Abstract
Leptin exerts a permissive action on puberty by stimulating release of gonadotropin-releasing hormone (GnRH) in the hypothalamus. However, GnRH neurons lack leptin receptor (LepR), indicating that leptin must indirectly regulate these neurons. The Kiss1 gene produces kisspeptins that stimulate GnRH secretion. Because Kiss1 neurons express LepR and inactivation of Kiss1 causes hypogonadotropic hypogonadism, Donato et al., in this issue of the JCI, assessed whether deletion of LepR from Kiss1 neurons would prevent sexual maturation. Unexpectedly, mice lacking LepR in Kiss1 neurons had normal pubertal development and fertility. In contrast, deletion of LepR from the ventral premammillary nucleus, a region of the brain involved in sexual behavior, prevented puberty and fertility. These findings highlight the complex biology of leptin in reproduction.
Neuronal and hormonal basis of puberty
Puberty marks the attainment of sexual maturation and is accompanied by changes in body size and behavior characteristic of the transition from childhood to adulthood (1). Changes in the secretion of gonadotropin-releasing hormone (GnRH) are fundamental to reproductive maturation. GnRH neurons develop from the lining of the nose and migrate into the brain, spreading to the diagonal band of Broca, septum, vascular organ of the lamina terminalis, and preoptic hypothalamic area. GnRH is released from terminals in the median eminence at the base of the hypothalamus into the pituitary portal circulation and stimulates the synthesis and secretion of the gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH), which act on the ovaries and testes to regulate the secretion of sex steroids and production of eggs and sperm (1, 2). Sex steroids influence GnRH secretion via a feedback loop (Figure 1). In most mammals, the hypothalamic-pituitary-gonadal (HPG) axis is activated shortly before birth or just after and is followed by a decline in the plasma concentrations of gonadotropins and sex steroids for weeks or years depending on the species (2). After this period of quiescence of the HPG axis, puberty begins with high frequency and amplitude of pulsatile GnRH secretion, which leads to increased gonadotropin and sex steroid levels and culminates in gonadal, somatic, and behavioral maturation (2).
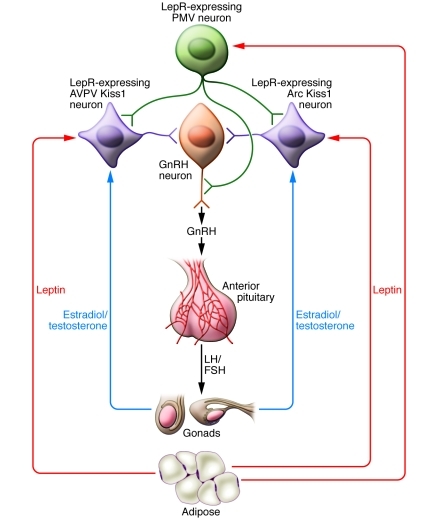
Figure 1. Schematic representation of Kiss1 and leptin signaling in mouse brain.
Kisspeptins are expressed by Kiss1 neurons in the anteroventral periventricular nucleus (AVPV) and arcuate nucleus (Arc), which innervate GnRH neurons. Sex steroids (estradiol and testosterone) exert feedback regulation on Kiss1 levels. At the onset of puberty, the sex steroids exert positive feedback regulation of Kiss1, increasing GnRH release into the pituitary portal circulation and thereby stimulating the secretion of gonadotropins and sex steroids as well as reproductive maturation. As indicated by the work of Donato et al. (12), although Kiss1 neurons express LepR, targeted deletion of Lepr in these neurons does not have an impact on puberty. Rather, leptin acts directly on the PMV and increases GnRH secretion through glutaminergic neurotransmission (12).
Although the factors that trigger the onset of puberty remain elusive, researchers have made tremendous progress in identifying permissive signals from peripheral organs and neuronal circuits that sense these signals (ref. 2 and Figure 1). Puberty can be disrupted by malnutrition, inflammation, hormonal imbalance, and other factors (2, 3). The discovery of leptin provided a plausible link between energy homeostasis and reproduction (4). Leptin is produced in adipose tissue, circulates in proportion to energy stores, and acts in a negative feedback manner in the hypothalamus and other regions of the brain to control feeding, energy expenditure, and neuroendocrine systems (4). Congenital leptin deficiency and loss-of-function mutations of leptin receptor (LepR) in patients and rodents result in a failure of pubertal maturation and infertility (4–6). In addition to inhibiting food intake and reducing body fat, leptin treatment restores puberty and fertility in congenital leptin deficiency (5, 7). Similarly, acquired leptin deficiency during fasting suppresses the HPG axis, and this can be reversed by leptin treatment (8, 9). Leptin also promotes puberty in normal animals (10, 11). These findings highlight the importance of leptin for energy homeostasis and reproduction. LepR has been located on anterior pituitary cells that produce LH and FSH, but evidence so far suggests that LepR is very sparse or lacking in GnRH neurons (4). Thus, the regulation of GnRH by leptin is likely to occur through other pathways in the brain. In this issue of the JCI, Donato et al. report on a series of elegant studies in mice to evaluate the role of Kiss1 as a mediator of the effects of leptin on puberty (12).
Leptin, Kiss1, and puberty
Various pathways in the brain have been proposed to mediate the activation of GnRH neurons during sexual maturation (1, 2). Depending on the species, neuropeptide Y (NPY) and γ-amino butyric acid (GABA) inhibit GnRH secretion, while glutamate stimulates it at the onset of puberty (13). LepR is located on neurons coexpressing proopiomelanocortin (POMC) and cocaine- and amphetamine-related transcript (CART) and NPY and agouti protein–related peptide (AGRP) in the arcuate nucleus; however, these neuropeptides do not seem to play major roles in the central action of leptin on the HPG axis (14, 15).
The Kiss1 gene was named by cancer biologists at Pennsylvania State University in Hershey, Pennsylvania, after Hershey’s Kisses chocolate (2). It encodes a 54–amino acid peptide (kisspeptin-54; also known as metastin) that is cleaved to shorter C-terminal kisspeptin-14, -13, and -10 peptides, which activate the receptor GPR54 (2). Kisspeptins and GPR54 are expressed in the placenta, pancreas, and brain. In the hypothalamus, Kiss1 is colocalized with LepR in the periventricular area and arcuate nucleus (2). Leptin-deficient ob/ob mice show reduced expression of Kiss1 in the hypothalamus, and this can be increased by leptin treatment (2). In wild-type mice, leptin increases levels of Kiss1, GnRH, gonadotropins, and sex steroids (2). Congenital or targeted inactivation of Kiss1 or Gpr54 results in failure of puberty and infertility (2). This genetic evidence, together with anatomical studies showing that Kiss1 neurons synapse on terminals of GnRH neurons and control the release of GnRH (16), suggests that kisspeptin-GPR54 signaling may mediate the permissive action of leptin on puberty and fertility.
To determine whether LepR signaling in Kiss1 neurons is critical for puberty, Donato et al. deleted Lepr from Kiss1 neurons in mice using Cre/loxP technology (12). Contrary to their prediction, the loss of leptin signaling in Kiss1 neurons had no impact on puberty, fertility, or litter size. Moreover, ablation of Kiss1 in the hypothalamus had no obvious effects on energy homeostasis, weight, or fat.
What’s the ventral premammillary nucleus got to do with it?
Donato and colleagues extended their quest for direct leptin targets to the ventral premammillary nucleus (PMV) (12), which mediates the effects of pheromones and various olfactory cues on sexual behavior (17). Previous studies by these researchers and others showed a high level of expression of LepR in the PMV (18, 19) and robust neuronal activation associated with reduced LH levels during fasting (18). The PMV provides innervation to GnRH neurons and hypothalamic neurons involved in energy homeostasis (19). Therefore, Donato and colleagues hypothesized that the PMV relays leptin signaling to the HPG axis during puberty (12). To address this possibility, they generated chemical lesions of PMV neuronal bodies while sparing the nerve tracts. In leptin-deficient female ob/ob mice, successful PMV neuronal body ablation did not affect food intake and weight, as compared with ob/ob mice with no PMV lesions. However, lesioning the PMV disrupted the ability of leptin to promote sexual maturation and increase LH levels (12). In further experiments, leptin signaling in the PMV was restored by microinjecting adeno-associated virus (AAV) expressing functional LepR in the PMV of db/db mice, which lack LepR. Reactivation of LepR in the PMV of female db/db mice did not affect food intake or weight, but it did improve sexual maturation and fertility. In contrast, there was no apparent improvement in sexual maturation and fertility when LepR was expressed in the PMV of male db/db mice (12).
Next, the role of glutamate as an excitatory signal from the PMV to GnRH neurons was studied (12). Using neuronal tract tracing, genetic mapping of LepR, and histological localization of GnRH neurons and vesicular glutamate transporter (vGluT2), Donato et al. showed that glutamate-positive neurons from the PMV innervate terminals of GnRH neurons in the base of the hypothalamus and median eminence and stimulate GnRH release. Major PMV projections were found to be present in areas of the hypothalamus where Kiss1 was expressed; however, reactivation of LepR in the hypothalamus of db/db mice did not affect the number of Kiss1 neurons or expression of Kiss1. Thus, leptin acting on PMV neurons restores sexual maturation in hypothalamic hypogonadism independent of Kiss1, without apparently altering energy balance.
Holy grail of puberty
The research of Donato and colleagues (12) illustrates the vexing issues facing researchers who study puberty (1, 2). Contrary to the notion that puberty is triggered by a single gene product or isolated metabolic cues, it is increasingly clear that multiple peripheral signals and sensing mechanisms in the brain control the onset and progression of puberty, fertility, and other reproductive parameters. Leptin, insulin, IGF, melatonin, and other hormones do not cause puberty (1–3). Instead, these function as “permissive signals” that interact with defined regions of the brain in a species-specific manner to allow or prevent the maturation of the HPG axis. The finding that ablation of Kiss1 did not affect sexual maturation or fertility does not diminish the potential role of Kiss1 in puberty. As with many genetic models, the phenotype may be modified by developmental compensation.
Donato and colleagues ought to be commended for carrying out a comprehensive series of experiments to illuminate the function of leptin in the PMV. In their previous studies in the rat, these investigators showed that neurons in the PMV express LepR and mediate the effects of low leptin levels on the HPG axis during fasting (18). The neuroanatomical and functional evidence in the current paper (12) support the notion that PMV neurons regulate the secretion of GnRH by releasing glutamate. Unlike neurons in the arcuate nucleus that respond to changes in leptin levels to regulate feeding and energy balance (4), the PMV has no apparent role in energy homeostasis. This result is consistent with previous studies showing that leptin is capable of restoring puberty and fertility independent of causing weight loss (7). Another area that deserves further study is why female, but not male, db/db mice respond to leptin signaling in the PMV. In this context, it is important to note that sex differences have been observed in the levels and activities of neuropeptides and other factors associated with reproduction (1–3). It is also noteworthy that most of the pregnancies resulting from LepR expression in the PMV of db/db mice ended in miscarriages, perhaps due to diabetes and other metabolic disorders not reversible by leptin in these mice (12).
The research of Donato and colleagues (12) extends our understanding of the complex circuitry linking leptin to energy homeostasis and reproduction. If confirmed in humans and other primates, these findings could potentially lead to novel diagnosis and therapies for disorders of puberty and fertility.
Acknowledgments
The Ahima laboratory is supported by funding from the NIH (RO1-DK062348 and PO1-DK049210).
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(1):34–36. doi:10.1172/JCI45813.
See the related article beginning on page 355.
References
- 1.Veldhuis JD, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26(1):114–146. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- 2.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martos–Moreno GA, Chowen JA, Argente J. Metabolic signals in human puberty: effects of over and undernutrition. Mol Cell Endocrinol. 2010;324(1–2):70–81. doi: 10.1016/j.mce.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21(3):263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 5.Farooqi IS, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. . N Engl J Med. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 6.Farooqi IS, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barash IA, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137(7):3144–3177. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 8.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 9.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111(9):1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138(2):855–858. doi: 10.1210/en.138.2.855. [DOI] [PubMed] [Google Scholar]
- 11.Chehab FF, Mounzih K, Lu R, Lim ME. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275(5296):88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- 12.Donato J, Jr, et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. . J Clin Invest. 2011;121(1):355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terasawa E, Luchansky LL, Kasuya E, Nyberg CL. An increase in glutamate release follows a decrease in gamma aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in the female rhesus monkeys. J Neuroendocrinol. 1999;11(4):275–282. doi: 10.1046/j.1365-2826.1999.00325.x. [DOI] [PubMed] [Google Scholar]
- 14.Hohmann JG, et al. Differential role of melanocortins in mediating leptin’s central effects on feeding and reproduction. Am J Physiol Regul Integr Comp Physiol. 2000;278(1):R50–R59. doi: 10.1152/ajpregu.2000.278.1.R50. [DOI] [PubMed] [Google Scholar]
- 15.Van de Wall E, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JT, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donato J, Jr, Cavalcante JC, Silva RJ, Teixeira AS, Bittencourt JC, Elias CF. Male and female odors induce Fos expression in chemically defined neuronal population. Physiol Behav. 2010;99(1):67–77. doi: 10.1016/j.physbeh.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Donato J, Jr, et al. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. . J Neurosci. 2009;29(16):5240–5250. doi: 10.1523/JNEUROSCI.0405-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leshan RL, Louis GW, Jo YH, Rhodes CJ, Münzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29(10):3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]