Abstract
Immune responses to hepatitis C virus (HCV) fail to clear the virus in most individuals. Why patients who are less likely to clear HCV infection have high plasma levels of CXCL10 (also known as IP-10), a chemokine that directs T cells to sites of infection, has long been unclear. In this issue of the JCI, Casrouge and colleagues shed light on this paradox by showing that CXCL10 in the plasma of many HCV patients is enzymatically processed to produce a CXCL10 receptor antagonist. These findings introduce a role for chemokine antagonism during HCV infection and unveil new avenues for improved HCV diagnosis and therapy.
Over 120 million persons worldwide have chronic HCV infection (1), which is a major cause of liver failure and hepatocellular carcinoma (2). Up to one-quarter of persons who are acutely infected with HCV spontaneously clear their infection, and the current standard of care — pegylated IFN-α (peg–IFN-α) and ribavirin — eliminates virus in only about half of those treated (3). This means that a substantial number of patients remain chronically infected with HCV. In these chronically infected individuals, HCV-specific T cells are ineffective at eradicating virus, yet are potent mediators of hepatocellular injury. Evidence presented in this issue of the JCI by Casrouge et al. (4) suggests that chemokine antagonism may contribute to this inability to clear HCV infection. Their data (4) also provide an explanation as to why high levels of the chemokine CXCL10 in the plasma or serum of an HCV-infected patient portend a poor response to peg–IFN-α and ribavirin (5–8).
Salient features of chemokines
Chemokines have a central role in inflammation and host defense. These small (8–17 kDa) cytokine-like molecules act to guide leukocytes along a concentration gradient toward lymphoid organs and sites of inflammation. They also play roles in embryogenesis, angiogenesis, and lymphoid organ development. Chemokines involved in inflammation are displayed on proteoglycans near the site of their production. Chemokines bind to G protein–coupled, seven-transmembrane receptors, of which there are almost twenty. CXCR3, the CXCL10 receptor, is expressed on activated T cells, NK cells, and some B cells (9). In the hepatic sinusoid, leukocyte recognition of chemokines triggers conformational changes in the integrins that they express on their surface, which are then able to mediate binding to endothelial ligands. These steps permit leukocyte transmigration to target tissue (Figure 1) (reviewed in ref. 10).
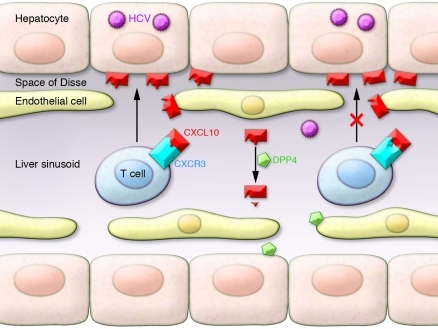
Figure 1. Model of chemokine antagonism in the HCV-infected liver.
CXCL10 produced in the infected liver recruits T cells from the blood to the infected hepatocyte via the liver sinusoid and the space of Disse (left). When processed by DPP4, CXCL10 becomes an antagonist of T cell recruitment (right). In this issue of the JCI, Casrouge and colleagues have shown that levels of this shortened antagonist form of CXCL10 are increased in many patients who fail to clear HCV (4), suggesting a role for chemokine antagonism in an ineffective anti-HCV response.
CXCL10 and liver disease
Among chemokines, CXCL10 plays a central role in liver inflammation, and it is expressed in the HCV-infected liver (11–13). Serum CXCL10 is also elevated during flares in HBV infection (14), in primary biliary cirrhosis, and in rheumatoid arthritis (15). In several independent studies, elevated serum/plasma levels of CXCL10 predict the failure of IFN-α–based HCV treatment (5–8).
Why a chemoattractant seemingly so potent as CXCL10 is elevated in patients who fail to clear HCV has been paradoxical. One possibility is that CXCL10 is overproduced in a futile attempt to draft pusillanimous T cells into the liver to combat infection. Indeed, chronic HCV infection is often associated with impaired function and reduced breadth of continuously activated, HCV-specific T cells (reviewed in ref. 16). However, in this issue of the JCI, data from Casrouge and colleagues suggest that CXCL10 may in fact be dissuading T cells from joining the fight (4).
Casrouge and colleagues performed a multianalyte profiling of patient plasma, confirming that CXCL10 levels are increased in patients that do not respond to anti-HCV therapy compared with those that do (4). They also observed that CXCL10 levels correlated with elevated numbers of circulating CXCR3+ cells. It had previously been proposed that the high levels of CXCL10 in patients who do not respond to anti-HCV therapy could act as an antagonist of T cell migration (5). Further, it has been reported that CXCL10 can be processed in vitro by dipeptidyl peptidase IV (DPP4; also known as CD26), which cleaves two amino acid residues from the amino terminus of CXCL10 and turns it into a CXCR3 antagonist (17), and that HCV patients have increased soluble DPP4 activity (18). However, distinguishing full-length from DPP4-processed CXCL10 in clinical samples has not been feasible until now.
After developing reagents to distinguish full-length from DPP4-processed CXCL10, Casrouge and colleagues found that, in many HCV-infected patients who do not respond to therapy, circulating CXCL10 is indeed processed into the shorter form (4). Importantly, patient blood had been collected in tubes containing a DPP4 inhibitor, as DPP4 remains active after blood collection. The authors demonstrated that before treatment, plasma DPP4 activity was higher in those individuals who went on to fail to respond to anti-HCV therapy than in patients who responded and healthy individuals. They also confirmed a previous report (17) showing that CXCL10 is cleaved by DPP4 in vitro. A DPP4 inhibitor, sitagliptin — which is used clinically in the treatment of type 2 diabetes — inhibited this cleavage. Using in vitro systems, Casrouge and colleagues showed that the full-length form of CXCL10, but not the short form, could direct the migration of CXCR3+ T cells (4). Short CXCL10 antagonized signaling by long CXCL10. Finally, they demonstrated that the short, antagonist form of CXCL10 predominates in the plasma of chronically infected patients who are destined to fail anti-HCV therapy; early virological responders were more likely than nonresponders to have undetectable amounts of short-form CXCL10. This latter finding will need to be confirmed in larger studies of patient cohorts carefully matched for liver function and inflammation. These results suggest that short-form CXCL10 in the plasma may antagonize T cell recruitment to the liver parenchyma (Figure 1).
Answers beget questions
The study by Casrouge et al. (4) raises new questions regarding HCV immunopathogenesis. Seemingly at odds with the study, plenty of T cells are present in the liver during HCV infection, even in those with elevated CXCL10 levels (19) and those with cirrhosis (13). It is unknown whether these T cells are all HCV-specific. One hypothesis to explain this discordance is that the short form of CXCL10 preferentially antagonizes T cells recognizing HCV peptides in an HLA-dependent context. Also, it is likely that chemotaxis of HCV-specific T cells to the liver depends upon the combinatorial effect of multiple chemokines with disparate roles and potencies. Of note, the importance of T cells in treatment-induced clearance of HCV remains controversial (16).
Importantly, the findings of Casrouge et al. (4) provide a rationale for the validation of short-form CXCL10 and DPP4 (which may be broad markers of inflammation) as clinically appropriate predictors of HCV clearance, potentially allowing for more individualized treatment decisions. It will also be useful to determine whether levels of DPP4 and short-form CXCL10 predict spontaneous resolution or chronic evolution of acute HCV infection. The successful validation of DPP4 and CXCL10 as predictors of spontaneous resolution could improve the early initiation of anti-HCV therapy in those who would most benefit, while sparing those who will clear HCV infection without treatment and those who will not respond to treatment from a therapy that has severe side effects and is therefore very poorly tolerated.
Hints about a link to diabetes?
Patients chronically infected with HCV are at increased risk of insulin resistance and frank type 2 diabetes (20, 21). Postprandial insulin secretion is controlled in part by the insulin secretagogue glucagon-like peptide–1 (GLP-1). The extremely short in vivo half-life of GLP-1 (1–2 minutes) is due to its inactivation by DPP4. The observation that DPP4 activity is increased in many HCV patients may provide clues about the pathogenesis of HCV-associated metabolic dysregulation. It is tempting to speculate that DPP4-mediated cleavage of GLP-1 may be partially responsible for HCV-related insulin resistance. Indeed, decreased levels of serum GLP-1 and increased levels of serum and liver DPP4 have been reported in HCV-infected patients relative to healthy volunteers (22). Intriguingly, levels of serum CXCL10 are also elevated in HCV patients with type 2 diabetes (23). Of course, much more work needs to be done to establish links between DPP4, CXCL10 levels, and type 2 diabetes. The next logical step is a comparison of levels of plasma DPP4 and GLP-1 in HCV-infected patients with and without impaired glucose tolerance or type 2 diabetes.
Acknowledgments
This work was supported in part by NIH grants R01AI60561 (to L.B. Dustin) and K08AI075301 (to E.D. Charles) and the Irma T. Hirschl/Monique Weill-Caullier Trust (to L.B. Dustin).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(1):25–27. doi:10.1172/JCI45610.
See the related article beginning on page 308.
References
- 1.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 2.Afdhal NH. The natural history of hepatitis C. Semin Liver Dis. 2004;24(suppl 2):3–8. doi: 10.1055/s-2004-832922. [DOI] [PubMed] [Google Scholar]
- 3.Heathcote EJ. Antiviral therapy: chronic hepatitis C. J Viral Hepat. 2007;14(suppl 1):82–88. doi: 10.1111/j.1365-2893.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 4.Casrouge A, et al. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J Clin Invest. 2011;121(1):308–317. doi: 10.1172/JCI40594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butera D, et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106(4):1175–1182. doi: 10.1182/blood-2005-01-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero AI, et al. Interferon (IFN)-γ-inducible protein 10: association with histological results, viral kinetics, and outcome during treatment with pegylated interferon-a-2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194(7):895–903. doi: 10.1086/507307. [DOI] [PubMed] [Google Scholar]
- 7.Lagging M, et al. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology. 2006;44(6):1617–1625. doi: 10.1002/hep.21407. [DOI] [PubMed] [Google Scholar]
- 8.Diago M, et al. Association of pretreatment serum interferon-γ inducible protein levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1-infected patients with chronic hepatitis C. Gut. 2006;55(3):374–379. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin S, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101(4):746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oo YH, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. J Autoimmun. 2010;34(1):45–54. doi: 10.1016/j.jaut.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Narumi S, et al. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol. 1997;158(11):5536–5544. [PubMed] [Google Scholar]
- 12.Harvey CE, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74(3):360–369. doi: 10.1189/jlb.0303093. [DOI] [PubMed] [Google Scholar]
- 13.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163(11):6236–6243. [PubMed] [Google Scholar]
- 14.Tan AT, et al. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol. 2010;52(3):330–339. doi: 10.1016/j.jhep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Nishioji K, et al. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin Exp Immunol. 2001;123(2):271–279. doi: 10.1046/j.1365-2249.2001.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119(7):1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proost P, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98(13):3554–3561. doi: 10.1182/blood.V98.13.3554. [DOI] [PubMed] [Google Scholar]
- 18.Andrieu T, et al. Similar increased serum dipeptidyl peptidase IV activity in chronic hepatitis C and other viral infections. J Clin Virol. 2003;27(1):59–68. doi: 10.1016/S1386-6532(02)00128-2. [DOI] [PubMed] [Google Scholar]
- 19.Zeremski M, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48(5):1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zekry A, McHutchison JG, Diehl AM. Insulin resistance and steatosis in hepatitis C virus infection. Gut. 2005;54(7):903–906. doi: 10.1136/gut.2004.059873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133(8):592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 22.Itou M, et al. Altered expression of glucagon-like peptide-1 and dipeptidyl peptidase IV in patients with HCV-related glucose intolerance. J Gastroenterol Hepatol. 2008;23(2):244–251. doi: 10.1111/j.1440-1746.2007.05183.x. [DOI] [PubMed] [Google Scholar]
- 23.Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab. 2009;5(1):26–34. doi: 10.1038/ncpendmet1027. [DOI] [PubMed] [Google Scholar]