Abstract
Epithelial ovarian cancer (EOC) cells often show increased activity of the PI3K/Akt pathway. In addition, we have previously shown that EOC ascites induce Akt activation in the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-sensitive EOC cell line, CaOV3, leading to TRAIL-mediated apoptosis inhibition. In this study, we investigated the role of Akt in intrinsic resistance to TRAIL, which is common in EOC cells. We report that Akt activation reduces the sensitivity of EOC cells to TRAIL. TRAIL-resistant SKOV3ip1 and COV2 cells were sensitized to TRAIL-induced apoptosis by PI3K or Akt inhibitors although inhibition of PI3K/Akt signaling pathway did not interfere with the recruitment and processing of caspase-8 to the death-inducing signaling complex. Conversely, overexpression of Akt1 in TRAIL-sensitive cells promoted resistance to TRAIL. Although the fact that TRAIL-induced caspase-8 activation was observed in both sensitive and resistant cell lines, Bid cleavage occurred only in sensitive cells or in SKOV3ip1 cells treated with LY294002. Bid expression was low in resistant cells and Akt activation downregulated its expression. Depletion of Bid by siRNA in OVCAR3 cells was associated with a decrease in TRAIL-mediated apoptosis. Overexpression of Bid only in SKOV3ip1 cells enhanced TRAIL-induced apoptosis. Simultaneous blockade of Akt pathway further increased TRAIL-induced apoptosis. Thus, Akt acts upstream of mitochondria and inhibits TRAIL-induced apoptosis by decreasing Bid protein levels and possibly inhibiting its cleavage.
Keywords: death receptors, ovarian carcinoma, resistance, PI3K/Akt pathway, TRAIL, Bid
Introduction
Resistance to chemotherapy is a major problem in epithelial ovarian cancer (EOC) treatment. Despite initial high response rate in patients with advanced EOC, most patients relapse with tumor that acquires resistance to chemotherapy (Yap et al., 2009). The tumor necrosis factor related-apoptosis-inducing ligand (TRAIL) holds great promise as an anti-cancer therapy because of its selective apoptosis-inducing action on tumor cells vs normal cells (Newsom-Davis et al., 2009). TRAIL-based therapies are now in phase I/II clinical trials (http://www.clinicaltrials.gov). TRAIL resistance in tumor cells, including EOC, may limit its therapeutic use (LeBlanc et al., 2002; Lane et al., 2004; Zhang and Fang, 2005). On binding to its receptors DR4 (TRAIL-R1) and DR5 (TRAIL-R2) TRAIL induces the formation of the death-inducing signaling complex (DISC) by recruiting Fas-associated death domain. Fas-associated death domain through its death effector domain recruits caspases-8/10, which assemble into the DISC (Kischkel et al., 1995; Bodmer et al., 2000). When recruited to the DISC, pro-caspases-8 is activated through proteolytic cleavage, resulting in active caspase-8 that may directly cleave downstream effector caspases (caspase-3, -6 and -7) leading to apoptosis. Alternatively, caspase-8 cleaves the BH3 domain-containing protein Bid, to generate a pro-apoptotic truncated form (tBid), which translocates to the mitochondrial membrane and triggers the intrinsic apoptosis pathway.
Although there are a number of means by which the association between TRAIL signaling cascade and the apoptotic machinery could be altered in TRAIL-resistant EOC cells, the phosphatidylinositol-3-kinase (PI3K)/Akt pathway is of particular interest because it is an important determinant of oncogenic transformation in EOC. The PI3K/Akt pathway is activated in a significant number of EOC cells (∼70%) because of amplification/overexpression/mutation (Bast et al., 2009). Activation of the PI3K/Akt pathway was shown to have a role in protecting EOC cells from chemotherapy-induced apoptosis (Page et al., 2000; Hu et al., 2002; Yang et al., 2006). Drugs and death receptor ligands rely on activation of apoptotic signaling pathways to destroy tumor cells. Indeed, the resistance to chemotherapy is because of the failure of tumor cells to undergo apoptosis. As shown by us and others, death receptor-mediated apoptosis is deficient in nearly 50% of ovarian cancer cell lines and primary tumors (Lane et al., 2004). Consequently, to fully exploit the potential of TRAIL, the problem of TRAIL resistance in EOC cells must be first understood.
Akt activation was shown to protect against TRAIL-induced apoptosis in several cell types, including melanomas and prostate, ovarian and non-small cell lung cancers (Chen et al., 2001; Nesterov et al., 2001; Kandasamy and Srivastava, 2002; Larribere et al., 2004; Kim and Lee, 2007; Lane et al., 2007). However, there are conflicting reports about the mechanisms by which Akt exerts its protective effect. In non-small cell lung and prostate cancer, Akt activation inhibited TRAIL-induced apoptosis by interfering with Bid cleavage by means of an unknown mechanism (Chen et al., 2001; Nesterov et al., 2001; Kandasamy and Srivastava, 2002). Inhibition of PI3K or AKT, completely blocked the anti-apoptotic effect of stem cell factor on TRAIL-induced apoptosis in melanoma cells (Larribere et al., 2004). FLIP did not appear to be involved but the precise mechanism was unclear. In TRAIL-sensitive EOC cells, inhibition of PI3K and Akt substantially inhibited the pro-survival effect of EOC ascites by a mechanism that involved upregulation of c-FLIPs (Lane et al., 2007).
Previous studies have shown that EOC cell susceptibility to TRAIL cannot be explained by the levels of TRAIL receptors (Cuello et al., 2001; Siervo-Sassi et al., 2003; Lane et al., 2004). TRAIL induces mitochondria-dependent apoptosis in EOC, a characteristic of the type II cells (Cuello et al., 2004). Bid has a central role in type II cells by connecting the mitochondria pathway of apoptosis to the extrinsic pathway (Newsom-Davis et al., 2009). Akt regulates the activity of Bad, Bax and X-linked inhibitor of apoptosis (XIAP) to promote survival (Datta et al., 1997; del Peso et al., 1997; Kandel and Hay, 1999; Dan et al., 2004). Akt may also upregulate the expression of anti-apoptotic proteins such as Bcl-2 (Du and Montminy, 1998). However, a recent study has shown that Akt activation does not affect the expression of Bcl-2, Bcl-XL and Bax (Lane et al., 2007). Thus, the mechanisms by which Akt confers protection and its action on the mitochondrial pathway in TRAIL-induced apoptosis remains to be fully understood in EOC cells.
In this study, we have examined the role of Akt and the mechanism of intrinsic TRAIL resistance in ovarian cancer cells. We report for the first time that Akt activation inhibits TRAIL-induced apoptosis by decreasing Bid expression. Furthermore, Bid depletion by RNA interference led to a decrease of TRAIL-induced apoptosis. These results show that Akt acts upstream of the mitochondria at the Bid level to inhibit TRAIL-induced cell death in EOC cells.
Results
TRAIL resistance is associated with Akt activation in EOC cells
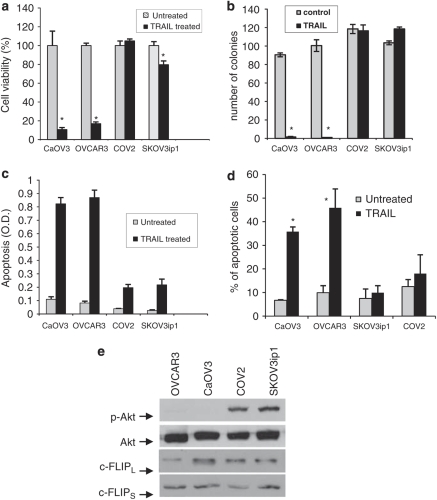
We have previously shown that ascites-mediated Akt activation protects CaOV3 cells against TRAIL-induced apoptosis (Lane et al., 2007). In addition, we showed that cell detachment decreased Akt activity and sensitized SKOV3ip1 and COV2 EOC cells to TRAIL (Lane et al., 2008). These data suggest that Akt may be an important regulator of TRAIL-induced apoptosis. In this study, we investigated whether Akt is indeed a mediator of TRAIL resistance. For our studies, four EOC cell lines were used. The cell lines were subdivided into sensitive (<20% cell viability; CaOV3 and OVCAR3 cells) and resistant (80% cell viability; COV2 and SKVO3ip1 cells) to TRAIL-induced cell death (Figure 1a). In sensitive cells, TRAIL treatment decreased the number of colonies by 10-fold whereas the number of colonies remained mostly unchanged in resistant cells (Figure 1b). TRAIL-induced apoptosis was significantly greater in sensitive cells when compared with COV2 and SKOV3ip1 cells as measured by oligosomal DNA fragmentation (Figure 1c) and the percentage of hypodiploid cells (Figure 1d).
Figure 1.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resistance correlates with Akt activation in ovarian cancer cells. Cell viability was assessed in four ovarian cancer cell lines by a short-term assay where cells were incubated with TRAIL (100 ng/ml) for 48 h and viability was assessed by XTT assay (a), and by a long-term assay where cells were treated with TRAIL (100 ng/ml) or left untreated (control) for 2 h and colonies were counted after 14 days (b). Apoptosis was measured by determining oligosomal DNA fragementation (c) and the percentage of apoptosis (d) was measured as percentage of hypoploid cells assessed by flow cytometry analysis during exposure to TRAIL (100 ng/ml) for 24 h. Data shown are means±s.e.m. derived from three independent experiments. (e) Expression/phosphorylation of Akt, FLIPL and FLIPS were analyzed by western blotting with appropriate antibodies. Representative immunoblot from three independent experiements. *P<0.001.
We examined the basal level of phospho-Akt in our panel of cell lines. As shown in Figure 1e, there was a positive correlation between high levels of Akt phosphorylation and resistance to TRAIL. Interestingly, however, there was no correlation between levels of c-FLIPS or c-FLIPL proteins, which inhibit caspase-8 activation at the DISC (Kruger et al., 2001; Panka et al., 2001; Panner et al., 2005), and the sensitivity of EOC cell lines to TRAIL-induced apoptosis.
Overexpression of Akt1 inhibits TRAIL-induced apoptosis in CaOV3 cells
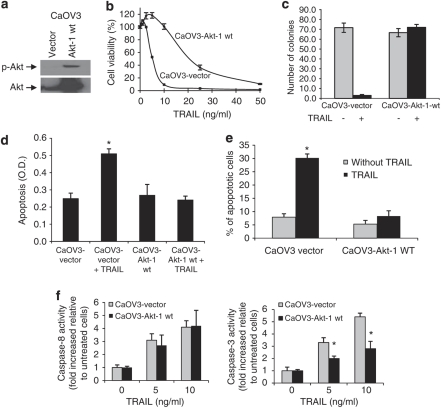
We determined whether Akt1 overexpression would exert a protective effect against TRAIL-induced apoptosis. CaOV3 cells overexpressing Akt1 (CaOV3-Akt-1 (wt)) or vector-transfected cells (CaOV3 (vector)) were validated by immunoblot (Figure 2a). Of note, the ectopic expression of Akt1 did not affect the protein levels of FLIPL and FLIPS (data not shown). CaOV3-Akt1 (wt) cells were significantly (P<0.001) more resistant to TRAIL compared with CaOV3 (vector) cells as indicated by a dose-response curve (Figure 2b) and colony formation after TRAIL exposure (20 ng/ml) (Figure 2c). However, the resistance of CaOV3-Akt1 (wt) cells can be overcome by higher concentrations of TRAIL (Figure 2b). We believe that this may be related to relatively low levels of pAkt in CaOV3-Akt1 (wt) cells despite high expression of total Akt. The level of cell death for CaOV3 (vector) cells was comparable to that of the parental CaOV3 cells (data not shown). Oligosomal DNA fragmentation and the percentage of hypodiploid cells were used to measure apoptosis. Akt1 overexpression decreased TRAIL-induced DNA fragmentation (Figure 2d) and the percentage of apoptosis (Figure 2e) to a level equal to untreated cells, suggesting that TRAIL-induced apoptosis is inhibited by expression/activation of Akt. Interestingly, despite showing resistance to TRAIL, CaOV3-Akt-1 (wt) cells showed similar levels of caspase-8 activity to control CaOV3 (vector) cells after TRAIL treatment (Figure 2f). However, caspase-3 activity was significantly lower in Akt 1-expressing CaOV3 cells as compared with control cells. These data suggest that Akt confers resistance to TRAIL-induced apoptosis by interfering downstream of caspase-8.
Figure 2.
Overexpression/phosphorylation of Akt inhibits TRAIL-induced apoptosis in CaOV3 cells. TRAIL-sensitive CaOV3 cells were stably transfected with empty vector (CaVO3 (vector)) or with Akt1-encoding construct (CaOV3-Akt-1 (wt)). (a) After selection, cells were analyzed for Akt expression and phosphorylation by immunoblot. Cell viability was assessed by short-term XTT assay in the presence of increasing concentration of TRAIL (b) or by long-term assay where cells were left untreated or exposed to TRAIL (20 ng/ml) for 2 h. After 14 days colonies were counted (c). Apoptosis was measured by determining oligosomal DNA fragementation (d) and the percentage of apoptosis (e) was measured as percentage of hypoploid cells assessed by flow cytometry analysis during exposure to TRAIL (20 ng/ml) for 24 h. Data shown are means±s.e.m. derived from three independent experiments. (f) Caspase-8 and caspase-3 activity were measured using fluorogenic substrates in CaOV3-vector and CaOV3-Akt-1 wt cells treated with TRAIL (0 to 10 ng/ml). Data shown are means±s.e.m. derived from three independent experiments. *P<0.001.
PI3K and Akt inhibitors enhance TRAIL-induced apoptosis in resistant cells
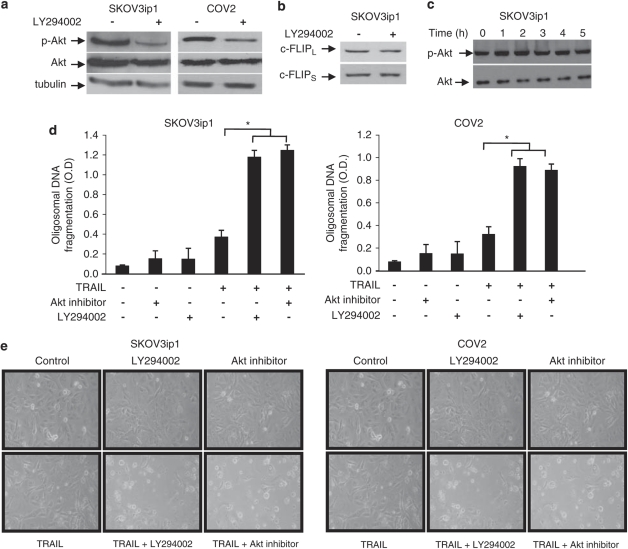
To further confirm the role of Akt, we assessed whether PI3K inhibitor LY294002 or Akt inhibitor blocked Akt activation and enhanced TRAIL-sensitivity in resistant cells. Treatment of SKOV3ip1 and COV2 cells with LY294002 (5 μ) for 90 min strongly blocked Akt phosphorylation (Figure 3a). Although we did not find any correlation between c-FLIPS or c-FLIPL protein levels and TRAIL sensitivity (Figure 1e), we assessed the effect of LY294002 on c-FLIPS or c-FLIPL expression to ensure that Akt activity does not affect their expression. Figure 3b shows no effect of PI3K inhibition on c-FLIPS or c-FLIPL protein levels in SKOV3ip1 cells. Similarly, the levels of pAkt and total Akt were not affected by TRAIL in SKOV3ip1 cells (Figure 3c). Pre-incubation of SKOV3ip1 or COV2 cells with LY294002 (5 μ) or Akt inhibitor (10 μ) sensitized these cells to TRAIL-induced apoptosis as shown by increased oligosomal DNA fragmentation (Figure 3d) and increased rounded dead cells (Figure 3e) compared with controls.
Figure 3.
Inhibition of the PI3K/Akt pathway sensitizes ovarian cancer cells to TRAIL-induced apoptosis. TRAIL-resistant SKOV3ip1 and COV2 cells were incubated with LY294002 (5 μ) for 60 min, after which immunoblot analysis was performed to determined (a) Akt-phosphorylation and (b) FLIPL and FLIPS expression. (c) SKOV3ip1 cells were treated with TRAIL (100 ng/ml) and Akt-phosphorylation was monitored for upto 5 h. Resistant cells were incubated with LY294002 (5 μ) or Akt inhibitor (10 μ) for 60 min, after which TRAIL (100 ng/ml) was added for 48 h. Apoptosis was determined by measuring oligosomal DNA fragmentation (d) and visualizing cells by optical microscopy (e). Data shown are representative of three independent experiments. Values are the means±s.e.m. *P<0.001.
Akt activation in human primary samples of EOC correlated with decreased TRAIL sensitivity
To determine whether Akt activation is correlated with decreased TRAIL sensitivity in clinical samples of EOC, we obtained primary samples of tumor cells from women having serous ovarian carcinoma. The sensitivity of primary ovarian tumors cells to TRAIL was determined by XTT assay as described previously (Lane et al., 2004), and expressed as IC50. The relative expression of pAkt in primary samples was assessed by western blotting and expressed as fold increased relative to pAkt levels in CaOV3 cells. pAkt levels in primary tumor samples correlated well and positively with increased TRAIL IC50, indicating that Akt activation confers resistance to TRAIL in vivo (n=12, Pearson's r=0.950; P<0.0001).
TRAIL resistance is downstream of caspase-8 but upstream of mitochondria
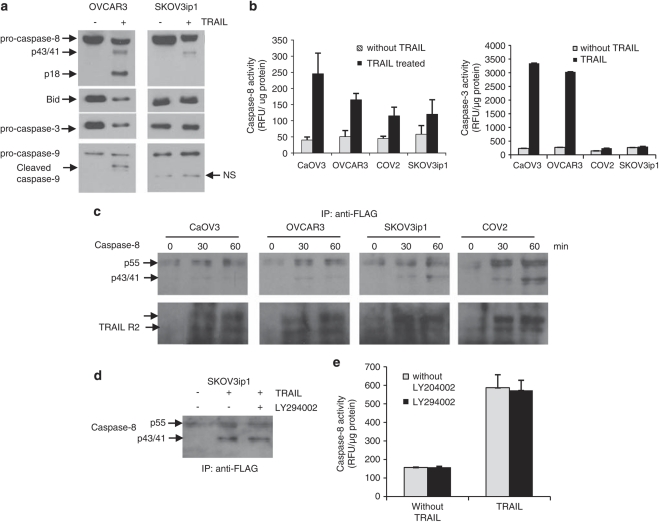
Analysis of caspase activation indicated that the pro-caspases were processed and cleaved fragments of caspase-8 (p43/41, p18) and caspase-9 (p35) was generated in TRAIL-sensitive OVCAR3 cells (Figure 4a). The generation of active caspase-8 and caspase-3 fragments was confirmed by measuring the cleavage of caspase-8 and caspase-3 substrates in OVCAR3 cells (Figure 4b). In contrast, only partially processed caspase-8 p43/41 fragments were detected in SKOV3ip1 and neither caspase-8 nor caspase-9 mature fragments were detected. The measurement of caspase-8 activity however, showed substantial caspase-8 substrate cleavage in SKOV3ip1 cells although to a lesser extend relative to OVCAR3 cells (Figure 4b). This indicated that caspase-8 is activated in SKOV3ip1 but probably at a level which is too low to be detected by an immunoblot. Caspase-3 activity was not, however, detected in SKOV3ip1 cells (Figure 4b). As binding of TRAIL to its receptors causes recruitment of pro-caspase-8 at part of the DISC, resulting in activation of caspase-8, we immunoprecipitated the DISC in cells treated with FLAG-tagged TRAIL. As shown in Figure 4c, pro-caspase-8 was recruited and cleaved at the DISC in a time-dependent manner in both sensitive and resistant cells. Of note, recruitment and processing of pro-caspase-8 at the DISC was not affected by LY294002 (Figure 4d). Similarly, caspase-8 activity in total cell lysate of SKVO3ip1 cells was not inhibited by LY294002 (Figure 4e) indicating that Akt blockade occurs downstream of caspase-8.
Figure 4.
Blockade of the TRAIL signaling cascade is located downstream of caspase-8 in resistant cells. (a) Caspase cleavage during TRAIL treatment (100 ng/ml) was assessed by immunoblot analysis after 24 h in TRAIL-sensitive CaOV3 and TRAIL-resistant SKOV3ip1. NS, nonspecific band. (b) Caspase-8 and caspase-3 activity in sensitive and resistant epithelial ovarian cancer (EOC cells) were measured by the cleavage of fluorogenic substrate IETD-AFC and DEDV-AFC after TRAIL treatment (100 ng/ml) for 24 h. (c) TRAIL receptors were immunoprecipitated using Flag-tagged recombinant TRAIL and anti-Flag M2 antibody at various times after TRAIL treatment. Co-immunoprecipitated DR5 (doublet) and caspase-8 (pro-caspase-8, p55 and cleavage fragments, p43/41) were revealed by immunoblotting. (d) SKOV3ip1 cells were incubated for 1 h with LY294002 (5 μ), after which caspase-8 was co-immunoprecipitated as described above following Flag-tagged TRAIL treatment for 1 h. (e) Caspase-8 activity was measured in SKOV3ip1 cells treated with LY204002 alone or in combination with TRAIL (100 ng/ml) for 24 h.
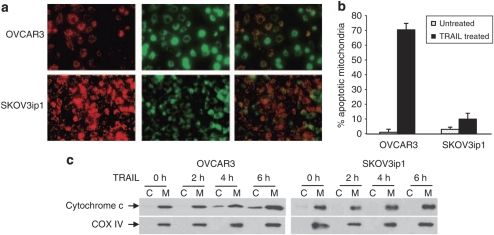
Mitochondrial activation is an essential event for efficient TRAIL-induced apoptosis in EOC cells (Cuello et al., 2004). We, therefore, examined the effect of TRAIL on mitochondrial outer membrane permeabilization and cytochrome c release in OVCAR3 and SKOV3ip1 cells. Mitochondrial outer membrane permeabilization was assessed by the uptake of a lipophilic cationic dye where red fluorescence represents intact mitochondria membrane and green fluorescence represents apoptotic mitochondria. Treatment of OVCAR3 cells with TRAIL, increased the number of green-labeled mitochondria (Figure 5a) and consequently the percentage of apoptotic mitochondria as compared with SKOV3ip1 cells (Figure 5b). Heavy membrane, enriched in mitochondria and cytosolic fractions, were isolated from OVCAR3 and SKOV3ip1 cells, after treatment with TRAIL. Cytochrome c was detected in the cytosol of OVCAR3 cells as early as 2 h after TRAIL treatment whereas cytochrome c was not detected in SKOV3ip1 even after 8 h of TRAIL treatment (Figure 5c). These results suggest the mitochondrial cell death pathway is inhibited in resistant cells.
Figure 5.
Lack of mitochondrial activation in TRAIL-resistant cells. (a) OVCAR3 and SKOV3ip1 cells were cultured for 24 h without TRAIL and the mitochondrial membrane integrity was assessed using MitoLight apoptotic detection kit staining. In treated cells, fresh culture medium containing TRAIL (100 ng/ml) was added for 5 h before subjected to MitoLight apoptotic detection kit staining. Only TRAIL-treated cells are shown. The red fluorescence (left panels) represents dimeric dye that has accumulated in the intact mitochondria membrane representing non-apoptotic cells. The green fluorescence (middle panels) represents cytoplasmic pools of monomeric-lipophilic-cationic dye indicating the the lack of ability of mitochondria to concentrate the dye and consequently shows apoptotic cells. Right panels represent overlays of left and middle panels. (b) Percentage of apoptotic mitochondria in OVCAR3 and SKOV3ip1 cells during TRAIL treatment. (c) OVCAR3 and SKOV3ip1 cells were treated with TRAIL for different times and levels of cytochrome c in cytosolic (C) and membrane (M) fractions were determined by western blot. COX IV was used as a mitochondrial marker and loading control.
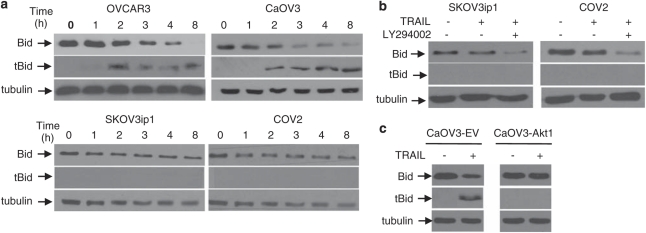
tBid is not detected in resistant cells
TRAIL-induced Bid cleavage generates a truncated form of Bid (tBid) that promotes the insertion of Bax into the mitochondrial outer membrane. As shown in Figure 6a, TRAIL (100 ng/ml) treatment resulted in a reduction in full-length Bid and the appearance of tBid overtime in sensitive cells but not in resistant cells suggesting that Akt interfere with caspase-8-mediated Bid cleavage. To further support this observation, SKOV3ip1 and COV2 cells were pre-incubated with LY204002 (5 μ) in the presence or absence of TRAIL. When TRAIL was combined with LY294002, there was a reduction of full-length Bid, but we did not detect tBid presumably, because the levels of tBid are too low to be detected by immunoblot (Figure 6b). Overexpression of Akt1 in CaOV3 cells prevented TRAIL-induced Bid cleavage (Figure 6c). These results suggest that Akt inhibits TRAIL-induced activation of the mitochondrial cell death pathway by preventing the accumulation of tBid at levels sufficient to induce apoptosis.
Figure 6.
Effect of Akt on Bid cleavage. (a) Immunoblot analysis for the assessment of Bid cleavage. Sensitive and resistant cells were treated with TRAIL (100 ng/ml) for various times and Bid cleavage was determined by the decrease of full-length Bid protein and the appearance of tBid on western blot using anti-Bid antibodies. (b) TRAIL-resistant SKOV3ip1 and COV2 cells incubated with LY294002 (5 μ) or left untreated for 1 h before adding TRAIL for 8 h. Cell lysates were analyzed by western blotting using the indicated antibodies. (c) CaOV3 cells expressing the empty vector (CaOV3-EV) or Akt1 (CaOV3-Akt1) were treated with TRAIL for 8 h. Cell lysates were analyzed as described above. Tubulin was used to ensure equal loading.
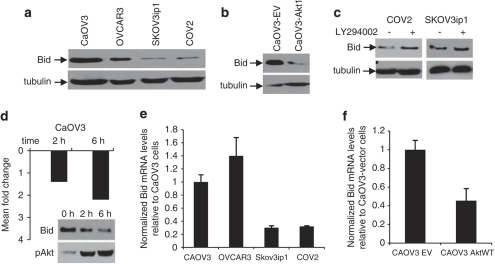
Akt activation decreases Bid protein levels
We compared levels of Bid protein in our TRAIL-sensitive and -resistant cell lines. The expression of Bid was lower in resistant cells, both, at the protein and transcriptional levels (Figures 7a and e). In contrast, the expression of Bcl-2, Bcl-XL, Mcl-1 and Bax did not correlate with TRAIL sensitivity as shown by immunoblot (Supplementary Figure S1 online). We next examined Bid expression in CaOV3-EV and CaOV3-Akt1 cells. The levels of Bid protein and mRNA were lower in Akt1 overexpressing cells relative to CaOV3-vector cells (Figures 7b and f). When Akt activation was inhibited by a PI3K-inhibitor, LY294002, in TRAIL-resistant cells, Bid expression was increased (Figure 7c). To further evaluate whether Akt inhibits Bid expression at the transcriptional level, we carried out Bid qRT–PCR at 2 and 6 h after exposure of CaOV3 cells to EOC ascites, which induce Akt activation within 30 min (Lane et al., 2010). As shown in Figure 7d, there was a decrease in Bid mRNA expression at 2 and 6 h. Taken together, these data suggest that Akt inhibits TRAIL-induced apoptosis by decreasing the expression of Bid.
Figure 7.
Akt downregulates Bid expression. (a) Western blot analysis of Bid expression in TRAIL-sensitive and -resistant cells. (b) Bid expression in CaOV3 cells expressing the empty vector (CaOV3-EV) or Akt1 (CaOV3-Akt1) assessed by western blot using anti-Bid antibodies. (c) SKOV3ip1 and COV2 were treated with LY294002 (5 μ) or incubated in standard medium for 1 h and Bid expression was assessed by western blot. Tubulin was used to ensure equal loading. (d) CaOV3 cells were exposed to EOC COV2 ascites (10% v/v) to induce Akt-phosphorylation and 2 and 6 h later, qRT–PCR analysis was performed for detecting Bid mRNA as described in Material and methods. Histogram represents mean-fold change of Bid mRNA levels relative to cells which were not exposed to ascites. Bid expression and Akt-phosphoryaltion were also assessed by western blot at 2 and 6 h to confirm that the decreased Bid at the protein level correlates with the phosphorylation of Akt. (e) Expression of Bid mRNA by qRT–PCR in our sensitive and resistant cell lines. Histogram represents of mean of normalized Bid mRNA and expressed relative to Bid mRNA levels in CaOV3 cells. (f) Histogram of mean of normalized Bid mRNA in CaOV3 (vector) and CaOV3-Akt1 (wt) cells. Bid mRNA levels are expressed relative to CaOV3 (vector) cells.
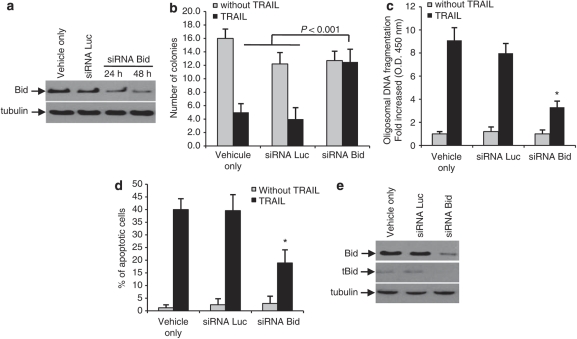
Depletion of Bid inhibits TRAIL-induced apoptosis
We examined the effect of Bid depletion by RNA interference on TRAIL-induced apoptosis in TRAIL-sensitive OVCAR3 cells. Knockdown of Bid by siRNAs decreased the levels of Bid at 24 and 48 h, whereas, the control siRNA (siRNA Luc) did not affect Bid expression at 48 h (Figure 8a). Bid depletion completely inhibited TRAIL-induced cell death, as evident, by the same number of colonies found in long-term assays in the presence or absence of TRAIL (20 ng/ml) (Figure 8b). Apoptosis was also significantly reduced, (P<0.001) as shown by further limited oligosomal DNA fragmentation (Figure 8c) and lower percentage of hypodiploid cells, in the presence of Bid siRNA (Figure 8d). Consistent with these observations, we were unable to detect tBid by immunoblot in Bid siRNA-transfected cells whereas tBid was detected with the control siRNA (Figure 8e). We tried to perform similar experiments in CaOV3 cells, but were not able to obtain consistent downregulation of Bid in these cells. This was probably related to the fact that CaOV3 cells are relatively resistant to siRNA transfection, as we did not achieve efficient transfection with the control fluorescent siRNA (siRNA Luc), either. In all, our data suggest Bid downregulation increased the resistance of OVCAR3 cells to TRAIL-induced apoptosis.
Figure 8.
Bid depletion inhibits TRAIL-induced apoptosis. (a) Expression of Bid analyzed by western blot in OVCAR3 cells incubated with transfectant only (vehicle), control siRNA (siRNALuc) for 48 h or Bid siRNA and cell lysates were obtained at 24 and 48 h after transfection. Bid expression was assessed by western blot. (b) Forty-eight hours after transfection, TRAIL (100 ng/ml) was added for 2 h, after which the number of colonies was determined by staining with crystal violet after 14 days. OVCAR3 cells were left untreated or treated with TRAIL (20 ng/ml) 48 h after the transfection with siRNAs for 48 h and apoptosis was measured by determining oligosomal DNA fragmentation 24 h after the addition of TRAIL (c) or by assessing the percentage of hypodiploid cells (d). Data shown are representative of three independent experiments. Values are the means±s.e.m. *P<0.001. (e) Bid expression in OVCAR3 cells transfected with siRNA as determined by western blot.
Bid overexpression promotes TRAIL-induced apoptosis in resistant cells
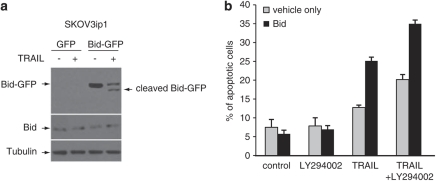
The requirement of Bid for TRAIL-induced apoptosis was confirmed by comparing the effects of stably expressing green fluorescent protein (GFP)-tagged Bid and GFP only in SKOV3ip1 cells. TRAIL treatment induced the cleavage of GFP-tagged Bid as shown by immunoblot (Figure 9a). However, the cleavage of endogenous Bid could not be detected as previously shown (Figure 6). TRAIL treatment also induced greater apoptosis in SKOV3ip cells overexpressing Bid compared with control cells (25.1±1.3 vs 12.7±0.8%, Figure 9b). Interestingly, the addition of LY294002 enhanced TRAIL-induced apoptosis in Bid-expressing SKOV3ip1 cells (35±1.5 vs 25.1±1.3%) suggesting that the inhibition of Akt phosphorylation enhanced Bid cleavage or that it alleviate a downstream blockade. The basal level of apoptosis in Bid-expressing SKOV3ip1 was unaffected by LY294002.
Figure 9.
Overexpression of Bid in resistant cells increases TRAIL-induced apoptosis. TRAIL-resistant SKOV3ip1 cells were stably transfected with GFP expression vector (GFP) or with GFP-tagged Bid vector (Bid-GFP). (a) After selection, cells were analyzed for Bid expression and cleavage in the presence or absence of TRAIL (200 ng/ml) by immunoblot. (b) The percentage of apoptosis was measured as percentage of hypoploid cells assessed by flow cytometry analysis during exposure to TRAIL (200 ng/ml) in the presence or absence of LY294002 (5 μ) for 24 h.
TRAIL resistance is not affected by XIAP downregulation
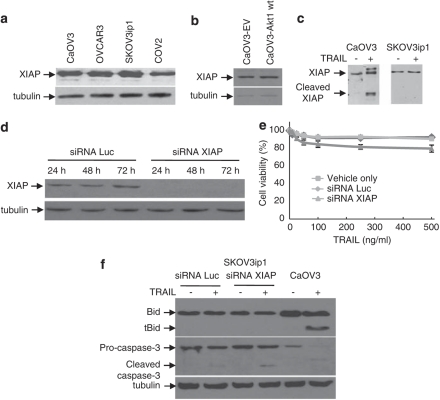
Bid and XIAP are substrates for caspase-3. In the presence of activated caspase-3, the full-length 53 kDa XIAP protein is depleted and concomitant generation of a 30 kDa fragment occurs (Deveraux et al., 1999). XIAP, the most potent caspase inhibitor of the IAP family, has been shown to regulate drug- and death receptor-induced apoptosis in EOC cells (Asselin et al., 2001; Lane et al., 2006). XIAP binds to the active form of caspase-3, but not to pro-caspase-3, and promotes its proteasomal degradation (Griffith et al., 1998; Deveraux et al., 1999; Lane et al., 2006). As XIAP regulates caspase-3 activity, it may also affect Bid cleavage. We therefore compared the levels of XIAP expression in sensitive and resistant cells and in CaOV3-Akt1 (wt) cells. There was no difference in XIAP expression between the cell lines (Figures 10a and b). As expected, XIAP was cleaved in TRAIL-sensitive CaOV3 cells but not in SKOV3ip1 cells (Figure 10c). To evaluate more specifically its contribution to TRAIL resistance, we downregulated XIAP expression with siRNA in SKOV3ip1 cells (Figure 10d). This treatment efficiently abolished XIAP expression but did not substantially sensitize cells to TRAIL (Figure 10e). In addition, XIAP downregulation had no effect on the cleavage of Bid and TRAIL-induced caspase-3 activation remained unaffected (Figure 10f). These data suggest XIAP do not significantly contribute to TRAIL resistance in SKOV3ip1 cells and thus,support our concept that Akt interfere with Bid processing upstream of the mitochondria.
Figure 10.
Effect of X-linked inhibitor of apoptosis (XIAP) depletion on TRAIL-induced apoptosis. (a) XIAP expression in sensitive and resistant cells determined by western blot. (b) XIAP expression in CaOV3-vector and CaOV3-Akt1 wt cells evaluated by Western blot. (c) CaOV3 and SKOV3ip1 cell were left untreated or were treated with TRAIL (100 ng/ml) for 24 h. Cell lysates were analyzed by western blot for XIAP expression. (d) SKOV3ip1 cells were transfected with control or XIAP siRNA. Cell lysates were obtained at 24, 48 and 72 h and XIAP expression was determined by western blot. (e) Transfected SKOV3ip1 cells were left untreated or were treated with increasing concentration of TRAIL after 48 h. Cell viability was assessed by XTT assay after 48 h. Data shown are representative of four independent experiments. Values are the means ± s.e.m. (f) Transfected SKOV3ip1 were left untreated or were treated with TRAIL (100 ng/ml) for 24 h. Cell lysates were analyzed for evidence of Bid cleavage and caspase-3 activation by western blot.
Discussion
In this study, we show that the PI3K/Akt signaling pathway regulates TRAIL-induced apoptosis in EOC cells. Akt activation mediates TRAIL resistance by decreasing Bid expression and possibly by inhibiting its cleavage. We also showed that Akt activation correlates with decreased TRAIL sensitivity in primary cultures of ovarian carcinoma suggesting the importance of Akt activation in clinical setting.
Because of the potential of TRAIL in antitumor treatment, considerable effort is being directed at understanding the determinant of TRAIL resistance in cancer cells. In addition, many tumor cells, and in particular EOC cells, appear, at least in vitro, resistant to TRAIL-induced apoptosis (Lane et al., 2004). Activation of the PI3K/Akt pathway is common in ovarian carcinoma (Bast et al., 2009). There have been several reports showing the role of Akt in EOC drug resistance (Page et al., 2000; Hu et al., 2002; Fraser et al., 2003; Westfall and Skinner, 2005; Yang et al., 2006; Mabuchi et al., 2007). However, the role of Akt in promoting survival against death receptor-induced apoptosis in EOC cells has not been clearly defined. A few reports suggested a role for the PI3K/Akt pathway in mediating TRAIL resistance. In two of these studies, Akt activation mediated by EOC ascites or serum from ovarian cancer patients inhibited TRAIL-induced apoptosis (Kang et al., 2004; Lane et al., 2007). Conversely, the inhibition of Akt phosphorylation in TRAIL-resistant SKOV3 cells enhanced TRAIL-induced apoptosis (Kim and Lee, 2005; Lane et al., 2008). Taken together, these studies suggested a role for Akt in the regulation of TRAIL-induced apoptosis. This study shows that overexpression of Akt1 in TRAIL-sensitive EOC cells, CaOV3 enhanced resistance to TRAIL whereas inhibition of PI3K or Akt decreased resistance to TRAIL-mediated cell death. Therefore, our data clearly established that the PI3K/Akt pathway has an important role in regulating TRAIL-induced apoptosis in EOC cells. Intrinsic resistance to TRAIL among ovarian cancer cells appears to be mediated, at least in part, by activation of this pathway. The clinical relevance of this observation was confirmed in primary cultures of tumor obtained from women suffering with advanced ovarian carcinoma. We established a statistical correlation between the levels of Akt-phosphorylation and TRAIL-IC50 in the primary cultures.
The antiapoptotic effect of Akt is believed to occur downstream of caspase-8 activation. Similarly, we found that Akt activation or its inhibition did not interfere with the recruitment and processing of pro-caspase-8 at the DISC. Activation of Akt inhibits apoptosis before mitochondrial cytochrome c release (Kandel and Hay, 1999). To promote release of cytochrome c, tBid translocates to the mitochondria where, in concert with pore-forming Bax and Bak proteins, it activates the mitochondria (Luo et al., 1998). Although it is well-established that Akt may function at the mitochondria by phosphorylating Bad which prevents its interaction with antiapoptotic proteins Bcl-2/Bcl-XL, there have been a number of reports, suggesting that Akt acts upstream of the mitochondria, although the site of action of Akt in the apoptotic cascade was not certain. In one study, Akt activation did not prevent Bid cleavage but inhibited Bak oligomerization and Bax activation, which are critical events in tBid-induced apoptosis (Majewski et al., 2004). In another report, although the authors could not identify the precise mechanism by which tBid was unable to activate Bax or Bak in TRAIL-resistant colon cancer cells despite the fact the tBid effectively translocated to the mitochondria (Ndozangue-Touriguine et al., 2008). In this study, we showed that Akt activation prevents the accumulation of caspase-8-induced tBid in TRAIL-resistant EOC cells, as shown by the observation that Akt overexpression in TRAIL-sensitive EOC cells prevents the detection of tBid. The fact that overexpression of Bid in SKOV3ip1 could induce apoptosis suggests that there was simply insufficient production of endogenous tBid after TRAIL treatment to directly activate BAK in these cells. The mechanism by which Akt interferes with the accumulation of tBid is not certain. Bid can be phosphorylated by casein kinase II leading to resistance to cleavage by caspase-8 and resistance to TRAIL-induced apoptosis (Desagher et al, 2001; Izeradjene et al., 2005). However, analyses of Bid phosphorylation by immunoblot in CaOV3 (vector) and CaOV3-Akt1 (wt), showed that ectopic Akt1 expression did not affect Bid phosphorylation (N. Goncharenko-Khaider, unpublished data) suggesting that Akt pathway does not block Bid cleavage by interfering with its phosphorylation. In addition, TRAIL-induced apoptosis in resistant EOC cells was not affected by the chemical inhibition of casein kinase II with apigenin or with emodin (lane D, unpublished data). It seems unlikely therefore that Akt affects Bid cleavage by interfering with Bid phosphorylation or by modulating the activity of casein kinase II. We cannot rule out the possibility that Akt does not directly interfere with Bid cleavage, but instead regulates tBid degradation or acts downstream of Bid to antagonize tBid activity at the mitochondria.
Our study reveals the critical importance of Bid expression levels to regulate TRAIL-induced apoptosis. We provide evidence that Akt transcriptionally regulates the expression of Bid. Firstly, EOC resistant-cells express lower levels of Bid protein (Figure 7). Secondly, PI3K inhibition increases Bid protein levels (Figure 7c). Finally, Akt activation increases mRNA Bid levels. The depletion of Bid by siRNAs significantly decreased TRAIL-induced apoptosis and prevented the detection of tBid in sensitive cells. Conversely, Bid overexpression in resistant cells increased apoptosis and led to tagged-Bid cleavage. Therefore, the Akt-mediated downregulation of Bid contributes to decreasing the sensitivity of EOC cells to TRAIL-induced apoptosis.
The antiapoptotic protein Mcl-1, a member of Bcl-2 family, has emerged as an important regulator of TRAIL sensitivity (Kim et al., 2008). Mcl-1 can bind to pro-apoptotic BH3-only proteins, such as Bim, Puma, and Bak, thereby inhibiting the activation of the mitochondria (Cheng et al., 2005). We have previously shown that ascites-induced Akt activation in CaOV3 cells does not affect the levels of pro-apoptotic proteins Bax and Bak and antiapoptotic proteins Bcl-2 and Bcl-XL (Lane et al., 2007). Consistent with these results, we showed in this study that expression of Mcl-1, Bcl-2 and Bcl-XL did not correlate with resistance to TRAIL.
Previous reports have suggested that in sensitive CaOV3 and HeLa cells, inhibition of TRAIL-induced apoptosis by Akt activation occurs by an upregulation of c-FLIP expression (Kang et al., 2004; Lane et al., 2007). Interestingly, we showed here that c-FLIP expression did not correlate with TRAIL sensitivity and c-FLIP expression was not affected by Akt inhibition in resistant cells. These data suggest that the mechanism by which Akt blocks the TRAIL signaling cascade may differ in sensitive vs resistant EOC cells. Akt activation in sensitive EOC cells may involve a dual resistance to TRAIL: a block at the DISC level through ascites-mediated upregulation of c-FLIPS (Lane et al., 2007) and a block at the Bid level because we showed that Akt1 overexpression in sensitive CaOV3 cells decreased Bid protein levels. In some context, XIAP has been shown to be a critical regulator of death receptor-induced apoptosis (Jost et al., 2009). Although the basal levels of XIAP were mostly comparable between sensitive and resistant cell lines, TRAIL treatment induced the cleavage of XIAP in sensitive cells, indicating that it is a consequence of cell death. However, the depletion of XIAP by siRNAs did not affect TRAIL-induced cell death, Bid levels and caspase-3 activation in SKOV3ip1 cells, ruling out a prominent role for XIAP in mediating TRAIL resistance.
In summary, we propose that binding of TRAIL to its cognate receptors induces caspase-8 activation at the DISC in both sensitive and resistant cells. In resistant cells, Akt activation decreases Bid protein levels and possibly interferes with its cleavage. The level of tBid generated is therefore below a threshold that is insufficient to trigger Bid-mediated Bax or Bak activation. The lack of efficient mitochondrial activation prevents the mitochondrial feedback loop required to fully activate caspase-8 in the cytosol. However, the low level of Akt activation in sensitive cells is insufficient to decrease Bid levels or to prevent tBid accumulation. In these conditions the amount of tBid produced is sufficient to fully activate the formation of Bax/Bak complexes resulting in activation of the mitochondria.
Materials and methods
Ovarian cancer tumor samples and cell culture
Tumor cells were isolated from EOC ascites fluids as described previously (Lane et al., 2004). Informed consent was obtained from all participants for this institutional review board-approved protocol. All samples were supplied by the Tumor Bank of the Réseau de Recherche en Cancer du Fond de Recherche en Santé du Québec, Canada. All tumor cell samples were used at low passage (<10). The human EOC cell line CaOV3 and OVCAR3 were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI-1640 (Wisent, St-Bruno, QC, Canada) supplemented with 10% fetal bovine serum and insulin (10 mg/l). CaOV3 cells were cultured in Dulbecco's modified Eagle medium/F12 (Wisent) supplemented with 10% fetal bovine serum and 2 m glutamine. COV2 cells were derived from ascetic fluids obtained from a patient suffering with a primary stage III serous ovarian carcinoma and were maintained in Dulbecco's modified Eagle medium/F12 (Wisent) supplemented with 20% fetal bovine serum. The SKOV3ip1 human EOC cell line was kindly provided by J. Price (MD Anderson Cancer Center, Houston, TX, USA) and maintained in Dulbecco's modified Eagle medium/F12 supplemented with 10% fetal bovine serum (Wisent).
Reagents
TRAIL was purchased from PeproTech. (Rocky Hill, NJ, USA). HRP-conjugated anti-mouse and -rabbit antibodies were purchased from Cell Signaling (Pickering, ON, Canada). Akt, caspase-3, caspase-9, COX IV, Bid and cytochrome c antibodies were also purchased from Cell Signaling. Antibodies for phospho-Akt (Ser-473 were obtained from Invitrogen (Biosource, Burlington, ON, Canada). Caspase-8 antibodies were obtained from R&D Systems (Minneapolis, MN, USA). Phenazine methosulfate and anti-tubulin antibody were obtained from Sigma. XTT reagents were from Invitrogen (Burlington, ON, Canada). LY294002 and Akt-specific inhibitor 1L-6-hydroxymethyl-chiro-inositol 2(R)-2-O-methyl-3-O-octadecylcarbonate were purchased from Sigma. Lipofectamine 2000 was purchased from Sigma (Oakville, ON, Canada).
Retroviral preparation and transduction efficiency
The pLPCXL retroviral vector construct endocing wildtype (wt) Akt1 was kindly provided by N. Rivard (University of Sherbrooke, Québec, Canada). Retroviral particles were generated by transfecting 293T cells with pLPCXL-Akt1, pVPack-VSV-G and pVPack-GP (Invitrogen) and used to infect CaOV3 cells. Pools of productively infected cells obtained by selection with puromycin (0,4 μg/ml) for 10 days were used for further analysis. In the case of stable expression of Bid, SKOV3ip1 cells were transfected with either pEGFPN1 vector (Clontech, Mountain view, CA, USA) or pEGFPN1 containing full-length Bid (cloned in EcoR1 site), selected in G-418 (1 mg/ml) for 10 days, and then used as a pooled.
Quantitative RT–PCR
Total RNA was extracted from CaOV3 cells using TRIzol reagent according to the manufacturer's protocol and subjected to reverse transcription using Promega (Madison, WI, USA) reverse transcriptase enzyme. The integrity of the cDNA was assessed by SRBR-base quantitative PCR, done on four housekeeping genes: MRPL19, PUM1, GAPDH and actin. Each sample was normalized to housekeeping gene levels. Primer sequences are available on request. Cycle conditions for all PCRs were as follows: an initial incubation of 2 min at 95 °C followed by 35 cycles at 94 °C for 30 s, 55 °C for 30 s, 72 °C for 60 s. The amplification was carried out by a 2-min incubation at 72 °C. PCR products quantification was performed as previously described (Klinck et al., 2008).
Cell viability assays
Short-term cell viability in the presence or absence of TRAIL was determined by XTT assay. Briefly, cells were plated at 20 000 cells/well in 96-well plates in complete medium. The next day, cells (confluence 60–70%) were treated with human TRAIL and incubated for 48 h. At the termination of the experiment, the culture media was removed and a mixture of phosphate-buffered saline and fresh media (without phenol red) containing phenazine methosulfate and XTT was added and kept for 30 min at room temperature. The absorbance was determined using a microplate reader at 450 nm (TecanSunrise, NC, USA). The percentage of cell survival was defined as the relative absorbance of untreated versus TRAIL-treated cells. For clonogenic survival assays, cells were plated in six-well tissue culture plates in standard medium. The next day, cells were treated with TRAIL (20–100 ng/ml) for 2 h, followed by several washes with standard medium. Then, cells were incubated in standard medium for 14 days. Cells were fixed and stained with crystal violet. The number of colonies, consisting of >25 cells, in triplicate was counted.
Apoptosis and caspase assays
Caspase-3 and caspase-8 fluorogenic protease assays were performed according the manufacturer's protocol (R&D Systems). In brief, 3 × 106 cells were lysed in 250 μl of cell lysis buffer, and total cell lysates were incubated with 50 μ of DEVD-AFC (caspase-3) or IETD-AFC (caspase-8) substrate for 1 h at 37 °C. Caspase-3 and caspase-8 activities were measured on a Versa Fluor fluoremeter (BioRad, Hercules, CA, USA). Protein concentration of the lysates was measured with Bio-Rad protein assay kit according to the manufacturer's recommendations. The release of nucleosomal DNA into the cytoplasm as a measure of apoptosis was determined using the Cell Death Detection ELISA Kit (Roche, Laval, Québec, Canada) according to the manufacturer's instruction. The absorbance was determined using a microplate reader at 410 nm (TecanSunrise). Propidium iodide staining for DNA fragmentation was done by fixing cells and staining them with propidium iodide for DNA analysis content as previously described (Lane et al., 2006). A total of 10 000 events were analyzed by flow cytometry and the percentage of hypodiploid cells was measured.
Western blot analysis
Cells were harvested and washed with ice-cold phosphate-buffered saline. Whole cell extracts were prepared in lysing buffer (glycerol 10%, Triton X-100 1%, TRIS 10 m pH 7.4, NaCl 100 m, EGTA 1 m, EDTA 1 m, Na4P2O7 20 m, NaF 1 m, Na3VO4 2 m and SDS 0.1%) containing protease inhibitors (0.1 m AEBSF, 5 μg/ml pepstatin, 0.5 μg/ml leupeptin and 2 μg/ml aprotinin) and cytosolic proteins were separated by 12% SDS–PAGE gels. Lysates for phosphorylated proteins were prepared in the presence of phosphatase inhibitors (100 m sodium fluoride, 100 μ sodium pyrophosphate and 250 μ sodium orthovanadate). Proteins were transferred to PVDF membranes (Roche) by electroblotting, and immunoblot analysis was performed as previously described (Lane et al., 2006). All primary antibodies were incubated overnight at 4 °C. Proteins were visualized by enhanced chemiluminescence (GE Healthcare, Baie d'Urfé, Québec, Canada).
Knockdown of Bid and XIAP
The Fluorescein-labeled Luciferase GL2 duplex was used as a control, the Bid siRNA was obtained from Dharmacon Research (Lafayette, CO, USA) and XIAP siRNA (Signal Silence XIAP siRNA) were purchased from Cell Signaling. Cells (6 × 104) were seeded in six-well plates and allowed to adhere for 24 h. Cells (50% confluent) were transfected with a mixture containing LipoFectamine 2000 (Invitrogen Life Technologies, Burlington, ON, Canada), optiMEM (Invitrogen) and Bid siRNA (10 μ) or XIAP siRNA (25 μ). The siRNAs/oligofectamine mixture was then added to the media of six-well plates containing cells. Cells were incubated for 4–6 h at 37 °C in a CO2 incubator and a medium containing fetal bovine serum was then added to it. After 48 h, TRAIL was added and cells were further incubated for another 48 h after which XTT assays were performed. For western blotting, the cells were lysed for 24 or 48 h post-transfection and total cell lysates were analyzed by immunoblot.
Statistical analysis
Statistical comparisons between two groups were performed using the Student's t-test and with ANOVA when comparing the data with more than two treatments groups. Statistical significance was indicated by P<0.05. The strength of the association between TRAIL IC50 and Akt phosphorylation levels in primary tumor samples was measured using Pearson's correlation coefficient parametric approach.
Acknowledgments
This study was supported by a Grant from the Cancer Research Society (A.P.). We wish to thank the Tumor Bank from the Réseau de Recherche en Cancer du Fond de Recherche en Santé du Québec, Canada for providing the primary tumor cell samples. We also thank Dr Louis Valiquette for his assistance with the statistical analyses.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc))
Supplementary Material
References
- Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862–1868. [PubMed] [Google Scholar]
- Bast RC, Jr, Hennessy B, Mills G. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, et al. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nature Cell Biol. 2000;2:241–243. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, et al. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–6083. doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- Cheng L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cuello M, Coasts AO, Darko I, Ettenberg SA, Gardner GJ, Nau MM, et al. N-(4-hydroxyphenyl) retinamide (4HPR) enhances TRAIL-mediated apoptosis through enhancement of a mitochondrial-dependent amplification loop in ovarian cancer cell lines. Cell Death & Differentiation. 2004;11:527–541. doi: 10.1038/sj.cdd.4401387. [DOI] [PubMed] [Google Scholar]
- Cuello M, Ettenberg SA, Nau MM, Lipkowitz S. Synergistic induction of apoptosis by the combination of TRAIL and chemotherapy in chemoresistant ovarian cancer cells. Gynecol Oncol. 2001;81:380–390. doi: 10.1006/gyno.2001.6194. [DOI] [PubMed] [Google Scholar]
- Dan HC, Sum M, Kaneko S, Feldman RI, Nicosia SV, Wang H-G, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-2-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Desagher S, Ose-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, et al. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase-8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999;18:5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- Fraser M, Leung BM, Yan X, Dan HC, Cheng JQ, Tsang BK. P53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res. 2003;63:7081–7088. [PubMed] [Google Scholar]
- Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- Hu L, Hoffmann J, Lu Y, Mill GB, Jaffe RB. Inhibition of phosphatidyl-inositol 3-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- Izeradjene K, Douglas L, Delaney A, Houghton JA. Casein Kinase II (CK2) enhances death-inducing signaling complex (DISC) activity in TRAIL-induced apoptosis in human colon carcinoma cell lines. Oncogene. 2005;24:2050–2058. doi: 10.1038/sj.onc.1208397. [DOI] [PubMed] [Google Scholar]
- Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DSC, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Srivastava RK. Role of phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in non-small cell lung cancer cells. Cancer Res. 2002;62:4929–4937. [PubMed] [Google Scholar]
- Kang YC, Kim KM, Lee KS, Namkoong S, Lee SJ, Han JA, et al. Serum bioactive lysophospholipids prevent TRAIL-induced apoptosis via PI3K/Akt-dependent cFLIP expression and Bad phosphorylation. Cell Death Differentiation. 2004;11:1287–1298. doi: 10.1038/sj.cdd.4401489. [DOI] [PubMed] [Google Scholar]
- Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- Kim KM, Lee YJ. Role of HER-2/neu signaling in sensitivity to tumor necrosis factor-related apoptosis-inducing ligand: enhancement of TRAIL-mediated apoptosis by amiloride. J Cell Biochem. 2005;96:376–389. doi: 10.1002/jcb.20512. [DOI] [PubMed] [Google Scholar]
- Kim YH, Lee YJ. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J Cell Biochem. 2007;100:998–1105. doi: 10.1002/jcb.21098. [DOI] [PubMed] [Google Scholar]
- Kim S-H, Ricci R, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer Res. 2008;68:2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Merhamann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (FAS/CD95)-associated proteins from a death-signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinck R, Bramard A, Inkel L, Dufresne-Martin G, Gervais-Bird J, Madden R, et al. Multiple alternative splicing markers for ovarian cancer. Cancer Res. 2008;68:657–663. doi: 10.1158/0008-5472.CAN-07-2580. [DOI] [PubMed] [Google Scholar]
- Kruger A, Schmitz I, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- Lane D, Cartier A, L'Espérance S, Côté M, Rancourt C, Piché A. Differential induction of apoptosis by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in human ovarian carcinoma cells. Gynecol Oncol. 2004;93:594–604. doi: 10.1016/j.ygyno.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Lane D, Côté M, Grondin R, Couture M-C, Piché A. Acquired resistance to TRAIL-induced apoptosis in human ovarian cancer cells is conferred by increased turnover of mature caspase-3. Mol Cancer Ther. 2006;5:509–521. doi: 10.1158/1535-7163.MCT-05-0362. [DOI] [PubMed] [Google Scholar]
- Lane D, Robert V, Grondin R, Rancourt C, Piché A. Malignant ascites protect against TRAIL-induced apoptosis by activating the PI3K/Akt in human ovarian carcinoma cells. Int J Cancer. 2007;121:1227–1237. doi: 10.1002/ijc.22840. [DOI] [PubMed] [Google Scholar]
- Lane D, Cartier A, Rancourt C, Piché A. Cell adhesion confers protection against TRAIL killing by activating the phosphatidylinositol 3-kinase-dependent pathway in human ovarian carcinoma cells. Int J Gynecol Oncol. 2008;18:670–676. [Google Scholar]
- Lane D, Goncharenko-Khaider N, Rancourt C, Piché A. Ovarian cancer ascites protects from TRAIL-induced cell death through αvβ5 integrin-mediated focal adhesion kinase and Akt activation. Oncogene. 2010;29:3519–3531. doi: 10.1038/onc.2010.107. [DOI] [PubMed] [Google Scholar]
- Larribere L, Khaled M, Tartare-Deckert S, Busca R, Luciano F, Bille K, et al. PI3K mediates protection against TRAIL-induced apoptosis in primary human melanocytes. Cell Death & Differentiation. 2004;11:1084–1091. doi: 10.1038/sj.cdd.4401475. [DOI] [PubMed] [Google Scholar]
- LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikos PI, Hensley HH, et al. RAD001 inhibits human ovarian cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13:4261–4270. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Robey RB, Hay N. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol Cel Biol. 2004;24:730–740. doi: 10.1128/MCB.24.2.730-740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndozangue-Touriguine O, Sebbagh M, Mérino D, Micheau O, Bertoglio J, Bérard J. A mitochondrial block and expression of XIAP lead to resistance to TRAIL-induced apoptosis during progression to metastasis of a colon carcinoma. Oncogene. 2008;27:6012–6022. doi: 10.1038/onc.2008.197. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem. 2001;276:10767–10774. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- Newsom-Davis T, Prieske S, Walczak H. Is TRAIL the holy grail of cancer therapy. Apoptosis. 2009;14:607–623. doi: 10.1007/s10495-009-0321-2. [DOI] [PubMed] [Google Scholar]
- Page C, Lin HJ, Jin Y, Castle VP, Nunez G, Huang M, et al. Overexpression of Akt/AKT can modulate chemotherapy-induced apoptosis. Anticancer Res. 2000;20:407–416. [PubMed] [Google Scholar]
- Panka DJ, Mamo T, Suhara T, Walsh K, Mier JW. Phosphatidylinositol 3-kianse/Akt activity regulates C-FLIP expression in tumor cells. J Biol Chem. 2001;276:6893–6896. doi: 10.1074/jbc.C000569200. [DOI] [PubMed] [Google Scholar]
- Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol. 2005;25:8809–8823. doi: 10.1128/MCB.25.20.8809-8823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siervo-Sassi RR, Marrangoni AM, Feng X, Winans M, Edwards RP, Lokshin A. Physiological and molecular effects of Apo2L/TRAIL and cisplatin in ovarian carcinoma cell lines. Cancer Letters. 2003;190:61–72. doi: 10.1016/s0304-3835(02)00579-7. [DOI] [PubMed] [Google Scholar]
- Westfall SD, Skinner MK. Induction of phosphatidylinositol 3-kinase sesnitizes ovarian cancer cells to carboplatin and allows adjunct chemotherapy treatment. Mol Cancer Ther. 2005;4:1764–1771. doi: 10.1158/1535-7163.MCT-05-0192. [DOI] [PubMed] [Google Scholar]
- Yang X, Frasier M, Moll UT, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Reviews Cancer. 2009;9:167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.