Abstract
Granulocytes are pivotal regulators of tissue injury. However, the transcriptional mechanisms that regulate granulopoiesis under inflammatory conditions are poorly understood. Here we show that the transcriptional coregulator B cell leukemia/lymphoma 3 (Bcl3) limits granulopoiesis under emergency (i.e., inflammatory) conditions, but not homeostatic conditions. Treatment of mouse myeloid progenitors with G-CSF — serum concentrations of which rise under inflammatory conditions — rapidly increased Bcl3 transcript accumulation in a STAT3-dependent manner. Bcl3-deficient myeloid progenitors demonstrated an enhanced capacity to proliferate and differentiate into granulocytes following G-CSF stimulation, whereas the accumulation of Bcl3 protein attenuated granulopoiesis in an NF-κB p50–dependent manner. In a clinically relevant model of transplant-mediated lung ischemia reperfusion injury, expression of Bcl3 in recipients inhibited emergency granulopoiesis and limited acute graft damage. These data demonstrate a critical role for Bcl3 in regulating emergency granulopoiesis and suggest that targeting the differentiation of myeloid progenitors may be a therapeutic strategy for preventing inflammatory lung injury.
Introduction
Granulocytes are important orchestrators of host defense responses. Their accumulation in target tissues must be strictly controlled, as too few granulocytes may not allow for adequate immunity and tissue repair, while too many granulocytes can promote tissue injury (1, 2). Under steady-state or homeostatic conditions, the number of circulating granulocytes is strictly maintained by low serum levels of G-CSF (3). Under inflammatory conditions, serum G-CSF concentrations rise, which results in rapid increases in granulocyte production in the bone marrow and a large expansion of granulocyte numbers in the periphery (4). This demand-driven or “emergency” granulopoiesis can also be regulated by other granulopoietic cytokines such as GM-CSF and IL-3 (5, 6).
Granulocytes arise from a small number of myeloid lineage-restricted progenitors within the bone marrow (7). HSCs eventually give rise to common myeloid progenitors (CMPs), which can differentiate into granulocyte-macrophage progenitors (GMPs) (8). Proliferation and differentiation of myeloid progenitors is required to support both homeostatic and emergency granulopoiesis. In response to saturating amounts of granulopoietic cytokines, myeloid progenitors divide more frequently to sustain the granulocyte output necessary to promote emergency granulopoiesis. However, the molecular mechanisms that differentially regulate these 2 states of granulopoiesis remain elusive. One hypothesis is that the expression patterns of transcription factors that control the proliferation of myeloid progenitors are differentially regulated to allow for accelerated granulocyte production. This has been observed for CAAT enhancer binding protein (C/EBP) family members (9, 10). For example, C/EBPα inhibits the proliferation of myeloid progenitors to a greater extent than does C/EBPβ. In response to high concentrations of granulopoietic cytokines, C/EBPα expression is attenuated, while C/EBPβ expression, which has been previously demonstrated to be required for emergency granulopoiesis, is upregulated. However, whether there are other transcription factors that specifically control emergency granulopoiesis remains largely unclear. For instance, despite their dominant role in regulating inflammatory responses, the expression patterns of the NF-κB family of transcription factors during emergency granulopoiesis is not well understood.
The IκB family member B cell leukemia/lymphoma 3 (Bcl3) was originally identified as a proto-oncogene (11). However, unlike other classical IκB family members, Bcl3 does not sequester NF-κB transcription complexes to the cytoplasm, but instead mainly resides in the nucleus. Here it is thought to play a critical role in counter-regulating inflammatory responses through limiting the transcription of NF-κB–dependent genes (12). For example, Bcl3 promotes IL-10–mediated inhibition of LPS-induced TNF-α expression in macrophages (13, 14). We have recently shown that Bcl3 can negatively regulate TLR4-mediated inflammatory gene expression by promoting the stability of NF-κB p50 homodimers, which can compete for κB elements otherwise occupied by pro-inflammatory RelA p65 and c-Rel–containing NF-κB transcription factor heterodimers such as NF-κB p50:p65 (15). In the absence of Bcl3 expression, mice become hyperresponsive to TLR signals and are incapable of achieving tolerance to repeated LPS challenge.
The fundamental mechanisms of human acute lung injury have been difficult to investigate, as clinical data are descriptive regarding the evolution of inflammatory changes inside and outside pulmonary tissue. As it concerns granulocyte-mediated lung injury there has been a lack of a model that allows the genetic dissection between lung-resident and peripheral responses in regulating emergency granulopoiesis. We have recently developed a vascularized and aerated orthotopic lung transplant model in the mouse, which recapitulates the ischemia-reperfusion induced acute graft injury observed in human lung recipients (16, 17). Utilizing this transplant model we selectively analyzed the role of Bcl3 in granulocyte production by studying the effects of wild-type lung engraftment into recipients that are Bcl3 deficient in the hematopoietic cell compartment, thereby eliminating the potential inflammatory contributions of Bcl3 in alveolar macrophages. Here, we report a new and unexpected role for Bcl3 in regulating emergency granulopoiesis. While Bcl3 expression is not required to maintain homeostatic granulopoiesis, we show that a lack of Bcl3 expression in lung recipient hematopoietic cells results in exacerbation of pulmonary tissue injury. Bcl3 transcripts accumulate in myeloid progenitors in response to G-CSF stimulation and act to limit their capacity to promote emergency granulopoiesis in an NF-κB p50–dependent manner.
Results
Pulmonary injury is exacerbated in Bcl3–/– lung graft recipients.
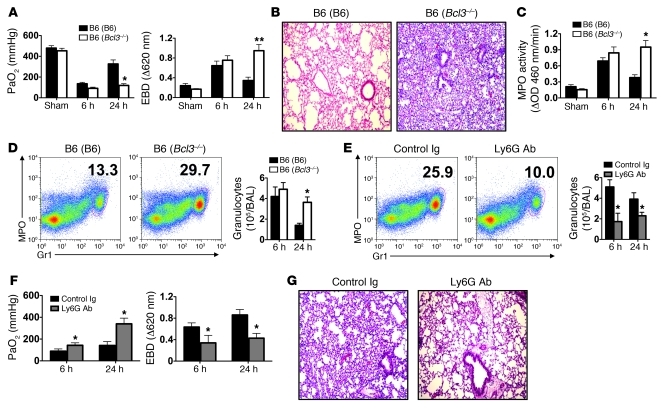
To examine the role of Bcl3 in emergency granulopoiesis, we reconstituted wild-type B6 mice with Bcl3–/– bone marrow (B6 [Bcl3–/–]), since Bcl3–/– mice have a primary defect in their stromal cells that prevents the full development of secondary lymphoid organs, while B6 (Bcl3–/–) mice have normal secondary lymphoid tissue (15, 18–20). B6 lungs were transplanted into syngeneic B6 (Bcl3–/–) or control B6 lung recipients reconstituted with wild-type B6 bone marrow (B6 [B6]) and evaluated for graft function and injury (Figure 1, A and B). Both B6 (B6) and B6 (Bcl3–/–) lung recipients had comparable graft function (PaO2) and pulmonary edema as measured by Evans Blue Dye (EBD) exclusion at 6 hours following transplantation. However, at 24 hours following transplantation, B6 → B6 (Bcl3–/–) lung recipients had significantly worse graft function and pulmonary edema as well as marked alveolar thickening and congestion when compared to B6 → B6 (B6) lung recipients. We also observed a significantly higher degree of myeloperoxidase (MPO) activity in graft tissue (Figure 1C) and a greater abundance of granulocytes in both lung grafts and airways after transplantation into B6 (Bcl3–/–) recipients at this time point (Figure 1D). Resting B6 (Bcl3–/–) mice had equivalent hematological parameters and no evidence of pulmonary neutrophilia when compared to B6 (B6) mice (Table 1 and Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI42596DS1). Importantly, these patterns of acute pulmonary injury were not unique to lung transplantation, as intratracheal LPS instillation resulted in worse lung injury and function and pulmonary neutrophilia in B6 (Bcl3–/–) when compared with B6 (B6) mice (Supplemental Figure 2). As granulocytes have been shown to be critical regulators of solid organ injury, we asked whether reduction of granulocytes in B6 (Bcl3–/–) lung recipients through administration of granulocyte-depleting antibodies (anti-Ly6G) would improve graft function and attenuate tissue injury. Anti-Ly6G treatment of B6 (Bcl3–/–) hosts reduced graft infiltration of granulocytes to levels seen in the grafts of B6 → B6 (B6) lung recipients (Figure 1, E and D). Such treatment of B6 (Bcl3–/–) lung recipients led to improvement in pulmonary function, reduced edema (Figure 1F), and preservation of lung architecture in the grafts (Figure 1G). Thus, granulocytes are major contributors of graft injury in B6 → B6 (Bcl3–/–) lung recipients.
Figure 1. Granulocytes promote lung graft injury in Bcl3-deficient recipients.
(A) Assessment of PaO2 (left) and exclusion of EBD (right) 6 and 24 hours following B6 → B6 (B6) or B6 → B6 (Bcl3–/–) lung engraftment. (B and C) Representative graft histology (original magnification, ×100) (n = 11) (B) and intragraft MPO activity (C) 24 hours following B6 → B6 (B6) or B6 → B6 (Bcl3–/–) lung engraftment. (D) Left: Representative FACS analysis (n = 5). Numbers denote percent abundance of granulocytes in graft tissue. Right: Granulocyte counts in BAL at 6 and 24 hours following B6 → B6 (B6) or B6 → B6 (Bcl3–/–) lung engraftment. (E) Left: B6 → B6 (Bcl3–/–) lung recipients were partially depleted for granulocytes with Ly6G-specific antibodies or treated with control Ig 4 hours prior to transplantation and assessed for percent abundance of granulocytes in graft tissue 24 hours after engraftment. Right: Granulocyte counts at 6 and 24 hours following engraftment in BAL. (F) Assessment of PaO2 (left) and EBD exclusion (right) at 6 and 24 hours following engraftment. (G) Representative lung graft histology (original magnification, ×100) of B6 → B6 (Bcl3–/–) recipients 24 hours after engraftment following treatment with either control Ig or Ly6G-specific antibodies (n = 4). Data are mean ± SD and, unless otherwise indicated, represent at least 3 independent experiments. *P < 0.05; **P < 0.01.
Table 1 .
Hematological parameters
Bcl3 negatively regulates emergency granulopoiesis.
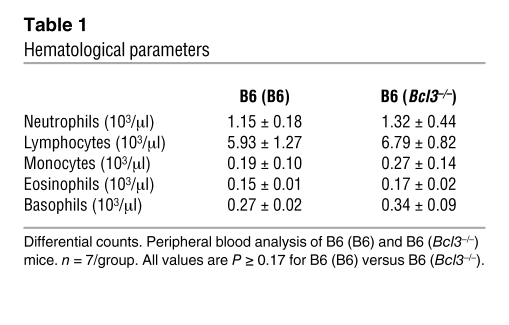
Next we set out to investigate mechanisms that could account for the increased accumulation of granulocytes and graft injury after lung transplantation into B6 (Bcl3–/–) recipients. We first evaluated whether recruitment into the graft was controlled by Bcl3 expression. To this end, equal numbers of purified CFSE-labeled wild-type B6 and Bcl3–/– granulocytes were adoptively transferred into B6 → B6 lung transplant recipients, and grafts were assessed for granulocyte accumulation (Figure 2A). We observed comparable accumulation of wild-type B6 and Bcl3–/– granulocytes in lung grafts, indicating that Bcl3 does not directly control granulocyte entry from peripheral blood into lung grafts. Moreover, granulocytes isolated from B6 (B6) and B6 (Bcl3–/–) mice were morphologically indistinguishable (Figure 2B). We then considered whether Bcl3 regulated granulocyte activation by comparing surface expression of adhesion molecules CD11b and CD62L (Figure 2C). At baseline, granulocytes in the peripheral blood of B6 (B6) and B6 (Bcl3–/–) mice expressed similar levels of CD11b and CD62L. Following engraftment with B6 lungs, B6 and Bcl3–/– blood granulocytes upregulated CD11b and shed CD62L to a comparable degree. Also, ROS burst in response to N-formyl methionyl-leucine-proline (f-MLP) or PMA stimulation was equivalent between B6 and Bcl3–/– resting and lung graft airway granulocytes (Figure 2D). As it has been previously shown that lung injury is exacerbated by increased granulocyte survival (21), we assessed granulocyte senescence in the airways of B6 (B6) to B6 (Bcl3–/–) lung recipients (Figure 2E). Surprisingly, despite greater accumulation of granulocytes in the airways of B6 → B6 (Bcl3–/–) lung recipients (Figure 1D vs. Figure 2E), we observed a moderate but significant decrease in their survival in the airways of B6 → B6 (Bcl3–/–) as compared with B6 → B6 (B6) lung recipients. We also measured the survival B6 and Bcl3–/– granulocytes ex vivo (Figure 2F). While spontaneous death was nearly equivalent for both types of granulocytes, stimulation of B6 granulocytes with G-CSF was more effective at increasing survival when compared with G-CSF stimulation of Bcl3–/– granulocytes.
Figure 2. Functional and phenotypic assessment of B6 and Bcl3-deficient granulocytes.
(A) Assessment of granulocyte recruitment into lung grafts. CFSE-labeled B6 or Bcl3–/– granulocytes (5 × 106) were assessed by FACS analysis in B6 → B6 lung grafts 3 hours following adoptive transfer. Numbers denote percent abundance of CFSE-labeled granulocytes within grafts. Results are representative of 3 independent experiments. (B) Nuclear morphology (original magnification, ×400) of B6 or Bcl3–/– granulocytes following Wright Giemsa stain. (C) Evaluation of granulocyte activation measured by CD11b and CD62L expression on granulocytes before (left) or 18 hours after (right) B6 → B6 (B6) or B6 → B6 (Bcl3–/–) lung engraftment. Results are representative of 5 independent experiments. (D) Measurement of f-MLP– or PMA-mediated ROS generation from bone marrow–derived or BAL-derived B6 or Bcl3–/– granulocytes 24 hours after engraftment. Data are representative of 3 independent experiments. (E) Survival in vivo of BAL granulocytes from B6 → B6 (Bcl3–/–) or B6 → B6 (B6) lung recipients 24 hours following transplantation depicted as a representative FACS plot (n = 6) (left) or as a scatter plot (right) showing percent abundance of Annexin V+ granulocytes. Data are representative of 7 independent experiments. (F) Survival ex vivo of B6 or Bcl3–/– granulocytes in the absence or presence of G-CSF (10 ng/ml) shown normalized to the number of Annexin V– cells at the initiation of culture. Data are representative of 2 independent experiments. Data represent mean ± SD. *P < 0.05.
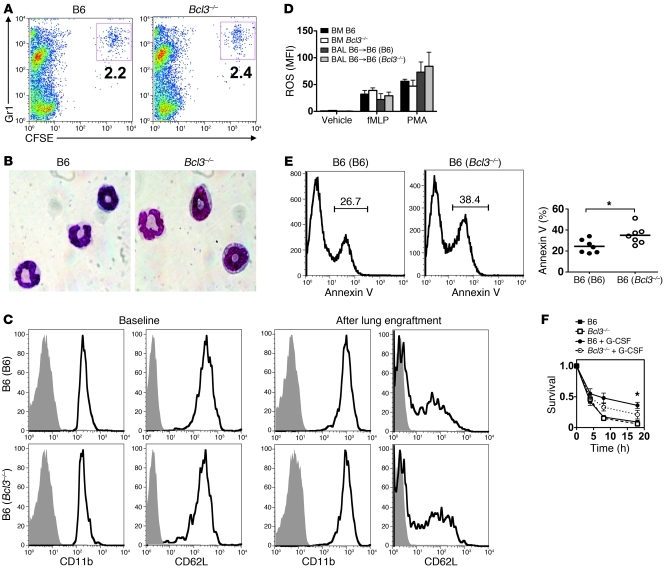
These findings prompted us to examine whether differences in granulocyte production played a role in the lung graft injury after transplantation into B6 (Bcl3–/–) recipients. We therefore assessed granulocyte accumulation in the peripheral blood following B6 → B6 (B6) and B6 → B6 (Bcl3–/–) lung transplantation (Figure 3A). Between 1 and 3 days after engraftment, B6 (Bcl3–/–) recipients had significantly more blood granulocytes than B6 (B6) recipients. We next examined the bone marrow of B6 → B6 (Bcl3–/–) lung recipients for evidence of augmented production of granulocytes (Figure 3B). One day after engraftment, there was an overall greater abundance of Gr1+ MPOlo-hi cells, along with a greater fraction of the mature Gr1+ MPOhi subset in the bone marrow of B6 → B6 (Bcl3–/–) lung recipients as compared with the bone marrow of B6 → B6 (B6) recipients. Thus, these data indicated that elevated granulocyte numbers in the blood of B6 → B6 (Bcl3–/–) lung recipients is due to augmented bone marrow granulopoiesis.
Figure 3. Bcl3 limits G-CSF–mediated emergency granulopoiesis.
(A) Left: Representative FACS analysis (n = 3). Numbers denote percent abundance of peripheral blood granulocytes at 24 hours following B6 → B6 (Bcl3–/–) or B6 → B6 (B6) lung engraftment. Right: Peripheral blood granulocyte numbers for indicated time points up to 72 hours after B6 → B6 (Bcl3–/–) or B6 → B6 (B6) lung transplantation (n = 4). (B) Representative FACS analysis (n = 3) of MPO+Gr1+ populations in the bone marrow of B6 (B6) or B6 (Bcl3–/–) mice 18 hours following the implantation of B6 lungs. Numbers indicate percent abundance of the indicated populations within the bone marrow. (C) Serum concentrations of indicated cytokines following B6 → B6 (Bcl3–/–) or B6 → B6 (B6) lung engraftment (n = 4). (D) Peripheral blood granulocyte numbers 24 hours following intravenous administration of 5 μg of G-CSF, GM-CSF, or IL-3 to either B6 (B6) or B6 (Bcl3–/–) mice (n = 5). Data represent mean ± SD. *P < 0.05; **P < 0.01.
The cytokines G-CSF, GM-CSF, and IL-3 are well characterized stimulators of granulocyte production. We next assessed concentrations of these cytokines in the peripheral blood of B6 (B6) and B6 (Bcl3–/–) mice, which received B6 lungs (Figure 3C). B6 (B6) and B6 (Bcl3–/–) lung recipients produced nearly equivalent amounts of granulopoietic cytokines. Notably, early after engraftment the serum concentration of G-CSF transiently increased almost 10-fold relative to pre-transplant levels. By contrast, GM-CSF and IL-3 serum concentrations showed only modest elevations following lung transplantation. To assess the functional significance of these observations, we administered recombinant G-CSF, GM-CSF, or IL-3 to B6 (B6) or B6 (Bcl3–/–) mice and measured granulocyte accumulation in the peripheral blood (Figure 3D). G-CSF–treated B6 (Bcl3–/–) mice accumulated approximately twice as many peripheral blood granulocytes as did B6 (B6) mice. By contrast, IL-3 and GM-CSF administration produced comparable increases of granulocytes in the peripheral blood of B6 (Bcl3–/–) mice relative to B6 (B6) mice. As granulocyte numbers in resting B6 (B6) and B6 (Bcl3–/–) mice are equivalent, our data collectively show that Bcl3 is a negative regulator of G-CSF–mediated emergency granulopoiesis.
Bcl3 controls myeloid progenitor mobilization.
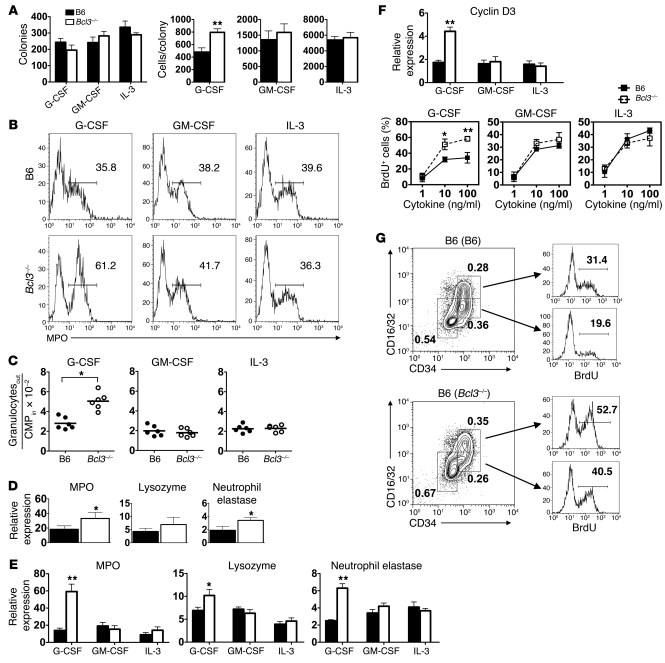
The finding that B6 (Bcl3–/–) mice have the capacity to produce more granulocytes could be due to greater numbers of myeloid progenitors within the bone marrow. To assess this possibility, we performed methylcellulose colony assays with bone marrow from B6 (B6) and B6 (Bcl3–/–) mice in the presence of G-CSF, GM-CSF, or IL-3 (Figure 4A). Bone marrow from both of these mice generated similar numbers of colonies irrespective of cytokine treatment, demonstrating that B6 (Bcl3–/–) and B6 (B6) mice have equivalent numbers of myeloid progenitors. However, unlike the case for GM-CSF or IL-3, G-CSF–stimulated Bcl3–/– bone marrow generated greater numbers of cells per colony relative to G-CSF–stimulated B6 bone marrow (Figure 4A). These data suggested that Bcl3–/– myeloid progenitors had an augmented capacity to produce granulocytes after stimulation with G-CSF. To confirm these findings, we used flow cytometry to sort CMPs from Bcl3–/– or B6 bone marrow, cultured them with G-CSF, GM-CSF, or IL-3, and assessed their differentiation into granulocytes. At 18 hours G-CSF–stimulated Bcl3–/– CMP cultures produced a markedly higher percentage of MPO+ cells relative to G-CSF–stimulated B6 CMP cultures (Figure 4B), while GM-CSF– or IL-3–stimulated Bcl3–/– and B6 CMP cultures generated similar percentages of cells that expressed MPO. By 3 days of culture there were significantly higher numbers of granulocytes in G-CSF–stimulated Bcl3–/– CMP cultures as compared with G-CSF–stimulated B6 CMP cultures (Figure 4C). By contrast, Bcl3–/– and B6 CMP cultures stimulated with either GM-CSF or IL-3 produced equivalent numbers of granulocytes. We also assessed granulocyte-associated gene expression in peripheral blood granulocytes isolated from G-CSF–treated B6 (B6) and B6 (Bcl3–/–) mice (Figure 4D). Compared with peripheral blood granulocytes from G-CSF–treated B6 (B6) mice, we observed significantly higher levels of MPO and neutrophil elastase transcript levels but comparable accumulation of lysozyme transcripts. In addition to elevations in transcript levels of MPO and neutrophil elastase, we also observed significantly increased accumulation of lysozyme transcripts in CMPs from G-CSF–treated B6 (Bcl3–/–) mice (Figure 4E). Of note, we detected relatively higher transcript accumulation of MPO and neutrophil elastase in CMPs isolated from G-CSF–treated B6 (Bcl3–/–) as compared with G-CSF–mobilized peripheral blood granulocytes from the same mice (Figure 4, E and D), indicating that Bcl3 has a more prominent role in gene expression in myeloid progenitors than in circulating granulocytes.
Figure 4. Proliferation and differentiation of Bcl3-deficient myeloid progenitors.
(A) Left: Methylcellulose colony count from B6 or Bcl3–/– bone marrow cells cultured with indicated cytokines. Right: Number of cells per colony. Data are representative of 2 independent experiments. (B) Representative FACS analysis (n = 4) of MPO expression in liquid CMP cultures following 18 hours of stimulation with indicated cytokines. Numbers within histograms indicate percent abundance of MPO+ cells. (C) Granulocyte output from CMP liquid cultures following 72 hours of stimulation with indicated cytokines. (D) Indicated transcript accumulation in peripheral blood granulocytes isolated from B6 (B6) or B6 (Bcl3–/–) mice 24 hours following a 5-μg injection of G-CSF. (E) Indicated transcript accumulation in CMPs isolated from B6 (B6) or B6 (Bcl3–/–) mice 24 hours following injection of 5 μg of indicated cytokines. (F) Bottom: CMP BrdU incorporation in response to indicated concentrations of G-CSF, GM-CSF, or IL-3 following 18 hours of liquid culture. Data are representative of at least 3 independent experiments. Top: Cyclin D3 transcript accumulation in CMPs isolated from B6 (B6) or B6 (Bcl3–/–) mice 24 hours following injection of 5 μg of indicated cytokines. (G) Representative (n = 3) BrdU incorporation of bone marrow Lin–Sca-1–c-Kit+ cells (GMPs; CD34+CD16/32+) and (CMPs; CD34+CD16/32–) in B6 →B6 (B6) or B6 (Bcl3–/–) lung recipients 24 hours following transplantation. Dot plot numbers indicate percent abundance. Data represent mean ± SD. *P < 0.05; **P < 0.01.
These observations raised the possibility that Bcl3 could also regulate the proliferation of myeloid progenitors. To address this question, we BrdU-pulsed CMP cultures that were stimulated with graded amounts of G-CSF, GM-CSF, or IL-3 (Figure 4F). Compared with B6 CMP cultures, Bcl3–/– CMP cultures had significantly higher percentages of BrdU+ cells following treatment with saturating concentrations of G-CSF. GM-CSF– and IL-3–treated B6 and Bcl3–/– cultures had comparable percentages of BrdU+ cells irrespective of cytokine concentration. Additionally, proliferative responses in G-CSF–stimulated Bcl3–/– CMPs were associated with elevated transcript levels of the cell cycle regulator cyclin D3 (Figure 4F). We then investigated myeloid progenitor proliferation in vivo by BrdU-pulsing B6 → B6 (B6) or B6 → B6 (Bcl3–/–) lung recipients and assessing BrdU incorporation in bone marrow–resident CMPs and GMPs 24 hours after engraftment (Figure 4G). Although B6 (B6) and B6 (Bcl3–/–) lung recipients had comparable bone marrow fractions of myeloid progenitors, we observed a markedly higher percentage of BrdU+ CMPs and GMPs in B6 (Bcl3–/–) as compared with B6 (B6) recipients. Thus, Bcl3 regulates both the differentiation and proliferation of myeloid progenitors during emergency granulopoiesis.
Regulation of Bcl3 expression.
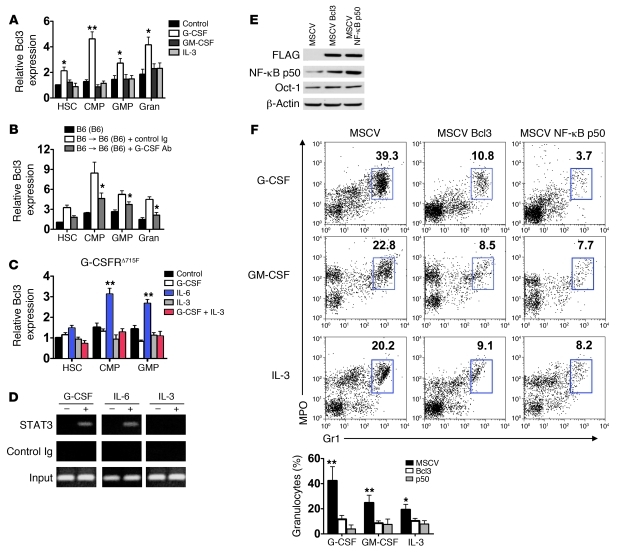
As Bcl3 is a co-transcriptional regulator, we considered the possibility that it differentially regulates the expression of genes that have been reported to have a role in G-CSF–mediated granulopoiesis (Supplemental Figure 3). In particular, the transcription factors C/EBPα and -β both have been shown to regulate granulopoiesis through promoting the differentiation of myeloid progenitors into granulocytes (10). However, we were not able to detect differences in the accumulation of C/EBPα and -β transcripts in B6 and Bcl3–/– myeloid progenitors. Additionally, transcript levels of critical components of the G-CSF signaling pathway, G-CSFR, SOCS3, and STAT3, were also similar in B6 and Bcl3–/– myeloid progenitors with or without G-CSF stimulation. We then considered whether Bcl3 expression in myeloid progenitors is modulated by cytokine stimulation. Purified B6 myeloid progenitors were stimulated with G-CSF, GM-CSF, or IL-3 and analyzed for Bcl3 transcript levels (Figure 5A). Notably, Bcl3 transcript accumulation increased approximately 4-fold in G-CSF–treated CMP cultures and was also significantly elevated in HSC and GMP cultures. By contrast, no significant changes in Bcl3 transcript levels were observed in myeloid progenitors following stimulation with GM-CSF or IL-3. Additionally, in mature granulocytes, Bcl3 transcript levels were also elevated after G-CSF but not GM-CSF or IL-3 treatment. To analyze changes in Bcl3 expression in vivo, we isolated RNA from myeloid progenitors and granulocytes in the airways of B6 (B6) recipients of B6 lungs that had been treated with G-CSF–neutralizing antibodies or control Ig (Figure 5B). Compared with control Ig–treated lung recipients, Bcl3 transcript levels were significantly attenuated after treatment with an anti–G-CSF antibody, indicating that G-CSF plays a prominent role in Bcl3 upregulation.
Figure 5. The dynamics and effects of Bcl3 expression in myeloid progenitors.
(A) Representative (n = 4) Bcl3 transcript expression in B6 myeloid progenitors or granulocytes (Gran) before (control) and after 18 hours of stimulation with 10 ng/ml of indicated cytokines in liquid culture. (B) Representative (n = 4) Bcl3 transcript level expression in myeloid progenitors and granulocytes purified from resting B6 mice, B6 → B6 (B6) treated with control Ig or G-CSF–specific antibodies 18 hours following transplantation. (C) Representative (n = 2) Bcl3 transcript accumulation in G-CSFRΔ715F myeloid cell progenitors. (D) Representative (n = 2) analysis of STAT3 association with Bcl3 promoter. Lin– B6 bone marrow cells were stimulated with indicated cytokines. Chromatin immunoprecipitation was then conducted with STAT3-specific or control antibodies, and amplification was performed with primers specific for an enhancer region of Bcl3. (E) Assessment of Bcl3 ectopic expression on NF-κB p50 protein accumulation. Lin– B6 bone marrow cells were transfected with MSCV, MSCV-Bcl3 (encoding N-FLAG Bcl3), or MSCV NF-κB p50 (encoding N-FLAG NF-κB p50). Nuclear protein was extracted, immunoblotted, and probed with FLAG–, NF-κB p50–, Oct-1–, and β-actin–specific antibodies. Results are representative of 3 independent experiments. (F) Top: Representative FACS analysis (n = 5). Numbers denote percent abundance of granulocytes in Lin– bone marrow cell cultures following 3 days of stimulation with indicated cytokines. Bottom: Mean percent abundance of granulocytes calculated from 5 independently conducted cultures derived from data in top panel. Data represent mean ± SD. *P < 0.05; **P < 0.01.
As these data suggested that G-CSF signaling enhances Bcl3 expression, we next analyzed the role of STAT3, a principal transcription factor that regulates granulopoiesis following G-CSFR engagement but is less critical for IL-3 receptor or GM-CSF receptor function (22–24). To accomplish this we isolated myeloid progenitors from mice with a cytoplasmic truncation in their G-CSFR (G-CSFRΔ715F mice), which attenuates G-CSF–mediated STAT3 activation (ref. 25 and Figure 5C). In contrast to wild-type B6 myeloid progenitors (Figure 5A), G-CSF did not upregulate Bcl3 transcript levels in G-CSFRΔ715F myeloid cultures even when IL-3, a strong STAT5 activator, was added (23). IL-6, however, which has been previously shown to activate STAT3 in G-CSFRΔ715F bone marrow cells, was able to upregulate Bcl3 transcript levels in G-CSFRΔ715F myeloid progenitors. To further confirm that STAT3 is involved in Bcl3 transcription, we performed chromosome immunoprecipitation assays. Lineage-depleted (Lin–) B6 bone marrow cells were stimulated with G-CSF, IL-6, or IL-3; chromosomal DNA was precipitated with STAT3-specific antibodies; and subsequent PCR amplification was conducted on a highly phylogenetically conserved distal enhancer region within intron 2 of Bcl3, which contains 3 STAT3 binding motifs (26, 27) (Figure 5D). We observed STAT3 binding to this enhancer region following stimulation with G-CSF or IL-6, but not with IL-3.
To analyze the role of Bcl3 transcript upregulation in myeloid progenitors, we ectopically expressed Bcl3 and a potential target of its co-transcriptional regulatory activity NF-κB p50 by retroviral-mediated gene transfer into Lin– B6 bone marrow cells (Figure 5E). Ectopically expressed Bcl3 and NF-κB p50 protein was clearly evident within B6 Lin– bone marrow cell nuclei. Moreover, consistent with our previous findings, enhanced Bcl3 expression resulted in an accumulation of NF-κB p50 protein (15). We then examined granulocyte production in Lin– B6 bone marrow cells ectopically expressing Bcl3 or NF-κB p50 following stimulation with G-CSF, GM-CSF, or IL-3 (Figure 5F). Irrespective of cytokine treatment, ectopic expression of Bcl3 or NF-κB p50 in Lin– bone marrow cells greatly reduced granulocyte production, indicating that the accumulation of either transcription factor is sufficient to inhibit granulopoiesis. However, ectopic expression of Bcl3 in NF-κB p50–deficient Lin– bone marrow cells did not markedly inhibit cytokine-mediated granulopoiesis, demonstrating a requirement for NF-κB p50 expression for Bcl3 to negatively regulate granulopoiesis (Supplemental Figure 4). Thus, the accumulation of Bcl3 protein attenuates the production of granulocytes in a NF-κB p50–dependent manner.
G-CSF blockade prevents lung graft injury in B6 (Bcl3–/–) recipients.
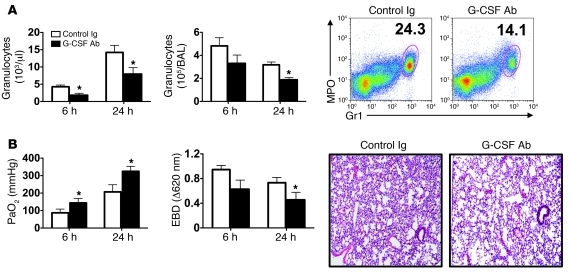
The observation that G-CSF is highly prevalent in the serum of lung transplant recipients led us to hypothesize that blocking G-CSF activity in B6 → B6 (Bcl3–/–) lung recipients can attenuate graft injury. Treatment of B6 (Bcl3–/–) recipients of B6 lungs with G-CSF–neutralizing antibodies significantly reduced granulocyte numbers in peripheral blood, airways, and lung tissue (Figure 6A) and was associated with marked improvement in lung function and graft histology as well as reduction in pulmonary edema (Figure 6B). These results demonstrate that blockade of G-CSF–mediated emergency granulopoiesis attenuates graft injury in B6 (Bcl3–/–) lung recipients.
Figure 6. G-CSF blockade prevents graft injury in B6 (Bcl3–/–) lung recipients.
(A) Granulocyte numbers in the peripheral blood (left) and BAL (middle) at 6 and 24 hours post-engraftment and a representative FACS analysis (24 hours; right) in which numbers indicate granulocyte percent abundance in graft tissue in B6 → B6 (Bcl3–/–) lung recipients treated with control Ig or G-CSF–specific antibodies (n = 10). (B) PaO2 (left) and EBD exclusion (middle) 6 and 24 hours post-engraftment and representative graft histology (right; original magnification, ×100) in B6 → B6 (Bcl3–/–) lung graft recipients 24 hours after engraftment treated with control Ig or G-CSF–specific antibodies (n = 10). Data represent mean ± SD. *P < 0.05.
Discussion
Our results reveal a previously unknown function for Bcl3. In addition to formerly described roles in secondary lymphoid organ development, cell survival, and inflammatory cytokine gene expression, we now demonstrate that Bcl3 expression is critical to controlling G-CSF–mediated emergency granulopoiesis (15, 28, 29). Excessive granulocyte accumulation has been shown to promote lung graft injury both in animal models and in the clinical setting (30, 31). Also, granulocytes play a key role in exacerbating LPS-mediated acute lung injury (32, 33). Interestingly, in humans, intra-bronchial LPS instillation results in G-CSF accumulation in the blood and bronchoalveolar lavage (BAL), which drives granulocyte accumulation in the airway (34). We observed a large accumulation of granulocytes in the peripheral blood and lung tissue of both B6 (Bcl3–/–) mice treated with LPS and B6 (Bcl3–/–) lung recipients, underscoring the role that Bcl3 plays in controlling granulopoiesis to prevent acute lung injury.
Our use of a transplant model to study the role of Bcl3 in acute lung injury has several advantages. First, lung ischemia reperfusion injury is associated with robust recruitment of granulocytes (16). Second, the relevance of this model is underscored by the high morbidity and mortality associated with this condition in the clinical setting (31). Third, by transplanting wild-type lungs into Bcl3-deficient recipients, this model allowed us to eliminate potential contributions of graft-resident Bcl3–/– alveolar macrophages to pulmonary injury. To this end, the earlier onset of lung injury following LPS treatment of B6 (Bcl3–/–) mice might be explained by previous observations of elevated LPS-mediated inflammatory cytokine synthesis by Bcl3-deficient lung-resident macrophages (14, 15). The improvement in pulmonary function and attenuation of graft injury in B6 (Bcl3–/–) recipients following granulocyte depletion or G-CSF blockade demonstrate the importance of Bcl3 in controlling granulocyte accumulation following lung transplantation. Interestingly, regulatory effects mediated by Bcl3 were not significant until 24 hours following transplantation, as graft injury and granulocyte numbers in the blood were nearly equivalent in B6 (Bcl3–/–) and B6 (B6) lung recipients at 6 hours following lung transplantation. These data indicate that Bcl3 does not control the rapid recruitment of granulocytes into the periphery, as would be the case for factors such as CXCR4, which negatively regulates the transit of mature granulocytes from the bone marrow into the blood (35). Consistent with this observation, granulocyte depletion or G-CSF blockade improved lung function to a comparable degree in B6 (Bcl3–/–) and B6 (B6) lung recipients at 6 hours following transplantation.
We additionally noted that granulocyte numbers along with G-CSF levels returned to nearly pre-operative levels 72 hours following lung transplantation in both B6 (B6) and B6 (Bcl3–/–) recipients, indicating that only a single transient phase of emergency granulopoiesis ensues following engraftment. However, Bcl3 expression may also be important in controlling granulocyte production in chronic lung diseases where emergency granulopoiesis is ongoing for extended time periods due to sustained G-CSF production (36). Also, the fact that a major effector function of IL-17+ T lymphocytes is the stimulation of G-CSF–mediated granulocyte production raises the possibility that Bcl3 control of granulopoiesis could play a critical role in regulating tolerance to self-antigens (37, 38). For example, in models of Type 1 diabetes the absence of Bcl3 expression in hematopoietic cells has been recently shown to exacerbate IL-17–associated destruction of islets (39).
Surprisingly, Bcl3 expression appears to be dispensable for granulocyte function as well as the maintenance of resting granulocyte numbers. This observation is in marked contrast to previously reported regulators of G-CSF–mediated granulopoiesis such as G-CSFR, STAT3, and SOCS3. For instance, G-CSFR promotes granulocyte survival and activation and is required for the homeostatic production of granulocytes (7). STAT3 promotes CXCR2-mediated chemotactic responses (40). SOCS3, a negative regulator of G-CSFR activation, inhibits G-CSFR–mediated survival and may be required to temper intrinsic granulocyte activity, as mice with a SOCS3-specific deletion in their hematopoietic cells develop solid organ damage from an inflammatory neutrophilia (41). However, consistent with previous reports of Bcl3 as a pro-survival factor (42), we observed that Bcl3 deficiency in activated granulocytes was associated with a decrease in their survival. Interestingly, a coupling of cell survival to proliferation has been previously observed for other pro-survival molecules such as Bcl2 (43). Moreover, Bcl3 expression in T lymphocytes promotes their survival while inhibiting proliferation and differentiation (29, 44).
To better understand how Bcl3 controls G-CSF–mediated emergency granulopoiesis, we analyzed differential and proliferative responses of myeloid progenitors. After only 18 hours, G-CSF–treated Bcl3–/– CMP cultures accumulated more MPO-expressing cells than equivalently stimulated wild-type B6 CMPs. In vivo, G-CSF–stimulated Bcl3–/– CMPs accumulated higher levels of transcripts associated with granulocyte function as compared with comparably stimulated B6 CMPs. We also observed the augmented transcript accumulation of the cell cycle regulator cyclin D3 in Bcl3–/– CMPs following G-CSF, but not GM-CSF or IL-3 stimulation. Consistent with this finding is a previous report of the cyclin D3 expression in myeloid progenitors during G-CSF–mediated emergency granulopoiesis (45). Moreover, we noted that enhancement of proliferation in Bcl3–/– CMP cultures was prominent only in response to high G-CSF concentrations, indicating a selective role for Bcl3 in attenuating granulocyte production under inflammatory conditions. However, as compared with CMPs, granulocyte function–associated transcripts accumulate to a larger extent in peripheral blood granulocytes isolated from G-CSF–treated B6 (Bcl3–/–) mice. Collectively, these observations support a model in which Bcl3 expression plays a predominant role in myeloid progenitors by tempering gene expression that is required for emergency granulopoiesis.
Depending on the inflammatory stimulus, Bcl3 gene expression may be regulated through different transcription factors. For example, it has been noted that rapidly after TLR engagement Bcl3 gene transcription is dependent on NF-κB activation, raising the possibility that Bcl3 expression can be regulated independently of STAT3 (46, 47). However, TLR stimulation has been also shown to lead to Bcl3 transcription in a STAT3-dependent manner through the autocrine effects of IL-6 and IL-10 production (13, 14). Likewise, in STAT3-hyporesponsive mutant G-CSFRΔ715F myeloid progenitors, we failed to observe Bcl3 transcript accumulation after stimulation with G-CSF. Moreover, we did not observe accumulation of Bcl3 transcripts following GM-CSF stimulation of myeloid progenitors. As the GM-CSF receptor has been reported to a be a strong activator of NF-κB but not STAT3 (48, 49), our data collectively support a model in which STAT3 activation is required to drive Bcl3 expression in myeloid progenitors. Interestingly, the ability of IL-6 to stimulate Bcl3 transcript accumulation in myeloid progenitors raises the possibility that this cytokine could play a role in regulating emergency granulopoiesis following lung ischemia reperfusion injury (50). However, unlike G-CSF, which is the primary regulator of granulocyte production, IL-6 also controls the production of monocytes from hematopoietic progenitors (51, 52). Moreover, IL-6 has also been shown to protect lung grafts from injury, as it reduces endothelial cell disruption, which inhibits granulocyte tissue sequestration (53). Therefore, a selective role for IL-6 in emergency granulopoiesis in the context of solid organ injury would be difficult to discern.
To confirm the functional significance of Bcl3 transcript accumulation in myeloid progenitors we ectopically expressed Bcl3 protein in bone marrow cells. Notably, we observed an attenuation of granulocyte formation in Bcl3 transgene–positive bone marrow cells that is not G-CSF specific and requires NF-κB p50 expression. Thus, these data suggest that when Bcl3 protein accumulates at sufficiently high levels it acts through transcriptional mechanisms that globally regulate granulopoiesis. In this context C/EBPs are likely targets of Bcl3-mediated effects on granulopoiesis, as their transcriptional activity is controlled by association with NF-κB p50 (12, 54). Also, C/EBPs are regulators of GM-CSF– and IL-3–dependent granulopoiesis (10). However, Bcl3 is unlikely to control granulopoiesis by directly regulating C/EBP expression. In the absence of Bcl3 we observed comparable C/EBPα or -β transcript levels irrespective of cytokine stimulation. In particular, the maintenance of C/EBPα expression in Bcl3–/– cells is notable; in a recent report NF-κB p50–/– mice were shown to have reduced levels of C/EBPα expression (55). Importantly, our data show that Bcl3 deficiency does not result in complete elimination of NF-κB p50 protein, indicating that there is still sufficient NF-κB p50 expression to maintain C/EBPα gene transcription. As we demonstrated that NF-κB p50 expression is required for Bcl3-mediated control of granulopoiesis, these data collectively suggest a mechanism by which altering NF-κB p50 levels in the nucleus is critical to control granulocyte production. Consistent with such a role is the observation that mutating the bZIP domain in C/EBPα to reduce the formation of C/EBPα:NF-κB p50 transcriptional complexes promotes granulopoiesis in myeloid progenitor cell lines (56, 57). In addition, C/EBPβ has an identical bZIP domain to C/EBPα, and coimmunoprecipitation studies have demonstrated an interaction between NF-κB p50 and C/EBPβ (58). Thus, it is conceivable that Bcl3 may act to stimulate the formation of NF-κB p50:C/EBP transcriptional complexes, which in turn promote gene expression patterns that limit granulocyte production.
A potential therapeutic role of inhibiting emergency granulopoiesis is substantiated by our demonstration that G-CSF blockade in B6 (Bcl3–/–) lung recipients significantly reduces graft injury and improves function. Moreover, G-CSF blockade effects on lung injury may also be favorably affected by the inhibition of granulopoiesis-independent functions of G-CSF such as the blunting of bone marrow mobilization (59), survival (60), or tissue migration of granulocytes (61). In summary, we have identified what we believe is a novel role for Bcl3 in negatively regulating G-CSF–mediated emergency granulopoiesis. Recent reports linking increased G-CSF serum levels or G-CSF treatment to acute lung injury in the clinical setting highlight the importance of understanding mechanisms that calibrate granulocyte production under emergency conditions (62, 63).
Methods
Mice.
C57BL/6J (B6) and NF-κB p50–/– (B6 background) mice were purchased from The Jackson Laboratory. Bcl3–/– mice (B6 background) were a gift from Y. Chen (University of Pennsylvania, Philadelphia, Pennsylvania, USA); G-CSFRΔ715F mice (B6 background) were a gift from D.C. Link (Washington University in St. Louis). All mice were maintained in a pathogen-free facility at Washington University School of Medicine. All experiments were reviewed and approved by the Animal Studies Committee of Washington University in St. Louis.
Lung transplantation, LPS administration, antibodies, and BrdU assays.
Orthotopic left vascularized lung transplants were performed as previously described (17). LPS (300 μg) from strain O111:B4 (Sigma-Aldrich) was administered down the airway in 100 μl of PBS. Granulocytes were partially depleted with a 250-μg dose of Ly6G-specific antibodies (1A8; Bio-X-Cell) administered intravenously 4 hours prior to surgery. G-CSF was neutralized with 200 μg of G-CSF–specific antibodies (Peprotech) administered intravenously 1 hour prior to surgery. In vivo 1 mg of BrdU was administered intraperitoneally 4 hours before sacrifice for analysis. In vitro BrdU was added to myeloid progenitor cultures at a final concentration of 10 μM, and cells were analyzed 30 minutes later for BrdU incorporation. BrdU assay was conducted by flow cytometric (FACS) analysis using BrdU-specific antibodies (Invitrogen) and permeabilization/fixation reagents from a BrdU Flow Kit (BD Biosciences) in accordance with the manufacturer’s recommendations.
Granulocyte purification, survival, and graft infiltration assay.
Granulocytes were purified from the bone marrow using a previously described 3-layer Percoll (GE Healthcare Life Sciences) gradient method optimized to collect mature neutrophils (64). Granulocyte preparations were routinely greater than 90% Gr1+MPO+ cells with high granularity as determined by side scatter. To measure granulocyte infiltration in lung grafts, freshly isolated bone marrow granulocytes were stimulated for 1 hour in 30 ng/ml of G-CSF, stained with 5 μM CFSE (Invitrogen), quenched in 50% FBS (Stem Cell Technologies)/PBS, and washed twice in PBS before adoptive transfer into B6 → B6 lung recipients. To measure their survival, freshly isolated bone marrow or BAL granulocytes were stained with Annexin V–FITC (BD Biosciences — Pharmingen) as per the manufacturer’s recommendations.
Bone marrow transplantation.
Bone marrow chimeras were created as previously described (65).
Granulocyte analysis.
BAL and lung tissue digest were prepared as previously described (16). Lung cell isolates were analyzed by FACS analysis through staining with Gr1 (RB6-8C5)–, CD11b (M1/70)–, and CD62L (MEL-14)–specific antibodies (ebioscience). Intracellular staining for MPO (8F4; Cell Sciences) was conducted after cell surface staining using a fixation/permeabilization reagent (ebioscience) in accordance with the manufacturers’ recommendations. Granulocytes were counted in the BAL and peripheral blood with a HEMAVET analyzer (Drew Scientific).
Methylcellulose colony assay.
Bone marrow suspensions were mixed with MethoCult M3234 (Stemcell Technologies) and treated with mouse recombinant cytokines (Peprotech) G-CSF (10 ng/ml), GM-CSF (10 ng/ml) or IL-3 (10 ng/ml). Colonies were counted on day 7 with an inverted microscope, and cells per colony were calculated by counting the total number of cells and dividing by the number of colonies.
Cell sorting, Lin– bone marrow cell preparation, and culture.
HSCs, CMPs, and GMPs were purified as previously described (8). Bone marrow cells were stained with the following biotin-conjugated antibody cocktail for the identification of lineage-specific markers: CD3e (145-2C11), CD4 (L3T4), CD8a (Ly-2), CD19 (eBio1D3), B220 (RA3-6B2), Gr-1 (RB6-8C5), Ter-119, CD49b (DX5), CD11c (N418), and CD11b (M1/70). Preparations were then stained with PerCP-Cy5.5–conjugated streptavidin, FITC-conjugated anti-CD34 (RAM34), APC-conjugated c-Kit (2B8), APC-750–conjugated Sca-1 (D7) and PE-conjugated CD16/32 (clone 93). All antibodies were obtained from ebioscience. Cells were then sorted on a custom configured Reflection Cell Sorter through a Lin–c-kit+Sca-1+ gate for HSCs, a Lin–c-kit+Sca-1–CD34+CD16/32– gate for CMPs and a Lin–c-kit+Sca-1–CD34+CD16/32+ gate for GMPs. Lin– bone marrow cells were prepared by 2 rounds of negative selection using the above described lineage-specific antibody cocktail and anti-biotin microbeads. Myeloid progenitors were cultured in round-bottom 96-well plates in medium containing StemPro 34 SFM (Gibco) supplemented with 20% FBS (Stem Cell Technologies) and indicated cytokines at 10 ng/ml.
Immunoblot.
Nuclear protein was prepared from 106 Lin– bone marrow cells using a Nuclear Extract Kit (Active Motif) in accordance with the manufacturer’s recommendations. For whole cell lysates, protein was prepared from an equivalent number of Lin– bone marrow cells. Protein extracts were then heat-denatured in SDS sample buffer (62.5 mM Tris pH 6.8, 2% SDS, 10% glycerol, 50 mM ETT, 0.01% bromophenol blue), resolved on a denaturing 10% SDS-PAGE gel, transferred to nitrocellulose membranes via XCell II blotter (Invitrogen), and probed with either FLAG (M2; Sigma-Aldrich), NF-κB p105/50 (Cell Signaling), Oct-1 (Abcam), or β-actin–specific (AC-15; Santa Cruz Biotechnology Inc.) antibodies. Immunoreactivity was detected with horseradish peroxidase–conjugated antibodies specific for either rabbit (Cell Signaling) or mouse (Cell Signaling) IgG and visualized on X-OMAT film (Kodak) by chemiluminescence (ECL kit; Amersham Biosciences).
Retroviral vector transduction and culture.
N-FLAG Bcl3 cDNA or N-FLAG NF-κB p50 cDNA, provided by Y. Chen, were subcloned into the EcoRI site located within a bicistronic expression cassette of pMSCV-NGFR (MSCV), a gift from W. Pear (University of Pennsylvania, Philadelphia, Pennsylvania, USA), which contains an internal ribosomal entry site that drives the simultaneous expression of a truncated form of the human nerve growth factor receptor (NGFR) to constructs pMSCV-Bcl3 or pMSCV- NF-κB p50. Retrovirus was packaged in Plat-E cells (Cell Biolabs) in a manner previously described (66), and high-titer retroviral supernatant was spin transfected with 5 μg/ml of polybrene into Lin– bone marrow cells stimulated in DMEM (Invitrogen), 20% FBS (Stem Cell Technologies), 10 ng/ml IL-3, 50 ng/ml IL-6 (Peprotech), and 100 ng/ml SCF (Peprotech) for 18 hours. Transfected bone marrow cells were reconstituted into lethally irradiated B6 or NF-κB p50–/– mice. Six to 10 weeks later, Lin– bone marrow cells were isolated and then purified for NGFR+ cells with NGFR-specific antibodies (BD Biosciences) and anti-biotin microbeads (Miltenyi Biotec). For culture, equal numbers of NFGR+ Lin– bone marrow cells were treated with G-CSF (10 ng/ml), GM-CSF (10 ng/ml), or IL-3 (10 ng/ml) for 3 days and then assessed for granulocyte accumulation with Gr1-, CD11b-, and MPO-specific antibodies.
Chromosomal immunoprecipitation assay.
Lin– bone marrow cells (106) were stimulated with 30 ng/ml of G-CSF, IL-6, or IL-3 for 30 minutes, resuspended in cell lysis buffer (5 mM PIPES, pH 8.1; 10 mM KCL; 0.5% Igepal; 10 mM PMSF, Sigma-Aldrich; 10 mg/ml aprotinin, Sigma-Aldrich; and 10 mg/ml leupeptin, Sigma-Aldrich), and nuclei were released with a Dounce Homogenizer. To prepare chromatin, nuclei were resuspended in nuclear lysis buffer (50 mM Tris-Cl, pH 8.1, 10 mM EDTA, 1% SDS, and protease inhibitors), incubated on ice for 20 minutes, and freeze-thawed twice in liquid nitrogen. Ruptured nuclei were sonicated at 50% power in a Branson 250 Sonicator for five 30-second pulses spaced by 1 minute. Chromatin DNA was cleared by 14,000-g centrifugation for 10 minutes and dialyzed into immunoprecipitation buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-Cl, pH 8.1, 167 mM NaCl, and protease inhibitors). Following an overnight incubation at 4°C with either STAT3 antiserum (PA-ST3; R&D Systems) or polyclonal rabbit IgG (Sigma-Aldrich), chromatin was immunoprecipitated with Protein A/G agarose (Santa Cruz Biotechnology Inc.), eluted off with 1% SDS, 0.1 M NaHCO3, and 0.01 mg/ml Herring sperm DNA, and re-equilibrated in 10 mM Tris EDTA buffer, pH 8.0. DNA was amplified with primers (sense 5′-TCCCCGACTGGCCAGGCTTC-3′; antisense 5′-AAGCGTCACCCGCTTGGCTC-3′) specific for the distal enhancer region within intron 2 of the mouse Bcl3 gene.
ROS and MPO assay.
Generation of ROS was assessed by FACS analysis by detection of Rhodamine 123 accumulation in cultured granulocytes following treatment with DHR123 (Invitrogen) and either 0.2 μg/ml PMA (Sigma-Aldrich), 0.5 μM f-MLP (Sigma-Aldrich), or vehicle for 10 minutes at 37°C. MPO lung tissue level activity was determined as previously described (67). Optical density was measured at 460 nm with a spectrophotometer (model PMQ II; Carl Zeiss). One unit of enzyme activity was defined as 1.0 optical density (AU) min–1mg protein–1 at room temperature.
ELISA.
Serum G-CSF, GM-CSF, and IL-3 concentrations were measured with Quantikine ELISA kits (R&D Systems).
EBD exclusion and lung function.
EBD was administered intravenously 4 hours prior to sacrifice, at which point lung grafts were excised and flushed with 20 ml of PBS. To extract EBD, lung tissue was homogenized in 5 ml of formamide. The homogenate was incubated at 37°C for 24 hours and centrifuged at 3,500 g for 30 minutes. The optical density of the supernatant was measured at 620 nm and expressed as milligrams of EBD per gram of wet lung weight. To assess graft function, arterial blood gases were measured using an iSTAT Portable Clinical Analyzer (iSTAT) at a FiO2 of 1.0 after the right pulmonary hilum was clamped for 5 minutes.
Real-time PCR.
FACS-sorted myeloid progenitors or Percoll-purified granulocytes were placed into TRIzol (Invitrogen), and RNA extraction was performed in accordance with the manufacturer’s instructions. Quantitative real-time RT-PCR was conducted on an ABI 7900 using TaqMan Gene Expression Assay system (Applied Biosystems) in accordance with manufacturer’s recommendations. Amplification of target sequences was conducted as follows: 50°C for 20 minutes and 95°C for 10 minutes, followed by 38–45 cycles of 95°C for 15 seconds and 60°C for 1 minute. All primers and MGB probes were purchased as kits from Applied Biosystems and can be identified in the following manner: C/EBPα (Mm01265914_s1), C/EBPβ (Mm00843434_s1), G-CSFR (Mm00432735_m1), STAT3 (Mm01219775_m1), SOCS3 (Mm01249143_g1), Bcl3 (Mm00504306_m1), cyclin D3 (Mm01273583_m1), MPO (Mm00447886_m1), lysozyme (Mm01238927_m1), neutrophil elastase (Mm00469310_m1), and 18S rRNA (Hs03003631_g1).
Statistics.
Data were analyzed using GraphPad Prism, version 5.0, and results are presented as mean ± SD. An unpaired 2-tailed Student’s t test was used to evaluate pairs of means for significance. Values of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Daniel C. Link and Marco Colonna of the Washington University School of Medicine for helpful discussions in the development of this manuscript. We also are grateful to William C. Eades, Jr., William G. Lamberton, and Jackie M. Hughes of the Siteman Cancer Center High Speed Cell Sorter Core for their technical assistance. D. Kreisel and A.E. Gelman are supported by a grant from the National Heart, Lung and Blood Institute (1R01HL094601).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(1):265–276. doi:10.1172/JCI42596.
References
- 1.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 2.Martin P, et al. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13(13):1122–1128. doi: 10.1016/S0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 3.Watari K, et al. Serum granulocyte colony-stimulating factor levels in healthy volunteers and patients with various disorders as estimated by enzyme immunoassay. Blood. 1989;73(1):117–122. [PubMed] [Google Scholar]
- 4.Cheers C, Haigh AM, Kelso A, Metcalf D, Stanley ER, Young AM. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988;56(1):247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalf D, Begley CG, Johnson GR, Nicola NA, Lopez AF, Williamson DJ. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986;68(1):46–57. [PubMed] [Google Scholar]
- 6.Metcalf D, et al. Hemopoietic responses in mice injected with purified recombinant murine GM-CSF. Exp Hematol. 1987;15(1):1–9. [PubMed] [Google Scholar]
- 7.Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity. 1996;5(5):491–501. doi: 10.1016/S1074-7613(00)80504-X. [DOI] [PubMed] [Google Scholar]
- 8.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, et al. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP alpha. Immunity. 2004;21(6):853–863. doi: 10.1016/j.immuni.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Hirai H, et al. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol. 2006;7(7):732–739. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- 11.Wulczyn FG, Naumann M, Scheidereit C. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-kappa B. Nature. 1992;358(6387):597–599. doi: 10.1038/358597a0. [DOI] [PubMed] [Google Scholar]
- 12.Dechend R, et al. The Bcl-3 oncoprotein acts as a bridging factor between NF-kappaB/Rel and nuclear co-regulators. Oncogene. 1999;18(22):3316–3323. doi: 10.1038/sj.onc.1202717. [DOI] [PubMed] [Google Scholar]
- 13.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169(5):2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 14.Kuwata H, et al. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood. 2003;102(12):4123–4129. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- 15.Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signaling by NF-kappaB p50 ubiquitination blockade. Science. 2007;317(5838):675–678. doi: 10.1126/science.1142953. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki M, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7(6):1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 17.Krupnick AS, et al. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 2009;4(1):86–93. doi: 10.1038/nprot.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Wang H, Claudio E, Brown K, Siebenlist U. A role for the IkappaB family member Bcl-3 in the control of central immunologic tolerance. Immunity. 2007;27(3):438–452. doi: 10.1016/j.immuni.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzoso G, et al. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. 1998;187(2):147–159. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz EM, Krimpenfort P, Berns A, Verma IM. Immunological defects in mice with a targeted disruption in Bcl-3. Genes Dev. 1997;11(2):187–197. doi: 10.1101/gad.11.2.187. [DOI] [PubMed] [Google Scholar]
- 21.Fialkow L, et al. Neutrophil apoptosis: a marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit Care. 2006;10(6):R155. doi: 10.1186/cc5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward AC, et al. Tyrosine-dependent and -independent mechanisms of STAT3 activation by the human granulocyte colony-stimulating factor (G-CSF) receptor are differentially utilized depending on G-CSF concentration. Blood. 1999;93(1):113–124. [PubMed] [Google Scholar]
- 23.Azam M, et al. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 1995;14(7):1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyle SE, Gasson JC. Characterization of the role of the human granulocyte-macrophage colony-stimulating factor receptor alpha subunit in the activation of JAK2 and STAT5. Blood. 1998;92(3):867–876. [PubMed] [Google Scholar]
- 25.McLemore ML, et al. STAT-3 activation is required for normal G-CSF-dependent proliferation and granulocytic differentiation. Immunity. 2001;14(2):193–204. doi: 10.1016/S1074-7613(01)00101-7. [DOI] [PubMed] [Google Scholar]
- 26.Brocke-Heidrich K, et al. BCL3 is induced by IL-6 via Stat3 binding to intronic enhancer HS4 and represses its own transcription. Oncogene. 2006;25(55):7297–7304. doi: 10.1038/sj.onc.1209711. [DOI] [PubMed] [Google Scholar]
- 27.Ge B, Li O, Wilder P, Rizzino A, McKeithan TW. NF-kappa B regulates BCL3 transcription in T lymphocytes through an intronic enhancer. J Immunol. 2003;171(8):4210–4218. doi: 10.4049/jimmunol.171.8.4210. [DOI] [PubMed] [Google Scholar]
- 28.Franzoso G, et al. Critical roles for the Bcl-3 oncoprotein in T cell-mediated immunity, splenic microarchitecture, and germinal center reactions. Immunity. 1997;6(4):479–490. doi: 10.1016/S1074-7613(00)80291-5. [DOI] [PubMed] [Google Scholar]
- 29.Rangelova S, Kirschnek S, Strasser A, Hacker G. FADD and the NF-kappaB family member Bcl-3 regulate complementary pathways to control T-cell survival and proliferation. Immunology. 2008;125(4):549–557. doi: 10.1111/j.1365-2567.2008.02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. Ischemia-reperfusion induces G-CSF gene expression by renal medullary thick ascending limb cells in vivo and in vitro. Am J Physiol Renal Physiol. 2004;286(6):F1193–1201. doi: 10.1152/ajprenal.00379.2002. [DOI] [PubMed] [Google Scholar]
- 31.Belperio JA, et al. CXCR2/CXCR2 ligand biology during lung transplant ischemia-reperfusion injury. J Immunol. 2005;175(10):6931–6939. doi: 10.4049/jimmunol.175.10.6931. [DOI] [PubMed] [Google Scholar]
- 32.Worthen GS, Haslett C, Rees AJ, Gumbay RS, Henson JE, Henson PM. Neutrophil-mediated pulmonary vascular injury. Synergistic effect of trace amounts of lipopolysaccharide and neutrophil stimuli on vascular permeability and neutrophil sequestration in the lung. Am Rev Respir Dis. 1987;136(1):19–28. doi: 10.1164/ajrccm/136.1.19. [DOI] [PubMed] [Google Scholar]
- 33.Bachmaier K, et al. E3 ubiquitin ligase Cblb regulates the acute inflammatory response underlying lung injury. Nat Med. 2007;13(8):920–926. doi: 10.1038/nm1607. [DOI] [PubMed] [Google Scholar]
- 34.O’Grady NP, et al. Local inflammatory responses following bronchial endotoxin instillation in humans. Am J Respir Crit Care Med. 2001;163(7):1591–1598. doi: 10.1164/ajrccm.163.7.2009111. [DOI] [PubMed] [Google Scholar]
- 35.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113(19):4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAllister F, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175(1):404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honkanen J, et al. IL-17 immunity in human type 1 diabetes. J Immunol. 2010;185(3):1959–1967. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- 38.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100(10):5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer S, Chen YH. Bcl-3, a multifaceted modulator of NF-kappaB-mediated gene transcription. Immunol Res. 2008;42(1–3):210–218. doi: 10.1007/s12026-008-8075-4. [DOI] [PubMed] [Google Scholar]
- 40.Panopoulos AD, et al. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108(12):3682–3690. doi: 10.1182/blood-2006-02-003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Croker BA, et al. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20(2):153–165. doi: 10.1016/S1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 42.Kashatus D, Cogswell P, Baldwin AS. Expression of the Bcl-3 proto-oncogene suppresses p53 activation. Genes Dev. 2006;20(2):225–235. doi: 10.1101/gad.1352206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Reilly LA, Huang DC, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J. 1996;15(24):6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 44.Bassetti MF, White J, Kappler JW, Marrack P. Transgenic Bcl-3 slows T cell proliferation. Int Immunol. 2009;21(4):339–348. doi: 10.1093/intimm/dxp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sicinska E, et al. Essential role for cyclin D3 in granulocyte colony-stimulating factor-driven expansion of neutrophil granulocytes. Mol Cell Biol. 2006;26(21):8052–8060. doi: 10.1128/MCB.00800-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez-Carrozzi VR, et al. A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell. 2009;138(1):114–128. doi: 10.1016/j.cell.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smale ST. Selective transcription in response to an inflammatory stimulus. Cell. 2010;140(6):833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebner K, Bandion A, Binder BR, de Martin R, Schmid JA. GMCSF activates NF-kappaB via direct interaction of the GMCSF receptor with IkappaB kinase beta. Blood. 2003;102(1):192–199. doi: 10.1182/blood-2002-12-3753. [DOI] [PubMed] [Google Scholar]
- 49.Matsuguchi T, Lilly MB, Kraft AS. Cytoplasmic domains of the human granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor beta chain (hbetac) responsible for human GM-CSF-induced myeloid cell differentiation. J Biol Chem. 1998;273(31):19411–19418. doi: 10.1074/jbc.273.31.19411. [DOI] [PubMed] [Google Scholar]
- 50.Liu F, Poursine-Laurent J, Wu HY, Link DC. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood. 1997;90(7):2583–2590. [PubMed] [Google Scholar]
- 51.Lieschke GJ, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–1746. [PubMed] [Google Scholar]
- 52.Jansen JH, Kluin-Nelemans JC, Van Damme J, Wientjens GJ, Willemze R, Fibbe WE. Interleukin 6 is a permissive factor for monocytic colony formation by human hematopoietic progenitor cells. J Exp Med. 1992;175(4):1151–1154. doi: 10.1084/jem.175.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farivar AS, Merry HE, Fica-Delgado MJ, McCourtie AS, Mackinnon-Patterson BC, Mulligan MS. Interleukin-6 regulation of direct lung ischemia reperfusion injury. Ann Thorac Surg. 2006;82(2):472–478. doi: 10.1016/j.athoracsur.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 54.Friedman AD. C/EBPalpha induces PU.1 and interacts with AP-1 and NF-kappaB to regulate myeloid development. Blood Cells Mol Dis. 2007;39(3):340–343. doi: 10.1016/j.bcmd.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D, Paz-Priel I, Friedman AD. NF-kappa B p50 regulates C/EBP alpha expression and inflammatory cytokine-induced neutrophil production. . J Immunol. 2009;182(9):5757–5762. doi: 10.4049/jimmunol.0803861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D, D’Costa J, Civin CI, Friedman AD. C/EBPalpha directs monocytic commitment of primary myeloid progenitors. Blood. 2006;108(4):1223–1229. doi: 10.1182/blood-2005-12-008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paz-Priel I, Ghosal AK, Kowalski J, Friedman AD. C/EBPalpha or C/EBPalpha oncoproteins regulate the intrinsic and extrinsic apoptotic pathways by direct interaction with NF-kappaB p50 bound to the bcl-2 and FLIP gene promoters. Leukemia. 2009;23(2):365–374. doi: 10.1038/leu.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein B, Cogswell PC, Baldwin AS., Jr Functional and physical associations between NF-kappa B and C/EBP family members: a Rel domain-bZIP interaction. Mol Cell Biol. 1993;13(7):3964–3974. doi: 10.1128/mcb.13.7.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17(4):413–423. doi: 10.1016/S1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 60.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100(3):854–861. doi: 10.1182/blood.V100.3.854. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen-Jackson H, Panopoulos AD, Zhang H, Li HS, Watowich SS. STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood. 2010;115(16):3354–3363. doi: 10.1182/blood-2009-08-240317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azoulay E, et al. Exacerbation by granulocyte colony-stimulating factor of prior acute lung injury: implication of neutrophils. Crit Care Med. 2002;30(9):2115–2122. doi: 10.1097/00003246-200209000-00027. [DOI] [PubMed] [Google Scholar]
- 63.Suratt BT, et al. Plasma granulocyte colony-stimulating factor levels correlate with clinical outcomes in patients with acute lung injury. Crit Care Med. 2009;37(4):1322–1328. doi: 10.1097/CCM.0b013e31819c14fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75(4):604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 65.Kreisel D, et al. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8(3):233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 66.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7(12):1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 67.Okazaki M, et al. Sphingosine 1-phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am J Transplant. 2007;7(4):751–758. doi: 10.1111/j.1600-6143.2006.01710.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.