Abstract
The pituitary-specific transcriptional factor-1 (PIT-1, also known as POU1F1), is an essential factor for multiple hormone-secreting cell types. A genetic defect in the PIT-1 gene results in congenital growth hormone (GH), prolactin (PRL), and thyroid-stimulating hormone (TSH) deficiency. Here, we investigated 3 cases of adult-onset combined GH, PRL, and TSH deficiencies and found that the endocrinological phenotype in each was linked to autoimmunity directed against the PIT-1 protein. We detected anti–PIT-1 antibody along with various autoantibodies in the patients’ sera. An ELISA-based screening revealed that this antibody was highly specific to the disease and absent in control subjects. Immunohistochemical analysis revealed that PIT-1–, GH-, PRL-, and TSH-positive cells were absent in the pituitary of patient 2, who also had a range of autoimmune endocrinopathies. These clinical manifestations were compatible with the definition of autoimmune polyendocrine syndrome (APS). However, the main manifestations of APS-I — hypoparathyroidism and Candida infection — were not observed and the pituitary abnormalities were obviously different from the hypophysitis associated with APS. These data suggest that these patients define a unique “anti–PIT-1 antibody syndrome,” related to APS.
Introduction
The pituitary-specific transcriptional factor-1 (PIT-1, also known as POU1F1), plays a pivotal role in regulating the expressions of growth hormone (GH), prolactin (PRL), and thyroid-stimulating hormone β (TSHβ). PIT-1 is essential for the differentiation, proliferation, and maintenance of somatotrophs, lactotrophs, and thyrotrophs in the pituitary (1, 2). Therefore, congenital abnormalities in the PIT-1 gene result in short stature and combined pituitary hormone deficiency (CPHD), characterized by GH, PRL, and TSH deficiencies (3). On the other hand, acquired CPHD is generally caused by various types of damage to the hypothalamic-pituitary region, resulting in impaired hormone secretion in a nonspecific pattern.
Autoimmune polyendocrine syndrome (APS) is defined by the occurrence of 2 or more autoimmune-based organopathies, including that of endocrine tissue, and is generally classified into 3 groups (4). APS-I is a rare disorder caused by defects in the autoimmune regulator (AIRE) gene (5, 6). The more common syndrome, APS-II, is less well defined and includes overlapping groups of disorders. It is strongly associated with polymorphic genes of the HLA. In immune dysfunction, polyendocrinopathy, and X-linked (IPEX) syndrome, the patients harbor mutations in the forkhead box P3 (Foxp3) gene that lead to severe autoimmunity and immune deficiency (7). APS patients produce various autoantibodies such as those against glutamic acid decarboxylase (GAD), insulin, and IA-2 in type 1 diabetes (8). In the case of autoimmune hypophysitis, several antigens including GH (9), α-enolase (10), and tudor domain containing protein-6 (TDRD6) (11) have been proposed as the candidates for the generation of antipituitary antibodies. However, their significance in the pathogenesis of hypophysitis remains unclear.
Here, we report the clinical manifestations of 3 adult patients with acquired CPHD exhibiting GH, PRL, and TSH deficiencies and circulating anti–PIT-1 antibodies.
Results
Three adult patients exhibited acquired GH, PRL, and TSH deficiencies
Case 1.
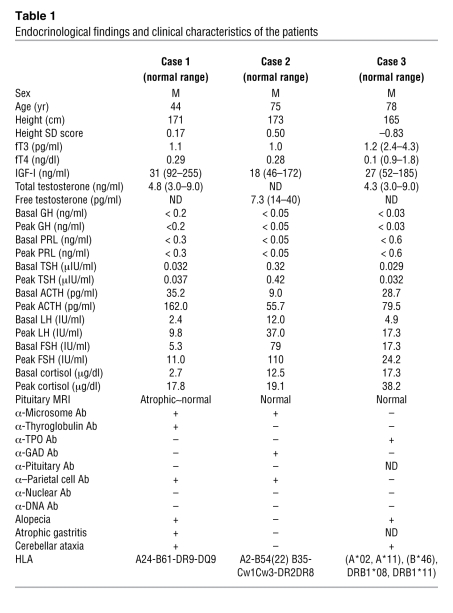
The proband was a 44-year-old man who experienced facial, finger, and arm edema for 2 years. His growth and pubertal development were within normal range, and he had 2 children. Endocrinological examinations revealed extremely low levels of serum TSH and free T4 (fT4), indicating secondary hypothyroidism (Table 1). The basal levels of serum GH and PRL were both undetectable. The GH response to insulin hypoglycemia was completely blunted. Furthermore, neither serum TSH nor PRL levels were increased after thyrotropin-releasing hormone (TRH) administration. The luteinizing hormone (LH) and follicle-stimulating hormone (FSH) responses to luteinizing hormone–releasing hormone (LHRH) were within normal range. While ACTH response to insulin hypoglycemia was exaggerated, the cortisol response was subnormal, suggesting subclinical primary adrenal insufficiency (Table 1). MRI demonstrated that the anterior pituitary was slightly atrophic (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI44073DS1). Atrophic gastritis was diagnosed by means of tissue biopsy. He manifested transient and mild cerebellar ataxia and taste disturbance.
Table 1 .
Endocrinological findings and clinical characteristics of the patients
Case 2.
A 75-year-old man was admitted to the Jikei University Hospital for glycemic control of diabetes mellitus (DM) (12). Because he was anti-GAD antibody–positive, he was diagnosed with slowly progressive IDDM (SPIDDM) and thereafter treated with insulin. Endocrinological examinations revealed low levels of serum TSH and fT4 (Table 1), indicating secondary hypothyroidism. The basal levels of serum GH and PRL were undetectable. The GH response to GHRH and of serum PRL and TSH to TRH were all blunted, respectively. The basal levels of LH and FSH and their responses to LHRH were elevated, and serum testosterone level was decreased, indicating primary hypogonadism (Table 1). MRI of the pituitary demonstrated no abnormalities (Supplemental Figure 1B). Unfortunately, the patient died in an accident. The autopsy was performed with written informed consent from his family.
Case 3.
A 78-year-old man was admitted to the Kanto Rosai Hospital for an evaluation of eyelid and leg edema that had persisted for several years. Endocrinological examinations revealed low levels of serum TSH and fT4, indicating secondary hypothyroidism (Table 1). The basal levels of serum GH and PRL were undetectable. The GH response to the GHRH-plus-arginine test was completely blunted. The TSH and PRL responses to TRH administration were also blunted. The responses of ACTH and cortisol to insulin hypoglycemia were normal, and the LH and FSH responses to LHRH were considered normal according to the patient’s age (Table 1). MRI of the pituitary demonstrated no abnormalities (Supplemental Figure 1C). He manifested transient and mild cerebellar ataxia.
All 3 patients in this study showed common endocrinological characteristics of combined GH, PRL, and TSH deficiency (Table 1), which we suspected would be related to PIT-1. However, clinical features such as normal height and adult-onset symptoms were not compatible with those of congenital CPHD resulting from abnormalities in the PIT-1 gene. As expected, we failed to detect any mutations in the PIT-1, PROP1, and HesX1 genes, which are reportedly related to these hormone deficits, in these patients. We further investigated the possible presence of an inhibitor of PIT-1–expressing cells in the sera. However, the patients’ sera failed to affect cell proliferation and PIT-1 transcriptional activity in GH3 cells (data not shown).
Specific anti–PIT-1 antibody was detected in the sera of these patients
Next, we investigated whether autoantibodies against the pituitary gland were present in the patients’ sera. To this purpose, we screened the patients’ sera for the presence of antibodies against mouse tissue extracts. Several nonspecific protein bands were detected in the sera when this was used as a primary antibody at a dilution of 1:500 (Supplemental Figure 2, A–D). However, further dilution of the sera allowed specific detection of an antigen in the extracts of the mouse pituitary and of GH3 cells that was not detected when the control serum was used (Figure 1, A–D). The molecular weight of this protein was approximately 33 kDa (Figure 1, A–C). We also detected a band of the same molecular weight in the lysates from the human pituitary (Figure 1E). Because the molecular weight of PIT-1 is 33 kDa, we speculated that this antigen might be PIT-1. Indeed, the 33-kDa band was specifically detected in the lysates from the cells or tissues that express PIT-1 protein, such as the rat anterior pituitary (Figure 1F and Supplemental Figure 3A). The patient’s serum detected both Pit, Oct, and Unc (POU) and the transactivation (TA) domain of PIT-1 (Supplemental Figure 3B), suggesting that the epitopes are widely distributed over the PIT-1 molecule. The intensity of the band corresponding to PIT-1 was substantially diminished when the sera were preincubated with recombinant human PIT-1 protein, indicating the specificity of the antibodies to PIT-1 (Supplemental Figure 3C). Human anterior pituitary cells from normal subjects were detected by immunohistochemistry with the patients’ sera as primary antibody as well as with the anti–PIT-1 antibody (Figure 2A). We further found that the anti–PIT-1 antibody is of IgG isotype (Supplemental Figure 3D), specifically IgG1 and IgG3 (Figure 2B). To confirm the specificity of the antibody for this syndrome, we established an anti–PIT-1 antibody–specific ELISA. As shown in Figure 3 and Supplemental Figure 3E, we failed to detect anti–PIT-1 antibodies in the sera of control subjects or the patients with pituitary tumor, hypophysitis, type 1 diabetes, autoimmune thyroiditis, APS-II, or autoimmune diseases such as SLE and RA (Figure 3).
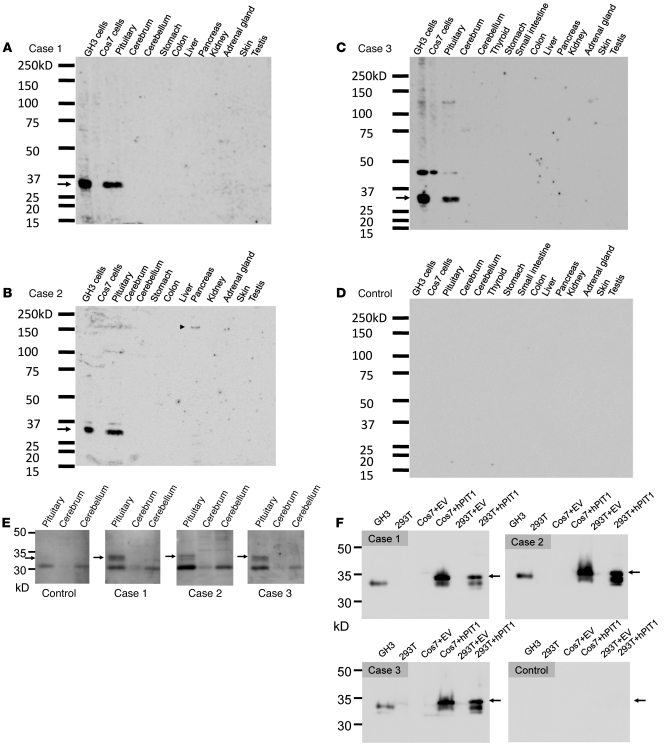
Figure 1. Immunoblotting analysis of mouse tissues and cell lysates.
The patients’ serum was used as a primary antibody (1:1000) to detect the autoantibody. (A–C) The patients’ sera specifically recognized a 33-kDa protein in the lysates from the pituitary and GH3 cells (arrow). (B) The serum of patient 2 recognizes a 150-kDa protein in the pancreas (arrowhead), but the size of this protein does not correspond to that of insulin (58 kDa) or GAD (65 kDa). (C) The serum of patient 3 recognized approximately 45 kDa protein as a nonspecific band in addition to the 33-kDa protein PIT-1. (D) Representative results of the healthy control subjects are shown. Neither the sera of 10 healthy control subjects nor those of 8 patients with pituitary adenoma and 6 patients with hypophysitis recognized the 33-kDa protein. (E) The patients’ sera detected the 33-kDa protein in the lysate from human pituitary. (F) The patients’ sera detected the PIT-1 protein in the lysates from GH3, hPIT-1–expressing Cos7, and 293T cells (arrows).
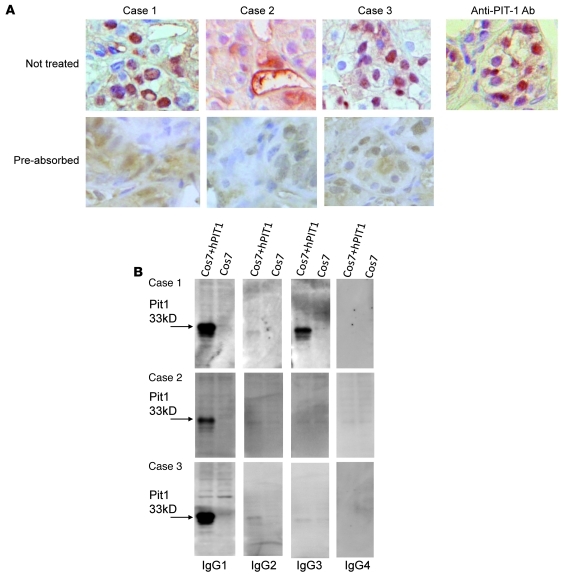
Figure 2. Patients’ sera detected natural form of PIT-1 by immunohistochemistry, and the antibodies were of IgG1 and IgG3 subtypes.
(A) Immunohistochemical analysis revealed that patients’ sera, as well as anti–PIT-1 antibody, recognize human anterior pituitary cells in the normal pituitary tissue. Original magnification, ×600. Preabsorption of the sera with rhPIT-1 substantially reduced the signal, indicating the specificity for PIT-1. (B) The anti–PIT-1 antibody belongs to the IgG1 and IgG3 fractions in case 1 and to the IgG1 fraction in cases 2 and 3 (B and Supplemental Figure 3E).
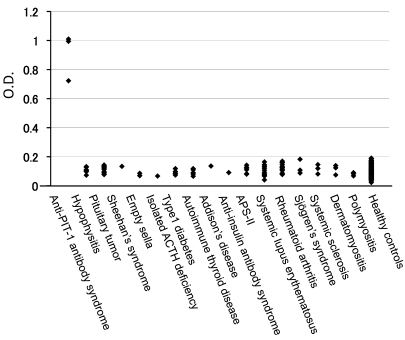
Figure 3. An anti–PIT-1 antibody–specific ELISA demonstrated that the anti–PIT-1 antibody is highly specific to these patients.
This antibody was not detected in control subjects or patients with other endocrine and autoimmune diseases, including APS-II.
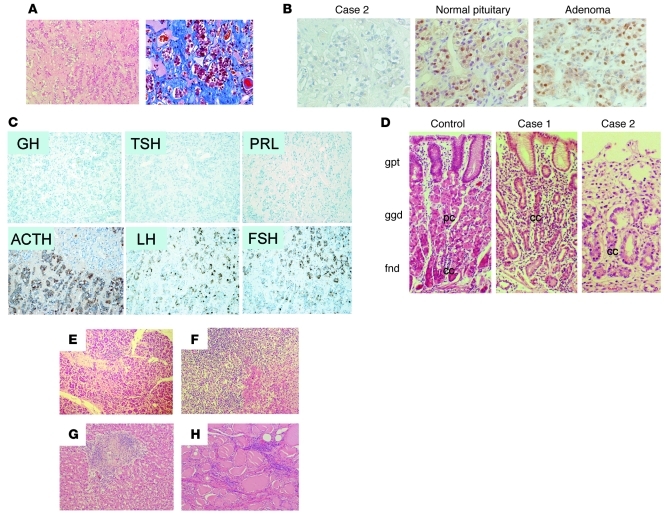
PIT-1–, GH-, PRL-, and TSH-positive cells were absent in the pituitary
We performed histological and immunohistochemical analyses of the autopsy specimens from patient 2. Histological analysis of the pituitary demonstrated that the number of adenohypophyseal cells was markedly reduced and that fibrosis was accompanied by moderate stromal infiltration of lymphocytes and plasma cells (Figure 4A). Immunohistochemical analysis revealed that the number of PIT-1– (Figure 4B), GH-, PRL-, and TSH-positive cells was markedly reduced despite the obvious presence of ACTH-, LH-, and FSH-positive cells (Figure 4C). In the stomach, atrophy of the villi, inflammatory infiltrate, and fewer parietal cells were observed (Figure 4D). In the pancreas, the exocrine tissues were atrophic and the number of islets was markedly reduced with lymphocytic infiltrations (Figure 4E and Supplemental Figure 4, A and B). In the adrenal glands, liver, and thyroid, disruption of the tissue structure and marked infiltration of lymphocytes and plasma cells were observed (Figure 4, F–H).
Figure 4. Histological analysis revealed the defect in PIT-1–positive cells and multiple endocrine organopathy.
(A) Microscopic analysis of H&E-stained sections of the pituitary tissue from patient 2. The number of adenohypophyseal cells is markedly reduced. The reduction is accompanied by infiltration of lymphocytes and plasma cells (left panel) and mild fibrosis in the stromal tissue (right panel: Azan staining). (B) Immunohistochemical analysis using an anti–PIT-1 antibody. In contrast to the normal pituitary or pituitary adenoma, no PIT-1–positive cells are observed in the pituitary in the case of patient 2. (C) Immunohistochemistry of the pituitary in the case of patient 2. GH-, PRL-, and TSH-positive cells are absent, despite the presence of ACTH-, LH-, and FSH-positive cells. (D) Histological analysis of the gastric mucosa from control and patients 1 and 2. In contrast to the control, no parietal cells were detected in patients 1 and 2. gpt, gastric pit; ggd, gastric glands; fnd, fundic glands; pc, parietal cells; cc, chief cells. Histological analysis of tissue of the pancreas (E), adrenal gland (F), liver (G), and thyroid (H) from patient 2 revealed inflammatory infiltration and disrupted tissue structure. Original magnification, ×100 (A, C); ×400 (B); ×200 (D); ×100 (E–H).
Discussion
Here, we report 3 adult patients with CPHD characterized by specific GH, PRL, and TSH deficiencies and circulating anti–PIT-1 antibodies. The normal growth and development of the patients strongly suggest that the condition was acquired because congenital GH or TSH deficiency results in severe growth retardation and developmental disabilities unless treated. Many different mutations of the PIT-1 gene have been found in families with complete GH and PRL deficiencies and variable defects in TSH secretion (2, 13, 14), in which the profile of hormonal impairment was similar to that of these patients. In animal models, both Snell (dw/dw) and Jackson (dw/dwJ) dwarf mice show GH, PRL, and TSH deficiencies caused by abnormalities in the Pit-1 gene (15). Histological analysis of these mice revealed total absence of somatotrophs, lactotrophs, and thyrotrophs (16), similar to what was observed in case 2. Taken together, these results strongly suggest the presence of acquired functional and structural impairment of PIT-1–expressing cells in our patients. We also suggest that the anti–PIT-1 antibody might be closely related to the occurrence of this acquired CPHD, not only as a marker, but also as a causal factor.
We found that anti–PIT-1 antibodies were present in these patients’ sera but not in the sera of control subjects or patients with various other diseases, indicating that the anti–PIT-1 antibody is highly specific to the disease. We could not detect any autoantibodies previously reported in common hypophysitis, such as anti-GH, anti–α-enolase, or anti-TDRD6 antibodies, by immunoblotting (Figure 1, A–D). Further, we did not detect any obvious pituitary abnormalities by MRI, such as an enlargement that is frequently observed in hypophysitis, indicating the difference from common hypophysitis. These findings indicate that the clinical and pathophysiological features of our patients are distinct from those of common hypophysitis observed in APS.
Histological analysis of tissues from patient 2 revealed various autoimmune endocrinopathies including insulitis, thyroiditis, and adrenalitis. The endocrinological findings in case 1 suggest the presence of subclinical primary adrenal insufficiency. In case 2, primary hypogonadism was observed and autoimmune adrenalitis was confirmed histologically. Autoimmune gastritis with disappearance of parietal cells was observed in cases 1 and 2. Various autoantibodies, such as those against microsomes, thyroglobulins, thyroid peroxidase (TPO), GAD, and parietal cells were detected (Table 1). Alopecia and cerebellar ataxia were also observed in these patients (Table 1). These results indicate that this syndrome is compatible with the definition of APS (8). In APS-I, mucocutaneous candidiasis and hypoparathyroidism are often observed early in development (17). In this aspect, our patients exhibited neither Candida infection nor abnormalities in the levels of serum calcium and PTH (data not shown), indicating that their conditions were obviously distinct from APS-I. APS-II has been reportedly associated with specific HLA haplotypes. The HLA haplotype of our patients was not the typical haplotype associated with APS-II (8). Rather, A24 and DR9 were detected in case 1 and B54 in case 2. Notably, DR4, DR9 (18), A24, and B54 (19) have been found to be associated with type 1 diabetes in Japanese populations. However, we could not find common HLA haplotypes among these patients.
Mechanistically, it is unlikely that PIT-1 protein is a direct target for the anti–PIT-1 antibodies because PIT-1 is a nuclear protein. One possibility is that immune intolerance to PIT-1 occurred by a still unknown reason, thereby provoking the attack of PIT-1–expressing cells by cytotoxic T cells through recognition of PIT-1 epitopes exposed with MHC (HLA) antigen on the cell surface. As a result, anti–PIT-1 antibodies would be produced. In terms of abnormal T cell function, lymphocytic infiltration of the pituitary, thyroid, adrenal gland, liver, and pancreas was observed. Similar mechanisms are speculated to explain the pathogenesis of type 1 diabetes (20). We have attempted to detect PIT-1–reactive T cells using an ELISPOT assay. Unfortunately, we have not been able to detect these cells so far (our unpublished observations), and this might be due to a lack of copresentation with the specific MHC antigen. Alternatively, it is interesting to note that the anti–PIT-1 antibody is of the IgG1 and IgG3 isotypes, which generally possess antigen-dependent cell-mediated cellular toxicity (ADCC). It is speculated that the mechanisms of ADCC may be related to the etiology.
In conclusion, this is the first report, to our knowledge, of adult CPHD characterized by specific GH, PRL, and TSH deficiencies and circulating anti–PIT-1 antibodies. Although further analysis is required to elucidate the mechanisms, we propose that this is a novel APS-related syndrome and have named it “anti–PIT-1 antibody syndrome.”
Methods
Patients.
The patients provided written, informed consent for the genetic, functional, and immunological analysis. All human and animal experiments were approved by the ethics committee of the Kobe University Graduate School of Medicine.
Hormonal assays.
An immunofluorometric assay was used to measure GH, PRL, TSH, ACTH, cortisol, fT3, and fT4
DNA sequencing and analysis.
Genomic DNA was prepared from blood leukocytes with a commercially available kit (Amersham). PCR was used to generate the products spanning exons and flanking intronic sequences of the PIT-1, PROP1, and HesX1 genes. DNA sequences were analyzed by direct sequencing.
Cell culture and cell proliferation assay.
Cos7 cells and 293T cells were obtained from Riken. AtT20 cells and GH3 cells were obtained from ATCC and maintained as previously described (1). MtT/s cells were a gift from K. Inoue (Saitama University, Saitama, Japan). These cells were cultured in DMEM containing 10% fetal calf serum. The sera of the patients or control subjects were added to the culture media at the concentrations of 1%, 5%, and 10%, and the cell proliferation was evaluated by using the MTS assay (Promega Japan) after 3 days. For PIT-1 overexpression, TOPO pcDNA3.1 containing the human PIT-1 gene was transfected into Cos7 or 293T cells by using FuGENE (Roche).
PIT-1 gene reporter assay.
The PIT-1 gene reporter assay was performed as previously described (21). Briefly, a reporter gene containing 7 copies of the PIT-1–binding sites from the rat PRL 5′ flanking region in a pGL3 basic vector (Promega) and CMV–hPIT-1 were transfected into COS7 cells. An empty vector and a vector containing the CRE-luc reporter gene were used as controls. After transfection with Fugene (Roche), the patients’ or control sera were added at concentrations of 1%, 5%, and 10% and the luciferase assay was performed (Promega).
Immunoblotting analysis.
Immunoblotting analysis was performed as previously described (22). The tissues were obtained from 8- to 12-week-old C57BL/6 mice or 8-week-old SD rats. Human pituitary was obtained from Biochain. Protein (20 μg) was loaded per lane. For the immunoblotting analysis, patients’ or control sera were used as primary antibody at dilutions of 1:200, 1:500, 1:1000, and 1:2000. HRP-conjugated goat anti-human IgG+A+M (H+L) (Zymed) was used as secondary antibody. The results with 1:500 are shown in Supplemental Figure 2, A–D. The IgG fraction was purified from serum by using the Melon Gel IgG Spin Purification Kit (Pierce). HRP-conjugated mouse anti-human IgG1-4–specific antibodies (Zymed) were used to detect IgG subtypes. For the PIT-1 absorption test, prior to use for immunoblotting, each serum sample was incubated on a 96-well plate coated with or without 100 μg/ml of rhPIT-1 in PBS overnight at 4°C. Immunoblotting was then performed as described above.
Immunohistochemistry.
The tissues were fixed in 10% buffered formaldehyde, dehydrated in graded ethanol, and embedded in paraffin. Microsections were stained with H&E. For immunohistochemical analysis, 4 μm paraffin-embedded tissue sections of autopsy tissues from patient 2 and samples from control and pituitary adenoma were used. The labeled streptavidin biotin (LSAB) method (DAKO) was used for detection. The following antibodies were used: anti–PIT-1 (1:100; sc-442; Santa Cruz Biotechnology Inc.), anti-GH (1:800; Dakopatts), anti-ACTH (1:1600; Dakopatts), anti-PRL (1:1000; Immunotech), anti–β subunit of LH (1:1000; Immunotech), anti-FSH (1:1000; Immunotech), anti-TSH (1:100; Chemicon), anti-CD20 (1:250; clone L26; DAKO), anti-CD3 (1:30; clone PS1; Novocastra), anti-CD4 (1:20, clone 1F6; Novocastra), and anti-CD8 (1:50, clone C8/144B; DAKO). For detection of PIT-1, the antigen was retrieved by autoclaving the sections in citric acid buffer.
Patients whose samples were used for ELISA.
The diagnoses of pituitary tumors and hypophysitis were confirmed by pathological analysis using tissue samples obtained during surgery. APS-II was diagnosed according to the criteria described previously (8). The diagnoses and detailed characteristics of each patient are described in Supplemental Table 1.
ELISA for quantification of anti–PIT-1 antibody.
Ninety-six–well microtiter plates (Nunc-Immuno Plate; Nalge Nunc International) were coated with 10 μg/ml of recombinant human PIT-1 protein (Santa Cruz Biotechnology Inc.). Serum was diluted 1:100 with PBS, added to the plate, and incubated for 1 hour. The binding of anti–PIT-1 antibody was detected by using HRP-conjugated goat anti-human IgG+A+M (H+L) and the ELISA POD substrate kit (Nakarai tesk).
Supplementary Material
Acknowledgments
We thank C. Ogata and K. Imura for their excellent technical assistance and H. Iwakabe for support. This work was supported in part by Grants-in-Aid for Scientific Research (B) (nos. 18390276, 20390262) for Scientific Research on Innovative Areas (no. 20200079) from the Ministry of Education, Culture, Sports, Science, and Technology; Grants-in-Aid for Scientific Research (Research on Hypothalamo-hypophyseal Disorders) from the Ministry of Health, Labor and Welfare; the Foundation for Growth Science; and the Takeda Science Foundation.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(1):113–119. doi:10.1172/JCI44073.
References
- 1.Ingraham HA, et al. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- 2.Cohen LE, Wondisford FE, Radovick S. Role of Pit-1 in the gene expression of growth hormone, prolactin, and thyrotropin. Endocrinol Metab Clin North Am. 1996;25(3):523–540. doi: 10.1016/S0889-8529(05)70339-X. [DOI] [PubMed] [Google Scholar]
- 3.Pfaffle RW, et al. Mutation of the POU-specific domain of Pit-1 and hypopituitarism without pituitary hypoplasia. Science. 1992;257(5073):1118–1121. doi: 10.1126/science.257.5073.1118. [DOI] [PubMed] [Google Scholar]
- 4.Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat Rev Endocrinol. 2010;6(5):270–277. doi: 10.1038/nrendo.2010.40. [DOI] [PubMed] [Google Scholar]
- 5.Finnish-German APECED Consortium. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17(4):399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 6.Nagamine K, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17(4):393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 8.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med. 2004;350(20):2068–2079. doi: 10.1056/NEJMra030158. [DOI] [PubMed] [Google Scholar]
- 9.Takao T, Nanamiya W, Matsumoto R, Asaba K, Okabayashi T, Hashimoto K. Antipituitary antibodies in patients with lymphocytic hypophysitis. Horm Res. 2001;55(6):288–292. doi: 10.1159/000050015. [DOI] [PubMed] [Google Scholar]
- 10.O’Dwyer DT, et al. Identification of the 49-kDa autoantigen associated with lymphocytic hypophysitis as alpha-enolase. J Clin Endocrinol Metab. 2002;87(2):752–757. doi: 10.1210/jc.87.2.752. [DOI] [PubMed] [Google Scholar]
- 11.Bensing S, et al. Pituitary autoantibodies in autoimmune polyendocrine syndrome type 1. Proc Natl Acad Sci U S A. 2007;104(3):949–954. doi: 10.1073/pnas.0610070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito T, et al. A case of acquired deficiency of pituitary GH, PRL and TSH, associated with type 1 diabetes mellitus. Endocr J. 2004;51(3):287–293. doi: 10.1507/endocrj.51.287. [DOI] [PubMed] [Google Scholar]
- 13.Pfaffle R, et al. Pit-1: clinical aspects. Horm Res. 1996;45 suppl 1:25–28. doi: 10.1159/000184824. [DOI] [PubMed] [Google Scholar]
- 14.Pfaffle RW, et al. GH and TSH deficiency. Exp Clin Endocrinol Diabetes. 1997;105 suppl 4:1–5. [PubMed] [Google Scholar]
- 15.Li S, Crenshaw EB 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 16.Roux M, Bartke A, Dumont F, Dubois MP. Immunohistological study of the anterior pituitary gland - pars distalis and pars intermedia - in dwarf mice. Cell Tissue Res. 1982;223(2):415–420. doi: 10.1007/BF01258498. [DOI] [PubMed] [Google Scholar]
- 17.Michels AW, Eisenbarth GS. Autoimmune polyendocrine syndrome type 1 (APS-1) as a model for understanding autoimmune polyendocrine syndrome type 2 (APS-2). J Intern Med. 2009;265(5):530–540. doi: 10.1111/j.1365-2796.2009.02091.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi K, et al. Association of HLA-A24 with complete beta-cell destruction in IDDM. Diabetes. 1993;42(7):1086–1093. doi: 10.2337/diabetes.42.7.1086. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, et al. Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care. 1993;16(5):780–788. doi: 10.2337/diacare.16.5.780. [DOI] [PubMed] [Google Scholar]
- 20.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10(7):501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 21.Okimura Y, Howard PW, Maurer RA. Pit-1 binding sites mediate transcriptional responses to cyclic adenosine 3′,5′-monophosphate through a mechanism that does not require inducible phosphorylation of Pit-1. Mol Endocrinol. 1994;8(11):1559–1565. doi: 10.1210/me.8.11.1559. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, et al. Biologically inactive growth hormone caused by an amino acid substitution. J Clin Invest. 1997;100(5):1159–1165. doi: 10.1172/JCI119627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.