Abstract
As an emerging maize (Zea mays) seedling senses light, there is a decrease in the rate of mesocotyl elongation, an induction of root growth, and an expansion of leaves. In leaf tissues, mesophyll and bundle sheath cell fate is determined, and the proplastids of each differentiate into the dimorphic chloroplasts typical of each cell type. Although it has been inferred from recent studies in several model plant species that multiple photoreceptor systems mediate this process, surprisingly little is known of light signal transduction in maize. Here, we examine two photomorphogenic responses in maize: inhibition of mesocotyl elongation and C4 photosynthetic differentiation. Through an extensive survey of white, red, far-red, and blue light responses among a diverse collection of germplasm, including a phytochrome-deficient mutant elm1, we show that light response is a highly variable trait in maize. Although all inbreds examined appear to have a functional phytochrome signal transduction pathway, several lines showed reduced sensitivity to blue light. A significant correlation was observed between light response and subpopulation, suggesting that light responsiveness may be a target of artificial selection. An examination of C4 gene expression patterns under various light regimes in the standard W22 inbred and elm1 indicate that cell-specific patterns of C4 gene expression are maintained in fully differentiated tissues independent of light quality. To our knowledge, these findings represent the first comprehensive survey of light response in maize and are discussed in relation to maize breeding strategies.
The transition from skotomorphogenic to photoautotrophic growth is a complex and highly regulated process that has been the subject of intense study (Nemhauser and Chory, 2002). However, in maize (Zea mays), little is known of the underlying mechanisms governing this transition (Vanderhoef and Briggs, 1978). Under current cultivation practices, maize seeds are sown within a few inches of the soil surface. Soon after germination, the shoot apex, sheathed by the coleoptile, is pushed through the soil by the elongating mesocotyl. Reduced root formation and unexpanded leaves facilitate this rapid upward movement of the shoot apex while expending the least amount of energy from seed reserves. At the soil surface, incident light represses mesocotyl elongation, induces leaf expansion, and promotes root formation. As cells are recruited into emerging leaf primordia, proplastids differentiate into the dimorphic bundle sheath (BS) and mesophyll (M) cell chloroplasts, and the photoautotrophic phase of sporophytic development begins.
Light is the most important environmental cue to signal the transition from skotomorphogenesis to photomorphogenesis. In higher plants, phytochromes, cryptochromes, phototropins, and UV-B photoreceptors enable the developing seedling to monitor the quality and flux of incident light (Kevei and Nagy, 2003). The photoreversible phytochromes mediate responses to red (R) and far-red (FR) regions of the spectrum including de-etiolation, leaf expansion, stem and petiole elongation, chloroplast differentiation, circadian rhythms, and time to flowering (Fankhauser, 2001; Kevei and Nagy, 2003). The blue (B)- and UV-A-absorbing cryptochromes (CRY1 and CRY2) mediate de-etiolation, entrainment of the circadian clock, chloroplast development, and time to flowering (Lin, 2002). Most recently, the identification of a third photoreceptor family, the phototropins, has revealed a central role for B in the regulation of chloroplast movement, regulation of stomatal aperture, phototrophic curvature, and hypocotyl elongation (Kagawa, 2003).
Despite the rapid progress in dissecting light signal transduction pathways in the model eudicot Arabidopsis, little is known of the molecular components of monocot seedling development. Phytochrome mutants have only been described recently in sorghum (Sorghum bicolor; Childs et al., 1997), barley (Hordeum vulgare; Hanumappa et al., 1999), rice (Oryza sativa; Izawa et al., 2000; Takano et al., 2001), and maize (Sawers et al., 2002). No mutations have yet been defined in B photoreceptors for any grass species. Furthermore, monocot and eudicot lineages diverged approximately 130 million years ago providing ample opportunity for genetic drift, polyploidization events, and artificial (human [Homo sapiens]) selection to facilitate the divergence of light signal transduction pathways between these two groups of plants (e.g. Hayama et al., 2003).
As one of the world's most agronomically important monocot grasses, maize has been under intense human selection for at least 5,000 to 6,000 years (Benz, 2001; Piperno and Flannery, 2001; Matsuoka et al., 2002b). Increasingly, breeding strategies strive to maintain high-density plantings while reducing shade avoidance responses that include elongation of the stem, increased internode length, and decreased yield (Tetio-Kagho and Gardner, 1988). Thus, identifying mechanisms for suppressing the photoreceptor-mediated responses to shade is one major objective of understanding light signal transduction pathways in maize.
In this study, we have characterized two aspects of photomorphogenic growth in maize seedlings, the inhibition of mesocotyl elongation and C4 photosynthetic differentiation. The inhibition of mesocotyl elongation by light is a well-characterized phenomenon in maize (Vanderhoef and Briggs, 1978; Vanderhoef et al., 1979; Iino, 1982; Barker-Bridgers et al., 1998) and is a quantitative measure of light response when seedlings are grown under well-defined light and temperature regimes. To examine the contribution of light on photosynthetic differentiation, gene expression changes were also examined in the two photosynthetic cell types of a maize seedling leaf. As a C4 plant, maize utilizes two anatomically and biochemically distinct cell types to fix carbon. The BS cells surrounding the vasculature contain centrifugally arranged chloroplasts with large starch granules and unstacked thylakoid membranes. The M cells lying adjacent to the BS contain randomly arranged chloroplasts with stacked thylakoids and little or no starch (Edwards and Walker, 1983). Both cell types accumulate a distinct set of photosynthetic enzymes and proteins that enable them to cooperate in the fixation of carbon (Sheen, 1999). Together, mesocotyl elongation and C4 photosynthetic differentiation provide two very different measures of light response in the photomorphogenic development of maize seedlings.
To further define the role of phytochromes in the regulation of maize seedling development, plants homozygous for the elm1 mutation were incorporated into the surveys of mesocotyl elongation and photosynthetic differentiation. The elm1 mutation conditions seedlings that are pale green with an elongated mesocotyl under all light conditions. In the field, elm1 plants tend to lodge and flower slightly earlier than the wild-type (Sawers et al., 2002). The elm1 mutant is impaired in phytochrome responses due to a block in phytochrome chromophore biosynthesis (Sawers et al., 2002) and, therefore, provides a means of examining phytochrome-mediated responses under any light regime.
In this study, we find that seedlings from 30 inbred lines of maize grown in the dark (D) or under continuous R, FR, B or white (W) light show highly variable photomorphogenic responses. Furthermore, significant differences in light response were observed between semitropical (ST) varieties and North American Cornbelt-derived stiff stalk (SS) and non-stiff stalk (NSS) subpopulations. These results are consistent with the theory that modern breeding practices have selected for lines with reduced light response (Sawers et al., 2002). We also show a limited role for light in C4 photosynthetic development. Although light has been implicated in the initiation of C4 development (Langdale et al., 1988b), we present evidence that B and R/FR light signaling pathways play a minor role in maintaining cell-specific patterns of gene expression. Finally, we present evidence that although the inhibition of mesocotyl elongation in elm1 seedlings was greatly impaired under all light treatments, the elm1 mutation had little effect on the cell-specific accumulation of photosynthetic enzymes in the BS and M cells.
RESULTS
Variation in Light-Mediated Repression of Mesocotyl Elongation
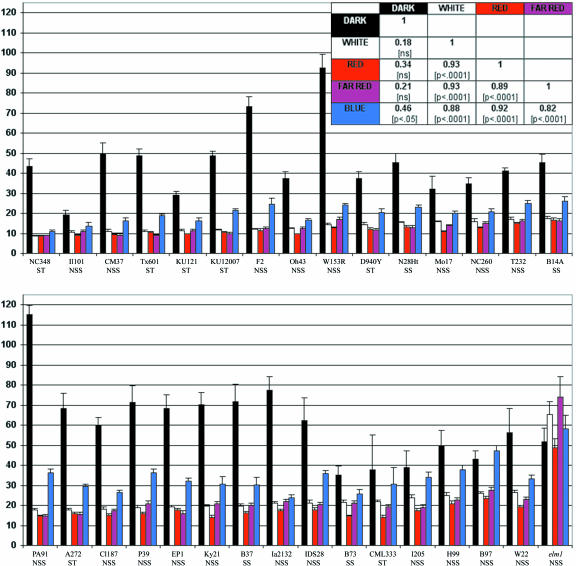
The light-mediated repression of mesocotyl elongation was used as a quantitative measure of light response among a diverse collection of maize inbred germplasm. The 30 lines represent three subpopulations (Remington et al., 2001), including four SS, 19 NSS, and seven tropical/ST lines (see Table I). Seedlings were germinated under continuous W, R, FR, B or D, and mesocotyl length was measured after 10 d. The range of variation among the lines is displayed in Figure 1, where lines are rank-ordered from shortest to longest mean mesocotyl length under W. Line means and ses are shown for each light treatment in Supplemental Table VI (available in the online version of this article at http://www.plantphysiol.org). A relatively short mesocotyl under a given treatment indicates that the seedling is highly responsive to that treatment, whereas longer mesocotyls indicate a weaker response. The variation in mean mesocotyl length among inbred lines was the greatest when plants were grown in D, where there was a 6-fold difference between the shortest (IL101) and longest (PA91) lines. Under W, there was a 3-fold difference in mean mesocotyl length between the shortest (NC348) and longest (W22) lines.
Table I.
Maize inbred germplasm used in experiments
| Inbred Name | Original Seed Sourcea | Bruthell Lab Accession No. | Originb | Subpopulationc |
|---|---|---|---|---|
| A272 | Buckler 3-A272-3 | DC01-008 | South Africa | ST |
| B14A | Goodman 496-3/99 | DC01-081 | CCB-B14 | SS |
| B37 | Goodman 497-2/99 | DC01-082 | CCB-B37 | SS |
| B73 | Goodman 504-2/99 | DC01-084 | CCB-B73 | SS |
| B97 | Buckler 95-B97-3 | DC01-006 | CCB | NSS |
| CI187 | Buckler 38-CI187-2 | DC01-003 | CCB | NSS |
| CM37 | Burr 000641-6(X) | DC01-024 | NCB | NSS |
| CML333 | Buckler 30-CML333-3 | DC01-007 | International Center for Development of Maize and Wheat (Mexico) | ST |
| D940Y | Buckler 39-D940Y-1 | DC01-002 | South Africa | ST |
| EP1 | Goodman 571-2/99 | DC01-107 | NCB | NSS |
| F2 | Goodman 572-3/99 | DC01-108 | NCB | NSS |
| H99 | Goodman 584-3/99 | DC01-114 | CCB | NSS |
| I205 | Goodman 592-1/99 | DC01-118 | CCB | NSS |
| IA2132 | Goodman 593-3/99 | DC01-119 | Sweet corn | NSS |
| IDS28 | Goodman 596-3/99 | DC01-120 | Popcorn | NSS |
| IL101 | Goodman 594-3/99 | DC01-122 | Sweet corn | NSS |
| KUI2007 | Goodman 7753-3/98 | DC01-130 | Suwan (Thailand) | ST |
| KUI21 | Goodman 7744-1/98 | DC01-127 | Suwan (Thailand) | ST |
| KY21 | Goodman 605-3/99 | DC01-131 | United States (South) | NSS |
| MO17 | Goodman 614-3/99 | DC01-134 | CCB-C103 | NSS |
| N28Ht | Goodman 633-3/99 | DC01-137 | CCB-SSS | SS |
| NC260 | Goodman 2680-1/99 | DC01-141 | CCB | NSS |
| NC348 | Buckler 92-NC348-3 | DC01-010 | Tuxpeno/Caribbean | ST |
| OH43 | Goodman 657-3/99 | DC01-153 | CCB-OH43 | NSS |
| P39 | Goodman 663-3/99 | DC01-155 | Sweet corn | NSS |
| PA91 | Goodman 664-3/99 | DC01-156 | CCB | NSS |
| T232 | Goodman 691-2/99 | DC01-162 | South Africa | NSS |
| TX601 | Buckler 71-TX601-4 | DC01-009 | Tuxpeno | ST |
| W153R | Goodman 714-3/99 | DC01-171 | CCB | NSS |
| W22 | Kermicle | TB97-161 | NCB | NSS |
Seed Sources: Ed Buckler (Cornell University), Major Goodman (North Carolina State University), Ben Burr (Brookhaven National Laboratory), and Jerry Kermicle (University of Wisconsin, Madison)
Origins: CCB, Central Corn Belt (United States); NCB, Northern Corn Belt (United States and Canada). Information provided by Major Goodman
Subpopulations: ST, SS, and NSS. Group assignments according to Remington et al. (2001), except for CM37, TX601, and W22 (J. Liu, personal communication)
Figure 1.
Mean mesocotyl lengths (in millimeters) ± ses of 30 maize inbred lines and the elm1 mutation (maintained in W22 germplasm). Subpopulation designations are given below the line name. Mesocotyls were measured in seedlings grown for 10 d under each continuous light treatment and ordered by length under W light. Each bar = line mean for a light treatment, including D, W, R, FR, and B. The inset table shows the correlations and corresponding significance levels for the inbred line means for all the light treatments.
The light responses of seedlings grown under R, FR, or B light of similar irradiance were strongly correlated with one another and with the response under W (see Fig. 1, inset). Thus, lines that had relatively short mesocotyls under W also had short mesocotyls under R, FR, and B. Furthermore, all of the inbreds examined in this survey were highly responsive to R and FR, suggesting that a phytochrome signaling pathway is operational in all of the inbreds examined. Among light treatments, mesocotyl elongation was inhibited the least by B. Mixedmodel analysis (Table II) revealed that all three of the main effects, light treatment, subpopulation, and line, each explained significant portions of the variation in mesocotyl length. The interactions among these effects are also significant, indicating that the effect of the light treatments varies among the subpopulations and among the lines. These data also indicate that lines within each subpopulation are heterogeneous with respect to their pattern of response.
Table II.
Mixed model analysis of the natural log of mesocotyl length in 30 maize inbred lines grown in growth chambers under continuous W, R, FR, and B light and in the D for 10 d
| Source | Numerator Degrees of Freedom (df) | Denominator df | F | P |
|---|---|---|---|---|
| Light Treatment | 4 | 9 | 495.18 | <0.0001 |
| Subpopulation | 2 | 12.2 | 73.61 | <0.0001 |
| Treatment × subpopulation | 8 | 71.9 | 4.67 | 0.0001 |
| Line (subpopulation) | 27 | 127 | 47.17 | <0.0001 |
| Treatment × line (subpopulation) | 108 | 635 | 6.18 | <0.0001 |
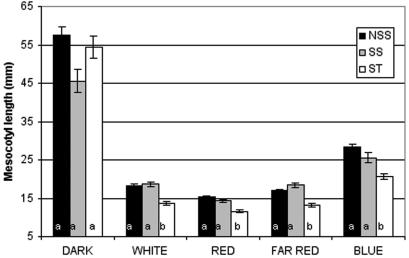
The current subpopulation designations in maize are based on variation at several SSR loci and likely reflect a history of artificial selection and genetic drift (Remington et al., 2001). As shown in Figure 2, the three subpopulations are highly variable and indistinguishable in the D. Furthermore, SS and NSS lines did not differ significantly from one another with respect to their overall pattern of response to light. However, in all of the light treatments, mesocotyls of seedlings of ST origin are shorter on average than those of the two temperate zone SS and NSS lines. For instance, the majority of the lines shown to be insensitive to B are derived primarily from either SS or NSS subpopulations (10 of 11). Together, these data suggest that North American subpopulations are less sensitive to light than the ST and tropical inbred lines.
Figure 2.
Mean mesocotyl lengths (in millimeters) ± ses for the three subpopulations represented in the maize inbred germplasm grown under five light treatments. Means groups (a or b) for each treatment-subpopulation combination were assigned by testing for differences among the least square means of fixed effects calculated by the mixed model analysis, with P values adjusted for multiple comparisons.
The elm1 mutant was included in this survey of light response to assay the contribution of phytochrome to mesocotyl elongation under each light treatment. The elm1 mutation was derived from a W22 inbred; thus, near-isogenic comparisons can be made between W22 and elm1. A separate mixed-model analysis was carried out with the mesocotyl length data collected on W22 and elm1. As expected, the two fixed effects, light treatment and line, were significant, as were the interaction between them. The means tests revealed that W22 and elm1 did not differ under D and B but were significantly different under W, R, and FR (data not shown). Comparing the response across the light treatments for each line individually, we find that in elm1, mesocotyl elongation is the same under all light environments, including D, whereas in W22, the pattern of response is more complex. In all of the pair-wise comparisons among the treatment means for W22, only three were significant: D versus R, D versus FR, and B versus R. These data indicate that although the B mediated inhibition of mesocotyl elongation is highly attenuated in the elm1 mutant, this effect is not significantly different from the response found in W22.
In 11 of the 30 lines surveyed, the means for the B and D treatments were not significantly different from one another (difference among least square means generated by a mixed-model analysis and tested using the Tukey-Kramer P values adjusted for multiple comparisons) and include B14A, B73, B97, CML333, H99, I205, IA2132, IL101, N28Ht, NC260, and W22. The apparent absence of a B response in these lines may reflect an insensitivity of some lines to the B fluence rate used. To further examine B responses in these lines, an additional survey was conducted at a higher B fluence rate (13 μmol m-2 s-1). Nine of the 11 nonresponsive lines, six responsive lines, and the elm1 mutant line were grown under high B for 10 d, and mesocotyl lengths were measured. The results of this analysis are shown in Tables III and IV and indicate that all lines tested respond to high B with the exception of elm1 and IL101. IL101 is consistently one of the shortest lines under all light treatments but also suffers quite high mortality (45.4% compared with 16% overall), reducing the sample size and the power to detect differences among the light treatments for this line. Thus, none of the inbred lines are completely nonresponsive to B but instead show varying sensitivities to B, as evidenced by the significant line × treatment effect in the ANOVA (Table III). For instance, mean mesocotyl length in B97 was not significantly different under D and low B, but under high B, mean mesocotyl length was significantly different from D (Table IV). However, some inbred lines showed no significant difference in response under high versus low B (B73, N28Ht, and W22). These results indicate that the inhibition of mesocotyl elongation may be mediated by low fluence B for some lines (e.g. B37, CI187, D940Y, EP1, IDS28, and PA91), whereas others require a high fluence of B to mediate this response (e.g. B14A, B73, B97, H99, IA2132, N28Ht, NC260, and W22).
Table III.
ANOVA of the natural log of mesocotyl length in 15 maize inbred lines and the mutant elm1 grown in growth chambers under continuous low-fluence B light, high-fluence B light, and in the D for 10 d Type III sums of squares used throughout.
| Source | df | F | P |
|---|---|---|---|
| Light treatment | 2 | 754.50 | <0.0001 |
| Line | 15 | 37.35 | <0.0001 |
| Line × treatment | 30 | 19.63 | <0.0001 |
| Error | 689 | — | — |
Table IV.
Least squares means and means tests for 15 maize inbred lines and elm1 mutant generated by the ANOVA shown in Table III Group: N, nonresponsive in original survey, i.e. line means for D vs. Low B were not different; R, responsive in original survey, i.e. line means for D vs. Low B were different.
| Inbred Line
|
Group
|
Least Squares Means
|
Probability Values for Tests of Inbred Line × Treatment Effect
|
||||
|---|---|---|---|---|---|---|---|
| D | Low B | High B | D vs. Low B | L vs. High B | D vs. High B | ||
| B14A | N | 3.77 | 3.24 | 2.80 | NSa | * | **** |
| B73 | N | 3.49 | 3.22 | 2.86 | NS | NS | **** |
| B97 | N | 3.72 | 3.84 | 3.14 | NS | **** | **** |
| H99 | N | 3.81 | 3.59 | 3.03 | NS | **** | **** |
| IA2132 | N | 4.04 | 3.57 | 2.75 | NS | **** | **** |
| IL101 | N | 2.97 | 2.50 | 2.26 | NS | NS | NS |
| N28Ht | N | 3.52 | 3.03 | 2.92 | NS | NS | **** |
| NC260 | N | 3.35 | 2.99 | 2.60 | NS | * | **** |
| W22 | N | 3.87 | 3.49 | 3.37 | NS | NS | ** |
| elm | N | 3.85 | 3.97 | 3.78 | NS | NS | NS |
| B37 | R | 4.40 | 3.36 | 3.23 | **** | NS | **** |
| CI187 | R | 4.16 | 3.38 | 2.59 | **** | **** | **** |
| D940Y | R | 4.45 | 3.17 | 2.32 | **** | **** | **** |
| EP1 | R | 4.16 | 3.33 | 2.62 | **** | **** | **** |
| IDS28 | R | 4.31 | 3.14 | 2.67 | **** | ** | **** |
| PA91 | R | 4.74 | 3.57 | 2.73 | **** | **** | **** |
NS, P > 0.05
P < 0.05
P < 0.01; ***, P < 0.001
P < 0.0001
As observed for light responses, mesocotyl elongation in the D is a highly variable trait among inbred lines examined (Fig. 1). However, mesocotyl length in all light environments (with the exception of B) is not correlated with mesocotyl length in D (Fig. 1, inset). This suggests that mesocotyl length in D and light is controlled independently. Nevertheless, the analysis of the elm1 mutant shows that mesocotyl length under R, FR, B, and W are not significantly different from D. This finding suggests that mesocotyl length in D represents a maximal elongation response in maize. These findings raise the important question of how “light responsiveness” should be defined. Many Arabidopsis researchers have used measures of mesocotyl inhibition to relate the mesocotyl length under a light condition to a mean measure of mesocotyl length in the D. For example, “relative inhibition” in Hennig et al. (2001) is calculated as:
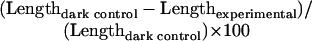 |
In Mazzella et al. (2001), the authors divide the mean hypocotyl length for each replicate in a light condition by the mean of all the replicate D controls from the same experiment. The 6-fold difference observed in mean mesocotyl length of D-grown seedlings suggests that mean mesocotyl length of D should be considered in determination of light responsiveness in maize because it may underlie the differences among lines.
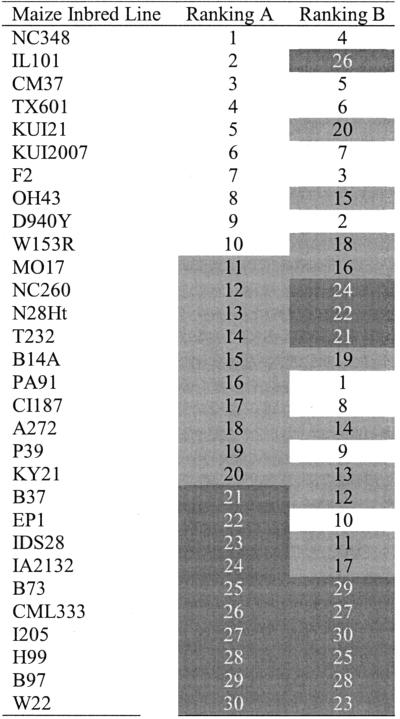
In Table V, the 30 maize inbreds used in our survey are rank ordered by two measures of response to W, mean mesocotyl length and mean mesocotyl length under W divided by mean length in the D. To highlight the differences in the rankings, the lines were grouped into three classes (white, gray, and black) based on rank order under each scheme. As shown, there is little correspondence between these two rankings. For instance, one of the most responsive lines (ranked 2) under W (IL101) was ranked as one of the least responsive lines (ranked 26) when mean D mesocotyl is used to calculate responsiveness. This variation in rankings highlights the importance of standardizing measures of light responsiveness in maize and of making near-isogenic comparisons between wild-type and mutant lines.
Table V.
Comparison of rankings of W response in 30 maize inbred lines using two different measures: ranking A, length of mesocotyl under W vs. ranking B, length under W divided by length in D
In both cases, a low ranking would signify a high response to W.
RNA Expression Levels of Three C4-Specific Transcripts in D-Shifted Plants
As a second measure of light responsiveness, we examined the patterns of photosynthetic gene expression in developing maize seedlings. Previous studies have indicated that R and B light regulate the accumulation of C4 photosynthetic transcripts in maize (Langdale et al., 1988b; Thomas et al., 1990; Purcell et al., 1995; Casati et al., 1998). However, to examine the effects of light quality on the maintenance of C4 photosynthesis, we first had to formulate an assay. The C4 photosynthetic pathway is not immediately established in emerging maize leaf tissues and is only exclusively utilized in blade tissues (Perchorowicz and Gibbs, 1980). Therefore, it was necessary to first grow seedlings for approximately 12 d under W to establish the C4 pathway. At this stage, plants were transferred to one of four light treatments or placed in D to examine the effects of specific light qualities on maintaining the C4 differentiated state.
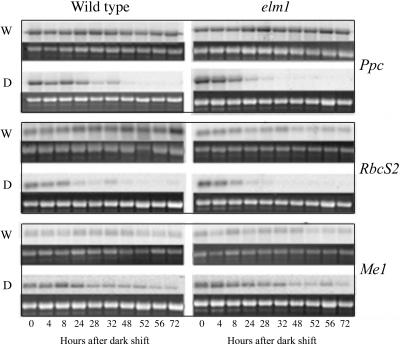
To examine the persistence of a light generated signal in maintaining BS and M cell-specific expression, transcripts encoding the C4 isoform of phosphoenolpyruvate carboxylase (Ppc), the small subunit of Rubisco (RbcS2), and NADP-malic enzyme (Me1) were examined after a transfer of the plants to D (Fig. 3). Transcripts for these genes had previously been shown to accumulate preferentially in M (Ppc) or BS (RbcS2 and Me1; Sheen and Bogorad, 1987; Langdale et al., 1988a). As shown in Figure 3, WT and elm1 plants that were maintained in W over the time course continued to accumulate high levels of each transcript. Plants shifted to D maintained high levels of transcripts for all three genes for at least 24 h. After approximately 24 h of D, Ppc transcript levels decreased dramatically and were maintained at a relatively low level. This finding is consistent with a previous study in maize that showed Ppc transcript levels are strongly decreased in abundance after a 24-h D shift (Thomas et al., 1990). Similarly, transcripts of RbcS2 were maintained at high levels during the first 8 h after a shift to darkness. After this time point, the transcript levels declined and were barely detectable by 48 h. Surprisingly, the levels of Me1 continued to be maintained at relatively high levels over the entire time course, suggesting that Me1 and RbcS2 transcript accumulation profiles are differentially regulated in BS cells.
Figure 3.
Northern-blot analysis of transcript accumulation in wild-type and elm1 seedlings. Total RNA was extracted from WT and elm1 seedlings grown in W light or after a shift to D. RNA was extracted from leaf tissue harvested at time points indicated. Approximately 5 μg of total RNA was used in northern-blot analysis. The 27S ribosomal band was used as a loading control and was visualized using ethidium bromide staining after membrane transfer. Gene-specific fragments of Ppc, RbcS2, and Me1 were hybridized to the filters according to the protocols described in “Materials and Methods.”
To examine the effects of a phytochrome deficiency on C4 gene expression, Ppc, RbcS2, and Me1transcript accumulation profiles were examined in the elm1 mutants. Transcripts for all three genes accumulated to similar levels in elm1 and WT plants grown under continuous W (see Fig. 3, time point 0). However, after 32 h of D growth, transcript levels of Ppc and RbcS2 were greatly reduced in elm1 seedlings but were still detectable in wild-type tissues. These results suggest that a fully functional phytochrome signal transduction pathway is not required for the accumulation of Ppc, RbcS2, and Me1 in W-grown seedling leaf tissue. However, it does suggest a role for phytochrome in maintaining transcript levels when plants are transferred from W to D.
R, B, and FR Contributions to Cell-Specific Patterns of C4 Gene Expression
Both Ppc (Sheen and Bogorad, 1987; Langdale et al., 1988a) and RbcS2 (Ewing et al., 1998), are differentially expressed in maize leaf tissue. Transcripts for RbcS2 accumulate in BS cells, whereas transcripts for Ppc accumulate in M cells. The results shown in Figure 3 show that at 48 h after a shift to D, Ppc and RbcS2 transcripts are not detectable in wild-type or elm1 mutant plants. This suggests that transcriptional or posttranscriptional controls that maintain Ppc and RbcS2 transcript levels in W light are no longer operational after 48 h D growth.
Therefore, to examine the effects of monochromatic light on the maintenance of cell-specific C4 gene expression profiles, plants were grown for 12 d under W light then shifted to R, FR, or B chambers for 48 h. At 48 h, M cell protoplasts and BS cell strands were isolated (see “Materials and Methods”). To control for the effects of extended enzymatic digestions in the isolation of M cell protoplasts, a stressed leaf control was included in which leaf strips were incubated in the protoplast isolation buffer for approximately 3 h, without the addition of enzyme. RNA was isolated from samples enriched in BS and M cells and from total leaf and stressed leaf control samples for northern-blot analysis.
As shown in Figure 4, Ppc transcripts preferentially accumulated in M cells in plants grown in W and were not detected in leaf tissues of plants that were shifted to D after 12 d of W growth. The slight decrease in the levels of Ppc in the stressed fractions (stressed leaf control) relative to total leaf indicates that the levels of Ppc transcript observed in the M fraction are an underestimation of the total Ppc transcript pool in M cells. After a transfer to B, R, or FR, wild-type plants continued to accumulate high levels of Ppc transcripts in M cells. The low levels of Ppc transcript observed in BS, under all light treatments, likely represent low levels of contaminating M and epidermal guard cells in the BS prep (data not shown). These data suggest that the Ppc promoter is responsive to B, R, and FR light and that the M cell-specific pattern of Ppc expression is not dependent on B-, R-, or FR-mediated repression of Ppc transcript accumulation in differentiated BS cells. Together, these results indicate that phytochrome and cryptochrome photoreceptors do not mediate the patterns of Ppc transcript accumulation in maize.
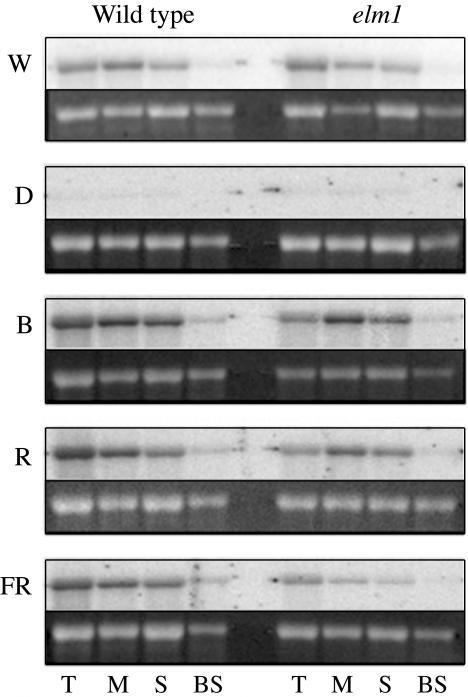
Figure 4.
Northern-blot analysis of Ppc transcript accumulation in wild-type and elm1 seedlings. Total RNA was extracted from leaf tissue (T), M protoplasts, stressed control (S), and BS cells of plants grown in W light for 12 d and shifted into D, B, R, and FR. Approximately 5 μg of total RNA was used in northern-blot analysis. The 27S ribosomal band is shown as a loading control.
To further investigate the role of phytochromes in the cell-specific expression of C4 genes, Ppc transcript profiles were also examined in elm1 mutants. As shown in Figure 4, Ppc transcripts accumulated to slightly lower levels in elm1 plants grown under B, R, or FR light relative to wild type, suggesting that phytochromes contribute to Ppc transcript accumulation in fully differentiated tissues. However, Ppc transcripts accumulated specifically in M cells of elm1 mutants under all light treatments, suggesting again that phytochromes do not play a significant role in the cell-specific accumulation of Ppc transcripts.
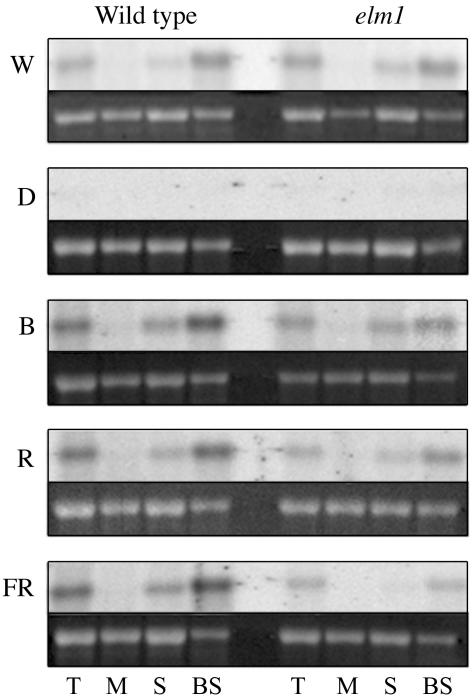
Previous reports have indicated that R is necessary for the accumulation of RbcS2 in the BS cells, whereas B is required for the repression of RbcS2 accumulation in M cells (Purcell et al., 1995). To examine the effects of light on the cell-specific accumulation of RbcS2, transcript accumulation profiles were examined in wild-type and elm1 plants. As was observed with Ppc transcripts, levels of RbcS2 were lower in elm1 mutants relative to wild-type plants under R and FR treatments (Fig. 5). It is important to note, however, that the levels of RbcS2 transcript decline significantly in the stressed leaf control relative to wild type under W, R, and FR treatments. This effect is particularly dramatic in elm1 plants grown under FR, where the very low levels of RbcS2 in the stressed leaf control preclude a determination of cell-specific gene expression in FR-grown elm1 mutants. Nevertheless, it is clear from the wild-type plants grown under R and FR and in elm1 mutants grown under R that the BS cell-specific pattern of RbcS2 accumulation is maintained in the absence of B.
Figure 5.
Northern-blot analysis of RbcS2 transcript accumulation in wild-type and elm1 seedlings. Total RNA was extracted from leaf tissue (T), M cells, stressed control (S), and BS cells of plants grown in W light for 12 d and shifted into D, B, R, and FR light. Approximately 5 μg of total RNA was used in northern-blot analysis. The 27S ribosomal band is shown as a loading control.
In summary, transcript accumulation profiles observed in wild-type and elm1 mutant plants strongly suggest that phytochromes do not contribute significantly to the maintenance of cell-specific patterns of Ppc and RbcS2 expression in photosynthetically differentiated tissues. Nevertheless, phytochromes do contribute to maintaining the levels of Ppc and RbcS2 transcripts under B, R, and FR light. Finally, B is not required to maintain the cell-specific expression of RbcS2 in wild-type or phytochrome-deficient maize plants.
DISCUSSION
To further our understanding of light signaling pathways in maize, we have exploited several recently developed genetic resources in maize. The inhibition of mesocotyl elongation was examined in a well-characterized set of maize inbreds representing the three major subpopulations of maize, SS, NSS, and ST that are utilized throughout the world in breeding programs. The domestication of maize is believed to have begun in Southern Mexico (Benz, 2001; Piperno and Flannery, 2001; Matsuoka et al., 2002b), and in molecular surveys of the maize germplasm utilized in this study, the ST and tropical accessions represent a greater pool of genetic diversity than the SS and NSS accessions (Remington et al., 2001; Matsuoka et al., 2002a). However, the ST and NSS lines are poorly differentiated groups. This likely reflects the fact that NSS lines represent a diverse group derived through the crossing of northern flints by southern dents. SS lines were derived from a relatively small subpopulation, and their divergence from ST and NSS lines is due primarily to genetic drift (Remington et al., 2001). We also have utilized the phytochrome deficient elm1 mutant to directly assess the contribution of phytochrome signaling pathways to the inhibition of mesocotyl elongation and the expression of photosynthetic genes. The elm1 mutant is defective in phytochrome responses due to a block in phytochrome chromophore biosynthesis that severely reduces the active phytochrome pools in maize (Sawers et al., 2002). Together, these resources have enabled us to perform a comprehensive survey of seedling light responses in this agronomically important plant species.
Mesocotyl Length Variation in Maize
Maize is grown throughout the world, where it has undergone selection under a range of light environments that vary in intensity, spectral quality, and duration. Because critical developmental responses throughout the plant life cycle are light dependent, perhaps it is not surprising to find the high degree of variation in light response among the 30 accessions examined. The significantly shorter mesocotyls detected in the tropical lines compared with the U.S./Canadian Cornbelt lines seem to indicate that a loss of light responsiveness at the seedling emergence stage has accompanied selection by breeders in northern temperate regions (Salamini, 1985). Whether this is part of a syndrome of overall loss of light sensitivity as maize was moved from the shortday growing conditions of subtropical Central America to the long-day conditions of the temperate zones has yet to be determined. Interestingly, a study of a wide geographic collection of Arabidopsis found that accessions from latitudes closer to the equator had longer hypocotyls than those from further north (Maloof et al., 2001), the opposite of the trend we have detected in maize. The authors speculate that plants grown at lower latitudes might compensate for the higher light intensity with a decreased sensitivity to light. Thus, light responses in maize, a crop plant that has been under strong artificial selection for thousands of years, appear to differ from those in a weedy species such as Arabidopsis. Differences in light responses between crop plants and weed species have been detailed previously and suggest that light response pathways may be an ideal target for crop improvement (Ballare, 1999; Ballare and Casal, 2000).
One possible point of divergence between Arabidopsis and maize light response is in their phytochrome signaling pathways. In Arabidopsis, the shade avoidance syndrome is primarily mediated through a phytochrome B signaling pathway (Aukerman et al., 1997; Devlin et al., 1998). In grasses, shade avoidance is characterized by acceleration of flowering at the expense of yield, increased internode elongation, and decreased branching or tillering (Casal et al., 1985; 1990; Casal, 1993; Ballare and Casal, 2000). In a natural environment, this syndrome is likely to provide plants with a competitive advantage (Ballare et al., 1987; Dorn et al., 2000; Gilbert et al., 2001). However, in a crop plant such as maize, breeding practices strive for high-density plantings of near-isogenic plants. Because shade avoidance would be detrimental to yield, artificial (human) selection is likely to drive the attenuation of shade avoidance responses. The correlation between R/FR responsiveness and subpopulation and the finding that phytochrome-deficient plants result in early flowering in maize (Sawers et al., 2002) is consistent with the notion that breeding practices have selected for reduced phytochrome response in maize.
However, several traits, including leaf/stem morphogenesis, shoot/root biomass allocation, tillering, plant height, and the redirectioning of leaf position in field-grown maize plants, are responsive to the ratio of R to FR (Kasperbauer and Karlen, 1994; Maddonni et al., 2002). Furthermore, a severe reduction in phytochrome response as observed in the elm1 mutant results in increased lodging (Sawers et al., 2002). Thus, the maintenance of some phytochrome responses is clearly beneficial under current cultivation practices. These findings suggest that phytochrome pathways have been maintained through the domestication process but that modern cultivation practices have selected for attenuation of some light responses.
In addition to phytochrome-mediated responses, we also examined the effects of B on mesocotyl elongation. Surprisingly, 11 of the 30 lines examined were nonresponsive to the B treatment given that was of similar energy to the R and FR treatments. However, most of these lines did respond to a higher fluence B. These altered sensitivities to B may be attributable to either variation at B photoreceptors or downstream components of the pathway. In Arabidopsis, cryptochromes and phototropins have been shown to contribute to B inhibition of hypocotyl elongation (Ahmad and Cashmore, 1993; Folta and Spalding, 2001). However, the fact that light response was similar across light treatments for each inbred line, including the low-B nonresponsive lines, suggests that down-stream components of the light signal transduction pathway may contribute to the underlying variation. Transcriptional regulators, such as COP1 and HY5 (Deng et al., 1991; Oyama et al., 1997), act down-stream of individual photoreceptors in Arabidopsis. Variation in the maize orthologs of these genes may contribute to the variation seen in B response and R- and FR-mediated light responses. Further experiments to examine irradiance-dependent effects of B on mesocotyl elongation will help clarify the molecular basis of B light responses in maize.
As shown in Figure 1 and Table IV, it appears that elm1 mutants are nonresponsive to B. In studies of chromophore deficient mutants of pea (Weller et al., 1997) and Arabidopsis (Liscum and Hangarter, 1993), B-mediated inhibition of hypocotyl elongation was also impaired but only to a limited degree. Although this could indicate a difference in monocot and dicot B signaling pathways, it is important to note that the parental W22 inbred line is one of the 11 lines that showed no significant difference in mesocotyl length in D versus low B and was one of the least responsive lines in high B. Thus, insensitivity to B observed in the elm1 mutant might reflect the attenuation of B responses in the W22 inbred and not the direct interactions between R/FR and B signaling pathways. Introgression of the elm1 mutant into a highly B-responsive line (e.g. NC348) should permit the examination of phytochrome contributions to B response in maize.
Influence of Light Quality on Photosynthetic Differentiation
Photosynthetic differentiation in maize results in the development of dimorphic and metabolically distinct BS and the M cells. These cells contain plastids with unique ultrastructure, enzymatic profiles, and biochemical activities that cooperate in C4 photosynthesis (Sheen, 1999). Several studies of C4 photosynthetic differentiation in maize have indicated that light plays a central role in the differentiation process. In particular, the accumulation of the photosynthetic genes Ppdk, Ppc, Me1, and RbcS2 have been shown to be under light control (Nelson et al., 1984; Sheen and Bogorad, 1986; Sheen and Bogorad, 1987; Schaffner and Sheen, 1991, 1992; Casati et al., 1998; Ewing et al., 1998; Kausch et al., 2001). However, the mechanisms by which light regulates cell-specific photosynthetic gene expression remain unresolved for any gene in maize.
Despite a severe deficiency in spectrophotometrically active phytochrome pools in the elm1 mutant (Sawers et al., 2002), we observed a relatively normal accumulation of Ppc, RbcS2, and Me1 under W. This result is consistent with previous studies of phytochrome chromophore-deficient mutants in Arabidopsis (Chory et al., 1989) and suggests a limited role for phytochrome in regulating C4 photosynthetic gene expression in mature, light-grown plants. In D-shifted plants, the relative amounts of transcripts of both Ppc and RbcS2 appear to decrease dramatically after approximately 24 h. The decrease in Ppc RNA pools is consistent with previous studies of Ppc accumulation in maize (Thomas et al., 1990) and indicates that these transcripts are relatively unstable in the absence of the light stimuli or that the rate of Ppc transcription is severely reduced in the D. Phytochrome may be one factor that contributes to the stability of Ppc and RbcS2 because the transcript pools decreased more rapidly in elm1 plants than wild type when shifted to D. In contrast, Me1 transcripts were maintained at relatively high levels throughout the time course (72 h). Although it is not clear what regulates the stability of Me1 (see Sheen and Bogorad, 1987), it appears as though active pools of phytochrome are not necessary to maintain transcript levels in D. Clearly, among photosynthetic genes, multiple modes of regulation are present to maintain or repress transcript levels during the normal growth of plants.
Another goal of this study was to examine the contribution of light quality to C4 photosynthetic gene expression. The analysis of wild-type and elm1 mutant seedlings under R, B, and FR light indicate that all three light qualities modulate the levels of Ppc and RbcS2 accumulation. This light regulation is attributable in part to the phytochrome signal transduction pathway because the levels of Ppc and RbcS2 are reduced in the phytochrome-deficient elm1 mutant under R and FR. Previous studies have demonstrated a role for R in inducing cell-specific expression of Ppc (Langdale et al., 1988a; Thomas et al., 1990; Schaffner and Sheen, 1992) and RbcS (Zhu et al., 1985; Purcell et al., 1995) after a shift from D to light growth. Here, we demonstrate the involvement of R, B, and FR light on maintaining the levels of Ppc and RbcS2 in fully differentiated C4 photosynthetic tissues.
Interestingly, phytochrome and putative cryptochrome signaling pathways do not appear to play a role in restricting RbcS2 to BS cells in mature leaf tissues. When plants were shifted from W to monochromatic R or FR light, we failed to detect ectopic RbcS2 message in M cells despite a phytochrome-mediated induction of gene expression in BS cells. This was somewhat surprising in light of a previous study demonstrating a role for B in repressing RbcS2 in M cells (Purcell et al., 1995). This discrepancy may reside in differences in experimental design. In the previous study, D plants containing etioplasts were shifted to B light, whereas we shifted W-grown plants with fully differentiated BS and M cell chloroplasts to B. Thus, the B regulation of M cell-specific RbcS2 expression accompanying the transition from skotomorphogenesis to photomorphogenesis may be different from the regulatory pathways mediating transcriptional control in fully differentiated leaves.
Although it is clear that phytochromes and possibly cryptochromes play a role in the induction of C4 gene expression patterns, other mechanisms of regulation may also contribute to cell-specific patterns of photosynthetic gene expression. Redox regulation (Karpinski et al., 1997), hormones (Sakakibara et al., 1998; Sheen, 1998), and photosynthetic metabolites (Jang and Sheen, 1994) all play a role in regulating photosynthetic gene expression in plants. Thus, in addition to direct light effects, multiple signaling mechanisms may be cooperatively responsible for the induction and maintenance of the distinctive C4 pattern of expression.
Ultimately, the cell-specific expression of Ppc and RbcS2 genes resides on the cis- and trans-acting factors controlling expression. Transient assays in maize M cells have determined regulatory regions of several C4 photosynthetic genes. For example, promoter fusion constructs transiently introduced into maize leaves have defined several important 5′ regions necessary for light-regulated, cell-specific gene expression of the RbcS2 gene (Schaffner and Sheen, 1991; Bansal et al., 1992; Viret et al., 1994; Purcell et al., 1995). Studies of the Ppc promoter have also revealed regulatory elements required for proper expression (Schaffner and Sheen, 1992; Kausch et al., 2001). Furthermore, nuclear-encoded transcription factors have been identified that bind specific regions of the RbcS promoter to repress M cell-specific expression (Xu et al., 2001). Transcriptional activators for Ppc also have been identified through in vitro-binding assays that may control light- and cell-specific patterns of expression (Morishima, 1998; Yanagisawa and Sheen, 1998; Yanagisawa, 2000). These studies suggest that transcription initiation may be a mechanism to regulate cell-specific gene expression. However, in vitro studies also suggest an important role for posttranscriptional control of nuclear gene expression (Viret et al., 1994; Purcell et al., 1995). Our finding that Me1 transcripts accumulated throughout a 72-h time course in D-shifted plants, whereas Ppc and RbcS2 transcripts were undetectable after 48 h, suggests that multiple regulatory mechanisms may mediate the cell-specific patterns of photosynthetic gene expression in maize.
Based on our results and previous studies, we propose a revised model of C4 differential gene expression, which involves two distinct mechanisms of regulation. The first relies on positional effects augmented by light cues, perceived by phytochrome and cryptochromes, to establish chloroplast morphology and initiate gene expression. Once the chloroplasts are fully functioning, maintenance of the pattern initiated by light signals is achieved by the photosynthetic capacity of the chloroplasts or by transport of sugars or hormones from the vasculature. Physiological differences between the dimorphic chloroplasts may condition different responses, which would also function to maintain the distinct patterns of gene expression. The use of mutants and tools of functional genomics including microarray analysis should provide greater insight into the complex regulatory networks underlying BS and M cell differentiation.
The morphological changes associated with adaptation to a light environment are complicated and involve the coordination of photoreceptors and downstream components. Here, we have examined two photomorphogenic responses in maize, the inhibition of mesocotyl elongation and C4 photosynthetic differentiation. We have shown that multiple photoreceptors contribute to light response in a diverse collection of maize germplasm and that a reduced light response is correlated with the development of early flowering (SS and NSS) inbreds. We have also shown that the maintenance of the C4 photosynthetic pathway in maize is not dependent on cell-specific light response pathways. This result may indicate a limited role for phytochromes in altering photosynthetic capacity in maize. However, a number of factors including plant architecture, flowering time, and resource allocation contribute to yield and are likely to be directly influenced by phytochrome signaling. The uncoupling of C4 photosynthesis from phytochrome control may provide an opportunity for artificial selection to act on phytochrome signaling networks that are not constrained to essential functions such as photosynthetic development. Together, these studies suggest that future work to define the function of downstream components of the R/FR and B response pathways in maize should prove fruitful in engineering an improved germplasm for this important crop plant.
MATERIALS AND METHODS
Germplasm
Thirty maize (Zea mays) inbred lines, representing wide genetic and geographic diversity, were grown in the mesocotyl survey. Table I provides details on the origin and subpopulation of these inbreds, as well as seed source information. Seed for the growth chamber experiments was obtained from pooled sib-matings and self-pollinations carried out in our 2001 summer nursery in Aurora, NY with plants grown from the original seed collections. Subpopulation assignments were made using a model-based approach based on SSR data (Remington et al., 2001).
Cultivation of Plant Material
Maize kernels were surface sterilized with a 10% (v/v) bleach solution for 15 min, rinsed thoroughly, and imbibed overnight in sterile water, before planting in 12 × six-cell Rootrainer trays (Hummert, Inc., St. Louis) filled with vermiculite. Three Rootrainer trays in a low-sided clear plastic tray that acted as a water reservoir were placed in a Percival model E-30LED growth chamber (Percival Scientific, Boone, IA) for B, R, and FR treatments. All LED light chambers are operated in a light-tight, air-conditioned darkroom that is illuminated with a green safelight. The W light treatments were performed in a walk-in growth chamber under incandescent and fluorescent lighting. Light fluences for the treatments were W, 3.7 μmol m-2 s-1; R, 1.8 μmol m-2 s-1 peak wavelength of 664 nm; FR, 1.2 μmol m-2 s-1, peak wavelength of 736 nm; low B, 1.3 μmol m-2 s-1, peak wavelength of 470 nm; and high B, 13 μmol m-2 s-1, peak wavelength of 470 nm. Fluence rates were measures with an IL1400A Radiometer (International Light, Inc., Newburyport, MA) equipped with a SEL033 silicon probe (detection range: 200-1,100 nm). Temperature was maintained at 28C. Seeds were randomly assigned positions in the trays and planted just below the surface. Seedlings were grown for 10 d, and the mesocotyls were then measured with Traceable digital calipers (Control Company, Friendswood, TX) to the nearest millimeter. For the gene expression analysis, seeds were treated as above and grown under a combination of incandescent and cool-white bulbs providing a fluence rate of approximately 100 μmol m-2 s-1 of continuous W. Plants were grown for 12 d or until emergence of the third leaf. They were then transferred into Percival Scientific model E-30LED light chambers for 72 h in one of four light conditions: D, R (4 μmol m-2 s-1), FR (4 μmol m-2 s-1), or B (7μmol m-2 s-1). Tissue was harvested under a green safe light at the following intervals after shift: 0, 4, 8, 24, 28, 32, 48, 52, 56, and 78 h. Second and third leaf tissue was harvested into liquid nitrogen and stored at -80C.
Experimental Design and Statistical Analysis
The program PROC MIXED in SAS (SAS/STAT Software version 8, SAS Institute Inc., Cary, NC) was used to analyze the mesocotyl length data in the main light survey (Table II; Figs. 1 and 2). A screen consisted of two identical planting arrays grown in two different light chambers during the same 10-d period. Thus, screen was included as a random factor in the mixed model analysis, with light treatment, subpopulation, and line as fixed effects. Mesocotyl lengths were natural log transformed to more closely approximate normality. Each of the five light conditions was replicated three times, and the data were pooled for analysis. Five seeds from each line were planted in every array. The mean sample size per line treatment combination was 12.5. Mesocotyl measurements of 1,883 seedlings were included in the data set. Line means and ses for all experiments are shown in Supplemental Table VI. Tests of differences among subpopulation × treatment and line × treatment effects were carried out using the least squares means with the P values adjusted for multiple comparisons using the Tukey-Kramer method. Correlations among treatment effects were generated by the program PROC CORR in SAS, with line as the replicate (inset in Fig. 1).
Seedlings of the elm1 mutant were also grown alongside the 30 inbreds in the light survey, and the treatment means and ses are shown in Figure 1. However, because the pattern of response of this mutant is so different from the wild-type inbreds, these data were not included in the first analysis. To examine the effect of the elm1 mutation, the same mixed model used to examine light response in the 30 inbreds was applied to elm and the near-isogenic W22 inbred.
A subsequent experiment examined the B response with the addition of a high-B treatment. A subset of the original 30 inbred lines, including six “responsive” and nine “nonresponsive” and the elm1 mutant line were grown for 10 d under high B. Responsiveness was determined by whether or not the treatment means for each line under D and low B were significantly different in the original light surveys, using the means tests described above. The program PROC GLM in SAS (SAS/STAT Software version 8) was used for this analysis (Table III). For the high-B treatment, two sets of seedlings were grown, with 12 seeds planted per line in each set, and the data were pooled for analysis. These data were combined with the D and low-B data collected in the original survey to complete the data set analyzed in Tables III and IV.
RNA-Blot Analysis
Total RNA was extracted from approximately 1 g of tissue as previously described (Van Tunen et al., 1988) and quantified using a DU 530 Spectrophotometer (Beckman, Fullerton, CA). Approximately 5 μg of RNA was fractionated on 6.8% (v/v) formaldehyde and 1.5% (w/v) agarose gel. The RNA was transferred onto Hybond N+ nylon membrane (Amersham Biosciences, Sunnyvale, CA) through capillary action in 20× sodium chloride and sodium citrate. Radiolabeled probes were synthesized from gel-extracted fragments derived from pNHMrbcS2, pNHMppc, and pNHMme1 (see below) with random hexamers and Klenow fragment (Promega, Madison, WI). In brief, approximately 50 μg of DNA template was labeled with Klenow fragment in a reaction mix containing 500 mm HEPES (pH 6.6), 12.5 mm MgCl2, 25 mm β-mercaptoethanol, 125 mm Tris (pH 8.0), and 50 μm each dNTPs and [32P]CTP for 1 to 2 h at 37C. Blots were prehybridized in Church and Gilbert (1984) buffer solution (250 mm NaPO4, 1% [w/v] bovine serum albumin, 7% [w/v] SDS, and 1 mm EDTA) for 1 to 2 h at 65°C. After replacing the buffer solution, probe was directly added to the blots and hybridized overnight at 65°C. Two low-stringency washes were performed for 5 min each at 65°C in buffer containing 40 mm NaPO4 and 5% (w/v) SDS, followed by two high-stringency washed for 10 min at 65°C in 40 mm NaPO4 and 1% (w/v) SDS buffer. Blots were placed on a phosphor screen (Amersham Biosciences) overnight and imaged on a Storm 840 phosphor imager (Amersham Biosciences).
RbcS2, Me1, and Ppc Probes
A genomic fragment of RbcS2 (GenBank accession no. Y09214) was PCR amplified from W22 DNA using primers 5′RbcS2 (GACCGTGGCTAGATCGAC) and 3′RbcS2 (CTACTAGTGGAATCAGAATCTGTT) and subcloned into pTOPO (Invitrogen, Carlsbad, CA) to create pNHMrbcS2. A genomic fragment of the Ppc gene (GenBank accession no. X15642) was amplified from W22 DNA using primers: 5′Ppc (GCTCAGGGACAAATACGTGG) and 3′Ppc (GTATAATATGCCAAGATTTTCCACTTG) and subcloned into pTOPO (Invitrogen) to create pNHMppc. An Me1-specific fragment was amplified from cDNA made from total RNA of light-grownW22 plants using PCR primers designed to Me1 (GenBank accession no. J05130): 5′Me1 (GATCGGGACATCTGGAGTTGG) and 3′Me1 (CAGGTACAATGCCTCTCCAGC) and subcloned into pTOPO (Invitrogen) to create pNHMme1.
Separated Cell Preparations
Seeds were surfaced sterilized, imbibed, and grown for 12 d under 100 μm m-2 s-1 W as detailed above. Plants were then placed in one of four light conditions (R, FR, B, or D) for 48 h. Tissue was harvested under dim-green safe lights for all light treatments except the W control, which was performed in ambient light. M and BS cells were then isolated as previously described with some modifications (Sheen and Bogorad, 1985).
M Cell Preparation
Approximately 5 g of leaf tissue (second and third leaves) were cut transversely into 1- to 2-mm strips and subjected to enzymatic digestion in enzyme buffer (20 mm MES [pH 5.5], 1 mm MgCl2, 0.6 m sorbitol, 2% [w/v] Cellulase Onazuka [Yakult Pharmaceuticals, Tokyo], and 0.1% [w/v] macerase [Calbiochem, San Diego]]. A stress control was also performed in which leaf strips were floated in enzyme buffer without cellulase and macerase. After 3 h at room temperature, the strips were filtered through a 120-μm nylon net (Millipore, Billerica, MA), washed with wash buffer (50 mm Tris [pH 7.5], 1 mm MgCl2, 0.6 m sorbitol, and 100 mm β-mercaptoethanol), and gently pressed with a stainless steel spoon for approximately 1.5 min or until the buffer solution turned noticeably green. The leaf material was filtered through a 60-μm nylon filter to remove leaf particles and cellular debris. The filtrate containing protoplasts were pelleted at 1,200g for 5 h, washed with wash buffer, and pelleted again. This final pellet was resuspended in 500 μL of wash buffer solution and dropped into liquid nitrogen in peel-away cups (VWR Scientific, Bridgeport, NJ).
BS Preparation
Approximately 4 g of leaf tissue (second and third leaves) were cut into 1- to 2-mm squares and subjected to three brief (10-s) pulses on low setting in a Waring blender (Waring Products, Torrington, CT) in BS buffer I (0.33 m sorbitol, 0.3 m NaCl, 0.01 m EGTA, 0.01 m dithiothreitol, 0.005 m diethyldithio carbamic acid, and 0.2 m Tris [pH 9.0]). The leaf solution was filtered through a 60-μm nylon net and subjected to three 1-h pulses on high, in BS buffer II (0.35 m sorbitol, 0.005 M EDTA, 0.1% [v/v] β-mercaptoethanol, and 0.05 m Tris [pH 8.0]). The solution was filtered through the nylon net between each pulse. After the final filtration, the BS strands (still on the net) were dried slightly on paper toweling and dropped into liquid nitrogen.
Supplementary Material
Acknowledgments
Statistical advice was provided by Francoise Vermeylen (Cornell University, Ithaca, NY). We gratefully acknowledge the generosity of Dr. Major Goodman (North Carolina State University, Raleigh) who was the source for the majority of the inbred lines utilized in this study. We would also like to thank Drs. Ben Burr (Brookhaven National Laboratory, Upton, NY), Ed Buckler (U.S. Department of Agriculture-Agricultural Research Service, Cornell University) and Jerry Kermicle (University of Wisconsin, Madison) for seed stocks and helpful discussions, and Dr. Terry Delaney (University of Burlington, VT), Dr. Ruairidh Sawers (Boyce Thompson Institute, Ithaca, NY), Ms. Moira Sheehan (Cornell University, Ithaca, NY), and Dr. Judy Kolkman (Boyce Thompson Institute, Ithaca, NY) for comments on the manuscript.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.029694.
This work was supported by the National Science Foundation (grant nos. IBN-0110297 to T.P.B. and GBN-9979516 to N.H.M.).
The online version of this article contains Web-only data.
References
- Ahmad M, Cashmore AR (1993) HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366: 162-166 [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA (1997) A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9: 1317-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballare CL (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4: 97-102 [DOI] [PubMed] [Google Scholar]
- Ballare CL, Casal JJ (2000) Light signals perceived by crop and weed plants. Field Crops Res 67: 149-160 [Google Scholar]
- Ballare CL, Sanchez RA, Scopel AL, Casal JJ, Ghersa CM (1987) Early detection of neighbor plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ 10: 551-557 [Google Scholar]
- Bansal KC, Viret JF, Haley J, Khan BM, Schantz R, Bogorad L (1992) Transient expression from cab-m1 and rbcS-m3 promoter sequences is different in mesophyll and bundle sheath cells in maize leaves. Proc Natl Acad Sci USA 89: 3654-3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Bridgers M, Ribnicky DM, Cohen JD, Jones AM (1998) Red-light-regulated growth: changes in the abundance of indoleacetic acid in the maize (Zea mays L.) mesocotyl. Planta 204: 207-211 [Google Scholar]
- Benz BF (2001) Archaeological evidence of teosinte domestication from Guila Naquitz, Oaxaca. Proc Natl Acad Sci USA 98: 2104-2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ (1993) Novel effects of phytochrome status on reproductive shoot growth in Triticum aestivum L. New Phytol 123: 45-51 [Google Scholar]
- Casal JJ, Deregibus VA, Sanchez RA (1985) Variations in tiller dynamics and morphology in Lolium mutiflorum Lam vegetative and reproductive plants as affected by differences in red/far-red irradiation. Ann Bot 56: 553-559 [Google Scholar]
- Casal JJ, Sanchez RA, Gibson D (1990) The significance of changes in the red/far-red ratio, associated with either neighbor plants or twilight, for tillering in Lolium multiflorum Lam. New Phytol 116: 565-572 [Google Scholar]
- Casati P, Drincovich MF, Andreo CS, Donahue R, Edwards GE (1998) UV-B, red, and far-red light regulate induction of the C4 isoform of NADP-malic enzyme in etiolated maize seedlings. Aust J Plant Physiol 25: 701-708 [Google Scholar]
- Childs KL, Miller FR, Cordonnier-Pratt MM, Pratt LH, Morgan PW, Mullet JE (1997) The sorghum photoperiod sensitivity gene, Ma3, encodes a phytochrome B. Plant Physiol 113: 611-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Peto CA, Ashbaugh M, Saganich R, Pratt L, Ausubel F (1989) Different roles for phytochrome in etiolated and green plants deduced from characterization of Arabidopsis thaliana mutants. Plant Cell 1: 867-880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81: 1991-1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XW, Caspar T, Quail PH (1991) cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev 5: 1172-1182 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Patel SR, Whitelam GC (1998) Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 10: 1479-1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LA, Pyle EH, Schmitt J (2000) Plasticity to light cues and resources in Arabidopsis thaliana testing for adaptive value and costs. Evolution 54: 1982-1994 [DOI] [PubMed] [Google Scholar]
- Edwards G, Walker D (1983) C3, C4: Mechanisms, and Cellular and Environmental Regulation, of Photosynthesis. University of California Press, Berkeley
- Ewing RM, Jenkins GI, Langdale JA (1998) Transcripts of maize RbcS genes accumulate differentially in C3 and C4 tissues. Plant Mol Biol 36: 593-599 [DOI] [PubMed] [Google Scholar]
- Fankhauser C (2001) The phytochromes, a family of red/far-red absorbing photoreceptors. J Biol Chem 276: 11453-11456 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471-478 [DOI] [PubMed] [Google Scholar]
- Gilbert IR, Jarvis PG, Smith H (2001) Proximity signal and shade avoidance differences between early and late successional trees. Nature 411: 792-795 [DOI] [PubMed] [Google Scholar]
- Hanumappa M, Pratt LH, Cordonnier-Pratt MM, Deitzer GF (1999) A photoperiod-insensitive barley line contains a light-labile phytochrome B. Plant Physiol 119: 1033-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422: 719-722 [DOI] [PubMed] [Google Scholar]
- Hennig L, Poppe C, Sweere U, Martin A, Schafer E (2001) Negative interference of endogenous phytochrome b with phytochrome a function in Arabidopsis. Plant Physiol 125: 1036-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M (1982) Inhibitory action of red light on the growth of the maize mesocotyl: evaluation of the auxin hypothesis. Planta 156: 388-395 [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Tokutomi S, Okuno K, Shimamoto K (2000) Phytochromes confer the photoperiodic control of flowering in rice (a short-day plant). Plant J 22: 391-399 [DOI] [PubMed] [Google Scholar]
- Jang JC, Sheen J (1994) Sugar sensing in higher plants. Plant Cell 6: 1665-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T (2003) The phototropin family as photoreceptors for blue light-induced chloroplast relocation. J Plant Res 116: 77-82 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9: 627-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperbauer MJ, Karlen DL (1994) Plant spacing and reflected far-red light effects on phytochrome-regulated photosynthate allocation in corn seedlings. Crop Sci 34: 1564-1569 [Google Scholar]
- Kausch AP, Owen TP Jr, Zachwieja SJ, Flynn AR, Sheen J (2001) Mesophyll-specific, light and metabolic regulation of the C4 PPCZm1 promoter in transgenic maize. Plant Mol Biol 45: 1-15 [DOI] [PubMed] [Google Scholar]
- Kevei E, Nagy F (2003) Phytochrome controlled signalling cascades in higher plants. Physiol Plant 117: 305-313 [DOI] [PubMed] [Google Scholar]
- Langdale JA, Rothermel BA, Nelson T (1988a) Cellular pattern of photosynthetic gene expression in developing maize leaves. Genes Dev 2: 106-115 [DOI] [PubMed] [Google Scholar]
- Langdale JA, Zelitch I, Miller E, Nelson T (1988b) Cell position and light influence C4 versus C3 patterns of photosynthetic gene expression in maize. EMBO J 7: 3643-3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C (2002) Blue light receptors and signal transduction. Plant Cell Suppl 14: S207-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP (1993) Photomorphogenic mutants of Arabidopsis thaliana reveal activities of multiple photosensory systems during light-stimulated apical hook opening. Planta 191: 214-221 [Google Scholar]
- Maddonni GA, Otegui ME, Andrieu B, Chelle M, Casal JJ (2002) Maize leaves turn away from neighbors. Plant Physiol 130: 1181-1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloof JN, Borevitz JO, Dabi T, Lutes J, Nehring RB, Redfern JL, Trainer GT, Wilson JM, Asami T, Berry CC et al. (2001) Natural variation in light sensitivity of Arabidopsis. Nat Genet 29: 441-446 [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Mitchell SE, Kresovich S, Goodman M, Doebley J (2002a) Microsatellites in Zea: variability, patterns of mutations, and use for evolutionary studies. Theor Appl Genet 104: 436-450 [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Vigouroux Y, Goodman MM, Sanchez GJ, Buckler E, Doebley J (2002b) A single domestication for maize shown by multilocus microsatellite genotyping. Proc Natl Acad Sci USA 99: 6080-6084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzella MA, Cerdan PD, Staneloni RJ, Casal JJ (2001) Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation of Arabidopsis development. Development 128: 2291-2299 [DOI] [PubMed] [Google Scholar]
- Morishima A (1998) Identification of preferred binding sites of a light-inducible DNA-binding factor (MNF1) within 5′-upstream sequence of C4-type phosphoenolpyruvate carboxylase gene in maize. Plant Mol Biol 38: 633-646 [DOI] [PubMed] [Google Scholar]
- Nelson T, Harpster MH, Mayfield SP, Taylor WC (1984) Light-regulated gene expression during maize leaf development. J Cell Biol 98: 558-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser J, Chory J (2002) Photomorphogenesis. In E Meyerowitz, C Somerville, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, pp 1-12 [DOI] [PMC free article] [PubMed]
- Oyama T, Shimura Y, Okada K (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 11: 2983-2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz JT, Gibbs M (1980) Carbon dioxide fixation and related properties in sections of the developing green maize leaf. Plant Physiol 65: 802-809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno DR, Flannery KV (2001) The earliest archaeological maize (Zea mays L.) from highland Mexico: new accelerator mass spectrometry dates and their implications. Proc Natl Acad Sci USA 98: 2101-2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell M, Mabrouk YM, Bogorad L (1995) Red/far-red and blue light-responsive regions of maize rbcS-m3 are active in bundle sheath and mesophyll cells, respectively. Proc Natl Acad Sci USA 92: 11504-11508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington DL, Thornsberry JM, Matsuoka Y, Wilson LM, Whitt SR, Doebley J, Kresovich S, Goodman MM, Buckler ESt (2001) Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proc Natl Acad Sci USA 98: 11479-11484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H, Suzuki M, Takei K, Deji A, Taniguchi M, Sugiyama T (1998) A response-regulator homologue possibly involved in nitrogen signal transduction mediated by cytokinin in maize. Plant J 14: 337-344 [DOI] [PubMed] [Google Scholar]
- Salamini F (1985) Photosensitivity in maize: evaluation, genetics, and breeding for insensitivity. In A Bandolini, F Salamini, eds, Breeding Strategies for Maize Production Improvement in the Tropics. Food and Agriculture Organization of the United Nations and Istituto Agronomico per L'Oltremare, Florence, Italy, pp 143-157
- Sawers RJ, Linley PJ, Farmer PR, Hanley NP, Costich DE, Terry MJ, Brutnell (2002) TP elongated mesocotyl1, a phytochrome-deficient mutant of maize. Plant Physiol 130: 155-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner AR, Sheen J (1991) Maize rbcS promoter activity depends on sequence elements not found in dicot rbcS promoters. Plant Cell 3: 997-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner AR, Sheen J (1992) Maize C4 photosynthesis involves differential regulation of phosphoenolpyruvate carboxylase genes. Plant J 2: 221-232 [DOI] [PubMed] [Google Scholar]
- Sheen J (1998) Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc Natl Acad Sci USA 95: 975-980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J (1999) C4 gene expression. Annu Rev Plant Physiol Plant Mol Biol 50: 187-217 [DOI] [PubMed] [Google Scholar]
- Sheen J, Bogorad L (1985) Differential expression of the ribulose bisphosphate carboxylase large subunit in bundle sheath and mesophyll cells of developing maize leaves is influenced by light. Plant Physiol 79: 1072-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J, Bogorad L (1986) Expression of the ribulose-1, 5-bisphosphate carboxylase large subunit gene and three small subunit genes in two cell types of maize leaves. EMBO J 5: 3417-3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen JY, Bogorad L (1987) Differential expression of C4 pathway genes in mesophyll and bundle sheath cells of greening maize leaves. J Biol Chem 262: 11726-11730 [PubMed] [Google Scholar]
- Takano M, Kanegae H, Shinomura T, Miyao A, Hirochika H, Furuya M (2001) Isolation and characterization of rice phytochrome A mutants. Plant Cell 13: 521-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetio-Kagho F, Gardner FP (1988) Responses of maize to plant-population density: I. Canopy development, light relationships, and vegetative growth. Agron J 80: 930-935 [Google Scholar]
- Thomas M, Cretin C, Vidal J, Keryer E, Gadal P, Monsinger E (1990) Light-regulation of phosphoenolpyruvate carboxylase mRNA in leaves of C4 plants: evidence for phytochrome control on transcription during greening and rhythmicity. Plant Sci 69: 65-78 [Google Scholar]
- Van Tunen AJ, Koes RE, Spelt CE, van Der Krol AR, Stuitje AR, Mol JNM (1988) Cloning of two chalcone flavanone isomerase genes from Petunia hybrida: coordinate, light regulated and differential expression of flavinoid genes. EMBO J 7: 1257-1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef LN, Briggs WR (1978) Red light-inhibited mesocotyl elongation in maize seedlings: I. The auxin hypothesis. Plant Physiol 61: 534-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef LN, Quail PH, Briggs WR (1979) Red light-inhibited mesocotyl elongation in maize seedlings. Plant Physiol 63: 1062-1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret JF, Mabrouk Y, Bogorad L (1994) Transcriptional photoregulation of cell-type-preferred expression of maize rbcS-m3: 3′ and 5′ sequences are involved. Proc Natl Acad Sci USA 91: 8577-8581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Murfet IC, Reid JB (1997) Pea mutants with reduced sensitivity to far-red light define an important role for phytochrome A in day-length detection. Plant Physiol 114: 1225-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Purcell M, Zucchi P, Helentjaris T, Bogorad L (2001) TRM1, a YY1-like suppressor of rbcS-m3 expression in maize mesophyll cells. Proc Natl Acad Sci USA 98: 2295-2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S (2000) Dof1 and Dof2 transcription factors are associated with expression of multiple genes involved in carbon metabolism in maize. Plant J 21: 281-288 [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Sheen J (1998) Involvement of maize Dof zinc finger proteins in tissue-specific and light-regulated gene expression. Plant Cell 10: 75-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YS, Kung SD, Bogorad L (1985) Phytochrome control of levels of mRNA complementary to plastid and nuclear genes of maize. Plant Physiol 79: 371-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.