Abstract
A specific light program consisting of multiple treatments with alternating red and far-red light pulses was used to isolate mutants in phytochrome A-dependent signal transduction in Arabidopsis seedlings. Because of their phenotype, the mutants were called eid (empfindlicher im dunkelroten Licht, which means hypersensitive in far-red light). One of the isolated mutants, eid6, is a novel recessive allele of the COP1 gene (constitutive photomorphogenic 1) that carries an amino acid transition in a conserved histidine residue of the RING finger domain. Mutant seedlings exhibited an extreme hypersensitivity towards all tested light qualities, but in contrast to known cop1 alleles, no constitutive photomorphogenic phenotype was detectable in darkness. Thus, the novel cop1eid6 allele seems to encode for a protein whose remaining activity is sufficient for the suppression of photomorphogenesis in dark-grown plants. In adult cop1eid6 plants, the development of the Cop1 phenotype is dominated by phytochrome B. Comparison of the phenotype of the novel cop1eid6 and the weak cop1-4 allele under continuous far-red light indicates that the RING finger and coiled-coil domains of COP1 are sufficient for some specific regulatory function in phytochrome A-dependent high irradiance responses.
Light is one of the major environmental factors that controls plant development. To perceive light, plants have evolved a large set of photoreceptors. Among these, the cryptochromes and phototropins are responsible for UV-A/blue light sensing, whereas the phytochromes predominantly regulate responses to red and far-red light (Neff et al., 2000). The phytochromes are dimeric proteins of approximately 125-kD subunits. Each monomer is covalently linked to a phytochromobilin chromophore, a linear tetrapyrrole. Phytochromes can exist in two different forms: the red light-absorbing Pr and the far-red light-absorbing Pfr form. Because the Pfr conformer is the physiologically active form, the photoreceptor can function as a red/far-red photoreversible light switch upon light pulse treatments for several physiological responses (Neff et al., 2000; Møller et al., 2002).
Phytochromes are encoded by a small multigene family of five different genes (PHYA to PHYE) in Arabidopsis (Clack et al., 1994). Phytochrome A (phyA) accumulates to a high level in the dark, and its Pfr form is rapidly degraded (Clough and Vierstra, 1997). The light-labile phyA mediates the very low fluence responses that can be induced by extremely low amounts of photons. Furthermore, phyA is also responsible for the so-called high irradiance responses (HIRs) that need high fluence rates of continuous far-red light to be fully induced. The light-stable phytochrome B (phyB) is the main photoreceptor to sense continuous red light and the classical red/far-red light photoreversible low fluence responses (Whitelam and Devlin, 1997; Neff et al., 2000; Møller et al., 2002).
A very drastic effect of light is seen during seedling development of higher plants. In darkness, seedlings follow a developmental program called skotomorphogenesis. They have a long hypocotyl, a hypocotyl hook, and small pale cotyledons. Upon irradiation, seedlings become de-etiolated. The induction of photomorphogenesis leads to the inhibition of hypocotyl elongation, opening of the hypocotyl hook, and unfolding of the cotyledons.
These drastic changes between skoto- and photomorphogenesis were used to screen for mutants in components of light signal transduction pathways (Hudson, 2000; Neff et al., 2000). The det (de-etiolated)/cop (constitutive photomorphogenesis)/fus (fusca) class (Chory et al., 1989; Deng et al., 1991; Miséra et al., 1994) of mutants was identified because of their de-etiolated seedlings phenotype even in darkness. In the last few years, considerable progress has been made in cloning and characterization of the respective genes. The det2 mutant is defective in the biosynthesis of brassinosteroids (Li and Chory, 1996). Another group carries mutations in components of the COP9 multiprotein complex. COP1, COP10, and DET1 do not belong to one of these groups (Hardtke and Deng, 2000; Møller et al., 2002).
The COP1 protein carries three structural modules: a RING finger motive, a coiled-coil domain, and multiple WD-40 repeats (Deng et al., 1992). COP1 is enriched in the nucleus in the dark and disappears from the nucleus in the light (von Arnim and Deng, 1994). The recessive nature of cop1 mutants and their light-grown phenotype in darkness suggest that the respective protein functions as a repressor of photomorphogenesis (Deng et al., 1991). RING finger proteins very often act as ubiquitin ligases, which target proteins for proteolysis in the proteasome (Tyers and Jorgensen, 2000). COP1 also functions in the regulation of ubiquitylation and protein stability. COP10, a COP1-interacting protein, shows homologies to ubiquitin-activating E2 proteins (Suzuki et al., 2002). HY5 and HYH, two bZIP transcription factors involved in light signaling, interact with COP1 and are degraded in darkness in a COP1-dependent pathway. HY5 is stabilized upon light treatments, most probably due to a reduced phosphorylation of the protein (Hardtke et al., 2000; Osterlund et al., 2000; Holm et al., 2002). The myb transcription factor LAF1, which is involved in phyA signal transduction, also interacts with the repressor and becomes destabilized by inducible overexpression of COP1 (Seo et al., 2003). Using an in vitro system, Seo et al. (2003) could demonstrate that COP1 catalyzes self-ubiquitylation and the ubiquitylation of LAF1.
To screen for hypersensitive mutants in phyA signaling, a screening program consisting of alternating 20-min red and far-red light pulses was used (Büche et al., 2000; Dieterle et al., 2001). In the wild type, the red light pulse causes a degradation of phyA that abolishes far-red light-induced HIR. phyB-5 seeds mutagenized with ethyl methylsulfonate were used for screening to exclude effects caused by phyB under the multiple red light treatment. With the screening program, seven different mutant lines were isolated that exhibited a strong photomorphogenic growth under the red/far-red light pulse treatment. The mutants formed six different complementation groups named eid1 to eid6 (empfindlicher im dunkelroten Licht, which means hypersensitive to far-red light; Büche et al., 2000). Here, we describe the physiological and molecular analysis of eid6. As shown below, a mutation in the COP1 gene was responsible for the observed Eid phenotype. Therefore, the novel allele is called cop1eid6 throughout the text.
RESULTS
Isolation and Genetic Analysis of the cop1eid6 Mutant
Seven different mutant lines were isolated that exhibited a strong photomorphogenic growth under a screening program consisting of alternating 20-min red and far-red light pulses (Büche et al., 2000). The screened cop1eid6 phyB-5 mutant was backcrossed to the phyB-5 background line and to the Landsberg erecta (Ler) wild type. F2 seedlings of both crosses exhibited a 3:1 segregation behavior for wild type: mutant phenotype that is characteristic for monogenic recessive mutations (Table I). F2 plants of the Ler backcrosses were used to isolate homozygous cop1eid6 mutants in a PHYB wild-type background.
Table I.
Segregation of crosses under different light conditions
| Cross | Treatment | F1 Phenotype | F2 Segregation (Etiolated:De-Etiolated) | Expected Ratio Etiolated: De-Etiolated | χ2 (χ299% = 6.64) |
|---|---|---|---|---|---|
| cop1eid6 phyB-5 × phyB-5 | Darkness | Etiolated | All:0 | All:0 | — |
| Weak red light | Etiolated | 488:183 | 3:1 | 1.39 | |
| phyB-9 × cop1eid6 phyB-5 | Darkness | Etiolated | All:0 | All:0 | — |
| Weak red light | Etiolated | 399:129 | 3:1 | 0.07 | |
| cop1eid6 phyB-5 × Ler | Darkness | Etiolated | All:0 | All:0 | — |
| Weak red light | Etiolated | 807:256 | 3:1 | 0.36 | |
| cop1eid6 × Ler | Darkness | Etiolated | All:0 | All:0 | — |
| Weak red light | Etiolated | 145:45 | 3:1 | 0.13 | |
| cop1eid6 phyB-5 × | Darkness | Etiolated | 612:240 | 3:1 | 5.53 |
| cop1—4 | Weak red light | De-etiolated | 0:368 | 0:All | — |
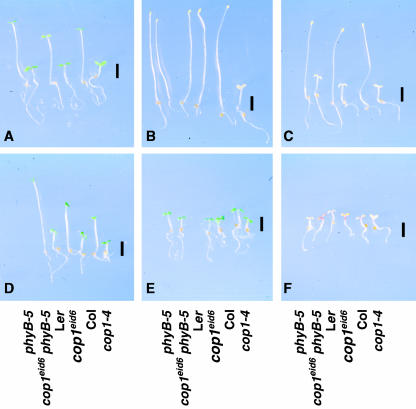
The cop1eid6 phyB-5 and the cop1eid6 mutant displayed a very strong phenotype under the screening program (Fig. 1A), whereas they did not exhibit a constitutive photomorphogenic development in darkness (Fig. 1B). cop1eid6 and cop1eid6 phyB-5 seedlings also showed an enhanced photomorphogenic response under continuous weak and strong red light (Fig. 1, C and D). Weaker effects were observed under strong continuous white and far-red light. Under continuous far-red light, cop1eid6 exhibited a clearly increased anthocyanin accumulation (Fig. 1, E and F).
Figure 1.
Seedling morphology of phyB-5, the screened cop1eid6 phyB-5 mutant, Ler wild type, cop1eid6 in Ler background, Columbia (Col) wild type, and cop1-4 in Col background. Seedlings were grown for 3 d after induction of germination. Bars = 2 mm. A, Seedlings grown under the screening program (alternating pulse treatments of 20 min of red followed by 20 min of far-red light). B, Seedlings grown in continuous darkness. C, Seedlings grown under weak continuous red light (15 nmol m-2 s-1). D, Seedlings grown under strong red light (14.3 μmol m-2 s-1). E, Seedlings grown under continuous white light (15 μmol m-2 s-1). F, Seedlings grown under strong far-red light (14 μmol m-2 s-1).
eid6 Carries a Mutation in the RING Finger Motif of the COP1 Gene
We mapped cop1eid6 using simple sequence length polymorphism and cleaved amplified polymorphic sequences (CAPS) markers (Konieczny and Ausubel, 1993; Bell and Ecker, 1994). No recombinants were observed with the COP1 CAPS marker after the analyses of 80 F2 plants homozygous for cop1eid6. Therefore, it could not be excluded that cop1eid6 is a novel COP1 allele with an unusual, non-constitutive photomorphogenic seedling phenotype.
To test whether cop1eid6 is a novel COP1 allele, the mutant was crossed with the weak recessive cop1-4 allele. The cop1-4 mutation created a stop codon at position 283 of the COP1 protein, which should result in the accumulation of a truncated version in mutant plants (McNellis et al., 1994a). Seedlings of the F1 and F2 generation of the cop1eid6 cop1-4 crossing displayed a de-etiolated phenotype under weak red light similar to their parental lines (Table I). This result clearly indicates that cop1eid6 and cop1-4 belong to the same complementation group because both mutants were recessive under selective light conditions when crossed with their respective wild type. In continuous darkness, seedlings of the F1 generation remained etiolated, and the F2 exhibited a 3:1 segregation for etiolated:de-etiolated seedlings. Thus, the constitutive photomorphogenic cop1-4 phenotype remained recessive in crosses between the weak cop1 allele and cop1eid6.
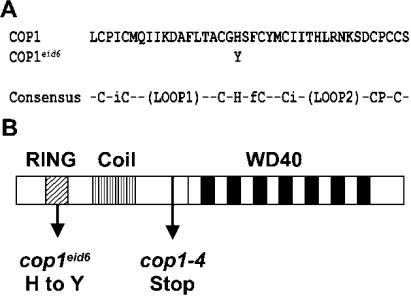
Sequencing of the COP1 gene from cop1eid6 revealed a single C to T base pair substitution at position 247 of the open reading frame. This mutation leads to a replacement of the His at position 69 of the COP1 protein by an aromatic Tyr residue (Fig. 2). His-69 is one of the highly conserved amino acids in the RING (really interesting new gene) finger motif of the COP1 protein (von Arnim and Deng, 1993). The RING finger domain comprises eight metal ligands formed by the consensus motif C3HC4 (Fig. 2). RING finger domains usually bind two zinc ions in a unique cross brace arrangement (Jackson et al., 2000).
Figure 2.
Mutations in the COP1 protein. A, Amino acid exchange in the cop1eid6 mutant: Amino acids 51 to 90 of the COP1 protein are given. The consensus of the RING finger motif is adapted from von Arnim and Deng (1993). Uppercase letters in the RING finger motif represent the eight conserved zinc ligands. Lowercase letters indicate other highly conserved amino acids. B, Schematic overview of the COP1 protein with the RING finger motif (RING), the coiled-coil domain (Coil), and the WD40 repeats (WD40). The arrows indicate the positions of mutations in cop1eid6 and cop1-4.
Adult cop1eid6 Plants Are Dwarf and Exhibit an Early Flowering Phenotype
Mutations in the COP1 gene are either homozygous lethal or lead to severe phenotypes in adult plants, such as dwarfism and early flowering (Deng and Quail, 1992; McNellis et al., 1994a). The cop1eid6 phyB-5 double mutant exhibited only a weak reduction in size when compared with its phyB-5 background (Fig. 3). In contrast, adult cop1eid6 single mutants were clearly smaller compared with wild-type plants and had a decreased vitality. Thus, they closely resembled the phenotype of the cop1-4 mutant under the same growth conditions (Fig. 3).
Figure 3.
Phenotypes of adult plants. Plants were grown for 35 d under long-day conditions (16 h of light/8 h of dark). Bar = 5 cm. Ler, Ler wild type. Col, Col wild type.
Another strong difference between cop1eid6 mutants and their respective background lines was observed for flower induction. Under short-day conditions (8 h white light: 16 h darkness), cop1eid6 phyB-5 and cop1eid6 always exhibited an early flowering phenotype (Table II). Under long-day conditions (16 h of white light and 8 h of darkness), no difference in the induction of flowering was observed between cop1eid6 phyB-5 and phyB-5 mutants, whereas cop1eid6 flowered earlier when compared with its Ler wild-type background. Even though flowering starts much earlier in both cop1eid6 lines, a weak effect of the applied photoperiod still remains because plants flower a little bit earlier under long-day conditions. cop1-4 also exhibited an early flowering phenotype under short- and long-day conditions, but no clear difference between the two photoperiods was detectable (Table II).
Table II.
Flowering time under short- and long-day conditions Values are means ± sd.
| Plants | Short Day
|
Long Day
|
||
|---|---|---|---|---|
| Rosette leaf no. | Days until flowering | Rosette leaf no. | Days until flowering | |
| phyB-5 | 16 ± 3 | 54 ± 3 | 5 ± 1 | 27 ± 4 |
| cop1eid6 phyB-5 | 7 ± 1 | 33 ± 1 | 5 ± 2 | 28 ± 4 |
| Ler wild type | 46 ± 5 | 59 ± 3 | 11 ± 2 | 32 ± 6 |
| cop1eid6 | 8 ± 1 | 36 ± 2 | 5 ± 1 | 33 ± 4 |
| Col wild type | 47 ± 3 | 56 ± 2 | 13 ± 2 | 32 ± 5 |
| cop1-4 | 10 ± 2 | 35 ± 2 | 9 ± 2 | 30 ± 4 |
Anthocyanin Accumulation
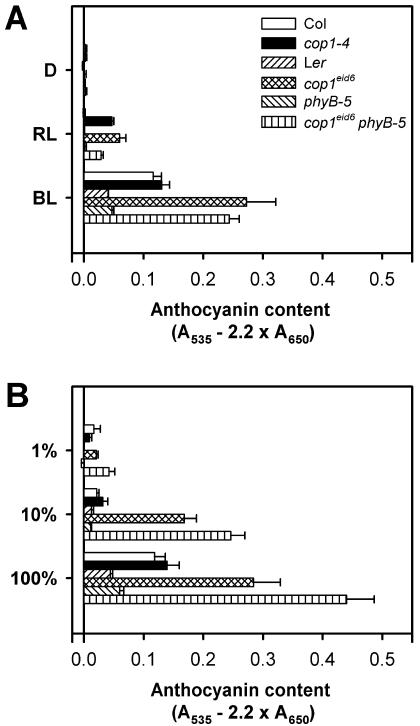
Anthocyanins accumulate in Arabidopsis seedlings under continuous far-red and blue light absorbed by phyA and blue light photoreceptors (Kunkel et al., 1996; Neff and Chory, 1998; Poppe et al., 1998). Anthocyanin accumulation was measured spectroscopically after extraction from wild-type, cop1-4, cop1eid6, and cop1eid6 phyB-5 seedlings grown under different light conditions. No anthocyanin was detectable in dark-grown seedlings (Fig. 4A). All examined cop1 mutants showed a light-dependent anthocyanin accumulation. Under strong continuous blue light, cop1eid6 accumulated a more than 5-fold higher anthocyanin level when compared with its Ler wild type, whereas cop1-4 exhibited the same response as seen with the corresponding Col background. Surprisingly, anthocyanin accumulation was detected under continuous red light in cop1eid6 and cop1-4 seedlings (Fig. 4A), whereas both wild types exhibited no response under this light quality. This red light effect seems to be partially dependent on the presence of phyB because the cop1eid6 phyB-5 double mutant exhibited a reduced anthocaynin level compared with cop1eid6.
Figure 4.
Analysis of anthocyanin accumulation under different light conditions. Seedlings were grown under different light conditions for 3 d after induction of germination before harvesting and anthocyanin extraction. Anthocyanin contents of different single and double mutants and their respective wild types are given. Error bars = se of the mean. A, Anthocyanin content of seedlings kept in darkness (D) and under strong continuous red (RL; 14.3 μmol m-2 s-1) or blue light (BL; 38.5 μmol m-2 s-1). B, Anthocyanin content of seedlings irradiated with different fluence rates of far-red light: 100%, 14 μmol m-2 s-1; 10%, 1.4 μmol m-2 s-1; 1%, 0.14 μmol m-2 s-1.
The highest level of anthocyanin accumulation was observed in the cop1eid6 phyB-5 double mutant under strong continuous far-red light (Fig. 4B). Ler, Col, and cop1-4 mutants reached only about one-half of the level observed for the double mutant (Fig. 4B). To further analyze this effect, fluence rate response curves were measured for anthocyanin accumulation under continuous far-red light. cop1eid6 clearly exhibited an increased sensitivity, whereas cop1-4 again behaved very similarly to its wild type. The highest sensitivity was seen for the cop1eid6 phyB-5 double mutant. Thus, phyB seems to reduce the effect of phyA on the regulation of anthocyanin accumulation.
Most cop1 alleles also exhibit a fusca phenotype (Miséra et al., 1994) that is characterized by a purple seed color caused by an enhanced anthocyanin accumulation in the embryo and the seedling. Both cop1eid6 and cop1-4 did not show such a phenotype (data not shown).
Hypocotyl Inhibition in cop1eid6 Is Extraordinarily Hypersensitive toward Light
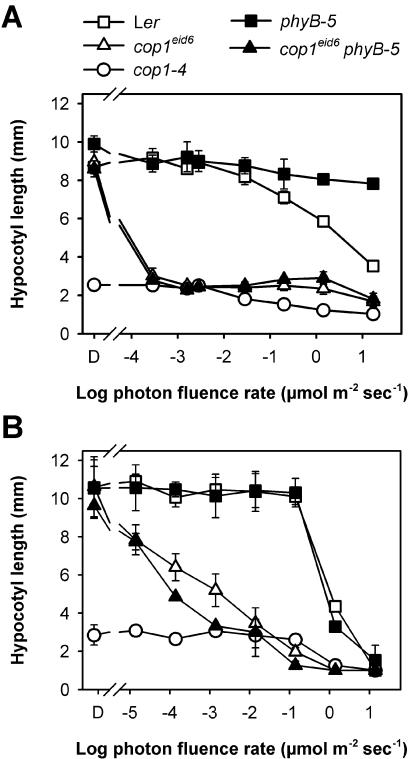
To further test the influence of light on the cop1eid6 mutant, fluence rate response curves for the inhibition of hypocotyl elongation were determined under continuous red and far-red light (Fig. 5A). Both the cop1eid6 mutant and the cop1eid6 phyB-5 double mutant showed a maximum hypocotyl inhibition even at the lowest fluence rates of red light used in the experiments. Thus, cop1eid6 was at least 4 orders of magnitude more sensitive compared with its Ler wild type. The hypocotyls of cop1-4 remained short even in darkness. Nevertheless, high fluence rates of red light were still able to cause a further reduction of hypocotyl length. Interestingly, further reduction of hypocotyl elongation started at about the same fluence rate as seen for the wild-type background. Thus, the red light HIR seems to be unaltered in cop1-4.
Figure 5.
Inhibition of hypocotyl elongation under different light conditions. Seedlings were irradiated at different fluence rates for 3 d after induction of germination. Error bars = se of the mean. A, Hypocotyl elongation under continuous red light. B, Hypocotyl elongation under continuous far-red light.
In continuous far-red light, cop1eid6 exhibited a clear log linear relationship between the photon fluence rate and the inhibition of hypocotyl elongation over 5 orders of magnitude (Fig. 5B). The cop1eid6 mutants were about 10,000 times more sensitive toward continuous far-red light compared with their corresponding background lines. Again, cop1-4 seedlings were short in darkness. A further inhibition of hypocotyl elongation was observed starting at about the same fluence rate that can induce an HIR in the respective wild type.
DISCUSSION
In this study, we describe the identification and characterization of cop1eid6, a mutant that exhibits an extremely increased light sensitivity. The mutated gene was identified by mapping analyses, allelism test after crossing with the cop1-4 mutant, and sequencing of the respective gene. These data clearly indicate that the phenotype of the mutant is caused by a point mutation in the COP1 gene. In contrast to all other known cop1 alleles, the cop1eid6 mutation does not lead to a constitutive photomorphogenic phenotype. Thus, the induction of photomorphogenesis in the cop1eid6 mutant remains strictly light dependent.
The lack of a dark phenotype indicates that cop1eid6 encodes a partially active COP1 protein. The activity of the mutated protein is obviously sufficient for the suppression of a photomorphogenic seedling development in darkness, but even minimal inputs from the photoreceptor systems can overcome the remaining COP1 activity. The remaining activity in darkness would also explain the partial dependence of flower induction on the photoperiod in cop1eid6 because its reduced activity might still be able to inhibit flower induction under long-night (short-day) conditions.
Because there is no visible phenotype in darkness, the cop1eid6 mutant is a good tool to study the interaction between COP1 and the different photoreceptors, which might function upstream of the protein. To date, this kind of analysis has been very difficult because known cop1 mutants are either lethal (cop1-5), show no light response (cop1-1), or show just a weak response (cop1-4) that is difficult to measure (McNellis et al., 1994a). With the cop1eid6 mutant, we were able to examine the COP1-dependent light regulation in the presence or absence of phyB under different light qualities. The clear difference in the adult phenotype of cop1eid6 and cop1eid6 phyB-5 plants and the increased anthocyanin accumulation under continuous blue and red light confirm former data by McNellis et al. (1994b) and Ang and Deng (1994), which demonstrated that COP1 functions downstream of phyB and blue light photoreceptors. The strong phyB effect in adult cop1eid6 plants is most probably due to a generally increased competence toward signals from this photoreceptor at elder stages of Arabidopsis development. Although adult phyB null mutants exhibit drastic changes in their morphology and in flowering time under normal light/dark conditions, all other photoreceptor mutants show more subtle changes, which only become detectable under specific light treatments (Whitelam and Devlin, 1997; Neff et al., 2000; Lin, 2002).
In most Arabidopsis ecotypes, light-dependent anthocyanin accumulation can only be induced by blue or UV light and by a phyA-dependent far-red light response (Kunkel et al., 1996; Neff and Chory, 1998; Poppe et al., 1998). In contrast, both cop1 alleles exhibited a red light-inducible accumulation of anthocyanin, which was absent in the respective wild types. The results for the cop1eid6 phyB-5 double mutant further indicate that this altered response is partially dependent on the presence of phyB. Therefore, the lack of a red light-induced anthocyanin accumulation in wild-type seedlings seems to be caused by an active, COP1-dependent suppression of signals and not by a general lack of competence toward input signals from phyB or other red light-sensing phytochromes.
The fluence rate response curves for hypocotyl elongation and anthocyanin accumulation under continuous far-red light clearly indicate that the cop1eid6 allele alters both phyA-dependent HIRs. In cop1-4, far-red light-dependent anthocyanin accumulation remains unaltered, and the onset of further reduction of hypcotyl elongation starts at about the same fluence rates as seen with the wild type. Thus, the truncated COP1-4 protein, which carries functional RING finger and coiled-coil domains, seems to be sufficient for a normal function in phyA-dependent HIR. Hoecker and Quail (2001) demonstrated that SPA1, an inhibitor of phyA-specific light responses (Hoecker et al., 1998, 1999), interacts with the coiled-coil domain of COP1. The truncated coiled-coil domain of SPA1 can also enhance the ubiquitin ligase activity of COP1 toward LAF1 (Seo et al., 2003). Because SPA1 also carries WD40 repeats, it is worthwhile to speculate that these repeats might replace the missing WD40 repeats of COP1 and that SPA1 is responsible for the phyA-specific reactions of a COP1-SPA1 ubiquitin ligase complex.
The cop1eid6 mutation might cause a more general effect compared with cop1-4 because it most probably alters the interaction with E2-ubiquitin conjugates in all COP1-dependent light reactions. The cop1eid6 mutation results in an exchange of a conserved His by a Tyr residue in the RING finger domain of COP1. Together with its zinc cofactor, the RING finger domain is responsible for the interaction with the E2-ubquitin conjugates, which are used to transfer the ubiquitin moiety to target proteins (Jackson et al., 2000; Tyers and Jorgensen, 2000). COP1 is able to catalyze self-ubiquitylation, and the ubiquitylation of LAF1 and ubiquitin ligase activity was abolished by mutations in two conserved Cys residues of the RING finger domain (Seo et al., 2003). His-69 mutated in cop1eid6 is an important residue in the zinc-binding pocket of COP1. Its replacement by a Tyr residue might result in a partially active ubiquitin ligase because the aromatic ring of this amino acid might still bind to the cofactor, although with reduced affinity. This partial activity is probably sufficient for the degradation of target proteins in darkness but might be to low to compensate for higher protein levels resulting from light-induced gene expression or for reduced affinities of COP1 substrates caused by light-dependent modifications. Alternatively, the mutation might lead to an increased auto-ubiquitylation rate, which should result in a more rapid turnover in the light.
The amino acid transition in the cop1eid6 RING finger domain might also change the subcellular localization of the protein because the altered region overlaps with the cytoplasmic localization signal and is close to a subnuclear localization motif (Stacey and von Arnim, 1999; Stacey et al., 1999). Thus, the mutated protein might be excluded more rapidly from its nuclear targets under light irradiation. The mutation might also alter the interaction with target proteins or with the phyB and cryptochrome photoreceptors, although it seems less plausible because these interactions are mainly restricted to the coiled-coil domain and the WD40 repeats (Hardtke et al., 2000; Osterlund et al., 2000; Hoecker and Quail, 2001; Wang et al., 2001; Yang et al., 2001; Holm et al., 2002).
MATERIALS AND METHODS
Plant Material, Growth Conditions, Light Sources, and Screening Program
The following ecotypes and mutants were used: Col, Ler (both obtained from Lehle Seeds, Tucson, AZ), phyB-5, phyB-9 (Reed et al., 1993), and cop1-4 (McNellis et al., 1994a).
For seedling analysis, seeds were sown on four layers of water-soaked filter paper in clear plastic boxes. A 48-h cold treatment at 4°C in darkness was followed by induction of germination for 2 h in white light and 22 h in darkness at 25°C. Afterward, the boxes were placed in the different light conditions for an additional 72 h. The used light sources and the performance of the screening are described by Büche et al. (2000).
Genetic Analysis
To obtain cop1eid6 single mutants, cop1eid6 phyB-5 was crossed with Ler wild type, and F1 plants were allowed to self-fertilize. F2 plants were analyzed using a CAPS marker (Konieczny and Ausubel, 1993). Genomic DNA was analyzed for the presence (phyB-5) or absence (PHYB) of a BsaBI restriction site in a PHYB-specific PCR product obtained by using the primers 5′-CGTGACGCGCCTGCTGGAATTGTT-3′ and 5′-TCCATTGATGCAGCCTCCGGCA-3′. Homozygous PHYB plants were self-pollinated, and the phenotype of the F3 generation was analyzed in weak red light (15 nmol m-2 s-1) and under the screening program to isolate homozygous PHYB cop1eid6 lines. A derived CAPS (Neff et al., 1998) marker was developed to detect the cop1eid6 mutation. PCR was performed with the primers 5′-ATTAAAGATGCTTTCCTCACGCTTGTGGT-3′ and 5′-AAAGCTAAGGACCAAACACAAATTACGAGT-3. This primer combination creates an AhdI restriction enzyme digestion site in the wild-type COP1 gene but not in cop1eid6. The COP1 dCAPS marker was used to confirm cosegregation between the Eid6 phenotype and the mutation in the respective COP1 allele.
Mapping and Sequencing
The cop1eid6 phyB-5 mutant (Ler) was crossed with phyB-9 (Col) for mapping analysis. Seedlings of the F2 generation that showed a de-etiolated phenotype in weak red light (15 nmol m-2 s-1) were transferred to soil. DNA was extracted from leaf tissue of individual F2 progenies according to Sawa et al. (1997). Mapping was performed using simple sequence length polymorphism markers and CAPS markers (Konieczny and Ausubel, 1993; Bell and Ecker, 1994). The phenotype of F3 seed batches was tested to confirm homozygosity of the individual F2 plants used for mapping. In total, 90 F2 plants were analyzed.
The coding region of the COP1 gene was amplified from genomic DNA as two fragments using the following pairs of oligonucleotides: 5′-CAAAAACCAAAATCACAATCGAAGAAATC-3′ with 5′-ACCGTACCGAAGAGAAGTCAAAAACCTT-3′ (5′-fragment) and 5′-CGTGATGATGAGCTGTTTGCCACTGCTG-3′ with 5′-CATGACCGATTCACATCACCGCATTTTGAT-3′ (3′-fragment). The fragments were subcloned into pBluescript KS vector (Stratagene, Amsterdam) using several internal restriction enzyme digestion sites. Sequencing was carried out at the DNA sequencing facility at the University of Freiburg (Germany) with the T3 and T7 sequencing primers.
Measurement of Hypocotyl Elongation and Determination of Anthocyanin Accumulation
Hypocotyl lengths were determined manually. Experiments were repeated at least three times independently. Means of three times (20 seedlings for each experiment) are shown.
For anthocyanin extraction, 60 seedlings were transferred to extraction buffer (17% [v/v] 1-propanol and 1% [v/v] concentrated HCl). The samples were boiled in a water bath for 1 min and cooled down on ice. After shaking overnight at 8°C in darkness, samples were centrifuged at 10,000g for 10 min. Anthocyanin content was determined spectroscopically as described by Schmidt and Mohr (1981). Data represent the mean of at least three independent experiments.
Detection of Flowering Time
Seeds were sown on soil and incubated for 2 d in darkness at 4°C. Afterward, they were transferred to climate chambers with either short-day (25°C, 8 h of white light and 16 h of darkness) or long-day (25°C, 16 h of white light and 8 h of darkness) conditions. Flowering time was recorded as the number of days and rosette leaves from the time when the seeds were transferred to light until the opening of the first flower bud. The experiment was repeated two to three times using six to 15 plants from each genotype in each experiment.
Distribution of Materials
Upon request, seeds of mutants will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this paper that would limit their use in noncommercial research purposes.
Acknowledgments
We thank Martina Krenz and Susanne Wagner for their excellent technical assistance, Xing-Wang Deng for the cop1-4 seeds, and Stefan Kircher, Tim Kunkel, and Katia Marrocco for critical reading of the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.028654.
This work was supported by the Deutsche Forschungsgemeinschaft (Signaltransduktionsmutanten der Photomorphogenese von Arabidopsis grant to E.S. and T.K.) and by the Fonds der Chemischen Industrie (grant to E.S.).
References
- Ang LH, Deng XW (1994) Regulatory hierarchy of photomorphogenic loci: allele-specific and light-dependent interaction between the HY5 and COP1 loci. Plant Cell 6: 613-628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR (1994) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19: 137-144 [DOI] [PubMed] [Google Scholar]
- Büche C, Poppe C, Schäfer E, Kretsch T (2000) eid1: a new Arabidopsis mutant hypersensitive in phytochrome A-depending high-irradiance responses. Plant Cell 12: 547-558 [PMC free article] [PubMed] [Google Scholar]
- Chory J, Peto C, Feinbaum R, Pratt L, Ausubel F (1989) Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell 58: 991-999 [DOI] [PubMed] [Google Scholar]
- Clack T, Matthews S, Sharrock RA (1994) The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol 25: 413-427 [DOI] [PubMed] [Google Scholar]
- Clough RC, Vierstra RD (1997) Phytochrome degradation. Plant Cell Environ 20: 713-721 [Google Scholar]
- Deng X-W, Caspar T, Quail PH (1991) cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev 5: 1172-1182 [DOI] [PubMed] [Google Scholar]
- Deng X-W, Matsui M, Wei N, Wagner D, Chu AM, Feldmann KA, Quail PH (1992) COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a Gb homologous domain. Cell 71: 791-801 [DOI] [PubMed] [Google Scholar]
- Deng X-W, Quail PH (1992) Genetic and phenotypic characterization of cop1 mutants of Arabidopsis thaliana. Plant J 2: 83-95 [Google Scholar]
- Dieterle M, Zhou YC, Schäfer E, Funk M, Kretsch T (2001) EID1, an F-box protein involved in phytochrome A-specific light signaling. Genes Dev 15: 939-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Deng X-W (2000) The cell biology of the COP/DET/FUS proteins: regulating proteolysis in photomorphogenesis and beyond? Plant Physiol 124: 1548-1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Gohda K, Osterlund MT, Oyama T, Okada K, Deng XW (2000) HY5 stability and activity in Arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J 19: 4997-5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker U, Quail PH (2001) The phytochrome A-specific signalling intermediate SPA1 interacts directly with COP1, a constitutive repressor of light signalling in Arabidopsis. J Biol Chem 276: 38173-38178 [DOI] [PubMed] [Google Scholar]
- Hoecker U, Tepperman JM, Quail PH (1999) SPA1, a WD-repeat protein specific to phytochrome A signal transduction. Science 284: 496-499 [DOI] [PubMed] [Google Scholar]
- Hoecker U, Xu Y, Quail PH (1998) spa1: a new genetic locus involved in phytochrome A-specific signal transduction. Plant Cell 10: 19-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M, Ma L-G, Qu L-J, Deng X-W (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16: 1247-1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson ME (2000) The genetics of phytochrome signalling in Arabidopsis. Sem Cell Dev Biol 11: 475-483 [DOI] [PubMed] [Google Scholar]
- Jackson PK, Eldridge AG, Freed E, Furstenthal L, Hsu JY, Kaiser BK, Reimann JDR (2000) The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol 10: 429-439 [DOI] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4: 403-410 [DOI] [PubMed] [Google Scholar]
- Kunkel T, Neuhaus G, Batschauer A, Chua NH, Schäfer E (1996) Functional analysis of yeast-derived phytochrome A and B phycocyanobilin adducts. Plant J 10: 625-636 [DOI] [PubMed] [Google Scholar]
- Li J, Chory J (1996) A role for brassinosteroids in light-dependent development in Arabidopsis. Science 272: 398-401 [DOI] [PubMed] [Google Scholar]
- Lin C (2002) Blue light receptors and signal transduction. Plant Cell 14: 207-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNellis TW, von Arnim AG, Araki T, Komeda Y, Miséra S, Deng X-W (1994a) Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell 6: 487-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNellis TW, von Arnim AG, Deng X-W (1994b) Overexpression of Arabidopsis COP1 results in partial suppression of light-mediated development: evidence for a light-inactivable repressor of photomorphogenesis. Plant Cell 6: 1391-1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miséra S, Müller AJ, Weiland-Heidecker U, Jürgens G (1994) The FUSCA genes of Arabidopsis: negative regulators of light responses. Mol Gen Genet 244: 242-252 [DOI] [PubMed] [Google Scholar]
- Møller SG, Ingles PJ, Whitelam GC (2002) The cell biology of phytochrome signalling. New Phytol 154: 553-590 [DOI] [PubMed] [Google Scholar]
- Neff MM, Chory J (1998) Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol 118: 27-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Fankhauser C, Chory J (2000) Light: an indicator of time and place. Genes Dev 14: 257-271 [PubMed] [Google Scholar]
- Neff MM, Neff JD, Chory J, Pepper AE (1998) dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J 14: 387-392 [DOI] [PubMed] [Google Scholar]
- Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462-466 [DOI] [PubMed] [Google Scholar]
- Poppe C, Sweere U, Drumm-Herrel H, Schäfer E (1998) The blue light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana. Plant J 16: 465-471 [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS. Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa S, Ito T, Okada K (1997) A rapid method for detection of single base changes in Arabidopsis thaliana using the polymerase chain reaction. Plant Mol Biol Rep 15: 179-185 [Google Scholar]
- Schmidt R, Mohr H (1981) Time-dependent changes in the responsiveness to light of phytochrome-mediated anthocyanin synthesis. Plant Cell Environ 4: 433-437 [Google Scholar]
- Seo HS, Yang JY, Ishikawa M, Bolle C, Ballesteros ML, Chua NH (2003) LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423: 995-999 [DOI] [PubMed] [Google Scholar]
- Stacey MG, Hicks SN, von Arnim AG (1999) Discrete domains mediate the light-responsive nuclear and cytoplasmic localization of Arabidopsis COP1. Plant Cell 11: 349-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey MG, von Arnim AG (1999) A novel motif mediates the targeting of the Arabidopsis COP1 protein to subnuclear foci. J Biol Chem 274: 27231-27236 [DOI] [PubMed] [Google Scholar]
- Suzuki G, Yanagawa Y, Kwok SF, Matsui M, Deng XW (2002) Arabidopsis COP10 is a ubiquitin-conjugating enzyme variant that acts together with COP1 and the COP9 signalosome in repressing photomorphogenesis. Genes Dev 16: 554-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M, Jorgensen P (2000) Proteolysis and the cell cycle: with this RING I do thee destroy. Curr Opinion Gen Dev 10: 54-64 [DOI] [PubMed] [Google Scholar]
- von Arnim AG, Deng X-W (1993) Ring finger motif of Arabidopsis thaliana COP1 defines a new class of zinc-binding domain. J Biol Chem 268: 19626-19631 [PubMed] [Google Scholar]
- von Arnim AG, Deng X-W (1994) Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell type specific modulation of its nucleocytoplasmic partitioning. Cell 79: 1035-1045 [DOI] [PubMed] [Google Scholar]
- Wang H, Ma L-G, Li J-M, Zhao H-Y, Deng XW (2001) Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294: 154-158 [DOI] [PubMed] [Google Scholar]
- Whitelam GC, Devlin PF (1997) Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ 20: 752-758 [Google Scholar]
- Yang H-Q, Tang R-H, Cashmore A (2001) The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13: 2573-2587 [DOI] [PMC free article] [PubMed] [Google Scholar]