Abstract
NKT cells in the mouse recognize antigen in the context of the MHC class I–like molecule CD1d and play an important role in peripheral tolerance and protection against autoimmune and other diseases. NKT cells are usually activated by CD1d-presented lipid antigens. However, peptide recognition in the context of CD1 has also been documented, although no self-peptide ligands have been reported to date. Here, we have identified an endogenous peptide that is presented by CD1d to activate mouse NKT cells. This peptide, the immunodominant epitope from mouse collagen type II (mCII707–721), was not associated with either MHC class I or II. Activation of CD1d-restricted mCII707–721–specific NKT cells was induced via TCR signaling and classical costimulation. In addition, mCII707–721–specific NKT cells induced T cell death through Fas/FasL, in an IL-17A–independent fashion. Moreover, mCII707–721–specific NKT cells suppressed a range of in vivo inflammatory conditions, including delayed-type hypersensitivity, antigen-induced airway inflammation, collagen-induced arthritis, and EAE, which were all ameliorated by mCII707-721 vaccination. The findings presented here offer new insight into the intrinsic roles of NKT cells in health and disease. Given the results, endogenous collagen peptide activators of NKT cells may offer promise as novel therapeutics in tissue-specific autoimmune and inflammatory diseases.
Introduction
The immune system responds to invading pathogens by recognizing their antigenic structures, while remaining unresponsive to self antigens. This simplified model, however, is challenged by the understanding that autoreactivity is a common feature of healthy organisms and a part of peripheral tolerance mechanisms. To generate animal models for autoimmune diseases, susceptible species are often immunized with self antigens. However, not all immunogenic self antigens have the capacity to provoke a pathogenic autoimmune response, and some of them confer protection (1). A widely accepted animal model for human rheumatoid arthritis is collagen-induced arthritis (CIA) in mice. CIA is induced by immunizing susceptible mouse strains with cartilage-derived triple helical type II collagen (CII). In mice expressing the MHC class II molecule H-2Aq, the disease-mediating epitope is an immunodominant glycopeptide derived from position 260–270 of rat CII (2). In comparison with heterologous CII, mouse CII, although capable of inducing arthritis, provokes a much weaker immune response to the immunodominant epitope and induces a lower incidence of arthritis (3). However, mouse CII immunization induces a recall proliferative response toward multiple epitopes in addition to the MHC class II–restricted CII260–270 peptide (4). The major epitopes share a common motif, with the strongest located at position 707–721 (mCII707–721). By investigating the immune response to this epitope, surprisingly, we found that this peptide was not MHC I/II restricted, but was binding and presented by CD1d, a cluster of differentiation glycoprotein. This led to the identification of a population of NKT cells that responded to mCII707–721 and were CD1d-restricted, TCRαβ+CD4+NK1.1+ cells.
CD1d is an alternative, MHC class I–like glycoprotein that presents antigens to NKT cells. The most extensively described population of CD1d-restricted NKT cells are reactive to the exogenous glycolipid α-galactosylceramide (αGalCer) (5). An endogenous ligand, isoglobotrihexosylceramide, has been shown to be involved in NKT cell autoreactivity (6). In addition to the association of CD1d with the binding and/or presentation of self and exogenous lipid or glycolipids (6), early reports have also indicated peptide-presenting capacity of CD1d (7–9).
Although the regulatory function of NKT cells is debated (5, 10), reports over the past decade have shown that both foreign and self-lipid–reactive CD1d-restricted NKT cells can regulate autoimmune diseases, moderate graft rejection, assist in host defense against infection, and promote tumor rejection, in spite of their relatively small population size (10–13). The immune regulatory function of CD1d-restricted NKT cells is ascribed to their capacity for rapidly releasing large quantities of cytokines, including IL-4 and IFN-γ (10). In the current report, we show that the mCII707–721–specific NKT cells are competent to produce IL-4, IFN-γ, IL-17A, TNF-α, and TGF-β upon peptide stimulation. However, the regulatory function of mCII707–721–specific NKT cells is likely through the Fas-FasL–mediated killing of activated T cells, not by cytokine production. Prevaccination with the mCII707–721 peptide resulted in downregulation of both Th1- and Th2-mediated immune responses and tissue inflammation in vivo. To our knowledge, this is the first report showing the existence of a CD1d-presented endogenous peptide with the capacity to activate immune-suppressive NKT cells. The results have implications for antiinflammatory vaccines for human autoimmune disorders.
Results
Collagen peptide mCII707–721 activates CD4+TCRαβ+ T cells in an MHC I/II–independent manner.
We immunized B10.Q mice with mCII707–721 and observed a strong and specific immune response, as measured by rechallenge of LN cells (LNCs) in vitro with mCII707–721 (Figure 1A). No response was induced in the absence of antigen (media) or by using a nonrelated peptide control, the mouse collagen type I α 1 chain peptide mCI707–721, which shares high homology with mCII707–721. mCI707–721 was chosen as the negative control because this 15-aa peptide is similar to mCII707–721 in size and sequence, differing in only 4 aa positions. It also does not elicit any T cell proliferation. Purified protein derivative (PPD) has been used as a positive control (recall antigen) for accuracy of immunization and proliferation, given that it was used for all the in vivo assays. PPD reactivity showed a proliferation index (mean ± SD) of 12.8 ± 2.1. We also immunized C57BL/6 mice (H2b, 6 mice per group) with mCII707–721 and observed a similar immune response as with B10.Q (proliferation indexes: media = 1; mCI707–721 = 1.25 ± 0.23; mCII707–721 = 3.68 ± 0.32, P ≤ 0.001 versus each negative and positive control; PPD = 12.78 ± 2.09). This indicates that the mCII707–721–reactive response is not strain specific. However, to control for possible strain-specific effects, the remainder of the study utilized only mice of B10.Q background.
Figure 1. Immunodominant mouse collagen type II peptide mCII707–721 is CD1d restricted and activates CD4+TCRαβ+ NKT cells.
(A) Significant proliferative response was observed after in vitro peptide stimulation of LNCs from mCII707–721–immunized mice (WT B10.Q). Controls are medium or control peptide mCI707–721. Proliferation index (PI) was calculated by normalizing all cpm values to media control as 1. (B) PI in response to mCII707–721 in KO mice, all B10.Q background. Proliferation was significantly reduced compared with WT littermates in Tcrab–/–, CD4–/–, and CD1d–/– mice, indicating that the mCII707–721 peptide response depends on CD1d-restricted CD4+TCRαβ+ T cells, but not classical MHC I/II presentation. (C) PI in response to mCII707–721 in B10.Q LNCs. Blocking with anti–MHC II and anti–MHC I antibodies produced similar levels of PI compared with mCII707–721 with control antibody. PI was calculated by normalizing all cpm values to media control as 1. (D) In vitro rechallenge response to mCII707–721, abrogated by anti-CD1 (1B1, 10 μg/ml) or anti-NK1.1 (PK136, 10 μg/ml), indicating that CD1d-dependent NKT cells were operative in the response. PI was calculated by normalizing all cpm values to media control as 1. Data are mean ± SD, n = 6. **P ≤ 0.01; ***P ≤ 0.001.
We characterized the T cell response toward the mCII707–721 peptide, using KO mice of genotypes MHC II–/–, TAP1–/–, CD1d–/–, Tcrab–/–, Tcrgd–/–, CD4–/–, and CD8–/–, all backcrossed to the same genetic background, B10.Q. These were immunized and LNCs were rechallenged with or without antigens in vitro. Proliferative response to mCII707–721 required TCRαβ expression, CD4+ T cells, and CD1d, but was not dependent on TCRγδ expression or CD8+ T cells (Figure 1B). No difference in proliferation was observed among the various KO mice and WT B10.Q mice in response to control peptides used in the assay (data not shown).
We investigated potential antigen-presenting molecules for mCII707–721 using MHC II–/– mice and TAP-deficient mice (TAP1–/–), which lack transporter associated with antigen processing, important for presentation of MHC I–restricted peptides. By utilizing these mice (Figure 1B), as well as monoclonal antibodies to block MHC class I and II (Figure 1C), sustained proliferation to mCII707–721 was observed. These data indicate that the mCII707–721 response was neither MHC II– nor MHC I–restricted. An MHC II–binding assay also verified that the mCII707–721 peptide did not bind to H-2q (data not shown). These results demonstrate that the T cell response to mCII707–721 was dependent on CD4 and TCRαβ, but unexpectedly, did not involve MHC I or II presentation.
mCII707–721 binds to CD1d and activates NKT cells.
Mice were immunized with mCII707–721, and recall antigen reactivity of LNCs or splenocytes was measured 10–13 days after immunization. As shown in Figure 1B and Figure 1D, the proliferative response to mCII707–721 was essentially completely diminished when mice genetically lacking CD1d-dependent NKT cells (CD1d–/– mice) were investigated or monoclonal antibodies to CD1d or NK1.1 were added to cultures prior to restimulation with mCII707–721. No effect on mCI707–721 or PPD was observed (data not shown). Similarly, depletion of NK1.1+ cells, or antibody blocking of CD1d in vivo prior to subsequent immunization with mCII707–721, resulted in reduced proliferative response to mCII707–721 (data not shown). Thus, the response to mCII707–721 was confirmed to be CD1d restricted.
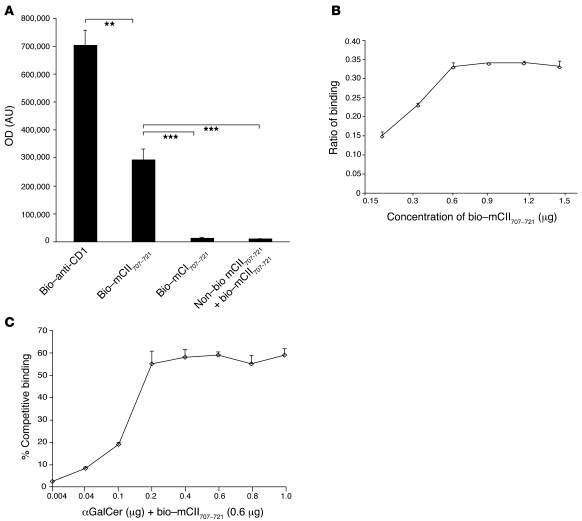
The binding capacity of CD1d for mCII707–721 was determined using recombinant soluble dimeric mouse CD1d immunoglobulin and biotinylated mCII707–721. Significant binding of biotinylated mCII707–721 to CD1d was observed, while no binding of the negative control, biotinylated mCI707–721, was detected. Excess nonbiotinylated mCII707–721 peptide effectively competed for binding of biotinylated mCII707–721 to CD1d (Figure 2A). Figure 2B shows that the interaction was dose dependent and saturable. Using αGalCer with biotinylated mCII707–721 revealed that at certain concentrations it competes for the binding to CD1d, indicating partial competition for the binding site (Figure 2C). PBS (non-CD1d-Ig dimer) is used for negative control.
Figure 2. mCII707–721 binds CD1d.
(A) The binding capacity of mCII707–721 to CD1d was determined using ELISA plates coated with CD1d-Ig dimer, with biotin-labeled anti-CD1d (bio–anti-CD1) (positive control), biotin-labeled mCII707–721 peptide, or biotin-labeled mCI707–721 (negative control). Excess nonlabeled mCII707–721 peptide with biotin-labeled mCII707–721 peptide was used for competitive binding. Significant differences were seen in wells with biotin-labeled mCII707–721 peptide compared with negative control or excess nonlabeled peptide. (B) mCII707–721 binding to CD1d was concentration dependent, with a maximum binding capacity of 0.6 μg/ml. Ratio of binding is the fluorometer OD of the sample divided by the positive control OD. (C) αGalCer and biotinylated-labeled mCII707–721 peptide compete for binding to CD1d. Biotinylated-labeled mCII707–721 peptide binding to CD1d is considered as 100% binding. Data are mean ± SD, n = 3–4. **P ≤ 0.01; ***P ≤ 0.001.
mCII707–721 activates a heterogeneous NKT cell population.
To investigate the frequency of NKT cell expansion in response to mCII707–721, WT B10.Q mice were treated in vivo with mCII707–721 and CFA and 13 days later, splenocytes were analyzed. mCII707–721 is capable of significantly inducing NKT cell expansion (gated on CD4+NK1.1+ T cells) compared with all other control groups, namely vehicle-treated (CFA), mCI707–721, and naive mice (Figure 3, A and B).
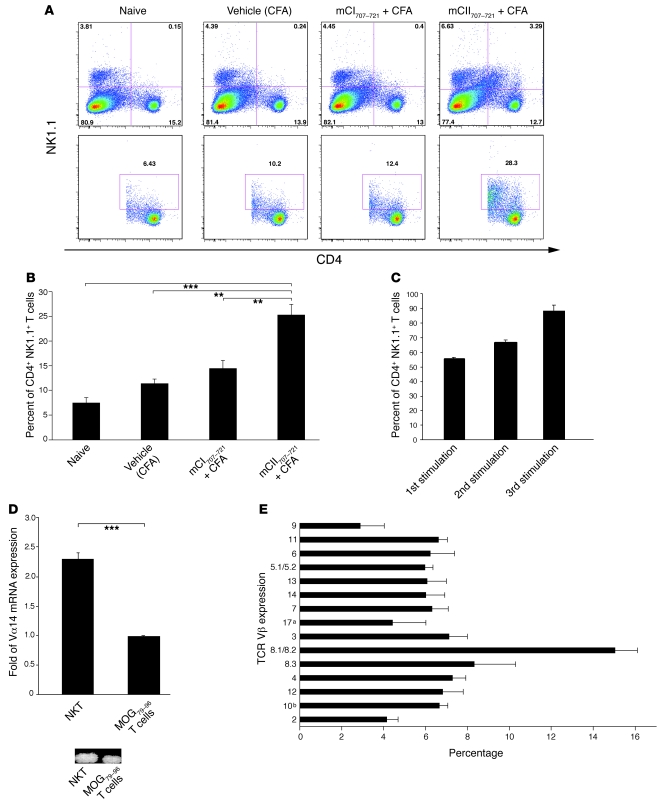
Figure 3. mCII707–721 induces NKT cell expansion.
(A and B) Splenocytes were taken from naive WT (B10.Q) mice and in vivo CFA-, mCI707–721–, and mCII707–721–treated mice. Single-cell suspensions were made and then stimulated with 100 μg/ml mCII707–721 for 48 hours. n = 3 mice per group. (A) A representative FACS staining shows total gated lymphocytes (upper row) and gated CD4+NK1.1+ NKT cells (lower row). (B) Percentage of CD4+NK1.1+ NKT cells from indicated groups. Data are mean ± SD, n = 3. **P ≤ 0.01; ***P ≤ 0.001. (C) Proportion of NK1.1+ T cells in cell line during the first stimulation of LNCs (57%), and after 2 (65%) and 3 (88%) in vitro stimulation cycles with mCII707–721. NK1.1+ T cells enriched after each stimulation, indicating response and proliferation after mCII707–721 exposure. Data are mean ± SD. n = 3. (D) mCII707–721–specific cell line characterized for TCR usage after 3 stimulation cycles with a significantly higher proportion of Vα14-Jα18 by real-time qPCR compared with control BQMOG79–96–specific T cells (top panel). Vα14 PCR product in agarose gel is shown (bottom panel). Data are mean ± SD. n = 3. ***P ≤ 0.001. (E) Splenocytes were taken from mCII707–721–immunized mice treated with 100 μg/ml mCII707–721 for 48 hours. FACS staining shows TCR Vβ usage. Percentages were computed relative to the sum of all of the 15 chains. Data are mean ± SD, n = 3.
To further characterize the mCII707–721–responding T cells, a peptide-specific cell line was established by subjecting LNCs from mice immunized with mCII707–721 to different cycles of restimulation and resting. Expression of the NK1.1 marker was confirmed after each round of antigenic stimulation. Figure 3C shows that 57% of the CD4+ mCII707–721–specific T cells in the primary cell line were NK1.1+ T cells. This percentage increased to 65% after the second stimulation and to 88% after the third, indicating enrichment of NKT cells upon antigenic stimulation.
NKT cells can utilize a diverse combination of Vα and Vβ chains, with evidence suggesting that any of the Vβ chains can pair with the Vα14 invariant chain (14). The TCR Vα usage of the mCII707–721–specific NKT line was characterized by real-time quantitative PCR (qPCR) and FACS. A conventional T cell line specific to the CNS self-antigenic peptide myelin oligodendrocyte glycoprotein 79–96 (MOG79–96) was included for comparison. The mCII707–721–specific NKT cell line showed higher expression of Vα14-Jα18 than the conventional T cell line by real-time quantitative PCR (qPCR) (Figure 3D). Using a panel of Vβ TCR antibodies to stain mCII707–721–specific NKT cells showed that these cells utilize polyclonal and diverse Vβ chains (Figure 3E). Although some skewing toward usage of Vβ8.2 is seen, mCII707–721 reactivity is heterogeneous, not clonal.
Activation of mCII707–721–reactive NKT cells requires TCR and costimulatory signaling.
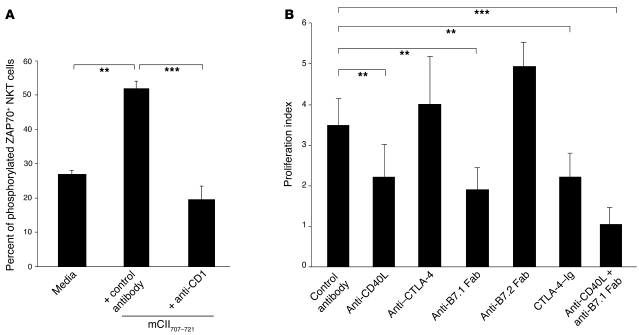
To examine TCR signaling in the mCII707–721–specific response, we investigated ZAP-70 phosphorylation in the mCII707–721–specific NKT cell line. NKT cells stimulated with mCII707–721 expressed phosphorylated ZAP-70 on the cell membrane, indicating TCR engagement and signaling (Figure 4A). Furthermore, blocking CD1d by adding a neutralizing antibody to the culture medium completely inhibited the appearance of phosphorylated ZAP-70 on the cell surface. Thus, interaction among CD1d, mCII707–721, and TCR is operative in the activation of mCII707–721–specific NKT cells.
Figure 4. mCII707–721–specific NKT cell activation engages TCR and requires costimulation.
(A) Activation of mCII707–721–specific NKT cells operated through TCR signaling. Percentage of phosphorylated ZAP70-positive cells in NK1.1+ T cells by FACS shows a significant increase over nonstimulated cells upon mCII707–721 stimulation in vitro, with abrogation by blocking anti-CD1 (1B1, 10 μg/ml). (B) Costimulatory signals for proliferation after in vitro stimulation of LNCs from mCII707–721–immunized mice. Blocking with antibodies to costimulatory proteins significantly reduced proliferation. Data are mean ± SD, n = 4. **P ≤ 0.01; ***P ≤ 0.001.
The requirement for costimulatory signals in the activation of mCII707–721–specific NKT cells was studied using antibodies or fusion proteins to block the B7- and CD40-dependent pathways in peptide-stimulated LNC cultures from mCII707–721–immunized mice. Blocking either the CD40/CD40L or the B7/CD28 pathways led to significant inhibition of the proliferative response (Figure 4B) and showed that B7.1, but not B7.2, was the crucial ligand for CD28 on the NKT cells. The data support the interpretation that B7.1-CD28 and CD40-CD40L, in addition to CD1d-TCR ligation, are the necessary signals for mCII707–721–induced NKT cell activation.
mCII707–721–specific NKT cells produce cytokines.
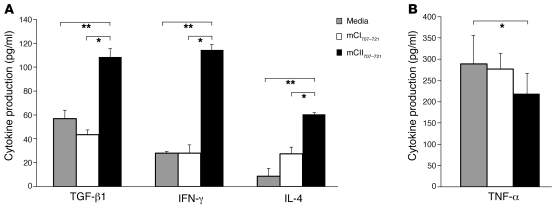
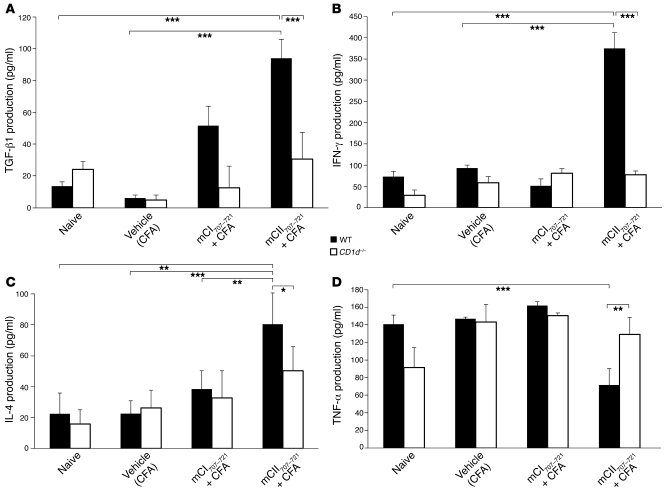
Cytokine production by the mCII707–721–specific NKT cells was measured by ELISA for IFN-γ, IL-2, IL-4, IL-10, TGF-β1, and TNF-α in peptide-stimulated LNC cultures from mCII707–721–immunized mice. TGF-β1, IFN-γ, and IL-4 production was significantly increased compared with cells stimulated with the negative control media and mCI707–721 (Figure 5A). IL-2 and IL-10 production was undetectable (data not shown). Interestingly, TNF-α production was significantly reduced upon mCII707–721–specific NKT cell activation (Figure 5B). The kinetics of cytokine production resembled those of conventional T cells, i.e., peaking at 72 hours after restimulation in vitro and showing no early burst of cytokines (data not shown).
Figure 5. mCII707–721–specific NKT cells produce cytokines.
(A) mCII707–721–specific cytokine production in primary LNCs from mCII707–721–immunized WT mice measured by ELISA. Significantly elevated levels of TGF-β1, IFN-γ, and IL-4 (n = 3) in response to mCII707–721 are seen, but (B) TNF-α levels were lowered. Data are mean ± SD, n = 6. *P ≤ 0.05; **P ≤ 0.01.
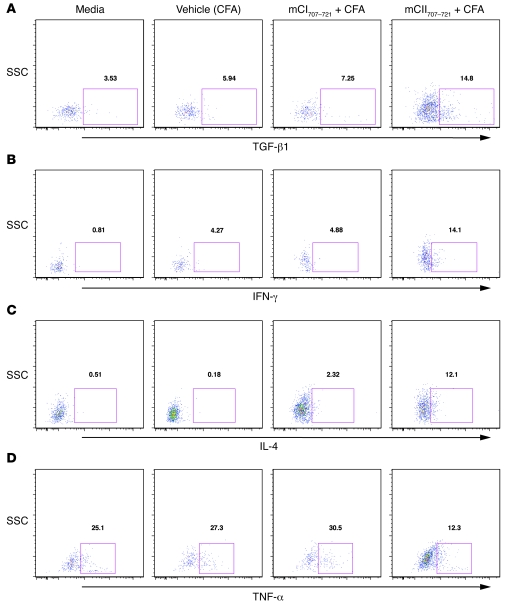
To investigate whether NKT cells are the direct source of cytokine production, we treated WT B10.Q mice with mCII707–721 plus CFA in vivo. As control groups, mice were treated with mCI707–721 plus CFA; an additional group received only vehicle (CFA), and finally a group of mice did not receive any treatment (naive). Splenocytes were dissected and single-cell suspensions treated in vitro with mCII707–721 for 48 hours. Cells were then stained for intracellular cytokines and analyzed using FACS. As shown in Figure 6, mCII707–721–specific NKT cells are capable of intracellular production of TGF-β1, IFN-γ, IL-4, and TNF-α. However, the levels of TNF-α intracellularly (Figure 5B) exhibited a trend similar to the levels seen as assessed by ELISA (Figure 7D), i.e., reduction in the in vivo mCII707–721–treated group compared with controls.
Figure 6. mCII707–721–specific NKT cells produce cytokines intracellularly.
Intracellular staining indicates that mCII707–721 induces TGF-β1, IFN-γ, and IL-4 production (A–C), but not increased TNF-α (D). (A–D) Splenocytes were taken from mCI707–721– and mCII707–721–immunized mice, then stimulated with mCII707–721 for 48 hours. Anti-CD3 (10 μg/ml) and anti-CD28 (2 μg/ml) with brefeldin A were added to cell cultures for 5 hours before staining. FACS profile shows intracellular staining of TGF-β1, IFN-γ, IL-4, and TNF-α. Cells were gated on CD4+NK1.1+ cells.
Figure 7. mCII707–721–specific NKT cells produce cytokines.
(A–D) Higher TGF-β1, IFN-γ, and IL-4 production and lower TNF-α production were seen from mCII707–721–immunized splenocytes compared with all other control groups in WT mice but not in CD1d–/– mice. Spleens were taken from CFA, mCI707–721–, and mCII707–721–immunized mice, then treated with mCII707–721 for 48 hours. Supernatants were taken for ELISAs of TGF-β1, IFN-γ, IL-4, and TNF-α. Data are mean ± SD, n = 5 mice per group. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Cytokine production of mCII707–721–specific NKT cells is CD1d dependent.
Splenocytes from similarly treated B10.Q WT and CD1d–/– mice were analyzed for the production of cytokines in response to mCII707–721. As depicted in Figure 7, A–C, only WT mice that were treated in vivo with mCII707–721 showed significant capacity to produce TGF-β1, IFN-γ, and IL-4, as measured by ELISA. Moreover, mCII707–721–specific TGF-β1, IFN-γ, and IL-4 production was significantly reduced in CD1d–/– compared with WT mice, though interestingly, a significantly lower TNF-α production was observed (Figure 7D).
mCII707–721–specific NKT suppression of T cells is Fas-FasL dependent.
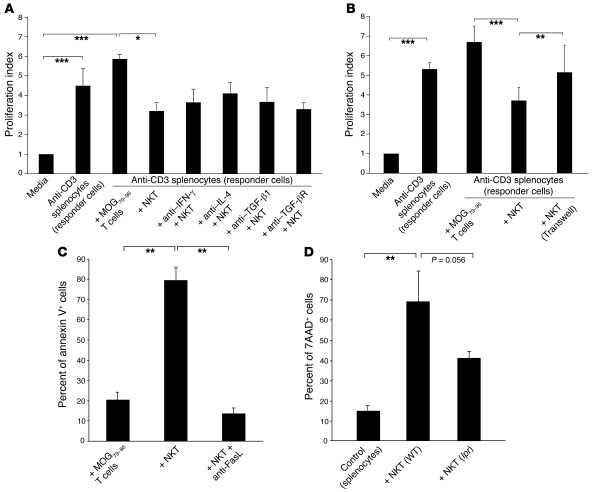
In vitro suppression of T cells was investigated by activating syngeneic splenocytes with plate-bound anti-CD3 (here referred to as responder cells), which were then cocultured with the mCII707–721–specific NKT cell line. Responder cells proliferated vigorously as indicated by a high proliferation index (Figure 8). However, mCII707–721–specific cells significantly inhibited proliferation of anti-CD3–stimulated responder T cells, compared with the control conventional MOG79–96 T cell line. Since the mCII707–721–specific NKT cells produced TGF-β1, IFN-γ, and IL-4 upon activation, we assessed the role of these cytokines in T cell suppression. To block these cytokines, neutralizing antibodies were added to mCII707–721–specific NKT cell cultures prior to coculture with anti-CD3–activated splenocytes. The neutralizing antibodies did not affect the significant decrease in responder T cell proliferation (Figure 8A), indicating that the suppressive effect of mCII707–721–specific NKT cells was independent of their cytokine production.
Figure 8. mCII707–721–specific NKT cells have a suppressive function.
(A and B) Suppressor assay was performed using in vitro–stimulated anti-CD3 splenocytes (responder cells) from B10.Q mice, cocultured for 64 hours with either mCII707–721–specific NKT cell line or B10.Q-restricted MOG79–96–specific T cell line (control) at a 1:1 ratio. Proliferation index was calculated by normalizing all cpm values to media control as 1. (A) Blocking IFN-γ, IL-4, TGF-β1, and TGF-β receptor with antibodies (20 μg/ml) had no effect on NKT cell suppression compared with isotype-matched controls; therefore suppression did not operate through cytokine release from mCII707–721–specific NKT cells. Data are mean ± SD, n = 3. *P ≤ 0.05; ***P ≤ 0.001. (B) Significantly reduced proliferation of anti-CD3–stimulated responder cells was observed when cultured with the mCII707–721–specific NKT cells (with or without Transwell system) compared with control cells. Proliferation of the responder cells was restored when a Transwell system inhibited cell-cell contact. Data are mean ± SD, n = 2–3. **P ≤ 0.01; ***P ≤ 0.001. (C) Cell death by annexin V+ FACS analysis of cocultured splenocytes (CFSE labeled), showing that the mCII707–721–specific NKT cell line induced significantly elevated cell death levels compared with the control cells. Anti-FasL (20 μg/ml) before coculturing revealed that splenocyte killing was mediated by FasL interaction. Data are mean ± SD, n = 3. **P ≤ 0.01. (D) Splenocytes (CFSE labeled) from WT mice stimulated with plate-bound anti-CD3 and cocultured for 48 hours with NKT cells purified from mCII707–721–immunized WT mice or lpr mice. Cell death was determined by 7AAD staining. Data are mean ± SD, n = 10 mice per group. **P ≤ 0.01.
Since these soluble cytokines seem to exert no major effects in the context of mCII707–721–specific NKT cell suppression, we investigated to determine whether cell-cell contact was required. As depicted in Figure 8B, the suppressive function of the mCII707–721–specific NKT cells was dependent on cell-cell contact, since separating direct cell-cell interaction using a Transwell system abrogated their suppressive effect.
We investigated whether mCII707–721–specific NKT cells could induce apoptosis, as measured by annexin V staining of anti-CD3–activated responder cells. Upon coculture, mCII707–721–specific NKT cells significantly induced apoptosis of anti-CD3–activated responder cells (Figure 8C). As Fas-FasL is one of most common mechanisms for induction of apoptosis (15), we used FasL-blocking antibody to determine whether Fas-FasL is involved in apoptosis induction. Cell death induced by mCII707–721–specific NKT cells was Fas-FasL dependent, since adding FasL-blocking antibody to the cultures inhibited apoptosis (Figure 8C). To confirm this result, we purified mCII707–721–specific NKT cells from mCII707–721–immunized Fas receptor–deficient mice (lpr mice). Anti-CD3–activated responder cells were cocultured with mCII707–721–specific NKT cells from lpr or WT mice, and cell death quantified as 7-aminoactinomycin D–positive (7AAD-positive) cells by FACS. Figure 8D shows that the cytotoxic function of mCII707–721–specific NKT cells was Fas-FasL dependent.
IL-17A is not required for mCII707–721–specific NKT function.
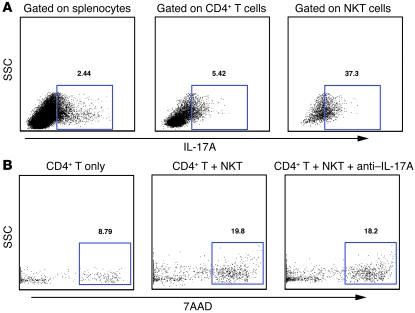
NKT cells are reported to produce IL-17 (16), so we stained splenocytes from mCII707–721–immunized mice for IL-17A. Upon immunization with mCII707–721, 37.3% of NKT cells produced IL-17A (Figure 9A). However, IL-17A did not appear to play a role in the suppressive function of these cells. CFSE-labeled CD4+ T cells were cocultured with mCII707–721–specific NKT cells in an anti-CD3–coated plate, with or without neutralizing IL-17A antibody. After 48 hours, cell death was analyzed with 7AAD staining on gated CD4+ T cells. The percentage of 7AAD+ T cells was similar with or without IL-17A blocking, suggesting that IL-17A was not involved in mCII707–721–specific NKT cells’ suppressive function (Figure 9B).
Figure 9. IL-17A from mCII707–721–specific NKT cells does not affect suppression.
(A) FACS with CD4, NK1.1, IL-17A antibodies and LIVE-DEAD marker from B10.Q WT mouse splenocytes 10 days after immunization with mCII707–721. One representative FACS is shown, with the percentage of IL-17A cells in CFSE-labeled splenocytes, CD4+ T cells, and CD4+ NK1.1+ NKT cells. (B) Single-cell spleen suspensions from B10.Q WT mice immunized with mCII707–721 stained for CD4 and NK1.1, purified by FACS. CD4+ T cells were stimulated with plate-bound anti-CD3 and cocultured for 48 hours with NKT cells with or without anti–IL-17A. Cell death was determined by 7AAD staining.
mCII707–721–specific NKT cells prevent Th1- and Th2-mediated responses.
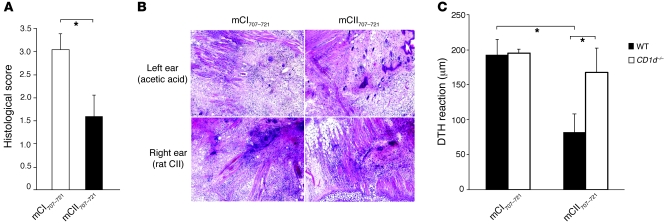
The in vivo suppressive capacity of mCII707–721–specific NKT cells was investigated using B10.Q mice vaccinated with mCII707–721 or the negative control peptide mCI707–721, prior to induction of a delayed-type hypersensitivity (DTH) reaction. Mice vaccinated with mCII707–721 developed significantly less inflammation than the control group (Figure 10, A and B). Moreover, the antiinflammatory effect of mCII707–721 vaccination was dependent upon activation of CD1d-restricted mCII707–721–specific NKT cells, since lack of this population in CD1d–/– mice resulted in significant abrogation of the antiinflammatory effect (Figure 10C). These results support the in vitro findings that CD1d-restricted mCII707–721–specific NKT cells suppress T cell activation and hence inhibit Th1-polarized DTH inflammation.
Figure 10. mCII707–721–specific NKT cells suppress Th1 cell–mediated immune responses.
(A and B) In vivo activation of mCII707–721–specific NKT cells significantly reduced Th1-driven inflammation due to DTH induced by rat CII, assessed by histopathological scoring of tissue inflammation. Data are mean ± SD, n = 6 mice per group. *P ≤ 0.05. Original magnification, ×100. (C) DTH response in WT (B10.Q) is significantly lower than in CD1d–/– mice. While mCII707–721 vaccination dampens DTH reaction, the mCI707–721–negative control peptide is inadequate to exert antiinflammatory effects. DTH response (ear swelling) was calculated by subtracting the thickness of the right ear from that of the left ear. Data are mean ± SEM, n = 4–5 mice per group. *P ≤ 0.05.
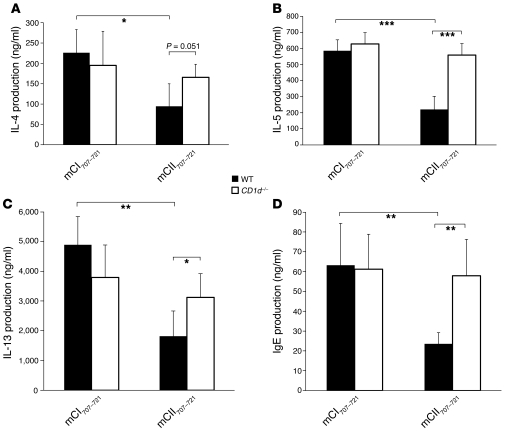
We next studied the effect of CD1d-restricted mCII707–721 NKT cells on the Th2-mediated immune response. Mice were vaccinated as above and then to provoke a Th2 response were injected with OVA emulsified in alum. Interestingly, a downregulated Th2 response was observed in the mCII707–721–vaccinated group compared with control. A significant decrease in IL-4, IL-5, and IL-13 production was detected in bronchoalveolar lavage fluid (BALF) after OVA rechallenge in WT mice. Moreover, we observed that vaccination with mCII707–721 repressed IgE production in BALF of WT mice. Furthermore, mCII707–721–specific NKT cell activation and the antiinflammatory effects of such activation were determined to be CD1d restricted, as CD1d–/– mice lacked such capacity (Figure 11). Taken together, the observations show that the mCII707–721–specific CD1d-restricted NKT cells are involved in immune regulation of both Th1-mediated cellular responses and Th2-mediated humoral immune responses.
Figure 11. Activation of mCII707–721–specific CD1d-restricted NKT cells in vivo significantly reduces Th2-mediated responses to OVA.
(A–D) Expression of IL-4, IL-5, IL-13, and IgE were all significantly reduced in BALF of mCII707–721–vaccinated B10.Q mice compared with all control-vaccinated groups of mice. CD1d–/– mice lack such mCII707–721–mediated antiinflammatory effects. Data are mean ± SD, n = 4–5 mice per group. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Activation of mCII707–721–specific NKT cells ameliorates CIA.
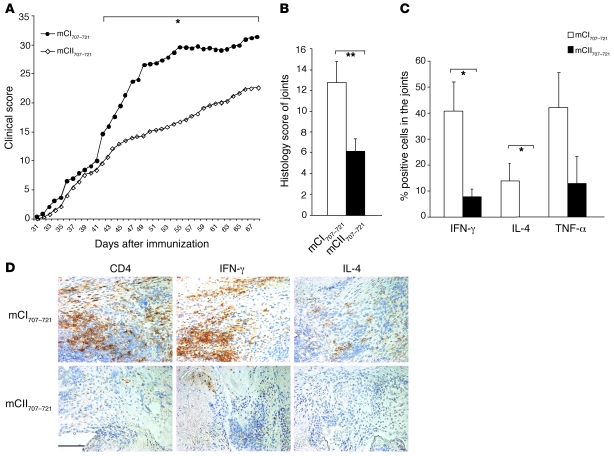
The mCII707–721 peptide is a major epitope in mouse collagen type II, so mCII707–721–specific cells might be crucial for regulating CIA. To test our hypothesis, B10.Q mice were vaccinated with either mCII707–721 or control peptide 10 days prior to CIA induction. We found that mCII707–721 vaccination ameliorated the severity of arthritis compared with the control (Figure 12A). The incidence in the mCII707–721–treated group was 74% compared with 93% in the control group. Both clinical signs of disease and histopathological studies revealed that mCII707–721–vaccinated mice had less joint inflammation than control peptide–vaccinated mice (Figure 12B). Prevention of inflammation in these mice was associated with significantly reduced numbers of CD4+ T cells in synovial infiltration (mean ± SD; 12 ± 3 vs. 37 ± 7, n = 10, P ≤ 0.05) and reduced production of IFN-γ, IL-4, and TNF-α (Figure 12, C and D), as determined by joint tissue immunohistochemistry staining. These results support the possibility of controlling arthritis by activation of mCII707–721–specific CD1d-restricted NKT cells.
Figure 12. Activation of mCII707–721–specific NKT cells suppresses arthritis.
(A) Prevaccination of B10.Q mice with mCII707–721 significantly reduced severity by CIA clinical scoring compared with control peptide (mCI707–721) vaccination (14 mice per mCI707–721 group, 19 mice per mCII707–721 group; *P ≤ 0.05). (B) Histological arthritic scores by H&E staining of joints. Data are mean ± SD, n = 5. **P ≤ 0.01. (C) Histological scores of percentage of IFN-γ–, IL-4–, and TNF-α–positive cells were significantly reduced in the mCII707–721–vaccinated group. Data are mean ± SD, n = 5. *P ≤ 0.05. (D) Suppression of arthritis was associated with reduced CD4+ T cell–infiltrating cells, IFN-γ–, and IL-4–producing cells by immunohistochemistry staining of joints. Red-brown color shows positively stained cells. Scale bar: 100 μm.
Vaccination with mCII707–721 ameliorates EAE.
The DTH and OVA sensitization experiments indicated that the suppressive quality of the mCII707–721–specific NKT cells was not antigen or tissue specific. Therefore, we evaluated the effect of vaccination with mCII707–721 on EAE, a well-described Th1-mediated autoimmune disease affecting the CNS and a widely used experimental model for MS. EAE was induced in B10.Q WT mice vaccinated with mCII707–721 by immunization with MOG79–96. EAE progression was significantly suppressed in mCII707–721–vaccinated mice, resulting in low mean clinical scores compared with the control group vaccinated with mCI707–721 (Figure 13A). Although no differences were seen between the 2 groups in disease incidence (both groups had 89% disease), affected mice in the mCII707–721–vaccinated group recovered entirely, while control group mice exhibited lingering symptoms up to 1 month after disease induction. In support, the CNS of mCII707–721–vaccinated mice showed significantly less demyelination than control mice (Figure 13, B and C), which correlated well with the clinical findings and the general inflammation observed in the CNS (data not shown).
Figure 13. Activation of mCII707–721–specific NKT cells suppresses EAE neurological deficits.
(A) Prevaccination of B10.Q mice with mCII707–721 significantly reduced EAE clinical scoring compared with control peptide (mCI707–721) vaccination. n = 9–10 mice per group. *P ≤ 0.05; **P ≤ 0.01. (B and C) Demyelination in CNS was significantly reduced in mCII707–721–vaccinated mice. (B) Representative sections of spinal cord from a mCII707–721–vaccinated mouse (left) and control (right). Arrows show demyelinated areas in white matter. Original magnification, ×25. (C) Demyelination was calculated as mm2 of demyelination per 100 mm2 spinal cord, n = 3 mice per group. Data are median ± SD. **P ≤ 0.01.
These data strongly support the capacity of mCII707–721–specific NKT cells to suppress a variety of inflammatory conditions, not limited to the Th1/Th2 paradigm nor restricted to collagen-tissue specificity.
Discussion
CD1d-restricted NKT cells exert profound and diverse regulatory effects in disease. They have deservedly earned the distinction of bridging the innate and adaptive immune systems, showing capacity for antiinflammation activity.
However, despite almost 2 decades of study, research on natural ligands of NKT cells has not resulted in identification of any self-peptide ligands that could activate NKT cells. Many experimental systems and clinical models have focused on exogenous glycolipids (such as αGalCer) presented by CD1d and highly potent in activation of NKT cells. Although this approach has been important to the characterization of NKT cells’ properties and functions, the biology of endogenous antigen–presentation by CD1d and activation of CD1d-dependent NKT cells remains largely unknown. The discovery of endogenous ligands, including self-peptide ligands that bind to CD1d, can shed light on the innate physiological functions of CD1d-dependent NKT cells. Our results support the idea that self-peptide reactivity could be considered part of the intrinsic immune function of NKT cells to maintain tolerance to self in cases of tissue inflammation.
We show here for what we believe is the first time a population of CD4+TCRαβ+ NKT cells with specificity for a self-tissue peptide that binds to CD1d. These CD1d-restricted mCII707–721–specific NKT cells constitute an important component of the peripheral tolerance maintenance mechanism in that they protect against tissue-specific autoimmunity. Although mCII707–721–specific NKT cells are part of the peripheral pool of immune cells, they require activation via CD1d–self-antigen–TCR signaling to become competent immune regulators. Significantly, they suppress a range of inflammatory and autoimmune conditions.
Although CD1d has a large hydrophobic groove more suitable for the binding of glycolipids, studies have reinforced the notion that peptides can bind (9). How the mCII707–721 structurally fits into this groove is not clear. Other studies have shown that a CD1d-restricted peptide can elicit a cellular immune response in vivo (17), leading the researchers to opine that intracellular murine CD1d might normally be associated with peptides and therefore peptide presentation may be a true physiologic function of murine CD1d (8).
Here, we determined that mCII707–721 does bind to CD1d and has the capacity to be presented to NKT cells. In support of this observation, CD1d presentation of mCII707–721 to NKT cells upregulated phosphorylated ZAP-70, a tyrosine kinase that is crucial for productive stimulation of T cells through TCR signaling after activation of TCR/CD3 (18). We found that mCII707–721–specific NKT cells required costimulatory signals via B7.1/CD28 and CD40/CD40L pathways, supporting earlier studies on NKT cell activation requirements (19). The limited usage of Vα and the Vβ chain diversity in the TCR of NKT cells, combined with a nonpolymorphic CD1d, suggest that the CD1d-restricted NKT cells may recognize a single or conserved set of antigens. Although some skewing toward Vα14-Jα18, Vβ8.2 TCR usage was observed in mCII707–721–reactive NKT cells, this response is far from being a clonal response. Instead, the mCII707–721 peptide activates a heterogeneous NKT cell population, as identified with a panel of Vβ TCR that showed it to be polyclonal and diverse. Nonetheless, variations in CD1d-restricted antigen presentation could arise from differences in expression, trafficking, processing, or loading of antigens (20).
We show that antigen-specific production of TGF-β1, IFN-γ, and IL-4 by CD1d-restricted mCII707–721–specific NKT cells resembles conventional T cells in the adaptive immune response. IL-17 production is associated with CD4+NK1.1+ invariant NKT cells (21) and is suggested to be pathogenic in several autoimmune diseases (22, 23). However, the pathogenic role of IL-17–producing Th17 cells has also received challenges (24), and the regulatory role for regulatory T cells has been shown to be independent of their influence on Th17 (25). Still, a potential influence for IL-17–producing NKT cells on the regulation of autoimmune diseases could be plausible. In the present study, we found CD1d-restricted mCII707–721–specific NKT cells produced IL-17A, but it was not involved in their suppressive function. In support, CD1d-dependent NKT cells’ regulation of arthritis is IL-17 independent (26).
Of note, the mCII707–721–specific NKT cells suppressed anti-CD3–activated T cells in a cell-cell contact–dependent manner. Cytokines including IL-4, IL-10, IL-13, and TGF-β1 have been suggested as important mediators in NKT cell suppressive functions (10, 27), so suppression mediated by a final release of cytokines upon cell interaction could be feasible. However, we detected no role for cytokine production by the mCII707–721–specific NKT cells in their in vitro–suppressive effects on T cells since neutralizing these cytokines did not prevent the suppressive effects. In our previous work, the regulatory role of CD1d-restricted NKT cells in EAE was associated with TGF-β1 production in the CNS (28). While mCII707–721–specific NKT cells produce substantial levels of TGF-β1 upon restimulation, the suppressive function of this cell population on T cell proliferation is independent of TGF-β1–TGF-β receptor interaction. This does not rule out the possibility that NKT cells could exert their regulatory functions in inflamed tissues via TGF-β1 secretion, since NKT cells may target cell types other than T cells. Interestingly, mCII707–721–specific NKT cells induced FasL-mediated apoptosis in activated T cells, similar to the killing of tumor cells by αGalCer-activated NKT cells (29). Consistent with other studies that show regulation of T cells by NKT cells through the use of Fas-FasL (30, 31), our data suggest that this could be one pathway by which physiologically relevant self-antigen–reactive NKT cells limit autoreactive T cells and prevent chronicity of tissue inflammation. We show that cytotoxicity of NKT cells against activated T cells was completely Fas-dependent in vitro and partially so in vivo. It is thus possible that when the Fas/FasL pathway is defective, other pathways could operate to mediate mCII707–721–specific NKT cell–mediated suppression. This possibility is supported by studies in a myelomonocytic leukemia cell line, in which human NKT cells were shown to utilize multiple cytotoxic mechanisms, including perforin/granzyme-B, TNF-α, FAS-L, and TRAIL in vitro (32).
With clear indications that the self mCII707–721–specific NKT cell population could suppress activated T cell responses and dampen inflammation, we investigated whether it could also regulate tissue-specific inflammation and autoimmunity in mice. Using a Th1-driven DTH assay and a Th2-driven antigen-induced airway inflammation, we found, unexpectedly, that mCII707–721–specific NKT cell activation reduced both the Th1-mediated DTH reaction and the Th2 cytokine response, and lowered IgE production. Thus, CD1d-restricted mCII707–721–specific NKT cells regulate both Th1-driven states and diminish humoral Th2 responses. Vaccination of mice with mCII707–721 prior to CIA induction significantly ameliorated arthritis, as evinced by lower clinical and histopathological scores. Significantly lower joint inflammation was accompanied by reduction of all tested cytokines but no alteration of the Th1/Th2 profile of cells infiltrating the joints. In accordance, recently we reported that general activation of CD1d-dependent NKT cells in WT mice prevents arthritis in different models of joint inflammation without induction of a cytokine shift, while CD1d-deficient mice lacking this population are defective in suppressing joint inflammation (26).
Given that NKT cells can either suppress or activate immunity, their therapeutic use necessitates a thorough understanding of their intrinsic biology. Limitations in this regard have been revealed by αGalCer or its synthetic analogs, which despite some success in early clinical trials have on the whole been less promising (33–35). This could perhaps be attributed to the fact that these substances are not found in self tissues and hence fail to replicate the natural course of NKT cell activation in disease. mCII707–721–specific NKT cells may be able to overcome these obstacles and achieve fuller potential as treatments for autoimmune diseases and cancer.
A natural question emerging from the currently reported results regards the tissue specificity of the autoimmune suppressive function of activated mCII707–721–specific NKT cells. Therefore, we investigated its effect on inflammation of the CNS via an EAE model. Mice vaccinated with mCII707–721 prior to induction of EAE showed significantly reduced clinical symptoms and demyelination compared with the control group. We surmise that even if the population of NKT cells is self-collagen specific, their suppressive function is not necessarily restricted to arthritis and cartilage tissue but can operate in other tissue-specific autoimmune and inflammatory diseases.
The question of how the mCII707–721–specific NKT cells regulate autoimmunity in multiple models, specifically with regard to their effects on autoreactive T cells, is of interest. This ability is consistent with properties of conventional regulatory T cells (36) as well as antigen-specific regulatory T cells generated in inflamed tissues such as those of the CNS (37). Antigen-nonspecific responses of NKT cells, or bystander suppression, could involve different mechanisms. One of the widely discussed ideas is the ability of NKT cells, even in a non–self-antigen–specific manner (e.g., αGalCer or its analogs), to regulate multiple autoimmune diseases via their capacity to produce an early burst of cytokines and deviation of the Th1/Th2 axis (reviewed in ref. 38). However, the suppressive function of mCII707–721–specific NKT cells differs since we detected a cell-cell–mediated mechanism. This is consistent with an earlier report (39) in which it was shown that NKT cells inhibited the differentiation of naive autoreactive T cells into effector cells. Here we have shown that the suppressive function of mCII707–721–specific NKT cells is to a great extent via the engagement of the Fas/FasL pathway. This supports a model whereby activation of autoreactive NKT cells could inhibit further effector T cell activation via a direct cytolytic effect.
Alternatively, the suppressive function of the NKT cells may also be mediated by differential engagement of or effects on tissue-specific APCs such as macrophages (40), mature/immature DCs (41), and/or B-1 B cells (42). The ability of NKT cells to modulate the function of APCs, as discussed in a recent review (6), may therefore play an important role in shaping the outcome of the immune response. Hence, differential engagement of APCs in different tissues, as the consequence of mCII707–721–specific NKT cell activation, may also serve a broad immune regulatory function.
The results of the present study support the contention that self-antigenic peptide-reactive NKT cells could be an important part of the peripheral tolerance maintenance mechanisms. Their physiological role may be to maintain tissue homeostasis through induction of cell-cell–mediated cytotoxic action on activated lymphocytes and to dampen inflammation regardless of the Th profile of its source. This is in contrast to the rapid release of cytokines traditionally assigned to the mode of action of NKT cells. Given that the joint is prone to wear and tear, the presence of this peptide-specific NKT cell population in the periphery could be part of the native physiology that might partly explain the relative resistance to mouse type II collagen–induced arthritis that is observed. As a general phenomenon across a range of disease states, such cells could act rapidly toward any danger signal evoked by pathogens or inflammatory conditions. The effect would be to prevent chronicity of the inflammation and hence sustain immune balance and tissue homeostasis. Given the high degree of conservation of NKT cells among species, therapeutic immunization with an NKT-activating peptide may hold promise for clinical benefit in human inflammatory diseases.
Methods
Mice.
Animals were kept and bred at the animal facilities of the University of Lund or the University of Copenhagen. All animal experiments were reviewed and approved by both of these universities’ institutional review boards, located in Lund and Copenhagen, respectively. All studies with mCII707–721 were performed on WT male C57BL/10.H-2q (B10.Q) mice (originated from Jan Klein, University of Tubingen, Tubingen, Germany). KO mice were backcrossed to this background to produce well-controlled genetic strains to avoid strain-specific effects that might influence the results.
Tcrab–/– or Tcrgd–/– mice were the results of targeted germ-line mutations on 129 and backcrossed to C57BL/6J (The Jackson Laboratory). These were backcrossed to the B10.Q background for 6 generations. Subsequent intercrossing of mice heterozygous or homozygous for mutated TCR yielded homozygous (αβ or γδ T cell deficient) and heterozygous (normal T cell phenotype) littermates. T cell phenotypes were determined by flow cytometry analysis of blood cells using mAbs against either TCRαβ (H57-597-FITC; Pharmingen) or TCRγδ (GL3-PE; Pharmingen).
CD4- and CD8-deficient founders backcrossed to C57BL/6J mice (43) were backcrossed to B10.Q for 10 generations, and positive offspring were intercrossed twice to yield littermates homozygous for the WT gene, homozygous for the disrupted gene, or heterozygous, as determined by flow cytometry with anti-CD4 (Gk1.5-PE; BD) and anti-CD8 (53-6.7-biotin: BD Biosciences — Pharmingen) mAbs.
MHC II–deficient and TAP-deficient mice (originally received from Diana Mathis, Institute of Genetics and Molecular and Cellular Biology, University of Strasbourg, Strasbourg, France) were backcrossed to C57BL/6J for 20 generations and then backcrossed to B10.Q for 3 generations. The offspring deficient in targeted genes were intercrossed once to yield homozygous KO or WT, and heterozygous littermates. lpr mice of H-2q haplotype were received from Saleh Ibrahim (Department of Immunology, Rostock University).
CD1d–/– mice (44) on a C57BL/6J background (over 20-generation backcross) were backcrossed to B10.Q for more than 5 generations. Animals were sex and age matched (8–20 weeks) for all experiments.
Media and reagents.
All cells were grown in cDMEM, DMEM with Glutamax-1 (GIBCO BRL, Life Technologies) supplemented with 10 mM HEPES buffer, 10% heat-inactivated FCS, 0.16 μM/ml penicillin, 0.03 μM/ml streptomycin, and 50 μM 2-mercaptoethanol. Growth was in a humidified incubator at 37°C with 7.5% CO2. All reagents were from Sigma-Aldrich, unless otherwise noted.
All peptides were from Schäfer. Mouse collagen type II peptide mCII707–721 (PPGANGNPGPAGPPG) and negative control mouse collagen type I peptide mCI707–721 (PPGPSGNAGPPGPPG) differ at positions 710 (proline to alanine), 711 (serine to asparagine), 714 (alanine to proline), and 717 (proline to alanine). Biotinylated peptides were used in binding assays.
Proliferation assays, mCII707–721–specific, and myelin-specific T cell lines.
B10.Q male mice, aged 8–14 weeks, were immunized in the hind paws and tail base with 200 μl of a 1:1 emulsion of 100 μg of either mCII707–721 or mCI707–721 in PBS and CFA containing Mycobacterium tuberculosis H37Ra (Difco). For some experiments, B10.Q and a panel of KO mice on a B10.Q background were used. Draining LNs or spleens were collected on days 10–13 after immunization, and a single-cell suspension was prepared in PBS by passing through a sieve. Cells were washed and resuspended in cDMEM.
To measure T cell proliferation in response to mCII707–721, cells were cultured in quadruplicate in round-bottom 96-well plates (Nunc) at 5 × 105 cells/well and stimulated with 100 μg/ml mCII707–721 or mCI707–721 and/or media only or PPD for 4 days in the presence of 1 μCi 3H-thymidine per well in the final 12 hours of culture. 3H-thymidine incorporation was measured in a beta scintillation counter (Matrix 96 Direct Beta Counter; Packard). mCII707–721–specific and myelin-specific T cell lines were established as previously published (37).
Blocking antibodies were anti-CD40L (20 μg/ml); anti–CTLA-4 (5 μg/ml); anti-B7.1 Fab-fragment (20 μg/ml); anti-B7.2 Fab-fragment (20 μg/ml); CTLA-4-lg fusion protein (10 μg/ml); and isotype controls (10 μg/ml).
CD1d-binding assay.
Maxisorp plates (Nunc) were coated with recombinant soluble dimeric mouse CD1d-lg dimer (BD Biosciences — Pharmingen) at 4 μg/well. Biotin-labeled mCII707–721 (0.6 μg/well) was added and incubated at 37°C for 2 hours. After washing, plates were incubated with europium-avidin followed by enhancement buffer according to the manufacturer’s instructions and fluorescence intensity measured on a fluorometer (Wallac Oy EG & G). Biotin-labeled anti-CD1d was a positive control, and biotin-labeled mCI707–721 was the negative control. Nonlabeled mCII707–721 in excess or varying amounts of αGalCer was used to assess competitive binding.
Ratio of binding for mCII707–721 to CD1d was calculated by dividing the fluorometer OD value by the OD value for the positive control, for different mCII707–721 concentrations.
FACS.
Standard FACS procedures and analysis were followed (45). Antibodies were from BD Biosciences — Pharmingen unless stated otherwise and used at 1–5 μg/ml, and were PE-Cy5–anti-CD4 (GK1.5), FITC–anti-TCR-β (H57-597), mouse Vβ TCR screening panel (cat. no. 557004), PE–anti–IL-4 (11B11), PE–anti–IFN-γ (XMG1.2), biotin–anti–TGF-β1 (A75-3.1), streptavidin-PE (cat. no. 554061), APC–anti–TNF-α (MP6-XT22), PE–anti–IL-17A (TC11-18H10), PE–anti-CD49b (DX5), FITC– and PE–anti-NK1.1 (Pk136).
Real-time PCR.
To detect TCR Vα14-Jα18 expression, total RNA was extracted from the NKT cell line using a QIAGEN kit, reverse transcribed, and amplified and quantified by SYBR Green detection. Primers were Vα14-Jα18 primer, forward: 5′-TGGGAGATACTCAGCAACTCTGG-3′; reverse: 5′-CAGGTATGACAATCAGCTGAGTCC-3′; and constant region housekeeping forward: 5′-CCTCTGCCTGTTCACCGACTT-3′; reverse: 5′-TGGCGTTGGTCTCTTTGAAG-3′. PCR was 10 minutes at 95°C, 40 cycles of 95°C for 10 seconds, decreasing by 20°C/s to 60°C, and 60°C for 60 seconds. The melting curve was 20°C/s to 95°C, cooling at 20°C/s to 60°C, and heating at 0.2°C/s to 95°C with fluorescence collection at 0.2°C intervals.
ELISA.
ELISAs were performed according to the manufacturers’ instructions. ELISA kits were used for IL-13 and IgE (BD Biosciences) and IL-4, IL-5, IFN-γ, TNF-α, and TGF-β1 (R&D Systems).
Purification of NKT cells.
Single-cell suspensions were washed and resuspended in 2% FCS in PBS with APC-CD4 and PE-NK1.1 for 20 minutes at 4°C. Cells were washed with 2% FCS in PBS and analyzed or purified using FACSAria. CD4+ T cells and CD4+NK1.1+ NKT cells were purified with FACSAria.
In vitro suppressive assays.
Splenocytes from B10.Q mice or a conventional B10.Q MOG79–96–specific T cell line were activated by culturing on plate-bound anti-CD3 at 5 μg/ml and used as responder cells by coculturing at a 1:1 ratio with mCII707–721–specific NKT cells. Proliferation was measured by 3H-thymidine incorporation. When splenocytes were responder cells, the B10.Q MOG79–96–specific cells were used as controls. Blocking antibodies were added to the NKT cells for 30 minutes before coculture.
A Transwell insert of 0.4 μm (Falcon) was used for separation of mCII707–721–specific NKT and responder cells. Soluble molecules, but not cells, could pass through the insert.
For annexin V staining, cells were incubated in binding buffer with 5 μl of annexin V–PE at room temperature for 5 minutes in the dark and washed. Positive cells were monitored by FACS. Some experiments used 7AAD instead of annexin V.
Vaccination with mCII707–721, OVA challenge, induction of DTH, CIA, and EAE.
Mice were immunized in the flank with 100 μg mCII707–721 emulsified 1:1 in CFA 10 days prior to disease induction. Control mice were similarly immunized with mCI707–721 and/or CFA (vehicle).
DTH response.
B10.Q mice and CD1d KO mice received an intradermal injection at the tail base with 100 μg of CII emulsified in CFA (Difco). At 13 days after immunization, mice received an injection of 20 μg CII in 0.05 M acetic acid in the outer right ear and just acetic acid in the outer left ear as a negative control. Additional control groups vaccinated with mCI707–721 and/or CFA (vehicle) were included when specified. After 48 hours, the DTH response was measured using a KrSplin caliper and calculated as the difference in swelling (thickness) of the right and left outer ears. Histopathological evaluation of inflammation in the outer ears was also performed.
OVA sensitization and BALF.
B10.Q and CD1d KO mice received an intraperitoneal injection on days 0 and 4 with 50 μg OVA with 5 mg alum. At day 13, mice were challenged with 50 μg of OVA intranasally. Mice were sacrificed 24 hours later and BALF collected for ELISA.
Induction and evaluation of CIA.
Male B10.Q mice, 8–12 weeks of age, were immunized intradermally at the tail base with 100 μg rat collagen emulsified in CFA (Difco) and boosted at day 35 with 50 μg rat CII emulsified in incomplete Freund adjuvant (Difco). Clinical scoring was performed as described (46). For histopathological and immunohistochemical analyses, ankle joints were removed on day 31 after collagen immunization (47). For immunohistochemistry, cryosections were stained with biotinylated antibodies to IFN-γ (Ani8), IL-4 (BVD6-24G2), TNF-α (MP6-XT3), and CD4 (L3T4). ExtrAvidin-peroxidase and diaminobenzidine (50 mg/ml; Saveen Biotech) were used for detection, with background hematoxylin staining. For cytokines, 0.1% saponin was used at all steps after fixation. Stained infiltrating cells were counted in the synovium by randomly selecting 6 areas per section and calculating the mean of positive cells per animal.
Induction and evaluation of EAE.
B10.Q mice were immunized for induction of EAE, as described (37).
For demyelination, the lumbar part of spinal cords from 3–4 mice per group was collected at day 40 after MOG immunization and fixed in 4% formaldehyde and then as described (48). Results are the mean from 4 sections.
Statistics.
Statistical evaluation was performed using StatView software. The Mann-Whitney test was used to analyze differences in clinical scores; otherwise a Student’s unpaired 2-tailed t test was used.
Acknowledgments
This work has been supported by grants from the Danish Multiple Sclerosis Society; a generous grant from the Lundbeck Foundation, Denmark; the Danish Research Council — Medicine; the Swedish Rheumatism Association; Börje Dahlins Fond; the Swedish Research Council — Medicine; the Swedish Research Council — Natural Sciences; the Swedish Foundation for Strategic Research (SSF); the Crafoordska Foundation; the G-J Kocks Foundation; the Åke Wiberg Foundation; the Alfred ϖsterlunds Foundation; the Tore Nilson Foundation; the Konung Gustaf V:s 80 Årsfond; the M. Bergvalls Foundation; the Swedish Association of Neurologically Disabled; Trygg-Hansa; the Bibi and Nils Jensen Foundation; and the EU-supported project Masterswitch (HEALTH-F2-2008-223404). This publication reflects only the authors’ views. The European Community is not liable for any use that may be made of the information herein.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(1):249–264. doi:10.1172/JCI43964.
References
- 1.Issazadeh S, Kjellen P, Olsson T, Mustafa M, Holmdahl R. Major histocompatibility complex-controlled protective influences on experimental autoimmune encephalomyelitis are peptide specific. Eur J Immunol. 1997;27(6):1584–1587. doi: 10.1002/eji.1830270640. [DOI] [PubMed] [Google Scholar]
- 2.Brunsberg U, et al. Expression of a transgenic class II Ab gene confers susceptibility to collagen-induced arthritis. Eur J Immunol. 1994;24(7):1698–1702. doi: 10.1002/eji.1830240736. [DOI] [PubMed] [Google Scholar]
- 3.Holmdahl R, Jansson L, Larsson E, Rubin K, Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986;29(1):106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- 4.Bayrak S, et al. T cell response of I-Aq mice to self type II collagen: meshing of the binding motif of the I-Aq molecule with repetitive sequences results in autoreactivity to multiple epitopes. Int Immunol. 1997;9(11):1687–1699. doi: 10.1093/intimm/9.11.1687. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 6.Gapin L. iNKT cell autoreactivity: what is ‘self’ and how is it recognized? Nat Rev Immunol. 2010;10(4):272–277. doi: 10.1038/nri2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castano AR, et al. Peptide binding and presentation by mouse CD1. Science. 1995;269(5221):223–226. doi: 10.1126/science.7542403. [DOI] [PubMed] [Google Scholar]
- 8.Tangri S, Brossay L, Burdin N, Lee DJ, Corr M, Kronenberg M. Presentation of peptide antigens by mouse CD1 requires endosomal localization and protein antigen processing. Proc Natl Acad Sci U S A. 1998;95(24):14314–14319. doi: 10.1073/pnas.95.24.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Z, Castaño AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 1997;277(5324):339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114(10):1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Vliet HJ, et al. The immunoregulatory role of CD1d-restricted natural killer T cells in disease. Clin Immunol. 2004;112(1):8–23. doi: 10.1016/j.clim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Cardell S. The natural killer T lymphocyte: a player in the complex regulation of autoimmune diabetes in non-obese diabetic mice. Clin Exp Immunol. 2005;143(2):194–202. doi: 10.1111/j.1365-2249.2005.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Kaer L. alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5(1):31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 14.Mallevaey T, et al. T cell receptor CDR2 beta and CDR3 beta loops collaborate functionally to shape the iNKT cell repertoire. Immunity. 2009;31(1):60–71. doi: 10.1016/j.immuni.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata S. Fas-induced apoptosis, and diseases caused by its abnormality. Genes Cells. 1996;1(10):873–879. doi: 10.1046/j.1365-2443.1996.d01-214.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee KA, et al. A distinct subset of natural killer T cells produces IL-17, contributing to airway infiltration of neutrophils but not to airway hyperreactivity. Cell Immunol. 2008;251(1):50–55. doi: 10.1016/j.cellimm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Lee DJ, Abeyratne A, Carson DA, Corr M. Induction of an antigen-specific, CD1-restricted cytotoxic T lymphocyte response In vivo. J Exp Med. 1998;187(3):433–438. doi: 10.1084/jem.187.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan AC, et al. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14(11):2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uldrich AP, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175(5):3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brossay L, Tangri S, Bix M, Cardell S, Locksley R, Kronenberg M. Mouse CD1-autoreactive T cells have diverse patterns of reactivity to CD1+ targets. J Immunol. 1998;160(8):3681–3688. [PubMed] [Google Scholar]
- 21.Coquet JM, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105(32):11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenders MI, van den Berg WB. Translational mini-review series on Th17 cells: are T helper 17 cells really pathogenic in autoimmunity? Clin Exp Immunol. 2010;159(2):131–136. doi: 10.1111/j.1365-2249.2009.04039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fouser LA, Wright JF, Dunussi-Joannopoulos K, Collins M. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol Rev. 2008;226:87–102. doi: 10.1111/j.1600-065X.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 24.Haak S, et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119(1):61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Teige I, Ericsson I, Navikas V, Issazadeh-Navikas S. Suppression of EAE by oral tolerance is independent of endogenous IFN-beta whereas treatment with recombinant IFN-beta ameliorates EAE. Immunol Cell Biol. 2010;88(4):468–476. doi: 10.1038/icb.2009.111. [DOI] [PubMed] [Google Scholar]
- 26.Teige A, Bockermann R, Hasan M, Olofsson KE, Liu Y, Issazadeh-Navikas S. CD1d-dependent NKT cells play a protective role in acute and chronic arthritis models by ameliorating antigen-specific Th1 responses. J Immunol. 2010;185(1):345–356. doi: 10.4049/jimmunol.0901693. [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Seishima M. Regulation of the induction and function of cytotoxic T lymphocytes by natural killer T cell. J Biomed Biotechnol. 2010;2010:641757. doi: 10.1155/2010/641757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teige A, et al. CD1-dependent regulation of chronic central nervous system inflammation in experimental autoimmune encephalomyelitis. J Immunol. 2004;172(1):186–194. doi: 10.4049/jimmunol.172.1.186. [DOI] [PubMed] [Google Scholar]
- 29.Kawano T, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A. 1998;95(10):5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arase N, Arase H, Good RA, Onoe K. Contribution of host radioresistant T cells to the clonal elimination of minor lymphocyte stimulatory-1a reactive T cells in mouse bone marrow chimeras. Cell Immunol. 1994;156(1):13–23. doi: 10.1006/cimm.1994.1149. [DOI] [PubMed] [Google Scholar]
- 31.Moroda T, et al. Autologous killing by a population of intermediate T-cell receptor cells and its NK1.1+ and NK1.1- subsets, using Fas ligand/Fas molecules. Immunology. 1997;91(2):219–226. doi: 10.1046/j.1365-2567.1997.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17(6):1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Gabriel CL, Parekh VV, Van Kaer L. Invariant natural killer T cells: innate-like T cells with potent immunomodulatory activities. Tissue Antigens. 2009;73(6):535–545. doi: 10.1111/j.1399-0039.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- 34.Haeryfar SM. Invariant natural killer T cells in immune surveillance and tumor immunotherapy: perspectives and potentials. Arch Iran Med. 2008;11(2):186–195. [PubMed] [Google Scholar]
- 35.Van Kaer L. Natural killer T cells as targets for immunotherapy of autoimmune diseases. Immunol Cell Biol. 2004;82(3):315–322. doi: 10.1111/j.0818-9641.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 36.Taams LS, Akbar AN. Peripheral generation and function of CD4+CD25+ regulatory T cells. Curr Top Microbiol Immunol. 2005;293:115–131. doi: 10.1007/3-540-27702-1_6. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12(5):518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 38.Van Kaer L. Regulation of immune responses by CD1d-restricted natural killer T cells. Immunol Res. 2004;30(2):139–153. doi: 10.1385/IR:30:2:139. [DOI] [PubMed] [Google Scholar]
- 39.Novak J, Beaudoin L, Griseri T, Lehuen A. Inhibition of T cell differentiation into effectors by NKT cells requires cell contacts. J Immunol. 2005;174(4):1954–1961. doi: 10.4049/jimmunol.174.4.1954. [DOI] [PubMed] [Google Scholar]
- 40.Hegde S, Chen X, Keaton JM, Reddington F, Besra GS, Gumperz JE. NKT cells direct monocytes into a DC differentiation pathway. J Leukoc Biol. 2007;81(5):1224–1235. doi: 10.1189/jlb.1206718. [DOI] [PubMed] [Google Scholar]
- 41.Chen YG, et al. Activated NKT cells inhibit autoimmune diabetes through tolerogenic recruitment of dendritic cells to pancreatic lymph nodes. J Immunol. 2005;174(3):1196–1204. doi: 10.4049/jimmunol.174.3.1196. [DOI] [PubMed] [Google Scholar]
- 42.Campos RA, et al. Cutaneous immunization rapidly activates liver invariant Valpha14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198(12):1785–1796. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mak TW, Rahemtulla A, Schilham M, Koh DR, Fung-Leung WP. Generation of mutant mice lacking surface expression of CD4 or CD8 by gene targeting. J Autoimmun. 1992;5 suppl A:55–59. doi: 10.1016/0896-8411(92)90019-m. [DOI] [PubMed] [Google Scholar]
- 44.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275(5302):977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 45.Teige A, Bockermann R, Hasan M, Olofsson KE, Liu Y, Issazadeh-Navikas S. CD1d-dependent NKT cells play a protective role in acute and chronic arthritis models by ameliorating antigen-specific Th1 responses. J Immunol. 2010;185(1):345–356. doi: 10.4049/jimmunol.0901693. [DOI] [PubMed] [Google Scholar]
- 46.Holmdahl R. Genetics of susceptibility to chronic experimental encephalomyelitis and arthritis. Curr Opin Immunol. 1998;10(6):710–717. doi: 10.1016/S0952-7915(98)80093-9. [DOI] [PubMed] [Google Scholar]
- 47.Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of alpha-galactosylceramide. Arthritis Rheum. 2004;50(1):305–313. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 48.Teige I, et al. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol. 2003;170(9):4776–4784. doi: 10.4049/jimmunol.170.9.4776. [DOI] [PubMed] [Google Scholar]