed significantly higher rates of CO2 uptake at any position within the canopy. The leaves placed at the top of the canopy were only approaching saturation at the highest PPFD tested here. However, although the photosynthetic capacity of the leaves of the WT placed at intermediate or low positions within the canopy were virtually saturated by the lowest PPFD (200 μmol m-2 s-1), the transgenics retained a strong response to higher PPFD (Fig. 5A). The latter was particularly obvious for leaves placed at intermediate heights within the canopy, which in the transgenics resembled those leaves placed at the top of the canopy.
Figure 5.
Response of net CO2 uptake per unit area to PPFD (A) and actual net CO2 uptake per unit area (B) in leaves of different strata of potato plants of the WT or expressing the Arabidopsis PHYB transgene (Dara-5 and Dara-12). Plant density was 10 (A) or 20 (B) plants m-2. Data are means and se of four replicates.
At least at midday, the actual rate of CO2 uptake (i.e. the rate observed at each position with the actual PPFD reaching that point) was increased by the Arabidopsis PHYB transgene at least for upper and intermediate canopy strata (Fig. 5B). In summary, enhanced phyB levels decreased PPFD interception, particularly in the canopy with distant rows, increased maximum photosynthesis at all canopy strata and altered the vertical profile of PPFD in a way that favored upper leaves (with high photosynthetic potential) compared with lower leaves. Estimates of whole-canopy photosynthesis at midday predict approximately 80% increase in the transgenic crops cultivated with rows 35 cm apart. This difference appears significantly diluted at lower densities due to impaired PPFD interception.
Stomatal Conductance
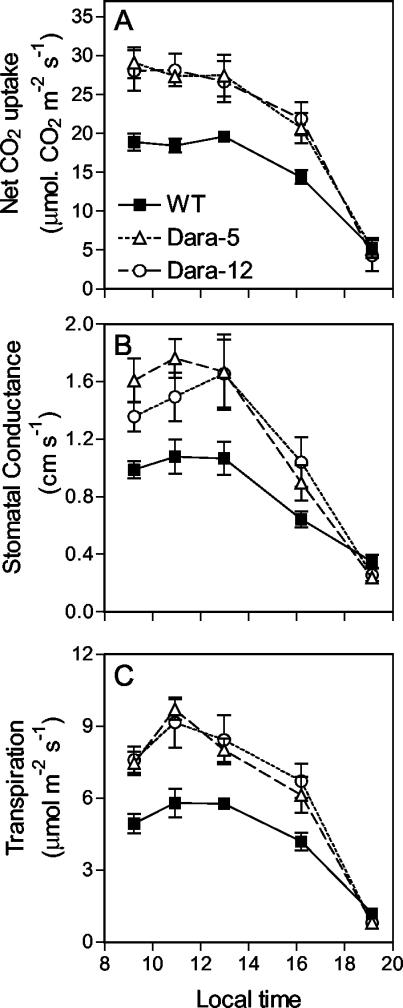
We also investigated the physiological basis of the differences in maximum photosynthesis. Both transgenic lines showed similarly high photosynthetic rates, but chlorophyll levels per unit leaf area were significantly greater in Dara-12 (17.49 ± 0.60 μg cm-2) than Dara-5 (12.6 ± 0.30 μg cm-2), which only marginally differed from the WT (10.77 ± 0.32 μg cm-2). Differences in specific leaf area were also smaller than those on photosynthesis rates (Dara-12, 5.4 ± 0.13 mg cm-2; Dara-5, 5.0 ± 0.15 mg cm-2; and WT, 4.5 ± 0.1 mg cm-2). This indicates that different rates of photosynthesis are not mainly the consequence of greener or thicker leaves. The daily time course of maximum net CO2 uptake in non-shaded leaves of the uppermost stratum showed larger differences between WT and transgenic plants around the central portion of the photoperiod (Fig. 6A; note that these rates were measured by using the PPFD source of the photosynthesis meter set at 1,300 μmol m-2 s-1). No differences were observed in darkness, indicating that the rate of mitochondrial respiration was not significantly affected by the Arabidopsis PHYB transgene (WT, -1.44 ± 0.08 μmol m-2 s-1; Dara-5, -1.65 ± 0.17 μmol m-2 s-1; Dara-12, -1.67 ± 0.35 μmol m-2 s-1). These observations indicate that the factor limiting photosynthesis at high irradiance was under diurnal control. The diurnal pattern observed for leaf stomatal conductance (Fig. 6B) is consistent with a major role in the control of maximum photosynthesis and the response to high phyB levels. The density of stomata was not significantly affected by the Arabidopsis PHYB transgene (WT, 133.65 ± 10.57 pores mm-2; Dara-5, 128.47 ± 12.75 pores mm-2; Dara-12, 141.97 ± 8.55 pores mm-2), indicating that enhanced CO2 uptake in the transgenics was largely due to increased aperture of the stomatal pore. Changes in leaf transpiration rates accompanied the effects of phyB levels on photosynthesis (Fig. 6C).
Figure 6.
Time course of maximum net CO2 uptake per unit area (A), CO2 conductance (B), and transpiration (C) in upper leaves of WT or transgenic potato expressing Arabidopsis PHYB (Dara-5 and Dara-12).
DISCUSSION
There are several reports showing that actual crop yield can be made closer to potential yield by transgenic expression of genes that increase plant defenses. Results showing yield increases in the field in transgenic plants with altered metabolism are scant (Taylor, 2001). Here, we demonstrate that PHYB, i.e. a gene linking development to the environment, can increase potential tuber yield of potato crops planted at high densities.
The analysis of the light environment in transgenic potato crops expressing the Arabidopsis PHYB transgene revealed a layer of complexity that was not envisaged at the plant level. Enhanced phyB levels not only altered plant responses to the light environment, but they also modified the light environment itself. Both transgenic lines showed reduced R to FR ratios of the horizontal radiation reflected on or transmitted by neighbor plants of the same row (Fig. 3), enhanced vertical attenuation of the PPFD (Fig. 4C), and delayed covering of the soil between rows and PPFD interception (Fig. 4, A and B).
Higher tuber yields in PHYB transgenics required high plant densities to be maximally expressed (Fig. 2). This is to some extent surprising because at high densities, the low R to FR ratios are predicted to reduce the proportion of phyB in the Pfr form and, therefore, the absolute difference between the WT and transgenics expressing Arabidopsis PHYB. However, density dependency is likely to reflect the reduced impact of delayed light interception in the transgenics when the space between rows was reduced (Fig. 4B). The possibility that overexpression of phytochromes could impair the ability of plants to “forage for light” had been predicted (Ballaré et al., 1995, 1997). The results presented here indicate that under certain conditions negative effects can be overcome by positive effects of increased phytochrome levels. An even more positive balance could at least be achieved theoretically by transgenic modification of genes acting downstream of phyB. These genes could control a subset of phyB-mediated responses and eventually induce the physiological and morphological responses with positive impact on yield while avoiding those with negative impact. The recent discovery of the PFT1 gene provides support for the hope on the existence of such specific genes downstream of phyB (Cerdán and Chory, 2003).
The genetic manipulation of canopy photosynthesis is recognized as one of the main avenues to increase crop yield (Horton, 2000). However, there is still little reliable information relating the modification of specific photosynthetic genes to performance under agricultural conditions (Dunwell, 2000). Maximum photosynthesis normally decays in older leaves within the canopy due to ontogenic and environmental factors (Constable and Rawson, 1980), and high phyB levels reduced the steepness of this decay (Fig. 5A). The higher rates of maximum photosynthesis per unit leaf area observed in the PHYB transgenics can be accounted for by the increased stomatal conductance (Fig. 6B). Thus, higher photosynthesis rates were accompanied by increased transpiration rates in the PHYB transgenics (Fig. 6C). Differences in leaf thickness, chlorophyll per unit area, or protein per unit area were relatively small and at most contributed weakly to differences in photosynthesis. High phyB levels apparently reduced the resistance of the stomatal pore to CO2 transport because the number of stomata was not significantly affected. Phytochrome effects on leaf conductance had been reported in physiological experiments performed in Phaseolus vulgaris (Holmes et al., 1986). The idea that reduced photomorphogenic responses could increase crop yield by shifting partitioning of photo-assimilates from the stem to harvestable organs does not provide an obvious explanation for the results observed here. Actually, the line expressing moderate levels of phyB (Dara-5) showed weaker effects on stem growth (final stem length reached WT levels in the field) and stronger effects on yield (Fig. 2; data not shown). The primary effect could be on tuber formation and the increased demand of photo-assimilates by the larger number of tubers. Control of maximum photosynthesis by the availability of sinks is well established (Paul and Foyer, 2001), and in some cases, it has been related to changes in stomatal conductance (Setter and Brun, 1980). In favor of this interpretation, tuber number increased more than tuber yield with the consequent reduction in weight per tuber. Increased photosynthesis would be necessary for the expression of this increased number of sinks.
One approach currently pursued by different seed companies to improve crop yield is to increase the number of plants per unit soil area. However, although at low plant densities, increasing the number of plants per unit area results in proportional increments in crop yield, above certain plant densities the marginal increment of yield with increased population decreases, approaching zero, and may eventually become negative (Tetio-Kagho, 1988; Sangoi et al., 2002). The results presented here suggest that enhanced PHYB levels could be used in breeding programs to shift optimum crop densities to higher values.
MATERIALS AND METHODS
Plant Material and Experimental Conditions
Plants of potato (Solanum tuberosum) cv Désirée were used as WT. The production and physiological characterization of two transgenic lines (Dara-5 and Dara-12) of the Desire background carrying the PHYB gene of Arabidopsis under the control of the 35 S promoter have been described previously (Thiele et al., 1999). These lines were propagated from tubers.
One experiment was conducted with potato plants grown in pots (65 L) containing a soil:sand mixture (1:1 [v/v]). Seed potatoes with an average diameter of 6 cm were used as starting material. Photoperiod at sowing was 14.17 h. Plants were watered as needed and fertilized with 12-5-14 N-P-K and Mg (Nitrofoska, Basf, Belgium). A complete randomized block design was used for both experiments with four true replicates.
Two experiments were conducted in the field of the Faculty of Agronomy of the University of Buenos Aires (34° 35′ S, 58° 29′W). Approximately 0.2 m3 m-2 soil and 0.2 m3 m-2 sand were added and mixed with the top 10 cm of the soil to obtain a texture adequate for tuber development. Seed potato with an average diameter of 6 cm was sown in plastic boxes containing a soil:sand mixture (1:1 [v/v]) and cultivated in a greenhouse until emergency. Plants were transplanted to field at a distance of 15 cm within the row. The distance between rows was 0.7 or 0.35 m (i.e. 10 or 20 plants m-2). Photoperiod at sowing (February) was 13.3 (first experiment) or 13.6 (second experiment) h. Plants were watered as needed with tap water and fertilized (10, 22, and 35 d after transplant) with 10 g m-2 of 12-5-14 N-P-K and Mg (Nitrofoska). A complete randomized block design was used for both experiments with four true replicates. All measurements were done in the middle row of each plot.
Western Blotting
The levels of PHYB were detected immunologically by using the antibodies anti-phyB Mab B6-B3 (Hirschfeld et al., 1998). Proteins were extracted from 100 mg of leaves taken from the upper strata of the canopy of field-grown plants by following the method of Martinez-García et al. (1999). PAGE and antibodies probing were conducted as described by Boccalandro et al. (2001). Band intensity (both PHYB-associated alkaline phosphatase activity and total protein Ponceau S reversible staining) was quantified with an imager system (Fluor-S Multimager, Bio-Rad, Hercules, CA). Data correspond to two independent biological replicates where PHYB and intensity relative to Ponceau S staining are expressed relative to WT values.
Description of the Light Environment
The PPFD was measured with a Li-188B sensor (Lincoln, NE) with its remote probe (line quantum sensor) placed at soil level either on the row or between rows facing upwards. For measurements on the row, the remote probe was also placed at different heights relative to the average top of the canopy: 0.8 (upper stratum), 0.5 (intermediate stratum), and 0.2 (lower stratum). The proportion of the soil covered by the canopy was measured using the implement used by Casal et al., 1986. The R to FR ratio was measured with a spectroradiometer (Skye Instruments Ltd, Llandrindod Wells Powys, UK) that senses the R and FR part of the solar spectrum with picks in 660 nm (R) and 730 nm (FR). The remote probe was placed horizontally at the base of the plants, facing toward neighbor plants within the row or in adjacent rows. Two measurements per block were averaged for the analysis.
Plant Growth and Development
The height of the stem was measured from the soil to the apex with a ruler. The final number of branches was recorded 45 d after emergence of the shoots (no new branches appeared afterward). The date of flower anthesis was recorded after daily observations for each plant and is expressed as the number of days after shoot emergence. The tubers were harvested 104 d after emergence of the shoot, stored at 10°C (for a maximum of 5 d), and weighted to the nearest of 0.1 g.
Leaf Photosynthesis and Conductance
Leaf CO2 exchange and conductance was measured using a closed infrared gas analysis system (LI-COR 6200). For each canopy level and genotype, two leaves per block were sampled. CO2 exchange at 160, 415, 930, and 1,290 μmol m-2 s-1 PPFD were measured for each sampled leaf, using a 0.25-L chamber attached to a regulated portable red light power (QB1 205LI-670, Quantum Devices Inc., Barneveld, WI).
For measurements of chlorophyll, a disc (0.5-cm diameter) was harvested from the leaves used to measure photosynthesis in each of the blocks and placed in 1 mL of N-N′-dimethyl formamide kept in vials wrapped in aluminum foil. The samples were stored in darkness at 4°C (for 3 d before absorbance measurements to calculate chlorophyll levels according to Inskeep and Bloom, 1985).
Acknowledgments
We deeply thank Antonio Hall for his helpful suggestions, comments, and discussion about data analysis and interpretation. We are grateful to Dr. Peter H. Quail for his kind provision of antibodies against PHYB. We also thank Matías Rugnone, Natalia Rossi, Alejandra Scanapieco, and Omar Boscacci for their excellent technical assistance and Miriam Izaguirre for collaboration in the edition of the manuscript.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.029579.
This work was supported by the University of Buenos Aires (grant no. G 067 to J.J.C.), by the National Research Council of Argentina (Consejo Nacional de Investigaciones Científicas y Técnicas grant no. PID 888 to J.J.C.), and by the Fundación Antorchas (grant no. 14116–16 to J.J.C.).
References
- Ballaré CL, Sánchez RA, Scopel AL, Casal JJ, Ghersa CM (1987) Early detection of neighbor plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ 10: 551-557 [Google Scholar]
- Ballaré CL, Scopel A, Sánchez RA (1995) Plant photomorphogenesis in canopies, crop growth and yield. Hortic Sci 30: 1172-1181 [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA (1989) Photomodulation of axis extension in sparse canopies. Plant Physiol 89: 1324-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247: 329-332 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA (1997) Foraging for light: photosensory ecology and agricultural implications. Plant Cell Environ 20: 820-825 [Google Scholar]
- Boccalandro HE, Mazza CA, Mazzella MA, Casal JJ, Ballaré (2001) Ultraviolet B radiation enhances a phytochrome B-mediated photomorphogenic response in Arabidopsis. Plant Physiol 126: 780-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Sánchez RA, Deregibus VA (1986) The effect of plant density on tillering: the involvement of R/FR and the proportion of radiation intercepted per plant. Environ Exp Bot 26: 365-371 [Google Scholar]
- Cerdán P, Chory J (2003) Regulation of flowering time by light quality. Nature 423: 881-885 [DOI] [PubMed] [Google Scholar]
- Constable GA, Rawson HM (1980) Effect of leaf position, expansion and age on photosynthesis, transpiration and water use efficiency of cotton. Aust J Plant Physiol 7: 89-100 [Google Scholar]
- Deregibus VA, Sánchez RA, Casal JJ (1983) Effects of light quality on tiller production in Lolium spp. Plant Physiol 72: 900-902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwell JM (2000) Transgenic approaches to crop improvement. J Exp Bot 51: 487-496 [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Tepperman JM, Clack T, Quail PH, Sharrock R (1998) Coordination of phytochrome levels in phyB mutants of Arabidopsis as revealed by apoprotein-specific monoclonal antibodies. Genetics 149: 523-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MG, Sager JC, Klein WH (1986) Sensitivity to far-red radiation in stomata of Phaseolus vulgaris L.: rhythmic effects on conductance and photosynthesis. Planta 168: 516-522 [DOI] [PubMed] [Google Scholar]
- Holmes MG, Smith H (1977) The function of phytochrome in the natural environment I characterization of daylight for studies in photomorphogenesis and photoperiodism. Photochem Photobiol 25: 533-538 [Google Scholar]
- Horton P (2000) Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture. J Exp Bot 51: 475-485 [DOI] [PubMed] [Google Scholar]
- Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a and b in n,n-dymethylformamide. Plant Physiol 77: 483-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S (1999) Multiple signaling pathways control tuber induction in potato. Plant Physiol 119: 1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SD, Heyer A, Dietze J, Prat S (1996) Phytochrome B mediates the photoperiodic control of tuber formation in potato. Plant J 9: 159-166 [Google Scholar]
- Khush GS (2001) Green revolution: the way forward. Nat Rev Genet 2: 815-822 [DOI] [PubMed] [Google Scholar]
- Libenson S, Rodriguez V, Sánchez RA, Casal JJ (2002) Low red to far-red ratios reaching the stem reduce grain yield in sunflower. Crop Sci 42: 1180-1185 [Google Scholar]
- Maddonni GA, Otegui ME, Andrieu B, Chelle M, Casal JJ (2002) Maize leaves turn away from neighbors. Plant Physiol 130: 1181-1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CC (1999) Crop scientists seek a new revolution. Science 283: 310-314 [Google Scholar]
- Martinez-García JF, Monte E, Quail PH (1999) A simple, rapid and quantitative method for preparing Arabidopsis protein extracts for immunoblot analysis. Plant J 20: 251-257 [DOI] [PubMed] [Google Scholar]
- McCormac AC, Wagner D, Boylan MT, Quail PH, Smith H, Whitelam GC (1993) Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome B-encoding cDNAs: evidence that phytochrome A and phytochrome B have distinct photoregulatory functions. Plant J 4: 19-27 [Google Scholar]
- Moffat AS (2000) Can genetically modified crops go “greener.” Science 290: 253-254 [DOI] [PubMed] [Google Scholar]
- Morgan DC, Smith H (1976) Linear relationship between phytochrome photoequilibrium and growth in plants under natural radiation. Nature 262: 210-212 [Google Scholar]
- Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52: 1383-1400 [DOI] [PubMed] [Google Scholar]
- Robson PRH, McCormac AC, Irvine AS, Smith H (1996) Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nat Biotechnol 14: 995-998 [DOI] [PubMed] [Google Scholar]
- Rousseaux MCH, Hall AJ, Sánchez RA (1999) Light environment, nitrogen content, and carbon balance of basal leaves of sunflower canopies. Crop Sci 39: 1093-1100 [Google Scholar]
- Sangoi L, Gracietti M, Rampazzo C, Bianchetti P (2002) Response of Brazilian maize hybrids from different eras to changes in plant density. Field Crop Res 79: 39-51 [Google Scholar]
- Schmitt J (1997) Is photomorphogenic shade avoidance adaptive? Perspectives from population biology. Plant Cell Environ 20: 826-830 [Google Scholar]
- Setter TL, Brun WA (1980) Stomatal closure and photosynthetic inhibition in soybean leaves induced by petiole girdling and pod removal. Plant Physiol 65: 884-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair T (1993) Crop yield potential and fairy tales. In D Buxton D, eds, International Crop Science I. Crop Science Society of America, Madison, WI, pp 707-712
- Smith H (1982) Light quality, photoperception and plant strategy. Annu Rev Plant Physiol 33: 481-518 [Google Scholar]
- Smith H (1992) The ecological functions of the phytochrome family: clues to a transgenic programme of crop improvement. Photochem Photobiol 56: 815-822 [Google Scholar]
- Smith H (2000) Phytochromes and light signal perception by plants: an emerging synthesis. Nature 407: 585-590 [DOI] [PubMed] [Google Scholar]
- Taylor DC (2001) Field testing transgenic rapeseed cv. Hero transformed with a yeast sn-2 acyltransferase results in increased oil content, erucic acid content and seed yield. Proc Natl Acad Sci USA 8: 317-322 [Google Scholar]
- Tetio-Kagho F (1988) Responses of maize to plant population density: I. Reproductive development, yield, and yield adjustments. Agron J 80: 935-940 [Google Scholar]
- Thiele A, Herold M, Lenk I, Quail PH, Gatz C (1999) Heterologous expression of Arabidopsis phytochrome B in transgenic potato influences photosynthetic performance and tuber development. Plant Physiol 120: 73-82 [DOI] [PMC free article] [PubMed] [Google Scholar]