Abstract
AIM: To develop a multiplex reverse transcription polymerase chain reaction (RT-PCR) method detecting circulating tumor cells in the peripheral blood of colorectal cancer (CRC) patients.
METHODS: Peripheral blood samples were collected from 88 CRC patients and 40 healthy individuals from the blood donors’ clinic and subsequently analyzed by multiplex RT-RCR for the expression of carcinoembryonic antigen (CEA), cytokeratin 20 (CK20) and epidermal growth factor receptor (EGFR) mRNA. The analysis involved determining the detection rates of CEA, CK20 and EGFR transcripts vs disease stage and overall survival. Median follow-up period was 19 mo (range 8-28 mo).
RESULTS: Rates of CEA, CK20 and EGFR detection in CRC patients were 95.5%, 78.4% and 19.3%, respectively. CEA transcripts were detected in 3 healthy volunteer samples (7.5%), whereas all control samples were tested negative for CK20 and EGFR transcripts. The increasing number of positive detections for CEA, CK20 and EGFR transcripts in each blood sample was positively correlated with Astler-Coller disease stage (P < 0.001) and preoperative serum levels of CEA (P = 0.029) in CRC patients. Data analysis using Kaplan-Meier estimator documented significant differences in the overall survival of the different CRC patient groups as formed according to the increasing number of positivity for CEA, CK20 and EGFR transcripts.
CONCLUSION: These data suggest that multiplex RT-PCR assay can provide useful information concerning disease stage and overall survival of CRC patients.
Keywords: Peripheral blood, Carcinoembryonic antigen, Cytokeratin 20, Epidermal growth factor receptor, Multiplex reverse transcription polymerase chain reaction
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies in the Western world. In Europe, CRC is the second most frequent cause of death from cancer, after lung cancer[1]. Although surgical resection followed by chemotherapy is the leading treatment option, approximately half of the patients eventually die due to distant metastases[2]. Overall survival is associated with the disease stage at the time of diagnosis, suggesting that early detection of disseminated disease may be of great significance[3]. Consequently, the development of new diagnostic methods that allow better definition of disease stage and better monitoring of disease progression is critical.
In the past years, various techniques have been used for the detection of circulating tumor cells (CTCs) derived from solid tumors in different body compartments, with variable results concerning their accuracy and clinical significance. Reverse transcription polymerase chain reaction (RT-PCR)-based methods have allowed the detection of CTCs in peripheral blood samples, which is characterized by great sensitivity compared to protein-based tumor marker estimations in serum[4]. However, controversial data have been reported, mainly associated with the choice of molecular tumor markers, cohort size and patient characteristics, as well as false-positive and false-negative results, caused by different PCR conditions and different sets of primers employed by different laboratories.
Among several molecular markers, cytokeratin 20 (CK20), carcinoembryonic antigen (CEA) and epidermal growth factor receptor (EGFR) are those more frequently used for CTC detection in CRC[5-7]. CK20 is a member of the intermediate filament protein family and a prominent component of the intestinal epithelium. CK20 expression is confined to gastrointestinal epithelium, urothelium, and Merkel cells of the epidermis, as well as malignancies that originate from the aforementioned sites[8-10]. Serum levels of CEA are commonly used in clinical practice for patient monitoring[11-14]. In addition, CEA and CK20 mRNA detection using RT-PCR methods have been widely investigated for the detection of CTCs in CRC patients[15-22]. Furthermore, EGFR has been suggested as a marker that is seldom expressed in hematopoietic cells[23-25], thus detection of EGFR over-expression in CRC patients has been correlated with the advanced clinical stages and disease progression in CRC patients[26-30].
The aim of the present study was to establish a multiplex PCR assay targeting the detection of CEA, CK20 and EGFR mRNAs in a single PCR reaction and to evaluate its possible clinical use in CRC patients.
MATERIALS AND METHODS
Subjects
Eighty eight patients (56 males and 32 females) with biopsy-proven primary CRC were enrolled in the study between June 2007 and June 2009. All patients underwent surgical resection for the primary tumor at the “Saint Savvas” Anticancer Hospital, the “G. Gennimatas” Athens General Hospital and the “Attikon” University Hospital, Athens, Greece. The mean age was 69 years (range 43-88 years). Peripheral blood samples were also collected from 40 healthy volunteers (20 men and 20 women), which were used as negative controls. Informed consent was obtained from all patients and healthy volunteers. The study was approved by the local Ethics Committee and conformed to the ethical standards of the Helsinki Declaration.
Cell lines and tissue samples
HT-29 CRC cells are known to express the 3 molecular markers used in our experiments and have been used in spiking experiments for the definition of the sensitivity of the assay[16,31-37]. HT-29 cells were maintained in Dulbecco’s modified Eagle’s medium/F-12 (Cambrex, Walkerville, MD, USA), supplemented with 10% heat-inactivated fetal bovine serum (Biochrom, Berlin, Germany) and 100 U/mL penicillin/streptomycin (Cambrex), at 37°C in a humidified atmosphere of 5% CO2.
CRC and matched normal adjacent tissue samples were obtained from 3 CRC patients during surgery, with the aim of confirming the expression of CEA, CK20 and EGFR mRNA in CRC specimens. The samples were collected in RNAlater solution (Ambion, Austin, TX) and subsequently homogenized (Ultra-Turrax T25; Thermo Fisher Scientific, Cheshire, UK) in 1 mL Tri Reagent TR-118 (MRC Inc. Cincinnati, OH, USA) at 500 g.
Blood sample preparation and RNA extraction
Three milliliters peripheral blood samples were taken preoperatively using a venous catheter into 3 mL EDTA-containing vacutainers (2 samples were obtained from each patient). All blood samples were processed within 4 h of collection. Each blood sample was added to 7.5 mL of Erythrocyte Lysis Buffer (ELB) (containing 155 mmol/L NH4Cl, 10 mmol/L KHCO3 and 0.1 mmol/L EDTA pH 7.4) and kept on ice for 45 min with occasional mixing by inversion every 5 min, so as to allow erythrocyte lysis. The samples were then centrifuged at 400 g for 10 min at 4°C and the supernatant was discarded. The pellet of nuclear blood cells was resuspended in 5 mL of ELB and kept on ice for an additional 5 min period in order to remove the remaining red blood cells. Next, the pellet was centrifuged at 400 g for 10 min at 4°C. The pellet was then homogenized in 1 mL Tri Reagent TR-118 (MRC Inc. Cincinnati, OH, USA) using a 5 mL syringe. Total cellular RNA was extracted using Tri Reagent TR-118, according to manufacturer instructions. The RNA pellet was diluted in diethylpyrocarbonate treated water; total RNA concentration and purity were determined by UV spectrophotometry (Biospec-nano, Shimadzu Biotech, Kyoto, Japan) and its quality was confirmed by amplification of cDNA for β-actin housekeeping gene.
RT-PCR
cDNA was synthesized using Moloney murine leukemia virus (M-MuLV) reverse transcriptase RNase H- (Finnzymes, Oy, Finland). Briefly, a mixture containing 1 μg of total RNA, 0.5 μg (25 μg/mL) oligo-dT(18) primer (Fermentas) and nuclease free water in a total volume of 15 μL was heated at 70°C for 5 min and then chilled in ice for another 5 min. The mixture was supplemented with 0.5 mmol/L deoxynucleotides (HT Biotechnology LTD), reverse transcriptase buffer containing 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 3 mmol/L MgCl2, and 10 mmol/L DTT, 40 U Human Placental RNase inhibitor (HT Biotechnology LTD) and finally 200 U M-MuLV reverse transcriptase up to a final volume of 20 μL; it was subsequently incubated at 37°C for 60 min. Table 1 presents the exact sets of primers used in our study. The sensitivity of the assay was determined in spiking experiments using serial dilutions of HT-29 CRC cells; 105, 104, 103, 102, 10, 1 and 0 cancer cells were added to different corresponding tubes, each containing 3 mL of blood taken from the same healthy subject. Spiking experiments were followed by erythrocyte lysis and RNA extraction, as described above.
Table 1.
Primer sequences used for reverse transcription polymerase chain reaction amplification of target transcripts
| Tumor marker | Sense | Antisense | PCR product size (bp) | Ref. |
| CEA | 5'-GGGCCACTGTCGGCATCATGATTG-3' | 5'-TGTAGCTGTTGCAAATGCTTTAAGGAAGAAGC-3' | 131 | [38] |
| CK20 | 5'-CAGACACACGGTGAACTATGG-3' | 5'-GATCAGCTTCCACTGTTAGACG-3' | 371 | [39] |
| EGFR | 5'-TCTCAGCAACATGTCGATGG-3' | 5'-TCGCACTTCTTACACTTGCG-3' | 474 | [40] |
CEA: Carcinoembryonic antigen; CK20: Cytokeratin 20; EGFR: Epidermal growth factor receptor; PCR: Polymerase chain reaction.
PCR conditions
Primer pairs were chosen so that the sequences were located at different exons and their specificity was confirmed using NCBI Blast (Table 1). PCR reaction was performed using Taq DNA Polymerase (Qiagen, Hilden, Germany) in a final reaction volume of 25 μL containing 1 × CoralLoad PCR Buffer (contains 15 mmol/L MgCl2), 1.67 mmol/L MgCl2 (final concentration), 200 μmol/L each dNTP and 2.5 U Taq DNA Polymerase; cDNA volume and primer concentrations varied depending on the marker and the sample examined. For the amplification of tissue sample cDNAs, primer concentrations and cDNA volumes were 0.4 μmol/L and 2 μL, respectively for all markers, while cycling conditions were 94°C for 3 min; 94°C for 1 min, 52°C for 1 min, 72°C for 1 min (36 cycles); 72°C for 10 min for CEA and CK20 cDNA amplifications then 94°C for 3 min; 94°C for 1 min, 57°C for 1 min, 72°C for 1 min (35 cycles); 72°C for 10 min for the amplification of EGFR cDNA. The concentrations and the cycling conditions concerning PCR reactions in serial dilutions of HT-29 cells for each marker were as follows: for the amplification of CEA cDNA, 0.08 μmol/L of primers and 1 μL of cDNA were used, while the cycling conditions were 94°C for 3 min, 94°C for 30 s, 58°C for 20 s, 72°C for 30 s (40 cycles); 72°C for 2 min; similarly, CK20 cDNA was amplified using 0.35 μmol/L of primers and 1 μL of cDNA, and PCR cycling conditions were 94°C for 3 min; 94°C for 30 s, 58°C for 20 s, 72°C for 30 s (36 cycles); 72°C for 2 min; finally, the primer concentration used for EGFR assays was 0.4 μmol/L and 2 μL of cDNA added to the PCR mix, while amplification took place at 94°C for 3 min; 94°C for 1 min, 50°C for 1 min, 72°C for 1 min (39 cycles); 72°C for 10 min. For the multiplex PCR reactions 2 μL of cDNA were used, adding primer concentrations as follows: CEA 0.06 μmol/L, CK20 0.15 μmol/L and EGFR 0.5 μmol/L. Cycling conditions were 94°C for 15 min; 94°C for 30 s, 58°C for 20 s, 72°C for 30 s (37 cycles); 72°C for 10 min. PCR products were analyzed by electrophoresis on a 2% agarose gel followed by ethidium bromide staining and then captured under ultraviolet light in a Kodak EDAS 290 imaging system (Carestream Health, Rochester, NY, USA).
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences Predictive Analytics Software (SPSS PASW Statistics ver. 18.0) (SPSS Inc., Chicago, IL). The potential correlation between the molecular marker expression and the clinical and pathological characteristics of the patients was tested by Spearman’s correlation. The overall survival rates were calculated using the Kaplan-Meier estimator and comparisons of survival curves between patient groups were carried out by the log-rank test. Overall survival was defined as the intermediate time interval between sampling and either death or last follow-up. To assess the independent prognostic significance of factors on overall survival, analysis was performed using the Cox proportional hazards regression analysis. A P value < 0.05 was considered to be statistically significant.
RESULTS
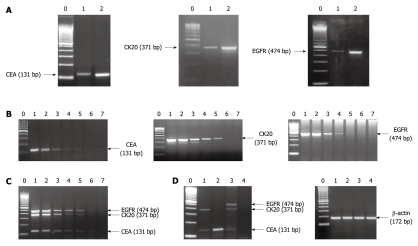
After CEA, CK20 and EGFR mRNA expression was confirmed in pairs of CRC and normal adjacent tissue samples, spiking experiments with HT-29 cells were conducted for establishing the sensitivity of the assay when each marker was run independently (Figure 1). The assay conditions were then accordingly normalized so as to allow the reproducible detection of 10 HT-29 cells in 3 mL of normal blood for all 3 markers examined by multiplex PCR reaction (Figure 1). This cut-off limit has been shown to be of clinical significance in our studies with prostate cancer patients[41-43]. The detection of each marker both in HT-29 cells and in tissue samples (Figure 1, Panel A) was followed by digestion of the PCR product using restriction enzymes (initial development, data not shown). An example of various detection patterns of CEA, CK20 and EGFR transcripts is presented in panel D of Figure 1.
Figure 1.
Detection of carcinoembryonic antigen, cytokeratin 20 and epidermal growth factor receptor mRNAs in colorectal cancer tissue samples, HT-29 colorectal cancer cells and colorectal cancer patient peripheral blood samples. A: Detection of carcinoembryonic antigen (CEA), cytokeratin 20 (CK20) and epidermal growth factor receptor (EGFR) mRNA in pairs of colorectal cancer (CRC) and normal adjacent tissue samples. Lane 0 = molecular weight marker (100 bp); lane 1 = normal tissue; lane 2 = CRC tissue; B: Assessment of the sensitivity of the reverse transcription polymerase chain reaction (RT-PCR) detections for CEA, CK20 and EGFR mRNA. Lane 0 = molecular weight marker (100 bp); lane 1 = 105 HT-29 cells in 3 mL of normal blood; lane 2 = 104 HT-29 cells in 3 mL of normal blood; lane 3 = 103 HT-29 cells in 3 mL of normal blood; lane 4 = 102 HT-29 cells in 3 mL of normal blood; lane 5 = 10 HT-29 cells in 3 mL of normal blood; lane 6 = 1 HT-29 cell in 3 mL of normal blood; lane 7 = normal blood; C: Assessment of the sensitivity of the multiplex RT-PCR detections for CEA (131 bp), CK20 (371 bp) and EGFR (474 bp) mRNA. Lane 0 = molecular weight marker (100 bp); lane 1 = 105 HT-29 cells in 3 mL of normal blood; lane 2 = 104 HT-29 cells in 3 mL of normal blood; lane 3 = 103 HT-29 cells in 3 mL of normal blood; lane 4 = 102 HT-29 cells in 3 mL of normal blood; lane 5 = 10 HT-29 cells in 3 mL of normal blood; lane 6 = 1 HT-29 cell in 3 mL of normal blood; lane 7 = normal blood; D: An example of multiplex RT-PCR-based detection pattern of tumor marker transcripts in the peripheral blood samples of CRC patients. Left: lane 0 = molecular weight marker (100 bp); lane 1 = patient sample positive for CEA (131 bp) and CK20 (371 bp) mRNA; lane 2 = patient sample positive for CEA (131 bp) mRNA; lane 3 = patient sample positive for CEA (131 bp), CK20 (371 bp) and EGFR (474 bp) mRNA; lane 4 = patient sample negative for all markers. Right: lane 0 = molecular weight marker (100 bp); lanes 1-4 = amplification of β-actin (172 bp) cDNA of the patient samples presented in the left side of this panel.
Out of the 40 healthy peripheral blood samples that were examined, 3 were positive for CEA transcripts, resulting in a specificity percentage of 92.5%. We were unable to correlate this finding with any particular clinical information on file of these volunteers. However, both CK20 and EGFR transcripts were absent from the analysis of healthy samples (specificity 100%). CRC patient characteristics are presented in Table 2.
Table 2.
Clinical and pathological characteristics of the colorectal cancer patients enrolled in the study
| No. of cases | |
| Total cases | 88 |
| Sex | 56 |
| Male | 32 |
| Female | |
| Stage (Astler-Coller) | |
| A | 4 |
| B | 26 |
| C | 27 |
| D | 31 |
| Lymph node metastasis | |
| No | 34 |
| Yes | 54 |
| Surgical intervention | |
| Resection | 83 |
| No intervention | 5 |
| Distant metastasis | |
| No | 57 |
| Yes (liver/lungs/both) | 24/2/5 |
| Age (yr), median (min-max) | 69 (43-88) |
| Tumor size (cm), median (min-max) | 4.0 (1.0-11.0) |
Cox regression analysis was performed by grouping CRC patients according to the number of detected transcripts using our assay, with the aim of comparing the clinical significance of such detection patterns in CRC patients. Six groups of CRC patients were defined: group 0: no marker expression; group 1: positive only for CEA; group 2: positive for CEA and CK20; group 3: positive for all three markers; group 4: patients of groups 1 and 2 and group 5: patients of groups 2 and 3. Disease stage was found to be an independent prognostic factor for survival when comparisons were performed between group 1 and group 2 (P = 0.005), between group 2 and group 3 (P = 0.005), between group 3 and group 4 (P = 0.003) as well as between group 1 and group 5 (P = 0.002). When group 1 and group 3 were compared, tumor size was found to correlate significantly with survival (P = 0.040); however, the analysis showed a suggestive prognostic significance of other factors, such as lymph node involvement (P = 0.088), stage (P = 0.066), sex (P = 0.062) and the positivity for all transcripts detection (P = 0.060). Cox regression analysis could not be performed for the comparison of group 0 with the other groups, since all 4 patients with negative detection for all 3 markers were still alive at the time of analysis.
Our analysis revealed that 84 of 88 CRC patients (95.5%) were positive for CEA transcripts; 69 of 88 (78.4%) were positive for CK20 transcripts and 17 of 88 (19.3%) were positive for EGFR transcripts. In particular, 3 of 4 (75%) stage A; 23 of 26 (88.5%) stage B; 27 of 27 (100%) stage C and 31 of 31 (100%) stage D patients were positive for CEA transcripts. In addition, 1 of 4 (25%) stage A, 18 of 26 (69.2%) stage B, 21 of 27 (77.8%) stage C and 29 of 31 (93.5%) stage D CRC patients tested positive for CK20 transcripts. The EGFR transcripts were detected in 17 of 88 (19.3%) patients: in 2 of 26 (7.7%) stage B; in 4 of 27 (14.8%) stage C and in 11 of 31 (35.5%) stage D. No EGFR transcripts were detected in stage A CRC patients. The correlation of PCR detections to disease stage are presented in Table 3.
Table 3.
Correlation of the number of multiplex reverse transcription polymerase chain reaction positive detections in each blood sample with disease stage in colorectal cancer patients n (%)
| CRC patients (n = 88) |
No. of positive marker detections in each blood sample |
|||
| 0 | 1 | 2 | 3 | |
| Stage A (n = 4) | 1 (25) | 2 (50) | 1 (25) | 0 (0) |
| Stage B (n = 26) | 3 (11.5) | 5 (19.2) | 16 (61.5) | 2 (7.7) |
| B1 (n = 9) | 1 (11.1) | 1 (11.1) | 7 (77.7) | 0 (0) |
| B2 (n = 17) | 2 (11.8) | 4 (23.5) | 9 (52.9) | 2 (11.8) |
| Stage C (n = 27) | 0 (0) | 6 (22.2) | 17 (63) | 4 (14.8) |
| C1 (n = 5) | 0 (0) | 1 (20) | 3 (60) | 1 (20) |
| C2 (n = 22) | 0 (0) | 5 (22.7) | 14 (63.6) | 3 (13.6) |
| Stage D (n = 31) | 0 (0) | 2 (6.5) | 18 (58.1) | 11 (35.5) |
Disease staging according to the modified Astler-Coller classification. A positive correlation was revealed using Spearman’s test (Spearman’s ρ = 0.396, P < 0.001). CRC: Colorectal cancer.
We documented that 84 CRC patients (95.5%) were positive by multiplex RT-PCR for at least one transcript (CEA); 69 (78.4%) were positive for at least two transcripts (CEA plus CK20), whereas 17 (19.3%) were positive for all 3 transcripts. Four (4.5%) patients showed no transcript detection. The association between clinical and pathological characteristics of patients with multiplex PCR-based detection of tumor markers was analyzed using Spearman’s correlation (Table 4). This analysis revealed that any of the 3 transcript detections was positively correlated with Astler-Coller stage of the disease, when examined singly (CEA: Spearman’s ρ = 0.264, P = 0.013; CK20: Spearman’s ρ = 0.306, P = 0.004; EGFR: Spearman’s ρ = 0.305, P = 0.004). In addition, positive detection of CK20 and CEA transcripts was positively correlated with serum CEA levels (CK20: Spearman’s ρ = 0.285, P = 0.014; CEA: Spearman’s ρ = 0.228, P = 0.050). Furthermore, a positive correlation was evident between positive detection of the three marker transcript panel and Astler-Coller stage (Spearman’s ρ = 0.396, P < 0.001), as well as serum CEA levels (Spearman’s ρ = 0.253, P = 0.029). Moreover, a suggestive (marginal) association was depicted when the positivity for all 3 transcript detections was analyzed vs lymph node involvement (Spearman’s ρ = 0.216, P = 0.055) (Table 4).
Table 4.
Analysis of the clinical and pathological characteristics in relation to the detection pattern of circulating tumor cells using the carcinoembryonic antigen, cytokeratin 20 and epidermal growth factor receptor multiplex assay n (%)
| Parameter |
No. of positive markers in each blood sample |
P-value | |||
| 0 | 1 | 2 | 3 | ||
| Age (yr) | |||||
| < 69 | 1 (2.4) | 7 (17.1) | 26 (63.4) | 7 (17.1) | |
| ≥ 69 | 3 (6.4) | 8 (17) | 26 (55.3) | 10 (21.3) | 0.963 |
| Sex | |||||
| Male | 3 (5.4) | 8 (14.3) | 32 (57.1) | 13 (23.2) | |
| Female | 1 (3.1) | 7 (21.9) | 20 (62.5) | 4 (12.5) | 0.294 |
| Stage (Astler-Coller) | |||||
| A + B | 4 (13.3) | 7 (23.3) | 17 (56.7) | 2 (6.7) | |
| C | 0 (0) | 6 (22.2) | 17 (63) | 4 (14.8) | |
| D | 0 (0) | 2 (6.5) | 18 (58.1) | 11 (35.5) | < 0.001 |
| Lymph node involvement1 | |||||
| ≤ 3 | 4 (7.1) | 11 (19.6) | 35 (62.5) | 6 (10.7) | |
| > 3 | 0 (0) | 4 (16) | 14 (56) | 7 (28) | 0.055 |
| Tumor size (cm)2 | |||||
| < 3 | 2 (10.5) | 2 (10.5) | 15 (79) | 0 (0) | |
| 3-5 | 1 (2.5) | 8 (20) | 21 (52.5) | 10 (25) | |
| > 5 | 1 (4.8) | 5 (23.8) | 11 (52.4) | 4 (19) | 0.774 |
| Differentiation3 | |||||
| Well + moderate | 4 (6.8) | 10 (17) | 36 (61) | 9 (15.2) | |
| Poor + no | 0 (0) | 4 (22.2) | 10 (55.6) | 4 (22.2) | 0.560 |
| Serum CEA (ng/mL)4 | |||||
| < 5 | 3 (10) | 9 (30) | 12 (40) | 6 (20) | |
| 5-10 | 0 (0) | 1 (7.1) | 11 (78.6) | 2 (14.3) | |
| > 10 | 0 (0) | 4 (13.3) | 17 (56.7) | 9 (30) | 0.029 |
P-values were calculated by Spearman’s test.
P-value calculation using data from 81 patients (7 missing values);
P-value calculation using data from 80 patients (8 missing values);
P-value calculation using data from 77 patients (11 missing values);
P-value calculation using data from 74 patients (14 missing values). CEA: Carcinoembryonic antigen.
Interestingly, all CRC patients who tested negative for all 3 tumor transcripts were alive at the time of verification. Of 15 patients who tested positive only for CEA, 14 were alive (93.3%) (follow-up from 9 to 26 mo). Thirty seven of 52 (71.2%) patients who tested positive for both CEA and CK20 were alive while 15 of them (28.8%) had died (follow-up from 1 to 28 mo). However, 10 of 17 patients who tested positive for all 3 transcripts had died (58.8%) and only 7 of them (41.2%) were alive (follow-up from 1 to 23 mo) at the time of analysis.
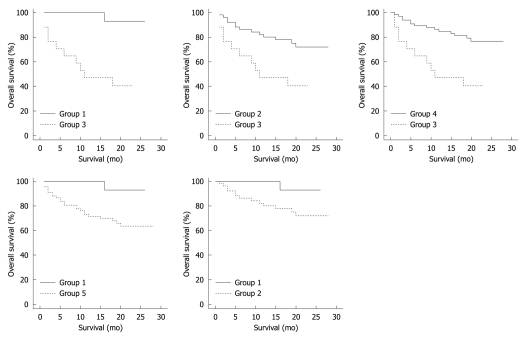
Kaplan-Meier plots comparing overall survival rates of groups 1, 2, 3, 4 and 5 are shown in Figure 2. Using log-rank test, such analysis revealed statistically significant differences between survival rates of CRC patient groups with different multiplex RT-PCR detection patterns. When patients with positive detection for only one marker (group 1) were compared to those positive for all three markers (group 3), a statistically significant difference in survival rates was observed (P = 0.002). Similarly, the comparison between the patient group with positive detections for two markers (group 2) and positive detections for all 3 markers (group 3) revealed significant differences in survival rates (P = 0.007). Likewise, significant survival differences were documented when survival of group 4 (groups 1 and 2) was compared to survival of group 3 (P = 0.001). In addition, significant differences in the survival of CRC patients were also found when group 1 was compared with group 5 (at least positive for 2 transcripts) (P = 0.041). No statistical significance was observed when group 1 was compared to group 2 (P = 0.116).
Figure 2.
Kaplan-Meier analysis comparing the overall survival of patient groups as developed according to transcript detection patterns for carcinoembryonic antigen, cytokeratin 20 and epidermal growth factor receptor. Group 1: Carcinoembryonic antigen (CEA) positive; Group 2: Positive for 2 markers (CEA and cytokeratin 20); Group 3: Positive for all markers; Group 4: Combination of groups 1 and 2; Group 5: Combination of groups 2 and 3. Log Rank test P values were as follows: Groups 1 vs 3: P = 0.002; Groups 2 vs 3: P = 0.007; Groups 4 vs 3: P = 0.001; Groups 1 vs 5: P = 0.041; Groups 1 vs 2: P = 0.116.
DISCUSSION
The advantages of multi-marker use for CTC detection in CRC patients have been previously described[5,44-46]. Several studies have shown that the use of more than one marker independently increases the sensitivity and the specificity of CTC detection which correlates with disease stage in CRC patients[47-51]. However, the application of separate PCR reactions for each marker can be impractical, time-consuming and costly. Conversely, the implementation of multiplex RT-PCR which allows the examination of multiple marker expression in a single reaction could be advantageous. The employment of multiplex RT-PCR on peripheral blood samples represents an easily applied, non-invasive technique for the detection of CTCs in cancer patients[52].
The aim of the present study was to develop first and then to test the clinical significance of a multiplex RT-PCR-based detection of three markers (CEA, CK20 and EGFR) in CRC patients. Since we used a short follow-up period, our main analysis was based on overall survival and not on progression-free survival.
Our multiplex PCR-based detections of CEA, CK20 and EGFR transcripts were characterized by very good specificity, since none of the 40 healthy donor samples was found positive for the expression of CK20 or EGFR mRNA and only 3 control samples tested positive for CEA transcripts. This revealed that our assay was of higher specificity than that of previous studies[30,31,53,54]. The observed false positive results in control subjects could be attributed to illegitimate transcription of CEA in mononuclear blood cells. Indeed, it has been reported that CEA mRNA is detected in peripheral blood CD34+ progenitor cells[55]. Based on our data, CEA detection was the most sensitive (expressed in 95.5% of the CRC patients); the positivity rate was higher than that observed by Guadagni et al[22] or by Ferroni et al[56]. This, however, may be due to clinicopathological characteristics of the enrolled patients in this study and our experimental setting. Moreover, our data suggested that EGFR is possibly a marker for advanced stage in CRC (expressed in 19.3% of the patients and mostly in advanced disease). CK20 detection corresponded to a detection rate of 78.4%, higher than that reported previously by other groups[15,31,57,58]. Again, these detection rate discrepancies among studies using the same mRNA markers can be attributed to patient characteristics, as well as to the particular experimental conditions used by each research group.
Herein, our results revealed that the increasing number of the transcripts detected in this experimental setting could distinguish certain patient groups having significantly different overall survival. However, the detection of 1 (CEA) vs the detection of 2 markers (CEA plus CK20) did not show any statistically significant difference in overall survival (P = 0.116), which may be attributed to the short follow-up period. Notably, none of the CRC patients who tested negative for all 3 markers died during follow-up. These results are in concert with the results of Wang et al[48], who examined a membrane array-based detection of 4 mRNA markers, including CEA and CK20, in 157 CRC patients and found that the positive detection of all 4 markers (human telomerase reverse transcriptase, CK19, CK20, and CEA) was associated with poor overall survival. Similar results were reported by Uen et al[47], analyzing the detection of the same transcripts with progression-free survival in CRC patients.
In addition, Spearman’s test revealed a positive correlation of positive detection for all 3 transcripts in our assay with higher disease stage and preoperative serum CEA levels. Furthermore, a suggestive correlation was also evident between positive detection of transcripts and higher level of lymph node involvement, suggesting that the higher number of tumor markers detected as positive by multiplex PCR assay is associated with higher levels of serum CEA and a larger amount of lymph node involvement, which are considered as indicators of advanced disease. This is in concert with the findings of Yeh et al[49]. Other studies have also examined the detection of CEA, CK20 or EGFR transcripts, however, using single PCR-based assays and reported that the higher number of positive detections correlated with disease stage[18,22,27,59] and serum CEA levels[38,60,61] or the presence of lymph node metastasis[58,62,63].
Herein, the increased number of positive multiplex PCR-based detections in each blood sample correlated with overall survival in CRC patients. Although the detection of the three marker panel was not found to be an independent prognostic factor of overall survival using Cox regression analysis, the log-rank test documented significant differences in overall survival rates among the patient groups formed according to the number of positive detections in each blood sample of CRC patients.
Nevertheless, our data suggested that our multiplex RT-PCR assay can provide useful information concerning CRC stage and overall survival. The combination of the mRNA markers in a single reaction could be of clinical value in the early detection of disseminated disease and monitoring of CRC patients, as it has been documented in other malignancies[5,41-45,64,65]. Future larger-scale studies and longer follow-up surveys would prove the clinical significance and prognostic importance of multiplex PCR-based detection of CTCs using CEA, CK20 and EGFR in CRC patients.
COMMENTS
Background
Colorectal cancer (CRC) is one of the most frequently diagnosed malignancies in both men and women. Overall survival is associated with the disease stage at the time of diagnosis, suggesting that early detection of disseminated disease may be of great significance. Detection of disseminated tumor cells in the peripheral blood of CRC patients could be promising for the early detection of disseminated disease and monitoring of CRC patients.
Research frontiers
Various techniques have been used for the detection of circulating tumor cells (CTCs) derived by solid tumors; among those, reverse transcription polymerase chain reaction (RT-PCR)-based methods have allowed the sensitive detection of CTCs in peripheral blood samples. Among several molecular markers, cytokeratin 20 (CK20), carcinoembryonic antigen (CEA) and epidermal growth factor receptor (EGFR) are those more frequently used for CTC detection in CRC using PCR-based methods.
Innovations and breakthroughs
Several studies have shown that the use of more than one marker independently increases the sensitivity and the specificity of the CTC detection in CRC patients. However, the application of separate PCR reactions for each marker can be impractical, while the implementation of multiplex RT-PCR allows the examination of multiple marker expression in a single reaction. The employment of multiplex RT-PCR in peripheral blood samples represents an easily applied, non-invasive technique for the detection of CTCs in cancer patients. The results showed that multiplex RT-PCR assay can provide useful information concerning CRC stage and overall survival.
Applications
The combination of mRNA markers in a single reaction could be of clinical value in the early detection of disseminated disease and monitoring of CRC patients. Future larger-scale studies and longer follow-up surveys would prove the clinical significance and prognostic importance of multiplex PCR-based detection of CTCs using CEA, CK20 and EGFR mRNA in CRC patients.
Terminology
CK20 is a member of the intermediate filament protein family and a prominent component of intestinal epithelium. CEA is a high molecular weight glycoprotein that plays a role in CRC metastasis. EGFR exerts control over normal cell growth and cancer pathogenesis in humans. Their mRNAs can be used as markers for the identification of CTCs in peripheral blood samples of CRC patients.
Peer review
This is a well written and produced paper with an important message. This original paper uses multiplex PCR assay for CK20, CEA and EGFR on blood samples to give information about CRC stage and prognosis. The authors have only studied small numbers of cases but the initial results are very encouraging.
Footnotes
Supported by The Ministry of Development of the Greek Government (GGET-AKMON)
Peer reviewers: Dr. John B Schofield, MB, BS, MRCP, FRCP, Department of Cellular Pathology, Preston Hall, Maidstone, Kent, ME20 7NH, United Kingdom; Yoshihisa Takahashi, MD, Department of Pathology, Teikyo University School of Medicine, 2-11-1 Kaga, Itabashi-ku, Tokyo 173-8605, Japan
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Mutch MG. Molecular profiling and risk stratification of adenocarcinoma of the colon. J Surg Oncol. 2007;96:693–703. doi: 10.1002/jso.20915. [DOI] [PubMed] [Google Scholar]
- 3.O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 4.Ghossein RA, Bhattacharya S. Molecular detection and characterisation of circulating tumour cells and micrometastases in solid tumours. Eur J Cancer. 2000;36:1681–1694. doi: 10.1016/s0959-8049(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 5.Tsouma A, Aggeli C, Pissimissis N, Lembessis P, Zografos GN, Koutsilieris M. Circulating tumor cells in colorectal cancer: detection methods and clinical significance. Anticancer Res. 2008;28:3945–3960. [PubMed] [Google Scholar]
- 6.Khair G, Monson JR, Greenman J. Epithelial molecular markers in the peripheral blood of patients with colorectal cancer. Dis Colon Rectum. 2007;50:1188–1203. doi: 10.1007/s10350-006-0875-9. [DOI] [PubMed] [Google Scholar]
- 7.Zieglschmid V, Hollmann C, Böcher O. Detection of disseminated tumor cells in peripheral blood. Crit Rev Clin Lab Sci. 2005;42:155–196. doi: 10.1080/10408360590913696. [DOI] [PubMed] [Google Scholar]
- 8.Moll R, Schiller DL, Franke WW. Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol. 1990;111:567–580. doi: 10.1083/jcb.111.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moll R, Löwe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140:427–447. [PMC free article] [PubMed] [Google Scholar]
- 10.Moll R, Zimbelmann R, Goldschmidt MD, Keith M, Laufer J, Kasper M, Koch PJ, Franke WW. The human gene encoding cytokeratin 20 and its expression during fetal development and in gastrointestinal carcinomas. Differentiation. 1993;53:75–93. doi: 10.1111/j.1432-0436.1993.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher RH. Carcinoembryonic antigen. Ann Intern Med. 1986;104:66–73. doi: 10.7326/0003-4819-104-1-66. [DOI] [PubMed] [Google Scholar]
- 12.Berman JM, Cheung RJ, Weinberg DS. Surveillance after colorectal cancer resection. Lancet. 2000;355:395–399. doi: 10.1016/S0140-6736(99)06552-6. [DOI] [PubMed] [Google Scholar]
- 13.Arnaud JP, Koehl C, Adloff M. Carcinoembryonic antigen (CEA) in diagnosis and prognosis of colorectal carcinoma. Dis Colon Rectum. 1980;23:141–144. doi: 10.1007/BF02587615. [DOI] [PubMed] [Google Scholar]
- 14.Harrison LE, Guillem JG, Paty P, Cohen AM. Preoperative carcinoembryonic antigen predicts outcomes in node-negative colon cancer patients: a multivariate analysis of 572 patients. J Am Coll Surg. 1997;185:55–59. doi: 10.1016/s1072-7515(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XW, Yang HY, Fan P, Yang L, Chen GY. Detection of micrometastasis in peripheral blood by multi-sampling in patients with colorectal cancer. World J Gastroenterol. 2005;11:436–438. doi: 10.3748/wjg.v11.i3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weitz J, Kienle P, Lacroix J, Willeke F, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res. 1998;4:343–348. [PubMed] [Google Scholar]
- 17.Fujita S, Kudo N, Akasu T, Moriya Y. Detection of cytokeratin 19 and 20 mRNA in peripheral and mesenteric blood from colorectal cancer patients and their prognosis. Int J Colorectal Dis. 2001;16:141–146. doi: 10.1007/s003840100286. [DOI] [PubMed] [Google Scholar]
- 18.Kienle P, Koch M, Autschbach F, Benner A, Treiber M, Wannenmacher M, von Knebel Doeberitz M, Büchler M, Herfarth C, Weitz J. Decreased detection rate of disseminated tumor cells of rectal cancer patients after preoperative chemoradiation: a first step towards a molecular surrogate marker for neoadjuvant treatment in colorectal cancer. Ann Surg. 2003;238:324–330; discussion 330-331. doi: 10.1097/01.sla.0000086547.27615.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232:58–65. doi: 10.1097/00000658-200007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadahiro S, Suzuki T, Maeda Y, Yurimoto S, Yasuda S, Makuuchi H, Kamijo A, Murayama C. Detection of carcinoembryonic antigen messenger RNA-expressing cells in peripheral blood 7 days after curative surgery is a novel prognostic factor in colorectal cancer. Ann Surg Oncol. 2007;14:1092–1098. doi: 10.1245/s10434-006-9289-0. [DOI] [PubMed] [Google Scholar]
- 21.Noh YH, Kim JA, Lim GR, Ro YT, Koo JH, Lee YS, Han DS, Park HK, Ahn MJ. Detection of circulating tumor cells in patients with gastrointestinal tract cancer using RT-PCR and its clinical implications. Exp Mol Med. 2001;33:8–14. doi: 10.1038/emm.2001.2. [DOI] [PubMed] [Google Scholar]
- 22.Guadagni F, Kantor J, Aloe S, Carone MD, Spila A, D'Alessandro R, Abbolito MR, Cosimelli M, Graziano F, Carboni F, et al. Detection of blood-borne cells in colorectal cancer patients by nested reverse transcription-polymerase chain reaction for carcinoembryonic antigen messenger RNA: longitudinal analyses and demonstration of its potential importance as an adjunct to multiple serum markers. Cancer Res. 2001;61:2523–2532. [PubMed] [Google Scholar]
- 23.Hildebrandt M, Mapara MY, Körner IJ, Bargou RC, Moldenhauer G, Dörken B. Reverse transcriptase-polymerase chain reaction (RT-PCR)-controlled immunomagnetic purging of breast cancer cells using the magnetic cell separation (MACS) system: a sensitive method for monitoring purging efficiency. Exp Hematol. 1997;25:57–65. [PubMed] [Google Scholar]
- 24.Leitzel K, Lieu B, Curley E, Smith J, Chinchilli V, Rychlik W, Lipton A. Detection of cancer cells in peripheral blood of breast cancer patients using reverse transcription-polymerase chain reaction for epidermal growth factor receptor. Clin Cancer Res. 1998;4:3037–3043. [PubMed] [Google Scholar]
- 25.Gradilone A, Gazzaniga P, Silvestri I, Gandini O, Trasatti L, Lauro S, Frati L, Aglianò AM. Detection of CK19, CK20 and EGFR mRNAs in peripheral blood of carcinoma patients: correlation with clinical stage of disease. Oncol Rep. 2003;10:217–222. [PubMed] [Google Scholar]
- 26.Gazzaniga P, Nofroni I, Gandini O, Silvestri I, Frati L, Aglianò AM, Gradilone A. Tenascin C and epidermal growth factor receptor as markers of circulating tumoral cells in bladder and colon cancer. Oncol Rep. 2005;14:1199–1202. [PubMed] [Google Scholar]
- 27.Giacomelli L, Gianni W, Belfiore C, Gandini O, Repetto L, Filippini A, Frati L, Aglianò AM, Gazzaniga P. Persistence of epidermal growth factor receptor and interleukin 10 in blood of colorectal cancer patients after surgery identifies patients with high risk to relapse. Clin Cancer Res. 2003;9:2678–2682. [PubMed] [Google Scholar]
- 28.Gazzaniga P, Gandini O, Giuliani L, Magnanti M, Gradilone A, Silvestri I, Gianni W, Gallucci M, Frati L, Aglianò AM. Detection of epidermal growth factor receptor mRNA in peripheral blood: a new marker of circulating neoplastic cells in bladder cancer patients. Clin Cancer Res. 2001;7:577–583. [PubMed] [Google Scholar]
- 29.Clarke LE, Leitzel K, Smith J, Ali SM, Lipton A. Epidermal growth factor receptor mRNA in peripheral blood of patients with pancreatic, lung, and colon carcinomas detected by RT-PCR. Int J Oncol. 2003;22:425–430. [PubMed] [Google Scholar]
- 30.De Luca A, Pignata S, Casamassimi A, D'Antonio A, Gridelli C, Rossi A, Cremona F, Parisi V, De Matteis A, Normanno N. Detection of circulating tumor cells in carcinoma patients by a novel epidermal growth factor receptor reverse transcription-PCR assay. Clin Cancer Res. 2000;6:1439–1444. [PubMed] [Google Scholar]
- 31.Wyld DK, Selby P, Perren TJ, Jonas SK, Allen-Mersh TG, Wheeldon J, Burchill SA. Detection of colorectal cancer cells in peripheral blood by reverse-transcriptase polymerase chain reaction for cytokeratin 20. Int J Cancer. 1998;79:288–293. doi: 10.1002/(sici)1097-0215(19980619)79:3<288::aid-ijc14>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Friederichs J, Gertler R, Rosenberg R, Nahrig J, Führer K, Holzmann B, Dittler HJ, Dahm M, Thorban S, Nekarda H, et al. Prognostic impact of CK-20-positive cells in peripheral venous blood of patients with gastrointestinal carcinoma. World J Surg. 2005;29:422–428. doi: 10.1007/s00268-004-7662-3. [DOI] [PubMed] [Google Scholar]
- 33.Giribaldi G, Procida S, Ulliers D, Mannu F, Volpatto R, Mandili G, Fanchini L, Bertetto O, Fronda G, Simula L, et al. Specific detection of cytokeratin 20-positive cells in blood of colorectal and breast cancer patients by a high sensitivity real-time reverse transcriptase-polymerase chain reaction method. J Mol Diagn. 2006;8:105–112. doi: 10.2353/jmoldx.2006.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch M, Kienle P, Sauer P, Willeke F, Buhl K, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M, Weitz J. Hematogenous tumor cell dissemination during colonoscopy for colorectal cancer. Surg Endosc. 2004;18:587–591. doi: 10.1007/s00464-003-9066-0. [DOI] [PubMed] [Google Scholar]
- 35.Molnar B, Ladanyi A, Tanko L, Sréter L, Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res. 2001;7:4080–4085. [PubMed] [Google Scholar]
- 36.Wharton RQ, Jonas SK, Glover C, Khan ZA, Klokouzas A, Quinn H, Henry M, Allen-Mersh TG. Increased detection of circulating tumor cells in the blood of colorectal carcinoma patients using two reverse transcription-PCR assays and multiple blood samples. Clin Cancer Res. 1999;5:4158–4163. [PubMed] [Google Scholar]
- 37.Lankiewicz S, Rother E, Zimmermann S, Hollmann C, Korangy F, Greten TF. Tumour-associated transcripts and EGFR deletion variants in colorectal cancer in primary tumour, metastases and circulating tumour cells. Cell Oncol. 2008;30:463–471. doi: 10.3233/CLO-2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva JM, Rodriguez R, Garcia JM, Muñoz C, Silva J, Dominguez G, Provencio M, España P, Bonilla F. Detection of epithelial tumour RNA in the plasma of colon cancer patients is associated with advanced stages and circulating tumour cells. Gut. 2002;50:530–534. doi: 10.1136/gut.50.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bostick PJ, Chatterjee S, Chi DD, Huynh KT, Giuliano AE, Cote R, Hoon DS. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol. 1998;16:2632–2640. doi: 10.1200/JCO.1998.16.8.2632. [DOI] [PubMed] [Google Scholar]
- 40.Mapara MY, Körner IJ, Hildebrandt M, Bargou R, Krahl D, Reichardt P, Dörken B. Monitoring of tumor cell purging after highly efficient immunomagnetic selection of CD34 cells from leukapheresis products in breast cancer patients: comparison of immunocytochemical tumor cell staining and reverse transcriptase-polymerase chain reaction. Blood. 1997;89:337–344. [PubMed] [Google Scholar]
- 41.Lembessis P, Msaouel P, Halapas A, Sourla A, Panteleakou Z, Pissimissis N, Milathianakis C, Bogdanos J, Papaioannou A, Maragoudakis E, et al. Combined androgen blockade therapy can convert RT-PCR detection of prostate-specific antigen (PSA) and prostate-specific membrane antigen (PSMA) transcripts from positive to negative in the peripheral blood of patients with clinically localized prostate cancer and increase biochemical failure-free survival after curative therapy. Clin Chem Lab Med. 2007;45:1488–1494. doi: 10.1515/CCLM.2007.301. [DOI] [PubMed] [Google Scholar]
- 42.Koutsilieris M, Lembessis P, Luu-The V, Sourla A. Repetitive and site-specific molecular staging of prostate cancer using nested reverse transcriptase polymerase chain reaction for prostate specific antigen and prostate specific membrane antigen. Clin Exp Metastasis. 1999;17:823–830. doi: 10.1023/a:1006783330996. [DOI] [PubMed] [Google Scholar]
- 43.Mitsiades CS, Lembessis P, Sourla A, Milathianakis C, Tsintavis A, Koutsilieris M. Molecular staging by RT-pCR analysis for PSA and PSMA in peripheral blood and bone marrow samples is an independent predictor of time to biochemical failure following radical prostatectomy for clinically localized prostate cancer. Clin Exp Metastasis. 2004;21:495–505. doi: 10.1007/s10585-004-3217-0. [DOI] [PubMed] [Google Scholar]
- 44.Nezos A, Pissimisis N, Lembessis P, Sourla A, Dimopoulos P, Dimopoulos T, Tzelepis K, Koutsilieris M. Detection of circulating tumor cells in bladder cancer patients. Cancer Treat Rev. 2009;35:272–279. doi: 10.1016/j.ctrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Panteleakou Z, Lembessis P, Sourla A, Pissimissis N, Polyzos A, Deliveliotis C, Koutsilieris M. Detection of circulating tumor cells in prostate cancer patients: methodological pitfalls and clinical relevance. Mol Med. 2009;15:101–114. doi: 10.2119/molmed.2008.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nezos A, Lembessis P, Sourla A, Pissimissis N, Gogas H, Koutsilieris M. Molecular markers detecting circulating melanoma cells by reverse transcription polymerase chain reaction: methodological pitfalls and clinical relevance. Clin Chem Lab Med. 2009;47:1–11. doi: 10.1515/CCLM.2009.009. [DOI] [PubMed] [Google Scholar]
- 47.Uen YH, Lin SR, Wu DC, Su YC, Wu JY, Cheng TL, Chi CW, Wang JY. Prognostic significance of multiple molecular markers for patients with stage II colorectal cancer undergoing curative resection. Ann Surg. 2007;246:1040–1046. doi: 10.1097/SLA.0b013e318142d918. [DOI] [PubMed] [Google Scholar]
- 48.Wang JY, Lin SR, Wu DC, Lu CY, Yu FJ, Hsieh JS, Cheng TL, Koay LB, Uen YH. Multiple molecular markers as predictors of colorectal cancer in patients with normal perioperative serum carcinoembryonic antigen levels. Clin Cancer Res. 2007;13:2406–2413. doi: 10.1158/1078-0432.CCR-06-2054. [DOI] [PubMed] [Google Scholar]
- 49.Yeh CS, Wang JY, Wu CH, Chong IW, Chung FY, Wang YH, Yu YP, Lin SR. Molecular detection of circulating cancer cells in the peripheral blood of patients with colorectal cancer by using membrane array with a multiple mRNA marker panel. Int J Oncol. 2006;28:411–420. [PubMed] [Google Scholar]
- 50.Conzelmann M, Linnemann U, Berger MR. Molecular detection of clinical colorectal cancer metastasis: how should multiple markers be put to use? Int J Colorectal Dis. 2005;20:137–146. doi: 10.1007/s00384-004-0640-2. [DOI] [PubMed] [Google Scholar]
- 51.Gervasoni A, Monasterio Muñoz RM, Wengler GS, Rizzi A, Zaniboni A, Parolini O. Molecular signature detection of circulating tumor cells using a panel of selected genes. Cancer Lett. 2008;263:267–279. doi: 10.1016/j.canlet.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Taback B, Chan AD, Kuo CT, Bostick PJ, Wang HJ, Giuliano AE, Hoon DS. Detection of occult metastatic breast cancer cells in blood by a multimolecular marker assay: correlation with clinical stage of disease. Cancer Res. 2001;61:8845–8850. [PubMed] [Google Scholar]
- 53.Wong IH, Yeo W, Chan AT, Johnson PJ. Quantitative relationship of the circulating tumor burden assessed by reverse transcription-polymerase chain reaction for cytokeratin 19 mRNA in peripheral blood of colorectal cancer patients with Dukes' stage, serum carcinoembryonic antigen level and tumor progression. Cancer Lett. 2001;162:65–73. doi: 10.1016/s0304-3835(00)00630-3. [DOI] [PubMed] [Google Scholar]
- 54.Vlems FA, Diepstra JH, Cornelissen IM, Ruers TJ, Ligtenberg MJ, Punt CJ, van Krieken JH, Wobbes T, van Muijen GN. Limitations of cytokeratin 20 RT-PCR to detect disseminated tumour cells in blood and bone marrow of patients with colorectal cancer: expression in controls and downregulation in tumour tissue. Mol Pathol. 2002;55:156–163. doi: 10.1136/mp.55.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fava TA, Desnoyers R, Schulz S, Park J, Weinberg D, Mitchell E, Waldman SA. Ectopic expression of guanylyl cyclase C in CD34+ progenitor cells in peripheral blood. J Clin Oncol. 2001;19:3951–3959. doi: 10.1200/JCO.2001.19.19.3951. [DOI] [PubMed] [Google Scholar]
- 56.Ferroni P, Roselli M, Spila A, D'Alessandro R, Portarena I, Mariotti S, Palmirotta R, Buonomo O, Petrella G, Guadagni F. Serum sE-selectin levels and carcinoembryonic antigen mRNA-expressing cells in peripheral blood as prognostic factors in colorectal cancer patients. Cancer. 2010;116:2913–2921. doi: 10.1002/cncr.25094. [DOI] [PubMed] [Google Scholar]
- 57.Katsumata K, Sumi T, Mori Y, Hisada M, Tsuchida A, Aoki T. Detection and evaluation of epithelial cells in the blood of colon cancer patients using RT-PCR. Int J Clin Oncol. 2006;11:385–389. doi: 10.1007/s10147-006-0590-5. [DOI] [PubMed] [Google Scholar]
- 58.Dandachi N, Balic M, Stanzer S, Halm M, Resel M, Hinterleitner TA, Samonigg H, Bauernhofer T. Critical evaluation of real-time reverse transcriptase-polymerase chain reaction for the quantitative detection of cytokeratin 20 mRNA in colorectal cancer patients. J Mol Diagn. 2005;7:631–637. doi: 10.1016/S1525-1578(10)60597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen C, Hu L, Xia L, Li Y. Quantitative real-time RT-PCR detection for survivin, CK20 and CEA in peripheral blood of colorectal cancer patients. Jpn J Clin Oncol. 2008;38:770–776. doi: 10.1093/jjco/hyn105. [DOI] [PubMed] [Google Scholar]
- 60.Friederichs J, Gertler R, Rosenberg R, Dahm M, Nekarda H, Holzmann B, Siewert JR. Correlation of CK-20-positive cells in peripheral venous blood with serum CEA levels in patients with colorectal carcinoma. World J Surg. 2007;31:2329–2334. doi: 10.1007/s00268-007-9149-5. [DOI] [PubMed] [Google Scholar]
- 61.Vlems FA, Diepstra JH, Punt CJ, Ligtenberg MJ, Cornelissen IM, van Krieken JH, Wobbes T, van Muijen GN, Ruers TJ. Detection of disseminated tumour cells in blood and bone marrow samples of patients undergoing hepatic resection for metastasis of colorectal cancer. Br J Surg. 2003;90:989–995. doi: 10.1002/bjs.4161. [DOI] [PubMed] [Google Scholar]
- 62.Iinuma H, Okinaga K, Egami H, Mimori K, Hayashi N, Nishida K, Adachi M, Mori M, Sasako M. Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int J Oncol. 2006;28:297–306. [PubMed] [Google Scholar]
- 63.Guo J, Xiao B, Zhang X, Jin Z, Chen J, Qin L, Mao X, Shen G, Chen H, Liu Z. Combined use of positive and negative immunomagnetic isolation followed by real-time RT-PCR for detection of the circulating tumor cells in patients with colorectal cancers. J Mol Med. 2004;82:768–774. doi: 10.1007/s00109-004-0590-8. [DOI] [PubMed] [Google Scholar]
- 64.Mitropapas G, Nezos A, Halapas A, Pissimissis N, Lembessis P, Sourla A, Vassilopoulos P, Koutsilieris M. Molecular detection of tyrosinase transcripts in peripheral blood from patients with malignant melanoma: correlation of PCR sensitivity threshold with clinical and pathologic disease characteristics. Clin Chem Lab Med. 2006;44:1403–1409. doi: 10.1515/CCLM.2006.260. [DOI] [PubMed] [Google Scholar]
- 65.Karavitaki N, Lembessis P, Tzanela M, Vlassopoulou V, Thalassinos N, Koutsilieris M. Molecular staging using qualitative RT-PCR analysis detecting thyreoglobulin mRNA in the peripheral blood of patients with differentiated thyroid cancer after therapy. Anticancer Res. 2005;25:3135–3142. [PubMed] [Google Scholar]