Abstract
AIM: To determine whether the association of β-blockers with endoscopic treatment is superior to endoscopic treatment alone for the secondary prophylaxis of oesophageal variceal bleeding.
METHODS: Randomised controlled trials comparing sclerotherapy (SCL) with SCL plus β-blockers (BB) or banding ligation (BL) with BL plus BB were identified. Main outcomes were overall and 6, 12 and 24 mo rebleeding rates, as well as overall and 6, 12 and 24 mo mortality. Two statistical methods were used: Yusuf-Peto, and Der Simonian and Laird. Inter-trial heterogeneity was systematically taken into account.
RESULTS: Seventeen randomised controlled trials were included, 14 with SCL and 3 with BL. Combination β-blocker and endoscopic treatment significantly reduced rebleeding rates at 6, 12 and 24 mo and overall [odds ratio (OR): 2.20, 95% confidence interval (CI): 1.69-2.85, P < 0.0001] compared to endoscopic treatment alone. Mortality at 24 mo was significantly lower for the combined treatment group (OR: 1.83, 95% CI: 1.16-2.90, P = 0.009), as well as overall mortality (OR: 1.43, 95% CI: 1.03-1.98, P = 0.03).
CONCLUSION: Combination therapy should thus be recommended as the first line treatment for secondary prophylaxis of oesophageal variceal bleeding.
Keywords: Oesophageal varices, Portal hypertension, Cirrhosis, Secondary prevention, β-blockers, Banding ligation
INTRODUCTION
Upper gastrointestinal bleeding from ruptured oesophageal varices is the main complication of portal hypertension and is one of the leading causes of death in patients with cirrhosis. It is estimated that 70% of patients who have survived a first episode of variceal bleeding subsequently rebleed[1]. Secondary prophylaxis of variceal bleeding is thus of utmost importance for these patients.
The use of β-blockers alone has been shown to decrease the risk of recurrent bleeding in cirrhotic patients[2-4]. Endoscopic treatments [sclerotherapy (SCL) and banding ligation (BL)] are also effective in preventing rebleeding in patients with portal hypertension. It has been demonstrated that sclerotherapy decreases the risk of recurrent bleeding and improves survival in cirrhotic patients compared to placebo[5,6]. Banding ligation is currently the endoscopic therapy of choice as it has been shown to be safer and more effective in preventing rebleeding than sclerotherapy[7-10]. However, sclerotherapy continues to be routinely used in a certain number of countries. Combining pharmacological and endoscopic treatment may be more effective than either treatment alone as they act through different mechanisms, thus enhancing each other’s therapeutic effect. Many randomised controlled trials have been undertaken to determine whether β-blockers have an additional beneficial role when combined with sclerotherapy or banding ligation to prevent rebleeding. The results of these studies are controversial. Sørensen[11] suggested that β-blockers and sclerotherapy had no additive effect in the prevention of variceal rebleeding, whereas a number of trials have reported favourable results when banding ligation is combined with β-blockers[12,13]. The recommendations of the American College of Gastroenterology[14] state that combined endoscopic and pharmacological therapy is the most effective for secondary prophylaxis, whereas the Baveno IV Consensus conference[15] recommends either β-blockers, banding ligation or both; the authors add that combined therapy is probably the best treatment, but that more trials are needed to prove this. The aim of this meta-analysis is therefore to determine whether the association of β-blockers with endoscopic treatment (sclerotherapy or banding ligation) leads to decreased rebleeding rates and improved survival in patients with portal hypertension compared to endoscopic treatment alone.
MATERIALS AND METHODS
Source of trials
All randomised controlled trials comparing endoscopic treatment alone (sclerotherapy or banding ligation) with a combination of endoscopic treatment associated with β-blockers for the prevention of variceal rebleeding were identified. Trial were retrieved using MEDLINE (1950 to October 2009) and Web of Science (1900 to October 2009), by using the terms “secondary prophylaxis variceal bleeding, endoscopic therapy, β-blockers, sclerotherapy and variceal rebleeding”. Conference abstracts from the American Association for the Study of the Liver, European Association for the Study of the Liver, the Digestive Disease Week, the United European Gastroenterology Week and the French National Society of Gastro-enterology between 1980 and 2009 were manually searched. The list of published articles or abstracts was verified and completed through an in depth study of the references quoted in each article. All trials included were peer-reviewed (full articles published in peer reviewed journals or abstracts selected by a peer committee). The final search was performed on April 30, 2010.
Study selection
The trials selected for this meta-analysis met the following criteria: (1) randomised controlled trials published as abstracts, letters to the editor or peer-reviewed articles; (2) patient population: patients presenting portal hypertension (with or without cirrhosis) and oesophageal varices which had previously bled and had not received prior β-blocker or endoscopic treatment; and (3) interventions: treatment with sclerotherapy or banding ligation alone or concomitant treatment with β-blockers (administered from the start of endoscopic treatment) and sclerotherapy or banding ligation; in parallel treatment groups. We chose to exclude studies which associated β-blockers with nitrates or studies in which β-blockers were commenced only after completion of endoscopic therapy. No language restrictions were applied.
Data extraction and quality assessment
Data extraction and quality assessment were performed by three independent reviewers (Ségalas-Largey F, Oberti F and Blanc P). Discrepancies were resolved through discussion before analyses. Trial quality was evaluated using criteria established by Poynard[16] and Nicolucci et al[17], simplified and adapted for portal hypertension by Pagliaro et al[18]. For each trial, two separate scores were calculated using Poynard’s and Pagliaro’s criteria; each score was expressed as a percentage of the highest score possible. In addition, we noted for each trial randomisation, investigator blinding, estimation of sample size and intention-to-treat analysis.
Data synthesis and analysis
Primary end-points were all-cause rebleeding rates and mortality at 6, 12 and 24 mo, as well as overall rebleeding and mortality rates as reported at end of follow-up. All-cause rebleeding was defined as upper gastrointestinal bleeding of any source. We chose this as the primary outcome as endoscopic treatment of oesophageal varices may induce bleeding related to oesophageal ulcers. Results were determined at different time points in order to take into account variations in follow-up duration between trials. For each time point, raw data was extracted from studies when available using Kaplan-Meier survival curves.
A meta-analysis including all trials (sclerotherapy and banding ligation) was performed, as well as subgroup analyses of trials using sclerotherapy and trials using banding ligation.
Statistical methods
All results were expressed as odds ratio (OR), with a 95% confidence interval (CI). Results were first calculated using a fixed-effect model (Collins et al[19]). Heterogeneity was calculated using Breslow-Day’s test. When significant heterogeneity was found, both the OR and P-heterogeneity were subsequently calculated using a random-effect model (DerSimonian et al[20]). This model takes into account heterogeneity by providing a more conservative estimate of treatment effect with wider confidence intervals in order to adjust for inter-trial variability. P-heterogeneity was initially considered significant if < 0.05; however, when P-heterogeneity tended towards significance (i.e. between 0.05 and 0.10), we preferred to take the precaution of calculating results using the random-effect model. The percentage of variability beyond chance was estimated using the I2 statistic[21]. Publication bias was assessed using the Egger Test[22] and represented graphically using funnel plots[23] plotting the natural log of the OR vs its standard error. The Trim and Fill analysis for publication bias was performed using Duval and Tweedie’s methods[24]. Additionally, the failsafe number according to Orwin’s formula[25] was calculated, which represents the number of non-significant studies which would be necessary to reduce the effect size to a non-significant value. All analyses were performed using Comprehensive Meta-analysis software (version 2.2.048 New Jersey, USA, 2008).
RESULTS
Study identification and selection
Our search identified 229 potentially relevant references. Two hundred and six reports were excluded when it was obvious they did not meet inclusion criteria. A total of 23 reports were considered for detailed analysis. Four studies were excluded as they associated β-blockers with nitrates[26-29]. Two studies were excluded as they started β-blocker therapy after completion of endoscopic therapy[30,31]. Seventeen studies were included in the meta-analysis.
Study characteristics
Study characteristics are reported in Table 1. Fourteen trials[32-45] comparing sclerotherapy with combination sclerotherapy plus β-blockers were included with a total of 925 patients. Among these 925 patients, 887 were analysed: 435 were treated by sclerotherapy alone and 452 by sclerotherapy plus β-blocker. Thirty-eight patients from the trial of Elsayed et al[34] were not included as they were excluded from the study after randomisation for various reasons (non compliance, loss to follow up, shunt surgery). Ten studies were published as full articles[33-37,40-44], three in abstract form[32,39,45] and one as a letter to the editor[38]. One study was published in Spanish[33].
Table 1.
Study characteristics
| Author | Country | Publication type | n | Length of follow-up (mo) |
| Combination therapy vs sclerotherapy | ||||
| Westaby et al[36] | United Kingdom | Article | 53 | 6 |
| Jensen et al[37] | Denmark | Article | 31 | 12 |
| Bertoni et al[38] | Italy | Letter | 28 | 2 |
| Gerunda et al[39] | Italy | Abstract | 60 | 6 |
| Lundell et al[40] | Sweden | Article | 41 | 8 |
| Kanazawa et al[32] | Japan | Abstract | 43 | 27 |
| Vinel et al[41] | France | Article | 74 | 3 |
| Acharya et al[42] | India | Article | 114 | 24 |
| Avgerinos et al[43] | Greece | Article | 85 | 23.9 |
| Villanueva et al[33] | Spain | Article | 40 | 26 |
| Vickers et al[44] | United Kingdom | Article | 73 | 24 |
| Elsayed et al[34] | Egypt | Article | 178 | 21 |
| Benedeto-Stojanov et al[45] | Yugoslavia | Abstract | 65 | 39 |
| Dowidar et al[35] | Egypt | Article | 40 | 16.2 |
| Combination therapy vs banding ligation | ||||
| Abdel-Rahim et al[46] | Egypt | Abstract | 50 | < 3 |
| Lo et al[12] | China | Article | 122 | 12 |
| de la Peña et al[13] | Spain | Article | 84 | 16 |
Three articles comparing banding ligation with combined banding ligation and β-blocker therapy were included, with a total of 256 patients[12,13,46]. Among these 256 patients, 252 were analysed. Four patients from the trial of de la Peña et al[13] were excluded after randomisation due to withdrawal (3 patients) or diagnosis of lymphoma (one patient). One hundred and twenty-five were treated with banding ligation alone and 128 with banding ligation plus β-blockers. Two trials were published as full articles[12,13] and one in abstract form[46].
Patient characteristics are shown in Table 2. Chronic liver disease was the most common cause of portal hypertension in all studies and was mainly due to alcohol abuse in 9 trials[12,33,36-41,44], to viral infection in 3 trials[12,42,43] and to schistosomiasis in 2 trials[34,35]. The aetiology of liver disease was not reported in three studies[32,45,46]. In 8 studies, only patients with cirrhosis were included[32,33,37,39-41,43,45]. Most patients had mild or severe liver disease in 9 studies[12,13,34-38,40,44]. However, in the study by Avgerinos et al[43], the majority of patients were Child’s class A, whereas in 3 trials Child’s class C patients were not included[33,39,42]. Severity of liver disease was either incompletely reported or not mentioned at all in 5 studies[32,39,41,45,46]. Variceal size was medium or large for the majority of patients in 11 trials[12,13,33,35-38,41-44] and was not reported in 5 others[32,34,39,40,45]. Sclerotherapy was performed with polidocanol in 5 studies[37,38,40-42], ethanolamine in 6 studies[33-36,43,44] and aetoxisclerol in one study[45]. The sclerosing agent was not reported in 2 trials[32,39]. Banding ligation was performed with multi-band ligators at intervals varying from 10 to 21 d, until complete variceal eradication was achieved. Propranolol was the β-blocker used in 12 trials[32,34-37,40-46] and nadolol in 5 trials[12,13,33,38,39]. The time to eradication of oesophageal varices was reported in 13 trials[12,13,33-38,40-44]. In the sclerotherapy plus β-blocker group the mean time to eradication of varices was slightly shorter (166 d-5.7 sessions) than in the sclerotherapy group (171 d-6 sessions). For patients treated with banding ligation, eradication was achieved in 44 d (3.3 sessions) vs 42 d (3.15 sessions) in the banding ligation plus β-blocker group. β-blockers were administered from the start of sclerotherapy until eradication in 7 studies[35-41], whereas in 5 studies[32-34,42,44], β-blockers were given both during and following variceal eradication. Follow-up duration for sclerotherapy studies ranged from 2 to 39 mo (mean 16.6 mo). Follow-up duration for banding ligation studies ranged from < 3 to 16 mo (mean 10.3 mo). Overall follow-up duration ranged from 2 to 39 mo (mean 15.4 mo).
Table 2.
Combination therapy vs banding ligation
| Author | Mean age (yr) | Men (%) | Non cirrhotic portal hypertension (%) | Alcoholic liver disease (%) | Child class A-B-C (%) | Size of varices small-medium-large (%) |
| Combination therapy vs sclerotherapy | ||||||
| Westaby et al[36] | 48.6 | 62 | 17 | 43 | 16-43-41 | 0-34-66 |
| Jensen et al[37] | 46.5 | 87 | 0 | 84 | 26-45-29 | 3-32-65 |
| Bertoni et al[38] | 59.1 | 64 | 0 | 57 | 32-32-36 | 7-46.5-46.5 |
| Gerunda et al[39] | NA | NA | 0 | 50 | NA | NA |
| Lundell et al[40] | 56.4 | 54 | 0 | 63 | 22-27-51 | NA |
| Kanazawa et al[32] | NA | NA | 0 | NA | NA | NA |
| Vinel et al[41] | 55.7 | 78 | 0 | 89 | NA | 0-32-68 |
| Acharya et al[42] | 34.7 | 85 | 11 | 7 | 60-40-0 | 0-0-100 |
| Avgerinos et al[43] | 58.2 | 72 | 0 | 26 | 74-19-7 | 8-45-47 |
| Villanueva et al[33] | 56.8 | 57.5 | 0 | 50 | 30-70-0 | 7.5-62.5-30 |
| Vickers et al[44] | 55.1 | 59 | 15 | 40 | 26-51-23 | 7-26-64 |
| Elsayed et al[34] | 43.0 | 84 | 0 | NA | 55-29-16 | NA |
| Benedeto-Stojanov et al[45] | NA | NA | 0 | NA | NA | NA |
| Dowidar et al[35] | 46.0 | 90 | 0 | 0 | 45-50-5 | 20-72.5-7.5 |
| Combination therapy vs banding ligation | ||||||
| Abdel-Rahim et al[46] | NA | NA | NA | NA | NA | NA |
| Lo et al[12] | 52.0 | 77 | 0 | 30 | 19-47-34 | 0-42-58 |
| de la Peña et al[13] | 60.0 | 75 | 0 | 66 | 15-56-29 | 21-54-25 |
NA: Not available.
Quality assessment
Variables defining study quality are presented in Table 3. All trials were correctly randomised. Three trials were double-blinded[12,37,42] and six trials were single-blinded[13,35,38,40,43,44]. Sample size was calculated in six trials. Six studies[12,40-44] were performed on intention-to-treat basis. The quality scores determined by Poynard’s criteria and by Pagliaro’s criteria were strongly correlated.
Table 3.
Combination therapy vs sclerotherapy
| Study | Random- isation | Investigator blinding | Estimate of sample size | Intention to treat analysis | Poynard’s quality score (%) | Pagliaro’s quality score (%) |
| Combination therapy vs sclerotherapy | ||||||
| Westaby et al[36] | Yes | No | No | No | 31 | 35 |
| Jensen et al[37] | Yes | Double blinded | No | No | 65 | 67 |
| Bertoni et al[38] | Yes | Single blinded | No | No | 54 | 44 |
| Gerunda et al[39] | Yes | No | No | No | 19 | 12 |
| Lundell et al[40] | Yes | Single blinded | No | Yes | 50 | 61 |
| Kanazawa et al[32] | Yes | No | No | No | 19 | 18 |
| Vinel et al[41] | Yes | No | Yes | Yes | 73 | 43 |
| Acharya et al[42] | Yes | Double blinded | Yes | Yes | 92 | 96 |
| Avgerinos et al[43] | Yes | Single blinded | Yes | Yes | 77 | 83 |
| Villanueva et al[33] | Yes | No | No | No | 55 | 67 |
| Vickers et al[44] | Yes | Single blinded | Yes | Yes | 83 | 74 |
| Elsayed et al[34] | Yes | No | No | No | 50 | 31 |
| Benedeto-Stojanov et al[45] | Yes | NA | NA | NA | 29 | 8 |
| Dowidar et al[35] | Yes | Single blinded | No | No | 54 | 50 |
| Combination therapy vs banding ligation | ||||||
| Abdel-Rahim et al[46] | Yes | NA | NA | NA | 8 | 17 |
| Lo et al[12] | Yes | Single blinded | Yes | Yes | 81 | 85 |
| de la Peña et al[13] | Yes | No | Yes | No | 69 | 82 |
NA: Not available.
Outcome measures
All-cause rebleeding: The all-cause rebleeding rate was reported in all studies except for the trial of Lundell et al[40]. For sclerotherapy trials, 162 patients rebleed in the sclerotherapy group (39.2%) whereas 110 patients (25.4%) rebleed in the sclerotherapy plus β-blocker group. Four sclerotherapy trials[32,34,37,41] found rebleeding rates to be significantly lower in the sclerotherapy plus β-blocker group; the difference was not significant for the remaining 10 studies. For banding ligation studies, 49 patients bled in the banding ligation only group (39.5%), whereas 22 patients bled in the banding ligation plus β-blocker group (17.2%). Two banding ligation studies found rebleeding rates to be significantly lower in the banding ligation plus β-blocker group[12,13]. The third study[46] also found bleeding rates to be lower in this group, without specifying whether the difference was significant or not. When sclerotherapy and banding ligation studies were pooled, a total of 211 patients (39.3%) bled in the endoscopic therapy only group, and 132 (23.5%) bled in the endoscopic therapy plus β-blocker group.
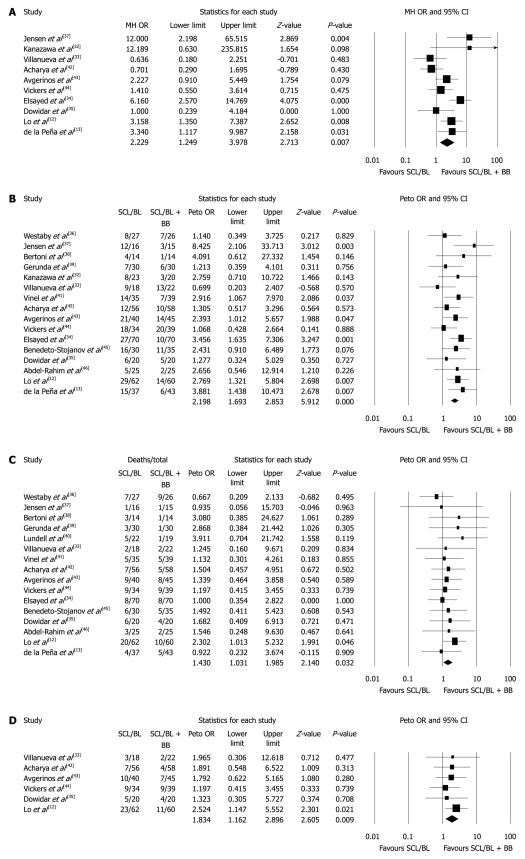
When sclerotherapy and banding ligation trials were pooled for meta-analysis (Table 4), rebleeding rates were significantly lower in the endoscopic therapy plus β-blocker group at 6 and 12 mo, and for overall end-of-follow-up bleeding rates (at 6 mo, OR: 1.70, 95% CI: 1.24-2.34, P = 0.01; at 12 mo, OR: 2.22, 95% CI: 1.25-3.99, P = 0.007; overall OR: 2.20, 95% CI: 1.69-2.85, P < 0.0001). At 24 mo, the decrease in rebleeding in favour of the endoscopic plus β-blocker group was at the limit of significance (OR: 1.67, 95% CI: 0.99-2.81, P = 0.05). All funnel plots were symmetrical; the failsafe numbers were 8, 16 and 33, respectively, for bleeding rates at 6 and 12 mo and overall. The Egger tests were negative for publication bias (P = 0.47, P = 0.31, P = 0.39, respectively). Forest plots for 12 mo and overall rebleeding rates are shown in Figure 1A and B.
Table 4.
Meta-analysis comparing endoscopic therapy (sclerotherapy or banding ligation) with combined endoscopic and β-blocker therapy
| SCL/BL, n/N (%) | SCL/BL + BB, n/N (%) | No. of trials analysed | I2 | OR (95% CI) | P for heterogeneity | P-value | |
| All-cause rebleeding | |||||||
| 6 mo | 121/410 (29.5) | 83/428 (19.4) | 11 | 36 | 1.70 (1.24-2.34)1 | 0.11 | 0.01 |
| 12 mo | 142/376 (37.8) | 85/392 (21.7) | 10 | 62.3 | 2.22 (1.25-3.99)2 | 0.004 | 0.007 |
| 24 mo | 106/272 (40.0) | 80/285 (28.1) | 7 | 50 | 1.67 (0.99-2.81)2 | 0.08 | 0.05 |
| Overall | 211/537 (39.3) | 132/561 (23.5) | 16 | 12.8 | 2.20 (1.69-2.85)1 | 0.31 | < 0.0001 |
| Mortality | |||||||
| 6 mo | 39/309 (12.6) | 33/319 (10.3) | 9 | 0 | 1.23 (0.75-2.04)1 | 0.53 | 0.41 |
| 12 mo | 33/246 (13.4) | 30/259 (11.6) | 7 | 0 | 1.18 (0.69-1.99)1 | 0.79 | 0.55 |
| 24 mo | 58/230 (25.2) | 37/244 (15.2) | 6 | 0 | 1.83 (1.16-2.90)1 | 0.92 | 0.009 |
| Overall | 98/536 (18.3) | 76/560 (13.6) | 16 | 0 | 1.43 (1.03-1.98)1 | 0.97 | 0.03 |
Fixed effect model;
Random effect model. SCL: Sclerotherapy; BL: Banding ligation; BB: β-blockers; OR: Odds ratio; CI: Confidence interval.
Figure 1.
Meta-analysis for rebleeding at 12 mo (A), overall rebleeding (B), overall mortality (C) and mortality at 24 mo (D) for trials comparing combined endoscopic treatment (sclerotherapy or banding ligation) and β-blockers with endoscopic treatment alone. SCL: Sclerotherapy: BL: Banding ligation; BB: β-blockers; OR: Odds ratio; CI: Confidence interval.
Mortality: Mortality was reported in all studies except for the trial of Kanazawa et al[32]. In sclerotherapy trials, 71 patients died in the sclerotherapy group (17.2%), whereas 59 deaths occurred in the sclerotherapy plus β-blocker group (13.7%). In banding ligation trials, 27 patients died in the banding ligation group (21.8%), whereas 17 patients died in the banding ligation plus β-blocker group (13.3%). None of the sclerotherapy or banding ligation trials reported a significant difference in mortality between the two treatment arms. When sclerotherapy and banding ligation studies were taken together, a total of 98 patients died in the endoscopic therapy group (18.3%) and 76 died in the endoscopic therapy plus β-blocker group (13.6%).
No significant difference in mortality rates was present at 6 and 12 mo. However, at 24 mo a significant difference in mortality in favour of the combination therapy (endoscopy plus β-blocker) group appeared (OR: 1.83, 95% CI: 1.16-2.90, P = 0.009). Overall mortality rates were also significantly decreased in the combination therapy group (OR: 1.43, 95% CI: 1.03-1.98, P = 0.03), as shown in Table 4. Funnel plots seemed symmetrical, and the failsafe number for both mortality at 24 mo and overall mortality was 2. The Egger test did not find evidence of publication bias (P = 0.17 and P = 0.34, respectively). Forest plots for overall and 24 mo mortality are shown in Figure 1C and D.
Subgroup analyses
Sclerotherapy trials: A subgroup analysis was performed including sclerotherapy trials only. The rebleeding rates at 6, 12 and 24 mo were lower in the sclerotherapy plus β-blocker group, but the difference was not significant (Table 5). When overall end-of-follow-up rebleeding rates were taken into account, the difference was significantly lower in favour of the sclerotherapy plus β-blocker group (OR: 2.00, 95% CI: 1.49-2.69, P < 0.0001). The failsafe number was 54, the funnel plot seemed symmetrical and the Egger test did not indicate publication bias (P = 0.46). No significant difference in mortality rates was present at 6, 12, 24 mo or with overall end-of-follow-up mortality (Table 5).
Table 5.
Subgroup meta-analysis including trials comparing sclerotherapy with sclerotherapy and β-blockers
| SCL, n/N (%) | SCL + BB, n/N (%) | No. of trials analysed | I2 | OR (95% CI) | P for heterogeneity | P-value | |
| All-cause rebleeding | |||||||
| 6 mo | 91/311 (29.2) | 74/325 (22.8) | 9 | 5.8 | 1.36 (0.95-1.95)1 | 0.39 | 0.09 |
| 12 mo | 105/277 (38.0) | 69/289 (23.9) | 8 | 69.0 | 2.03 (0.97-4.25)2 | 0.002 | 0.06 |
| 24 mo | 61/173 (35.3) | 56/182 (30.8) | 5 | 45.7 | 1.26 (0.80-2.00)1 | 0.12 | 0.33 |
| Overall | 162/413 (39.2) | 110/433 (25.4) | 13 | 20.7 | 2.00 (1.49-2.69)1 | 0.23 | < 0.0001 |
| Mortality | |||||||
| 6 mo | 30/247 (12.1) | 29/259 (11.2) | 8 | 0 | 1.07 (0.61-1.87)1 | 0.57 | 0.82 |
| 12 mo | 21/184 (11.4) | 23/199 (11.6) | 6 | 0 | 0.98 (0.52-1.85)1 | 0.83 | 0.96 |
| 24 mo | 34/168 (20.2) | 26/184 (14.1) | 5 | 0 | 1.56 (0.89-2.73)1 | 0.97 | 0.12 |
| Overall | 71/412 (17.2) | 59/432 (13.7) | 13 | 0 | 1.33 (0.91-1.94)1 | 0.97 | 0.14 |
Fixed effect model;
Random effect model. SCL: Sclerotherapy; BL: Banding ligation; BB: β-blockers; OR: Odds ratio; CI: Confidence interval.
Banding ligation trials: A subgroup analysis was performed including the three banding ligation trials (Table 6). Outcomes were significantly in favour of banding ligation plus β-blockers concerning overall bleeding rates (OR: 3.16, 95% CI: 1.76-5.34, P < 0.0001). The failsafe number was at 4, and the Egger test did not indicate publication bias (P = 0.47). No significant difference was present for mortality. Analyses at different time-points were not performed due to insufficient data.
Table 6.
Subgroup meta-analysis including trials comparing banding ligation with banding ligation and β-blockers
| BL, n/N (%) | BL + BB, n/N (%) | No. of trials analysed | I2 | OR (95% CI) | P for heterogeneity | P-value | |
| Overall all-cause rebleeding | 49/124 (39.5) | 22/128 (17.2) | 3 | 0 | 3.16 (1.76-5.34)1 | 0.85 | < 0.0001 |
| Mortality | 27/124 (21.8) | 17/128 (13.3) | 3 | 0 | 1.78 (0.92-3.43)1 | 0.53 | 0.09 |
Fixed effect model. SCL: Sclerotherapy; BL: Banding ligation; BB: β-blockers; OR: Odds ratio; CI: Confidence interval.
DISCUSSION
The results of this meta-analysis show that the association of endoscopic treatment with β-blockers is more effective in secondary prophylaxis of oesophageal variceal bleeding than endoscopic treatment alone, with a significant decrease in rebleeding rates overall and at all time points (6, 12 and 24 mo). We have shown for the first time a significant decrease in mortality rates with combined β-blocker and endoscopic treatment over endoscopic treatment alone, with significantly decreased overall and 24 mo mortality rates. Banding ligation is currently the preferred endoscopic treatment for oesophageal varices as it has been shown to be superior to due to less recurrent bleeding, fewer complications, and lower mortality rates[7,9,10,47-49]. However, we chose to include studies using sclerotherapy in our meta-analysis, as it continues to be widely used in the developing world, where schistosomiasis, one of the leading causes of portal hypertension worldwide[50], and viral B cirrhosis are prevalent.
The superiority of combination β-blocker and endoscopic treatment in reducing rebleeding rates may be explained by their synergistic action, with β-blockers reducing portal hypertension, while sclerotherapy and banding ligation act focally on the oesophagus. Although overall rebleeding rates are significantly in favour of combination therapy for sclerotherapy trials alone, when studies using banding ligation are added to those using sclerotherapy, the decrease in rebleeding rates in the combination therapy group becomes significant at 6, 12 and 24 mo, and remains significant overall. These results are robust as the failsafe numbers (i.e. number of medium sized non significant studies needed to reduce the effect size to a non significant value) are relatively high (8, 16 and 33 for 6 mo, 12 mo and overall bleeding rates, respectively). This may be due to the fact that all the banding ligation studies were in favour of combination therapy concerning rebleeding rates[12,13]. Indeed, the subgroup analysis including banding ligation studies only was strongly in favour of combined treatment concerning bleeding rates (P < 0.0001). However, we are aware that the number of studies using banding ligation included in this meta-analysis is relatively low (n = 3) compared to the number of studies using sclerotherapy (n = 14). This difference is due to the fact that sclerotherapy is an older and more widespread technique compared to banding ligation, and that the highly significant results in favour of combined therapy in the three banding ligation studies would not have been conducive to the completion of further similar studies. Kumar et al[28] suggested that the pooling of sclerotherapy and banding ligation studies as in the meta-analysis of Gonzalez et al[51] would lead to inferior results for endoscopic therapy as sclerotherapy is known to be inferior to banding ligation for the treatment of variceal bleeding. We therefore decided to perform a subgroup analysis including only banding ligation trials as suggested by a recent editorial[52], which showed a significant decrease in rebleeding rates for banding ligation associated with β-blockers. Although only three studies were concerned, this result is relatively robust as four additional medium-sized non significant studies would be needed to render the result insignificant.
As with bleeding rates, a significant difference in mortality in favour of the combination β-blocker and endoscopic therapy group appeared when sclerotherapy and banding ligation studies were pooled. A significant difference was present overall and at 24 mo, perhaps due to the fact that the numbers of deaths at 6 and 12 mo were too low to reveal any significant statistical difference [at 6 mo: 39/309 (12.6%) for the endoscopic therapy group and 33/319 (10.3%) for the combined therapy group; at 12 mo 33/246 (13.4%) and 30/259 (11.6%), respectively]. It could be hypothesised that this decrease in mortality may be due to reduced bleeding rates, but one must keep in mind that although mortality rates are a standard end-point of all clinical trials and meta-analyses concerning variceal bleeding, it is difficult to attribute decreased mortality to decreased bleeding rates in a cirrhotic population prone to various life-threatening complications. β-blockers could have a beneficial effect on survival independent of their preventative effect on variceal bleeding as they act globally by reducing portal hypertension, and it has been suggested that they may reduce the frequency of complications of cirrhosis such as ascites, hepato-renal syndrome, portal hypertensive gastropathy[53,54] and spontaneous bacterial peritonitis[55,56]. However, the results concerning decreased mortality in the combination endoscopic and β-blocker therapy group must be interpreted with caution as the failsafe number is relatively low: only two additional medium-sized non significant studies would be necessary in order to render the results insignificant. Additional studies with long term follow-up, preferably using banding ligation, are necessary in order to confirm these results.
Three meta-analyses have been previously published concerning combination therapy. The meta-analyses of Gonzalez et al[51] and Ravipati et al[57] compared combination treatment with endoscopic treatment (sclerotherapy or banding ligation) alone, and found a significant difference in rebleeding rates in favour of combination therapy, whereas results were non-significant for mortality. The meta-analysis of Ravipati et al[57] included 11 sclerotherapy trials, compared to 14 trials (148 additional patients) in our meta-analysis. Cheung et al[58] performed a small meta-analysis including four trials comparing banding ligation with banding ligation plus β-blockers, and did not find any significant difference for either mortality or bleeding rates. The meta-analysis of Gonzalez et al[51] included 23 trials. However, in contrast to our meta-analysis, these three meta-analyses[51,57,58] included studies associating β-blockers with nitrates, as well as studies in which β-blockers were introduced only at the end of variceal eradication. We decided not to include these studies for both medical and methodological reasons. Firstly, there is no proof in current literature that the association of nitrates with β-blockers is superior to β-blockers alone concerning bleeding rates and mortality. One non-blinded trial found a significantly lower bleeding rate for patients treated with nadolol plus isosorbide mononitrate[59]; however, two larger, more recent double-blinded placebo controlled trials[60,61] were unable to confirm these results, with a greater number of side effects noted in the nitrate group[60]. This association, which is poorly tolerated in certain patients, has therefore not yet been recommended by either the Baveno IV Consensus[15], or the American College of Gastroenterology[14]. Secondly, optimal combination therapy would entail introducing β-blockers at the beginning of endoscopic treatment as they would protect against bleeding recurrence before complete eradication, by reducing portal hypertension. We therefore chose not to include studies which started β-blocker treatment after eradication. However, we included all studies which started β-blockers at the beginning of eradication regardless of whether β-blocker treatment was continued after eradication or discontinued at the end of the eradication programme, as so far no studies have shown a difference in superiority between these two treatment protocols. Thirdly, including studies in which β-blockers are associated with nitrates or started only after eradication may considerably increase intertrial heterogeneity due to differences in treatment protocols. This methodological aspect has been an object of criticism in a recent editorial concerning a previous meta-analysis[52]. We therefore did not include studies associating β-blockers with nitrates. Our stricter study inclusion criteria may lead to more precise conclusions concerning optimal treatment modalities.
The results of clinical trials and meta-analyses especially should always be interpreted with caution. A recent trial comparing banding ligation alone with banding ligation associated with drug therapy[28] did not find any significant difference in bleeding rates or mortality. However, in this trial drug therapy consisted of β-blockers associated with nitrates, as opposed to β-blockers alone, as in the trials included in the present meta-analysis. Our meta-analysis was limited by variations in quality of the studies included, and by lack of data concerning studies published as abstracts or letters, for which trial and patient characteristics were incomplete. The time over which data was included in this study was relatively large (1986-2005), which may contribute to inter-trial heterogeneity as improvements in patient care over time would affect bleeding and mortality outcomes. Data was insufficient to determine the effect of the date of trials on outcome. Likewise, subgroup analyses according to criteria such as Child’s class, origin of cirrhosis or variceal size were not performed due to incomplete data. We nonetheless decided to include abstracts and letters in our meta-analysis in order to ensure optimal data analysis (246 additional patients). Adverse events were difficult to evaluate as the quality of reporting varied and most trials did not distinguish serious from non serious events. Nine studies did not perform intention-to-treat analysis whereas 2 did not state whether this was the case or not, which may lead to overestimation of treatment effect. Calculation of outcome measures at different time points reduced heterogeneity due to varying follow-up durations, but these results were limited by the fact that not all studies expressed outcome measures according to follow-up time. Ideally, a meta-analysis would include individual patient data with updated follow-up, but in our case these data were not available and we therefore had to rely on group data provided by each study. Nevertheless, we aimed to ensure the robustness of our meta-analysis results by calculating the failsafe number for each significant result, which was not estimated in previous meta-analyses[51,57,58].
In conclusion, we show that combination endoscopic and β-blocker therapy is more effective than endoscopic therapy alone in secondary prophylaxis of variceal bleeding, with a significant decrease in bleeding rates and mortality. With banding ligation becoming more and more widespread, we believe that combination β-blocker and banding ligation therapy should be recommended as first line prophylactic treatment in cirrhotic patients who have already bled from oesophageal varices.
COMMENTS
Background
Upper gastro-intestinal bleeding from ruptured oesophageal varices is a frequent and serious complication of cirrhosis. It has been estimated that 70% of patients who survive a first variceal bleeding episode subsequently rebleed. Secondary prophylaxis of variceal bleeding is therefore crucial in the management of these patients.
Research frontiers
Both β-blockers and endoscopic treatment (alone or in combination) have been used to prevent variceal rebleeding. However, there is currently no consensus as to which treatments are the most effective in secondary prophylaxis of variceal bleeding.
Innovations and breakthroughs
This meta-analysis shows for the first time that mortality is significantly decreased when β-blockers are associated with either sclerotherapy or banding ligation compared to endoscopic therapy alone. Bleeding rates are also decreased with combination β-blocker and endoscopic therapy.
Applications
The results suggest that combination β-blocker and endoscopic therapy should be recommended as the first line treatment for the secondary prophylaxis of oesophageal variceal bleeding. Additional studies with long-term follow-up are needed to confirm the results concerning mortality.
Peer review
This topic is important and there is still a considerable difference of opinion in the literature. The authors have done an exacting job of describing the conditions of their meta-analysis so that the readers can easily put the findings into the context of their own practice.
Footnotes
Peer reviewers: Mercedes Susan Mandell, MD, PhD, Department of Anesthesiology, University of Colorado Health Sciences Ctr., 12401 E. 17th Ave, B113 Aurora, CO 80045, United States; Sri P Misra, Professor, Gastroenterology, Moti Lal Nehru Medical College, Allahabad 211001, India
S- Editor Shi ZF L- Editor O’Neill M E- Editor Ma WH
References
- 1.Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80:800–809. [PubMed] [Google Scholar]
- 2.Lebrec D, Poynard T, Hillon P, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. N Engl J Med. 1981;305:1371–1374. doi: 10.1056/NEJM198112033052302. [DOI] [PubMed] [Google Scholar]
- 3.Garden OJ, Mills PR, Birnie GG, Murray GD, Carter DC. Propranolol in the prevention of recurrent variceal hemorrhage in cirrhotic patients. A controlled trial. Gastroenterology. 1990;98:185–190. doi: 10.1016/0016-5085(90)91308-s. [DOI] [PubMed] [Google Scholar]
- 4.Terés J, Bosch J, Bordas JM, Garcia Pagán JC, Feu F, Cirera I, Rodés J. Propranolol versus sclerotherapy in preventing variceal rebleeding: a randomized controlled trial. Gastroenterology. 1993;105:1508–1514. doi: 10.1016/0016-5085(93)90158-9. [DOI] [PubMed] [Google Scholar]
- 5.de Franchis R, Primignani M. Endoscopic treatments for portal hypertension. Semin Liver Dis. 1999;19:439–455. doi: 10.1055/s-2007-1007131. [DOI] [PubMed] [Google Scholar]
- 6.D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475–505. doi: 10.1055/s-2007-1007133. [DOI] [PubMed] [Google Scholar]
- 7.Stiegmann GV, Goff JS, Michaletz-Onody PA, Korula J, Lieberman D, Saeed ZA, Reveille RM, Sun JH, Lowenstein SR. Endoscopic sclerotherapy as compared with endoscopic ligation for bleeding esophageal varices. N Engl J Med. 1992;326:1527–1532. doi: 10.1056/NEJM199206043262304. [DOI] [PubMed] [Google Scholar]
- 8.Laine L, el-Newihi HM, Migikovsky B, Sloane R, Garcia F. Endoscopic ligation compared with sclerotherapy for the treatment of bleeding esophageal varices. Ann Intern Med. 1993;119:1–7. doi: 10.7326/0003-4819-119-1-199307010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gimson AE, Ramage JK, Panos MZ, Hayllar K, Harrison PM, Williams R, Westaby D. Randomised trial of variceal banding ligation versus injection sclerotherapy for bleeding oesophageal varices. Lancet. 1993;342:391–394. doi: 10.1016/0140-6736(93)92812-8. [DOI] [PubMed] [Google Scholar]
- 10.Lo GH, Lai KH, Cheng JS, Hwu JH, Chang CF, Chen SM, Chiang HT. A prospective, randomized trial of sclerotherapy versus ligation in the management of bleeding esophageal varices. Hepatology. 1995;22:466–471. [PubMed] [Google Scholar]
- 11.Sørensen TI. Failure of combined efforts: propranolol and sclerotherapy do not add up to the prevention of variceal bleeding. J Hepatol. 1993;19:197–199. doi: 10.1016/s0168-8278(05)80570-7. [DOI] [PubMed] [Google Scholar]
- 12.Lo GH, Lai KH, Cheng JS, Chen MH, Huang HC, Hsu PI, Lin CK. Endoscopic variceal ligation plus nadolol and sucralfate compared with ligation alone for the prevention of variceal rebleeding: a prospective, randomized trial. Hepatology. 2000;32:461–465. doi: 10.1053/jhep.2000.16236. [DOI] [PubMed] [Google Scholar]
- 13.de la Peña J, Brullet E, Sanchez-Hernández E, Rivero M, Vergara M, Martin-Lorente JL, Garcia Suárez C. Variceal ligation plus nadolol compared with ligation for prophylaxis of variceal rebleeding: a multicenter trial. Hepatology. 2005;41:572–578. doi: 10.1002/hep.20584. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–2102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 15.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Poynard T. [Evaluation of the methodological quality of randomized therapeutic trials] Presse Med. 1988;17:315–318. [PubMed] [Google Scholar]
- 17.Nicolucci A, Grilli R, Alexanian AA, Apolone G, Torri V, Liberati A. Quality, evolution, and clinical implications of randomized, controlled trials on the treatment of lung cancer. A lost opportunity for meta-analysis. JAMA. 1989;262:2101–2107. [PubMed] [Google Scholar]
- 18.Pagliaro L, D’Amico G, Sörensen TI, Lebrec D, Burroughs AK, Morabito A, Tiné F, Politi F, Traina M. Prevention of first bleeding in cirrhosis. A meta-analysis of randomized trials of nonsurgical treatment. Ann Intern Med. 1992;117:59–70. doi: 10.7326/0003-4819-117-1-59. [DOI] [PubMed] [Google Scholar]
- 19.Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162–1173. doi: 10.1056/NEJM198805053181805. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Orwin R. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983:157–159. [Google Scholar]
- 26.Jain A, Kumar A, Tyagi P, Sharma B, Sarin SK. Endoscopic Variceal Ligation (EVL) Plus Propranolol (P) and Isosorbide Mononitrate (ISMN) Versus Endoscopic Variceal Ligation Alone in Secondary Prophylaxis of Variceal Bleeding: A Prospective Randomised Controlled Trial [Abstract] Am J Gastroenterol. 2006;101:S179. [Google Scholar]
- 27.Jha S, Kumar A, Sharma B, Sarin SK. Endoscopic variceal ligation (EVL) plus propranolol and isosorbide mononitrate versus EVL alone in secondary prophylaxis of variceal bleeding: a prospective RCT [Abstract] Hepatology. 2007;46:A250. [Google Scholar]
- 28.Kumar A, Jha SK, Sharma P, Dubey S, Tyagi P, Sharma BC, Sarin SK. Addition of propranolol and isosorbide mononitrate to endoscopic variceal ligation does not reduce variceal rebleeding incidence. Gastroenterology. 2009;137:892–901, 901.e1. doi: 10.1053/j.gastro.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad I, Khan AA, Alam A, Butt AK, Shafqat F, Sarwar S. Propranolol, isosorbide mononitrate and endoscopic band ligation-alone or in varying combinations for the prevention of esophageal variceal rebleeding. J Coll Physicians Surg Pak. 2009;19:283–286. [PubMed] [Google Scholar]
- 30.Lo GH, Lai KH, Lee SD, Tsai YT, Lo KJ. Does propranolol maintain post-sclerotherapy variceal obliteration? A prospective randomized study. J Gastroenterol Hepatol. 1993;8:358–362. doi: 10.1111/j.1440-1746.1993.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 31.Sollano J, Chan M, Ismael A, Babaran R, Lim E. Propranolol prevents rebleeding after variceal ligation [Abstract] Gastrointest Endosc. 2001;53:AB143. [Google Scholar]
- 32.Kanazawa H, Watari A, Matsusaka S, Tada N, Miyata K, Saitoh H, Yoshizawa M, Kuroda H, Kobayashi M. [Prospective controlled study of elective sclerotherapy plus oral propranolol for prevention of recurrent bleeding in cirrhotics with recent variceal hemorrhage] Nippon Shokakibyo Gakkai Zasshi. 1991;88:1341–1348. [PubMed] [Google Scholar]
- 33.Villanueva C, Martínez FJ, Torras X, Sáinz S, Soriano G, González D, Balanzó J. [Nadolol as an adjuvant to sclerotherapy of esophageal varices for prevention of recurrent hemorrhaging] Rev Esp Enferm Dig. 1994;86:499–504. [PubMed] [Google Scholar]
- 34.Elsayed SS, Shiha G, Hamid M, Farag FM, Azzam F, Awad M. Sclerotherapy versus sclerotherapy and propranolol in the prevention of rebleeding from oesophageal varices: a randomised study. Gut. 1996;38:770–774. doi: 10.1136/gut.38.5.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowidar N, Hafez A, Abdel Baki M. Endoscopic sclerotherapy of oesophageal varices due to hepatosplenic schistosomiasis. A randomized controlled trial evaluating the effect of adjuvant propranolol therapy. J Egypt Soc Parasitol. 2005;35:773–786. [PubMed] [Google Scholar]
- 36.Westaby D, Melia W, Hegarty J, Gimson AE, Stellon AJ, Williams R. Use of propranolol to reduce the rebleeding rate during injection sclerotherapy prior to variceal obliteration. Hepatology. 1986;6:673–675. doi: 10.1002/hep.1840060422. [DOI] [PubMed] [Google Scholar]
- 37.Jensen LS, Krarup N. Propranolol in prevention of rebleeding from oesophageal varices during the course of endoscopic sclerotherapy. Scand J Gastroenterol. 1989;24:339–345. doi: 10.3109/00365528909093057. [DOI] [PubMed] [Google Scholar]
- 38.Bertoni G, Fornaciari G, Beltrami M, Conigliaro R, Grazia Mortilla M, Ricci E, Castagnetti E, Bedogni G, Plancher AC. Nadolol for prevention of variceal rebleeding during the course of endoscopic injection sclerotherapy: a randomized pilot study. J Clin Gastroenterol. 1990;12:364–365. [PubMed] [Google Scholar]
- 39.Gerunda GE, Neri D, Zangrandi F, Merenda R, Granzotto P, Ancona E, Battaglia G, Patarnello E, Antoniozzi F, Primigniani M, et al. Nadolol does not reduce early rebleeding in cirrhotics undergoing endoscopic variceal sclerotherapy: a multicenter randomized controlled trial [Abstract] Hepatology. 1990;12:988. [Google Scholar]
- 40.Lundell L, Leth R, Lind T, Lönroth H, Sjövall M, Olbe L. Evaluation of propranolol for prevention of recurrent bleeding from esophageal varices between sclerotherapy sessions. Acta Chir Scand. 1990;156:711–715. [PubMed] [Google Scholar]
- 41.Vinel JP, Lamouliatte H, Cales P, Combis JM, Roux D, Desmorat H, Pradere B, Barjonet G, Quinton A. Propranolol reduces the rebleeding rate during endoscopic sclerotherapy before variceal obliteration. Gastroenterology. 1992;102:1760–1763. doi: 10.1016/0016-5085(92)91740-u. [DOI] [PubMed] [Google Scholar]
- 42.Acharya SK, Dasarathy S, Saksena S, Pande JN. A prospective randomized study to evaluate propranolol in patients undergoing long-term endoscopic sclerotherapy. J Hepatol. 1993;19:291–300. doi: 10.1016/s0168-8278(05)80585-9. [DOI] [PubMed] [Google Scholar]
- 43.Avgerinos A, Rekoumis G, Klonis C, Papadimitriou N, Gouma P, Pournaras S, Raptis S. Propranolol in the prevention of recurrent upper gastrointestinal bleeding in patients with cirrhosis undergoing endoscopic sclerotherapy. A randomized controlled trial. J Hepatol. 1993;19:301–311. doi: 10.1016/s0168-8278(05)80586-0. [DOI] [PubMed] [Google Scholar]
- 44.Vickers C, Rhodes J, Chesner I, Hillenbrand P, Dawson J, Cockel R, Adams D, O’Connor H, Dykes P, Bradby H. Prevention of rebleeding from oesophageal varices: two-year follow up of a prospective controlled trial of propranolol in addition to sclerotherapy. J Hepatol. 1994;21:81–87. doi: 10.1016/s0168-8278(94)80141-x. [DOI] [PubMed] [Google Scholar]
- 45.Benedeto-Stojanov D, Tasic T, Bjelakovic G, Nagorni A. Prevention of recurrent upper gastrointestinal bleeding withpropranolol in patients with cirrhosis undergoing endoscopicsclerotherapy. J Hepatol. 2000;32:A72. [Google Scholar]
- 46.Abdel-Rahim AY, Abdel-Ghany MS, El-Kholy B. Band ligation alone versus band ligation and propranolol in the management of bleeding oesophageal varices [Abstract] Am J Gastroenterol. 2000;95:A2442. [Google Scholar]
- 47.Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding. A meta-analysis. Ann Intern Med. 1995;123:280–287. doi: 10.7326/0003-4819-123-4-199508150-00007. [DOI] [PubMed] [Google Scholar]
- 48.Hou MC, Lin HC, Kuo BI, Chen CH, Lee FY, Lee SD. Comparison of endoscopic variceal injection sclerotherapy and ligation for the treatment of esophageal variceal hemorrhage: a prospective randomized trial. Hepatology. 1995;21:1517–1522. [PubMed] [Google Scholar]
- 49.Lo GH, Lai KH, Cheng JS, Lin CK, Huang JS, Hsu PI, Chiang HT. Emergency banding ligation versus sclerotherapy for the control of active bleeding from esophageal varices. Hepatology. 1997;25:1101–1104. doi: 10.1002/hep.510250509. [DOI] [PubMed] [Google Scholar]
- 50.Laosebikan AO, Thomson SR, Naidoo NM. Schistosomal portal hypertension. J Am Coll Surg. 2005;200:795–806. doi: 10.1016/j.jamcollsurg.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez R, Zamora J, Gomez-Camarero J, Molinero LM, Bañares R, Albillos A. Meta-analysis: Combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med. 2008;149:109–122. doi: 10.7326/0003-4819-149-2-200807150-00007. [DOI] [PubMed] [Google Scholar]
- 52.Ayoub WS, Nguyen MH. Combination of pharmacologic and endoscopic therapy for the secondary prevention of esophageal variceal bleeding. Gastrointest Endosc. 2009;70:665–667. doi: 10.1016/j.gie.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 53.Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902–908. doi: 10.1053/jhep.2003.50133. [DOI] [PubMed] [Google Scholar]
- 54.Pérez-Ayuso RM, Piqué JM, Bosch J, Panés J, González A, Pérez R, Rigau J, Quintero E, Valderrama R, Viver J. Propranolol in prevention of recurrent bleeding from severe portal hypertensive gastropathy in cirrhosis. Lancet. 1991;337:1431–1434. doi: 10.1016/0140-6736(91)93125-s. [DOI] [PubMed] [Google Scholar]
- 55.Cholongitas E, Papatheodoridis GV, Manesis EK, Burroughs AK, Archimandritis AJ. Spontaneous bacterial peritonitis in cirrhotic patients: Is prophylactic propranolol therapy beneficial? J Gastroenterol Hepatol. 2006;21:581–587. doi: 10.1111/j.1440-1746.2005.03982.x. [DOI] [PubMed] [Google Scholar]
- 56.Hoshino S, Shinoura S, Akamine H, Kikuchi K, Keida Y. Effect of propranolol for the prevention of spontaneous bacterial peritonitis. Am J Gastroenterol. 2000;95:A349. [Google Scholar]
- 57.Ravipati M, Katragadda S, Swaminathan PD, Molnar J, Zarling E. Pharmacotherapy plus endoscopic intervention is more effective than pharmacotherapy or endoscopy alone in the secondary prevention of esophageal variceal bleeding: a meta-analysis of randomized, controlled trials. Gastrointest Endosc. 2009;70:658–664.e5. doi: 10.1016/j.gie.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 58.Cheung J, Zeman M, van Zanten SV, Tandon P. Systematic review: secondary prevention with band ligation, pharmacotherapy or combination therapy after bleeding from oesophageal varices. Aliment Pharmacol Ther. 2009;30:577–588. doi: 10.1111/j.1365-2036.2009.04075.x. [DOI] [PubMed] [Google Scholar]
- 59.Merkel C, Marin R, Enzo E, Donada C, Cavallarin G, Torboli P, Amodio P, Sebastianelli G, Sacerdoti D, Felder M, et al. Randomised trial of nadolol alone or with isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Gruppo-Triveneto per L’ipertensione portale (GTIP) Lancet. 1996;348:1677–1681. doi: 10.1016/s0140-6736(96)05406-2. [DOI] [PubMed] [Google Scholar]
- 60.García-Pagán JC, Morillas R, Bañares R, Albillos A, Villanueva C, Vila C, Genescà J, Jimenez M, Rodriguez M, Calleja JL, et al. Propranolol plus placebo versus propranolol plus isosorbide-5-mononitrate in the prevention of a first variceal bleed: a double-blind RCT. Hepatology. 2003;37:1260–1266. doi: 10.1053/jhep.2003.50211. [DOI] [PubMed] [Google Scholar]
- 61.D’Amico G, Pasta L, Politi F, Vizzini G, Traina M, Caltagirone M, Patti R, Madonia S, Pagliaro L. Isosorbide mononitrate with nadolol compared to nadolol alone for prevention of the first bleeding in cirrhosis. A double-blind placebo-controlled randomized trial. Gastroenterol Int. 2002;15:40–50. [Google Scholar]