Abstract
Vaccines remain one of the most cost-effective public health measures. Despite ongoing efforts, protective vaccines against cancer and many infectious diseases, including malaria, tuberculosis, and HIV/AIDS, are still not in hand. Most investigators believe that to succeed against these difficult targets, vaccines that generate potent T cell responses are needed. In this issue of the JCI, Salek-Ardakani et al. show how the relative virulence of a virus/vaccine vector affects the memory CD8+ T cells generated and how the response may be enhanced. The work has important implications for the development of future vaccines that aim to trigger CD8+ T cell responses.
Disease prevention is the key to public health. One of the most cost-effective ways to prevent disease is through vaccination, an approach that has enabled successful control of many infectious diseases that were once common, including measles, diphtheria, and pertussis (whooping cough). In addition, a protective vaccine was key to the global eradication of smallpox, and it is believed that vaccinations may soon relegate polio to a historical memory. However, many infectious diseases, including malaria, tuberculosis, and HIV/AIDS, have proven to be formidable foes; despite intensive research efforts, we still do not have efficacious protective vaccines. Most believe that to be successful, such vaccines — as well as vaccines that protect against cancer — will need to generate potent T cell responses.
Poxviruses are members of a large family of DNA viruses that share a long history with vaccination. Cowpox virus was the active agent in Jenner’s pioneering approaches to preventing smallpox in the 18th century and is closely related to vaccinia virus, which was used as the vaccine that helped eradicate smallpox in the latter half of the 20th century. Remarkably, vaccinia virus remains one of the leading vector candidates to be used in the development of vaccines against challenging infectious diseases and cancers, largely as a result of the promise seen in the earliest studies that showed its utility as an expression vector (1, 2) and its ability to prime T cell responses to expressed foreign antigens (3). However, the laboratory strains of vaccinia virus, and even the old conventional human smallpox vaccines, are likely not ideal vaccine vectors from a safety perspective, especially in current times, when greater numbers of immunocompromised people are likely to be vaccinated or inadvertently exposed; even in normal individuals, what is considered an acceptable risk/benefit ratio has shifted. This has led to the pursuit of more attenuated and replication-incompetent poxvirus-based vaccine vectors that have significantly improved safety profiles. A recent achievement that highlights the promise of poxvirus-based vaccines is the somewhat unexpected results of the Thai HIV vaccine trial (4). In that study, vaccination with a recombinant Canarypox virus vector expressing three HIV proteins along with boosts of HIV envelope proteins showed modest protection from HIV acquisition. While some worry about the reproducibility of the effect found, others are clearly encouraged by the results and are seeking to understand and improve upon the immune responses generated by the vaccination strategy. Since vaccine-induced sterilizing immunity (defined as antibody and innate immune responses that prevent actual infection) is a high bar to reach against the problematic organisms that cause malaria, tuberculosis, and HIV/AIDS, future vaccines will need to generate potent antibody and cytotoxic T cell responses.
In this issue of the JCI, Salek-Ardakani et al. show how the relative virulence of a virus/vaccine affects the memory CD8+ T cells that are generated and how one might manipulate costimulatory molecules to enhance the CD8+ T cell response (5). The work has important implications for development of future vaccines that aim to target the CD8+ T cell response and the stimulatory molecules that might be candidates to generate such responses.
T cell responses to poxvirus vaccines
Over the past two decades, there has been an increasing number of publications of human clinical studies with poxviruses as vaccine vectors against cancer and infectious agents, which indicates that basic poxvirus research has made a transition to clinical investigations. Clearly, vaccination with vaccinia virus generates long-lasting T cell responses to poxvirus antigens. For example, vaccinia virus–specific CD4+ and CD8+ T cells can be detected 6–8 decades after a single smallpox vaccination (6). In fact, the long-lived humoral and cellular immunity generated by the conventional smallpox vaccines create an additional challenge when using orthopoxviruses (i.e., poxviruses in the same genus as vaccinia virus) as vaccine vectors to immunize against other diseases: preexisting immunity to the vector dampens immune responses, especially when using a standard dose of replication-competent vaccinia virus–based vaccine (e.g., ref. 7). Because of the rare but significant complications that can occur with conventional smallpox vaccines, such as progressive vaccinia in immunocompromised hosts or eczema vaccinatum in some patients with common skin diseases, more attenuated poxviruses vectors have been developed. However, finding the perfect balance between safety and immunogenicity is challenging. For example, modified vaccinia Ankara (MVA), which is highly attenuated and does not generate infectious progeny in most mammalian cells, has been a lead candidate vector; however, human immune responses to foreign proteins encoded by MVA have been inconsistent. In small phase 1 human trials using an identical recombinant MVA encoding HIV proteins, one trial of 9 subjects revealed that approximately 90% developed CD8+ T cell responses after two intradermal vaccinations with 5 × 107 virus units (8), whereas another trial of 8 subjects reported that none of the subjects developed measurable T cell responses after two intradermal vaccinations with 1 × 108 virus units (9). While these studies used different assays to define vaccine-induced T cell responses, it is clear that improving vaccine-induced CD8+ T cell responses in humans is needed.
Enhancing T cell responses
Although it might seem obvious that a more virulent virus results in more viral replication, which leads to better immune responses than those induced with attenuated viruses, Salek-Ardakani et al. asked whether there was a specific molecular mechanism engaged by the former but not the latter that could be harnessed to boost immunity generated by attenuated (and thus presumably safer) virus vaccines (5). Using a panel of vaccinia viruses with various levels of virulence in mice, the authors provide interesting data on how the relative virulence of a virus/vaccine affects the memory CD8+ T cells generated. When mice were infected with one of the more virulent viruses (i.e., laboratory vaccinia virus strain Western Reserve), it replicated longer and resulted in a larger pool of initially primed CD8+ T cells, and subsequently a larger memory CD8+ T cell pool, than did a less virulent virus (e.g., human smallpox vaccine Lister). To show the importance of the CD8+ T cell responses, genetically manipulated mice that lacked the ability to generate humoral immunity and thus relied exclusively on the CD8+ T cell response were shown to be protected from lethal vaccinia virus challenge only when vaccinated with the more virulent vaccine. The memory CD8+ T cells from mice vaccinated with the virulent vaccine were also the only ones that conferred protection against lethal challenge when passively transferred to naive mice prior to challenge.
Based on their prior study, in which they found that OX40 (also known as CD134) stimulation was important in determining the magnitude of antivaccinia CD8+ T cell responses (10), Salek-Ardakani et al. focused on this and other costimulatory molecules to define a mechanism of the improved memory CD8+ T cell responses observed with more virulent vaccinia viruses. They examined the role of a group of costimulatory molecules present on CD8+ T cells: OX40, CD27, and CD28. Experimentally, the authors found that in Ox40–/– mice, the memory CD8+ T cell pool was greatly diminished only in animals infected with the most virulent vaccinia virus, whereas the memory pools generated after administration of attenuated vaccines remained relatively low and unchanged (5). Similar results were generated in Cd27–/– mice. For the panel of viruses with varying virulence, the Cd28–/– mice gave memory CD8+ T cell responses similar to those seen in infected wild-type mice (albeit with slightly different kinetics), which indicated that for the panel of vaccinia viruses tested, there was a CD28-dependent phase of CD8+ T cell priming followed by a CD28-independent phase. Furthermore, by blocking CD27 in Cd28–/– mice, the authors found that CD27 was needed for normal CD28-independent priming of CD8+ cells. Based on these results, they proposed the model illustrated in Figure 1. As additional support for the role of OX40 in the development of a robust memory CD8+ T cell response, they used an antibody to stimulate OX40 and showed that this enhanced CD8+ T cell responses in mice infected with more and less virulent vaccinia viruses. Furthermore, the virus with lower virulence (which previously did not protect against lethal challenge), when used together with OX40 costimulation, generated CD8+ T cell responses that protected mice from lethal vaccinia virus challenge.
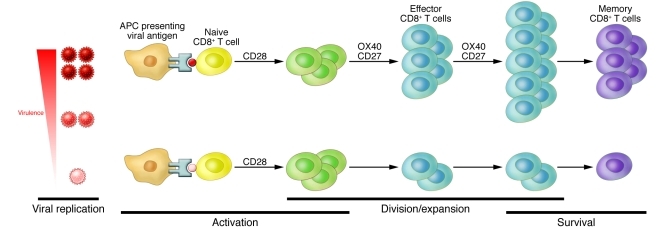
Figure 1. Viral virulence results in enhanced CD8+ T cell memory responses.
Compared with vaccination with a more attenuated virus (bottom), vaccination with a more virulent virus (top) causes stimulation of OX40 and CD27, which results in larger pools of effector and memory CD8+ T cells that can protect from subsequent lethal challenge.
Route of vaccination
Another recurrent issue observed by Salek-Ardakani et al. was that the route of vaccina virus vaccination had an impact on the quality of the immune responses generated (5). Their work described above focused on intraperitoneal vaccination. When the authors vaccinated mice by scarification with the less virulent vaccinia virus human smallpox vaccine strain Lister, they found that its replication, resulting CD8+ T cell responses (dependent upon OX40 and CD27, as well as CD28), and ability to protect genetically altered mice from lethal challenge in a CD8+ T cell–dependent fashion was similar to scarification with the more virulent strain of vaccinia virus. The fact that scarification leads to enhanced immune responses has been a recurring finding with vaccinia virus vaccines (11–13) and likely points to the special role the skin plays in initiating immune responses. Interestingly, the enhanced immune responses — especially T cell responses — are not just seen with replicating vaccinia virus in the skin, but also with replication-incompetent vaccinia virus vaccine vectors such as MVA. The use of the skin as a site for vaccine delivery, and its ability to generate improved immune responses, is being examined for other vaccine targets (e.g., ref. 14).
Questions and future challenges
While the work by Salek-Ardakani et al. (5) is intriguing and provides insights into a molecular mechanism of how vaccinia viruses with varying degrees of attenuation induce different levels of CD8+ T cell responses, and how one might use the information to enhance responses, numerous important questions remain. Will the molecular mechanism of enhanced CD8+ T cell responses found with vaccinia virus infections be the same for other vaccines and/or infectious agents? How translatable will the findings and interventions be from mice to humans? Can a similar intervention of OX40/CD28 stimulation enhance immune responses in subjects whose preexisting poxvirus immunity dampens vector replication and leads to poorer immune responses? Although there is published evidence that OX40 engagement results in better T cell responses generated by replication-incompetent poxvirus vaccines (15), is the magnitude of this response at a level that can protect from lethal challenge? If one approach to provide costimulatory activity is to express specific ligands from a replication-competent recombinant vaccinia virus vector, one must proceed judiciously. When investigators were interested in enhancing antibody responses during the early development of a poxvirus-based immunocontraceptive vaccine to be used in the wild to control rabbit populations in Australia, they first constructed an ectromelia (mousepox) virus that expressed IL-4 (16). Not only was the resulting virus more virulent in mouse strains that were usually resistant to ectromelia virus, but it also resulted in mortality in mice previously vaccinated with a normally protective vaccine.
The potential use of poxvirus-based vaccine vectors against infectious disease and cancer targets continues to show great promise. The work by Salek-Ardakani et al. (5) adds to an ever-expanding body of literature that provides a molecular understanding of adaptive immune responses to the virus as well as insights into how such knowledge can be used to enhance immune responses to poxvirus-based vaccines.
Acknowledgments
The author is supported in part by Public Health Service grants U01-AI077913, U01-AI066333, and U54-AI057168 (Middle Atlantic Regional Center of Excellence in Biodefense and Emerging Infectious Diseases) from the National Institute of Allergy and Infectious Disease.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(1):19–21. doi:10.1172/JCI45726
See the related article beginning on page 296.
References
- 1.Panicali D, Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackett M, Smith GL, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target for cross-reactive anti-influenza virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 5.Salek-Ardakani S, et al. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J Clin Invest. 2011;121(1):296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammarlund E, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 7.Cooney EL, et al. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991;337(8741):567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- 8.Mwau M, et al. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J Gen Virol. 2004;85(pt 4):911–919. doi: 10.1099/vir.0.19701-0. [DOI] [PubMed] [Google Scholar]
- 9.Goonetilleke N, et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol. 2006;80(10):4717–4728. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salek-Ardakani S, Moutaftsi M, Crotty S, Sette A, Croft M. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181(11):7969–7976. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClain DJ, et al. Immunologic responses to vaccinia vaccines administered by different parenteral routes. J Infect Dis. 1997;175(4):756–763. doi: 10.1086/513968. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16(2):224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilck MB, et al. Safety and immunogenicity of modified vaccinia Ankara (ACAM3000): effect of dose and route of administration. J Infect Dis. 2010;201(9):1361–1370. doi: 10.1086/651561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan SP, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Ngai N, Stone GW, Yue FY, Ostrowski MA. The adjuvancy of OX40 ligand (CD252) on an HIV-1 canarypox vaccine. Vaccine. 2009;27(37):5077–5084. doi: 10.1016/j.vaccine.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 16.Jackson RJ, Ramsay AJ, Christensen CD, Beaton S, Hall DF, Ramshaw IA. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J Virol. 2001;75(3):1205–1210. doi: 10.1128/JVI.75.3.1205-1210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]