Abstract
Bone morphogenetic protein (BMP) signaling has been linked to the development of pulmonary hypertension (PH). Inhibitors of differentiation (ID) proteins (ID1–4) are a family of basic helix-loop-helix transcription factors that are downstream targets of the BMP signaling pathway, but the role that ID proteins play in the development of PH is unknown. To address this, we evaluated pulmonary expression of ID proteins in a mouse model of hypoxia-induced PH. There is selective induction of ID1 and ID3 expression in hypoxic pulmonary vascular smooth muscle cells (VSMCs) in vivo, and ID1 and ID3 expression are increased by hypoxia in cultured pulmonary VSMCs in a BMP-dependent fashion. ID4 protein is barely detectable in the mouse lung, and while ID2 is induced in hypoxic peripheral VSMCs in vivo, it is not increased by hypoxia or BMP signaling in cultured pulmonary VSMCs. In addition, the PH response to chronic hypoxia is indistinguishable between wild type and Id1 null mice. This is associated with a compensatory increase in ID3 but not ID2 expression in pulmonary VSMCs of Id1 null mice. These findings indicate that ID1 is dispensable for mounting a normal pulmonary vascular response to hypoxia, but suggest that ID3 may compensate for loss of ID1 expression in pulmonary VSMCs. Taken together, these findings indicate that ID1 and ID3 expression are regulated in a BMP-dependent fashion in hypoxic pulmonary VSMCs, and that ID1 and ID3 may play a cooperative role in regulating BMP-dependent VSMC responses to chronic hypoxia.
Keywords: hypoxia, bone morphogenetic protein signaling, ID1, ID2, ID3, vascular smooth muscle cells, endothelial cells
genetic studies in patients with hereditary pulmonary arterial hypertension (HPAH) indicate that defective bone morphogenetic protein (BMP) type 2 receptor (BMPR2) signaling plays a critical role in promoting pulmonary hypertension (PH) and obliterative pulmonary vascular remodeling in this disease (16, 25). Studies in mice carrying heterozygous null and hypomorphic germ line Bmpr2 mutations show that these mice do not develop spontaneous PH, but they have increased susceptibility to PH in response to inflammatory mediators and serotonin (22, 38, 39) or to chronic hypoxia (9). Conditional deletion of Bmpr2 in endothelial cells (ECs) promotes spontaneous PH in a subset of affected mice (11), indicating that defective BMPR2 signaling plays a role in regulating EC function. However, interference with BMPR2 signaling in vascular smooth muscle cells (VSMCs) by overexpression of a dominant negative BMPR2 mutation also promotes spontaneous PH in mice (45). Furthermore, conditional deletion of the BMP type 1 receptor ALK3 in VSMCs reduces hypoxic pulmonary vascular remodeling and VSMC proliferation (7). These findings indicate that defective BMPR2 signaling influences both the EC and VSMC compartments in the pulmonary vasculature. However, the relationship between defective BMP signaling and vascular cell phenotypes in HPAH is complex and poorly understood (23).
One approach to explore this has been to investigate the downstream signaling pathways that mediate the effects of BMP receptor mutations in different pulmonary vascular cell types. These studies also enable us to interrogate PH-associated alterations in BMP signaling that occur in the pulmonary vasculature in the absence of BMPR2 mutations. Activation of the BMP receptors leads to COOH-terminal phosphorylation of the BMP activated SMADs (SMAD1, 5, and 8), their nuclear translocation, and transactivation of target genes (5). One well-characterized transcriptional target of BMP-activated SMADs is the gene encoding the basic helix-loop-helix protein inhibitor of differentiation 1 (ID1) (27, 40). ID proteins, of which there are four known members in mammals (ID1–4), lack a DNA binding domain and act as dominant-negative regulators of transcription by heterodimerization with DNA binding basic helix-loop-helix proteins (12). All four ID family members are targets of BMP signaling in a variety of different cell types (14, 15, 20, 37). In situ hybridization studies show that ID1, ID2, and ID3 have widely overlapping expression domains in many tissues of the developing embryo (many of which overlap with the embryonic expression domains of BMP-2), while ID4 is exclusively expressed in the central and peripheral nervous system (13, 35, 36). ID2 expression is distinct from ID1 and ID3 in the developing lung, where it is largely expressed in epithelial cells. ID1 and ID3 are mainly expressed in the lung mesenchyme (which includes primitive blood vessels) (13). Genetic loss of Id1 and Id3 alone in mice does not appreciably affect embryonic development. However, Id1/Id3 double null mice die midgestation with widespread abnormalities in the cerebral vasculature (24). These defects are reminiscent of the more generalized vascular remodeling defects seen in adult mice with 90% RNA interference knockdown of Bmpr2 expression in the germ line (19). This suggests that ID1 and ID3 could play a cooperative role in mediating BMPR2-dependent responses in the developing vasculature. ID1 has also been shown to play an essential role in mediating BMP-dependent effects in cultured ECs (migration and tube formation) (43) and in cultured human pulmonary artery smooth muscle cells (PASMC, proliferation) (48, 49). In addition, BMP-dependent induction of ID1 and ID2 expression is reduced in cultured PASMCs from HPAH patients carrying germ line BMPR2 mutations (48), and restoration of ID1 expression in these cells rescues BMP-dependent anti-proliferative responses that were observed in these studies (49). This suggests that ID proteins could play a role in mediating BMPR2-dependent effects in the pulmonary vasculature. However, these in vitro findings could be misleading, given the complex nature of the BMP signaling pathway in the intact vasculature (23), and definitive analyses of pulmonary vascular responses in the absence of ID1 expression in vivo have not been performed. In addition, while previous studies have shown that ID1 is dominantly expressed in pulmonary VSMCs in the adult lung (3, 8, 49), recent studies raise concern about the lack of specificity of commercial anti-ID1 antibodies used in these and other studies (30). Moreover, while lineage tracing studies indicate that ID2-positive cells give rise to pulmonary ECs in the adult (34), there is no additional published data on the distribution of other ID family members in the adult lung vasculature.
In these studies, we have carefully evaluated the expression pattern of ID family members in the pulmonary vasculature and determined how these are regulated in mice with hypoxia-induced PH. We confirmed specific immunoblotting and immunohistochemical staining of two recently developed rabbit monoclonal anti-ID1 and -ID3 antibodies in wild-type, Id1 and Id3 null mouse lung tissues and used these antibodies to demonstrate that ID1 and ID3 proteins are dominantly expressed in ECs, but that there is selective induction of ID1 and ID3 expression in hypoxic pulmonary VSMCs, both in vivo and in vitro. In addition, we evaluated the effect of ID1 deficiency in Id1 null mice on pulmonary vascular responses to chronic hypoxia. These studies demonstrate that ID1 is dispensable for mounting a normal PH and pulmonary vascular remodeling response to hypoxia. However, this is associated with a compensatory increase in ID3 expression in pulmonary VSMCs of Id1 null mice, suggesting that ID1 and ID3 may play a cooperative role in regulating pulmonary VSMC responses to chronic hypoxia.
MATERIALS AND METHODS
Mouse lines.
Germ line Id1 null mutant mice (46) bred on a C57Bl/6J; 129 genetic background were a gift from Robert Benezra (Memorial Sloan-Kettering Cancer Center). Genotyping for the Id1 null allele was performed by PCR of ear punch DNA, as described previously (46). Germ line Id3 null mutant mice (29) on a C57Bl/6 background were a gift from Yuan Zhuang (Duke University Medical Center). Genotyping for the Id3 null allele was performed by PCR of ear punch DNA using the following primers: common forward (CATTCTCGGAAAAGCCAGTC) and either wild-type reverse (CTCCCTCGCTCTTCTCTCCT) for a wild-type amplicon of 300 bp, or mutant reverse (GCCAGAGGCCACTTGTGTAG) for a mutant amplicon of 238 bp.
Experimental PH.
Nine- or eleven-week-old Id1 mutant mice and wild-type littermate controls were exposed to normoxia or 10% normobaric oxygen for 1 or 3 wk, as described (9). At completion, 12-wk-old mice were anesthetized with 375 mg/kg Avertin (Sigma) and ventilated by tracheotomy, and the chest was opened. Right ventricular (RV) systolic pressure (RVSP) and diastolic pressures (RVDP), and left ventricular (LV) diastolic pressure (LVDP) were measured by direct cardiac puncture using a pressure gauge needle. Pressure wave forms were visualized and measured in millimeters of mercury using a Digi-Med blood pressure analyzer with Gould printer (Supplemental Fig. S1; the online version of this article contains supplemental data). Mean RVSP was calculated from ≥15 s of stable measurement with heart rate >300 beats/min. RV and LV + septal (RV/LV+S) dry weights were determined to evaluate RV hypertrophy. Hematocrit was measured using a Drummond Scientific microhematocrit centrifuge, according to the manufacturer's instructions. All experimental methods were approved by Vanderbilt University's Institutional Animal Care and Use Committee.
Histological evaluation of the pulmonary vasculature.
After hemodynamic analysis, lungs were inflated and fixed in 10% formalin in PBS overnight before mounting in paraffin. While blinded to genotype and treatment condition, we assessed the percentage of muscularized peripheral vessels (Supplemental Fig. S2) and vascular cell proliferation in the pulmonary vasculature by two-color immunofluorescence staining for α-smooth muscle actin (α-SMA) and von Willebrand factor (vWF) or PCNA, respectively, as described (8). Vascular cell proliferation was assessed after 1 wk of hypoxia, because significant hypoxia-induced proliferation is not observed at the 3-wk time point. Alveolar density (number of alveoli/mm2) and peripheral vessel density (number of peripheral vessels/100 alveoli) were scored from 10 ×200 fields while blinded to genotype, as described (2).
Immunoblots.
Immunoblots were performed on lysates from whole lung, intrapulmonary artery (IPA) preparations and cell lysates, as indicated. IPAs were excised from freshly isolated left lung and separated from surrounding tissues under a dissecting microscope to evaluate protein expression in the pulmonary vasculature. These preparations contain continuous vessels corresponding to the first branch artery at the hilum through the third branch point of each subsequent artery (approximate diameter of 75 μm), allowing specific evaluation of proteins present in the arterial vasculature. We recovered 50–100 μg of protein from each IPA tree, sufficient for one to two immunoblots. Three commercially available anti-ID1 antibodies [rabbit monoclonal antibodies clone 195–14 and clone 37–2 (BioCheck), and rabbit polyclonal (Santa Cruz, SC-488)] were evaluated for use in immunoblot detection of ID1. Specificity for each antibody was confirmed by negative immunoblots on lung lysates from Id1 null mice (Supplemental Fig. S3). Immunoblots for ID1 expression in experimental lung tissue samples were performed using the anti-ID1 antibody clone 195–14. Additionally, rabbit polyclonal anti-ID2 and -ID4 antibodies (Santa Cruz, SC-489 and SC-491, respectively), rabbit monoclonal anti-ID3 clone 17–3 (BioCheck), rabbit polyclonal anti-COOH-terminal phospho-SMAD1/5/8 (Cell Signaling, 9511), mouse monoclonal anti-SMAD1 clone A-4 (Santa Cruz, SC-7965), and mouse monoclonal anti-β-actin antibody clone AC-74 (Sigma, A2228) were used. Specificity of the anti-ID3 antibody clone 17–3 was confirmed by negative immunoblots from Id3 null mice (Supplemental Fig. S4A). Appropriate species-specific goat polyclonal secondary antibodies (anti-mouse: Kirkegaard & Perry Laboratories, 04–18-06, and anti-rabbit: Cell Signaling, 7074) were utilized. A light-chain-specific secondary antibody (Jackson Immunolaboratories, 115–035-174) was used for detection of SMAD1 in whole lung lysates to resolve it from immunoglobulin heavy chain. Specific bands were quantified by densitometry and normalized to β-actin loading controls.
Immunohistochemistry.
Immunoperoxidase staining for ID1, ID2, and ID3 was performed on formalin-fixed, paraffin-embedded lung sections. Sections were heated in a vegetable steamer for 25 min in citrate antigen retrieval buffer (Biogenex) for all of the immunohistochemical and immunofluorescence stains, with the exception of vWF staining. For this, antigen retrieval was performed by incubating the tissue sections for 30 min at 37°C with 1 mg/ml trypsin (Sigma). Primary antibodies were detected with ABC amplification using horseradish peroxidase (HRP) conjugated streptavidin (Vector Laboratories) and diamino benzidine (Sigma) and counterstained with Mayer's hematoxylin (Fluka). Localization studies for ID protein expression in experimental lung tissue samples were performed using anti-ID1 clone 37–2, anti-ID2 clone 98–2, and anti-ID3 clone 17–3 rabbit monoclonal antibodies from Biocheck. In addition, we evaluated rabbit polyclonal anti-ID1 (SC-488) and anti-ID2 (SC-489) antibodies from Santa Cruz, and another rabbit monoclonal anti-ID1 antibody, clone 195–14 from Biocheck. Cells positive for ID1–3 protein expression were quantified from 10 ×200 fields while blinded to treatment and genotype. For these studies, we evaluated airway-associated vessels, separated into small (20–50 μm) and larger vessel sizes (50–100 μm). Vessels were only scored if in cross section, and counts were expressed as number of positive cells per vessel.
Immunofluorescence was performed on formalin-fixed, paraffin-embedded lung sections with the ID1–3 antibodies outlined above and detected with a rabbit IgG-specific biotin-conjugated secondary antibody, followed by streptavidin-conjugated HRP (ABC kit, Vector Laboratories). Cy3-labeled tyramide signal amplification was used to amplify the HRP signal (Perkin Elmer, NEL744), according to the manufacturers' instructions. VSMCs were colabeled using mouse monoclonal anti-α-SMA antibodies (clone 1A4, Sigma) and detected with anti-mouse FITC-conjugated secondary antibodies (Vector Laboratories, FI-2000). ECs were labeled using rabbit polyclonal anti-vWF antibodies (DAKO, A0082) and detected with rhodamine-conjugated, anti-rabbit secondary antibodies (Jackson Laboratories, 711–296-152). DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Cells were defined as VSMCs if α-SMA was positive, and ECs when located internally to α-SMA-positive smooth muscle cells. This method of defining ID1–3 expressing EC compartment of the pulmonary vasculature was validated in a separate series of experiments in which sequential lung tissue sections were colabeled with ID1–3 and α-SMA antibodies, or with α-SMA and vWF antibodies, as outlined above (Supplemental Figs. S5–S7). For these studies, we focused on small (20–50 μm) peripheral muscularized vessels distal to terminal bronchioles, since these are the major site of pulmonary vascular remodeling in hypoxic PH (26). While blinded to genotype and treatment conditions, ID1–3 positive cells were quantified in 10 ×200 fields of view. Vessels were only scored if in cross section. ID1–3 positive cells were expressed as compartment-specific cellular indexes (ID1–3 positive VSMC or EC/total VSMC or EC 4′,6-diamidino-2-phenylindole-stained nuclei).
Cell culture.
The isolation and characterization of PASMCs from wild-type mice has been described previously (8). PASMCs were cultured in DMEM (Gibco) containing 10% fetal bovine serum (Sigma). Cells were serum-starved for 24 h and then treated with 10 ng/ml recombinant human BMP-4 (R&D Systems) for an additional 4 h before lysis. To evaluate hypoxic responses, cells were serum starved for 24 h and exposed to 1 or 3% oxygen ± 200 ng/ml recombinant human Noggin (Alpha Diagnostic) under serum-free conditions in the Invivo2 400 Hypoxia Workstation for an additional 24 h before lysis.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism 5 with two-tailed t-test for pairwise comparisons and one-way ANOVA for multiple, between-group comparisons using Bonferroni correction for post hoc, pairwise comparisons. Minimal level of significance was set at P < 0.05.
RESULTS
Regulation of pulmonary ID1–3 expression levels by hypoxia.
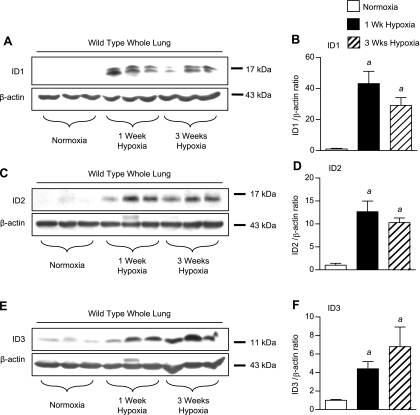
To evaluate the regulation of pulmonary ID proteins in response to hypoxia, we first performed immunoblot analyses of whole lung lysates from mice maintained under normoxic conditions or exposed to 1 or 3 wk of hypoxia. As reported previously (2, 8, 9, 22), this revealed activation of the BMP pathway by hypoxia, as indicated by increased COOH-terminal phosphorylation of the receptor-activated SMADs 1, 5, and 8 (pS1/5/8) (Supplemental Fig. S8, A and B). Expression of total SMAD1 tends to decrease after 3 wk of hypoxia, but this change is not statistically significant (Supplemental Fig. S8, A and C), indicating that the increase in phosphorylated SMAD1/5/8 represents activation of BMP signaling in the hypoxic lung. Increased BMP activation is correlated with a robust induction of pulmonary ID1 expression, peaking after 1 wk of hypoxia and persisting after 3-wk exposure to hypoxia (Fig. 1, A and B). This increase in ID1 expression was confirmed using two other commercially available anti-ID1 antibodies (Supplemental Fig. S3). All of these anti-ID1 antibodies detect a 15- to 17-kDa ID1 doublet that is lost in Id1 null lung lysates, confirming that both bands represent isoforms of ID1. Pulmonary ID2 (Fig. 1, C and D) and ID3 (Fig. 1, E and F) expression levels are also increased in wild-type mice following exposure to 1 and 3 wk of hypoxia. The anti-ID3 antibody used in these studies detects a single 13-kDa ID3 band in wild-type and not Id3 null mouse lungs (Supplemental Fig. S4A). In contrast, pulmonary ID4 expression is barely detectable in lung lysates from wild-type mice under normoxic conditions, and there is no significant increase in ID4 expression after 1 or 3 wk hypoxia (J. W. Lowery, data not shown).
Fig. 1.
Regulation of inhibitor of differentiation 1–3 (ID1–3) expression in the hypoxic lung. A: immunoblot for ID1 using rabbit monoclonal antibody clone 195–14 and β-actin in normoxic and 1-wk and 3-wk hypoxic wild-type whole lung lysates. B: densitometry of ID1 bands (doublet) relative to β-actin controls from A. C and D: immunoblot (C) and densitometry (D) for ID2 using the rabbit polyclonal antibody from Santa Cruz (SC-489) compared with β-actin in normoxic and 1-wk and 3-wk hypoxic wild-type whole lung lysates. E and F: immunoblot (E) and densitometry (F) for ID3 using rabbit monoclonal antibody clone 17–3 compared with β-actin in normoxic and 1-wk and 3-wk hypoxic wild-type whole lung lysates. Values are means ± SE. aP < 0.05 vs. wild-type normoxic control by one-way ANOVA with Bonferroni correction.
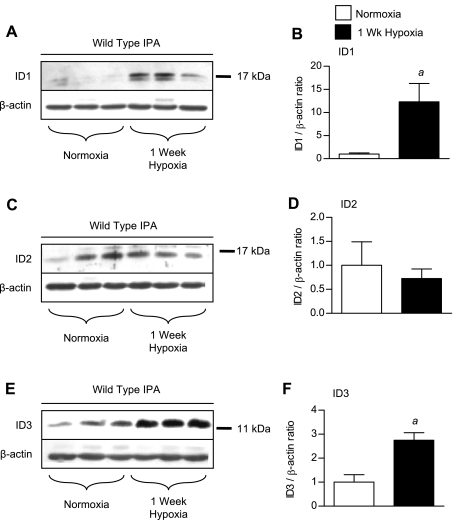
Since ID1–3 may be expressed in different cell types in the lung, we went on to evaluate their expression in the large-diameter pulmonary arterial vasculature (>75 μm diameter) by immunoblot using IPA preparations isolated from mice under normoxic or hypoxic conditions. Since total pulmonary ID1–3 levels are maximally induced after 1-wk exposure to hypoxia, these studies were performed in IPAs isolated from mice exposed to 1 wk of hypoxia. As seen in whole lung lysates, low-level expression of ID1 and ID3 in IPAs isolated from normoxic mice are upregulated after exposure to 1 wk of hypoxia (Fig. 2, A, B, E, and F). In contrast, ID2 expression in IPAs is not increased in response to hypoxia (Fig. 2, C and D).
Fig. 2.
Regulation of ID1–3 expression in hypoxic intrapulmonary artery (IPA) preparations. A: immunoblot for ID1 using rabbit monoclonal antibody clone 195–14, phospho-SMAD1/5/8 (pS1/5/8), and β-actin in normoxic and 1-wk and 3-wk hypoxic wild-type IPAs. B: densitometry of ID1 bands (doublet) relative to β-actin controls from A. C and D: immunoblot (C) and densitometry (D) for ID2 using the rabbit polyclonal antibody from Santa Cruz (SC-489) compared with β-actin in normoxic and 1-wk and 3-wk hypoxic wild-type IPAs. E and F: immunoblot (E) and densitometry (F) for ID3 using rabbit monoclonal antibody clone 17–3 compared with β-actin in normoxic, 1-wk hypoxic, and 3-wk hypoxic wild-type IPAs. Values are means ± SE. aP < 0.05 vs. wild-type normoxic control by one-way ANOVA with Bonferroni correction.
Localization of ID1–3 expression in the hypoxic lung.
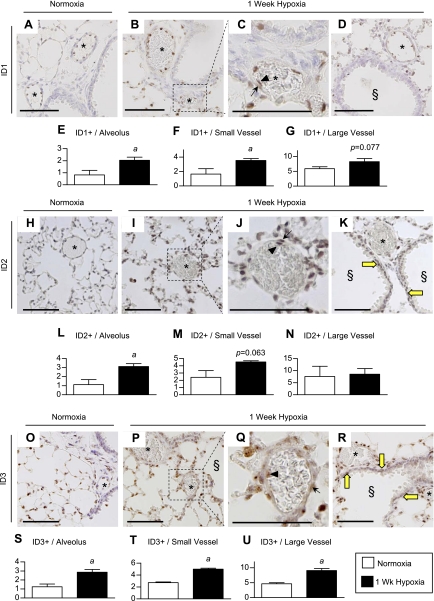
Having observed differential expression of ID1/3 and ID2 in whole lung vs. IPA preparations by immunoblot, we sought to determine their precise localization in the hypoxic lung by immunohistochemical analysis. We first evaluated the specificity of three different commercially available ID1 antibodies by comparing staining of lung sections from wild-type and Id1 null mice exposed to 1-wk hypoxia. The first antibody we tested, a rabbit polyclonal anti-ID1 antibody from Santa Cruz (C-20, SC-488), has been used extensively to evaluate ID1 expression domains in a variety of different tissues (1, 3, 4, 6, 8, 10, 17, 18, 30, 41, 44, 47, 52). This antibody gave weak but selective staining of pulmonary ECs in wild-type but not Id1 null mice (Supplemental Fig. S9, A, B, D, and E). However, this antibody also gave nonspecific cytoplasmic staining of bronchial and VSMCs in some of the muscularized, airway-associated vessels from wild-type and Id1 null lungs (Supplemental Fig. S9, C and F). Given this nonspecific staining of VSMCs, we went on to evaluate immunohistochemical staining of the lung using two other commercially available anti-ID1 rabbit monoclonal antibodies, clones 195–14 and 37–2 from BioCheck. Both of these antibodies have been validated in Id1 null mammary tissue, but there are no data on their use in mouse lungs (30). Clone 195–14 did not stain wild-type or Id1 null lung tissue (L. Anderson, data not shown). Clone 37–2, on the other hand, gave dominant nuclear staining in alveolar and EC compartments in wild-type but not Id1 null lung tissue (Supplemental Fig. S9, G and L). Using the validated rabbit monoclonal anti-ID1 clone 37–2 antibody, therefore, we found that ID1 is expressed in alveolar epithelial and pulmonary vascular ECs, but rarely in bronchial smooth muscle cells under either normoxic or hypoxic conditions (Fig. 3, A–D, Supplemental Fig. S10). Quantification of the number of ID1-positive alveolar and vascular cells in small airway-associated muscularized vessels (20- to 50-μm diameter) shows that both are significantly increased in response to hypoxia (Fig. 3, E and F). Larger vessels (50- to 100-μm diameter) also tend to have increased numbers of ID1-positive cells, but this increase is not statistically significant (P = 0.077; Fig. 3G). The majority of vessel-associated ID1-positive cells are ECs under both normoxic and hypoxic conditions (Fig. 3, A–D, and Supplemental Fig. S10). However, there is a significant increase in ID1-positive cells in the walls of larger vessels under hypoxic conditions (mean ± SE per vessel, normoxia: 0.158 ± 0.158 vs. hypoxia: 0.706 ± 0.122, two-tailed t-test, P < 0.03). These cells are located within the vessel wall and have elongated nuclei typical of VSMCs (Fig. 3C; arrow).
Fig. 3.
Localization of ID1–3 expression in the hypoxic lung. A–D: immunoperoxidase staining for ID1 in lung sections from normoxic and 1-wk hypoxic wild-type mice using rabbit monoclonal antibody clone 37–2. C: inset from B. E–G: quantification of ID1-positive nuclei in alveoli and in both small (20–50 μm) and larger airway-associated muscularized vessels (50–100 μm). H–K: immunoperoxidase staining for ID2 in lung sections from normoxic and 1-wk hypoxic wild-type mice using rabbit monoclonal antibody clone 98–2. J: inset from I. L–N: quantification of ID2-positive nuclei in alveoli and both small (20–50 μm) and larger airway-associated muscularized vessels (50–100 μm). O–R: immunoperoxidase staining for ID3 in lung sections from normoxic and 1-wk hypoxic wild-type mice using rabbit monoclonal antibody clone 17–3. Q: inset from P. S–U: quantification of ID3-positive nuclei in alveoli and both small (20–50 μm) and larger airway-associated muscularized vessels (50–100 μm). Asterisk (*) indicates vessels; arrows indicate vascular smooth muscle cell (VSMC) staining; arrowheads denote endothelial cell (EC) staining; § indicates muscularized airways; thick yellow arrows indicate smooth muscle staining in muscularized airways. Scale bar = 100 μm. Data are expressed as means ± SE (normoxia n = 4, 1-wk hypoxia n = 5). aP < 0.05 vs. wild-type normoxic control by two-tailed t-test.
Pulmonary ID2 expression was evaluated using two commercially available antibodies, a rabbit polyclonal antibody from Santa Cruz (SC-489) and a rabbit monoclonal antibody from Biocheck (clone 98–2). ID2 staining is more prominent using the anti-ID2 clone 98–2 antibody, but both antibodies showed similar vascular and dominant alveolar epithelial staining in hypoxic mouse lungs (Supplemental Fig. S11). In addition, ID2 staining is detected in bronchial epithelial and smooth muscle cell compartments of the lung using both antibodies under both normoxic and hypoxic conditions (Fig. 3K, yellow arrows; Supplemental Fig. S11, A and D). Therefore, while Id2 null mice were not available to evaluate antibody specificity, identical immunolocalization using two antibodies raised against different ID2 antigens suggest that ID2 localization using these antibodies is specific. Using the anti-ID2 clone 98–2 antibody, therefore, we show that there is a prominent increase in extravascular alveolar ID2-positive cells (Fig. 3L). The number of ID2-positive vascular cells also increases in small intrapulmonary airway-associated vessels (20- to 50-μm diameter) after 1-wk exposure to hypoxia, but these changes are not statistically significant (P = 0.063, Fig. 3M). Moreover, the number of ID2-positive vascular cells in larger airway associated vessels (50- to 100-μm diameter) does not change with hypoxia exposure (Fig. 3N). These findings are consistent with our immunoblot analyses of ID1 and ID2 expression levels in lung vs. IPA preparations, since they indicate that, unlike ID1, ID2 expression is dominantly induced in the extravascular compartment of the hypoxic lung.
To evaluate the expression pattern of ID3 in the mouse lung, we first confirmed ID3 staining specificity using the rabbit monoclonal anti-ID3 clone 17–2 from Biocheck by negative staining on Id3 null lung sections (Supplemental Fig. S4, B–G). Using this antibody we show that, like ID1, ID3 is detectable in alveolar epithelium and vascular cells under normoxic conditions, and that the number of ID3-expressing cells in both compartments is increased after 1-wk exposure to hypoxia (Fig. 3, O–U). Unlike ID1, but like ID2, ID3 is also expressed in bronchial smooth muscle cells under hypoxic conditions (Fig. 3R, yellow arrows).
ID1–3 expression and localization in peripheral pulmonary vasculature.
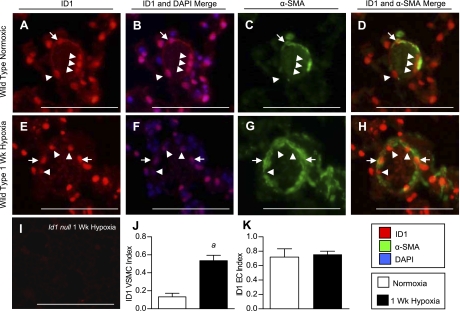
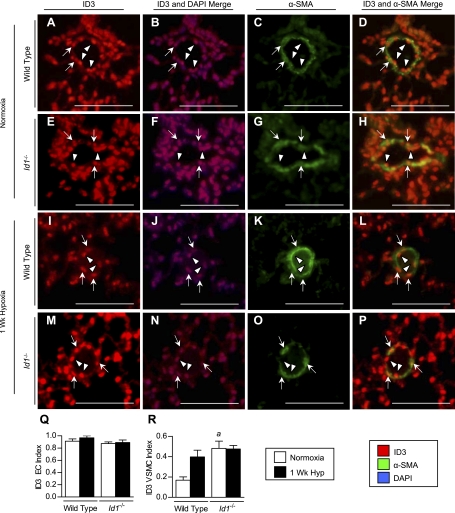
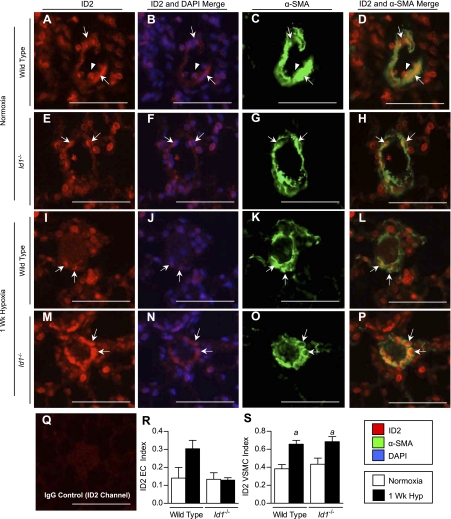
We also determined the cell types expressing ID1–3 in the small intrapulmonary resistance vessels (20- to 50-μm diameter and distal to terminal bronchioles), since these vessels are the main site of pulmonary vascular remodeling in hypoxic PH (26). For this, we performed double immunofluorescence analysis for ID1–3 and the smooth muscle cell marker α-SMA. Most ID1-expressing cells in these small peripheral vessels are inside the VSMC wall, consistent with their being ECs (Fig. 4, A–H, arrowheads). This was confirmed by immunolocalization of ID1 in sequential sections stained with the EC marker vWF (Supplemental Fig. S5). Quantification of cellular expression indexes under hypoxic conditions reveals a selective increase in the proportion of ID1-positive VSMCs, but not ECs, compared with normoxic lungs (Fig. 4, J and K). Similar results were obtained for ID3 (see Fig. 9, Q and R, Supplemental Fig. S6). In contrast, unlike ID1 and ID3, which are expressed in the majority of pulmonary ECs under normoxic conditions [the proportion of ID1- or ID3-positive ECs in peripheral pulmonary vessels (ID1/3 indexes, mean ± SE): ID1 0.712 ± 0.11; ID3 0.91 ± 0.03], ID2 is predominantly expressed in VSMCs and not ECs under normoxic conditions (ID2 EC index 0.14 ± 0.05; ID2 VSMC index 0.38 ± 0.04) (see Fig. 8, R and S, Supplemental Fig. S7). However, like ID1 and ID3, the number of ID2-positive VSMCs is increased after 1 wk of hypoxia. The number of ID2-positive ECs is also increased by hypoxia, but these changes are not statistically significant. These findings indicate that ID1–3 are upregulated in the VSMC compartment of the hypoxic pulmonary vasculature, but that the distribution of ID1/3 vs. ID2 expressing cells in VSMC and EC compartments of the peripheral pulmonary vasculature is distinct.
Fig. 4.
Vascular cell type-specific regulation of ID1 in hypoxia. A–H: immunofluorescence for ID1 using rabbit monoclonal antibody clone 37–2 (red) and mouse monoclonal anti-α-smooth muscle actin (α-SMA; green) in muscularized pulmonary vessels distal to terminal bronchioles from normoxic and 1-wk hypoxic wild-type mice. Nuclear staining with 4,6-diamidino-2-phenylindole (DAPI) is shown in blue. I: negative staining of 1-wk hypoxic lung from Id1 null mouse using anti-ID1 clone 37–2. J and K: quantification of cell-specific ID1 expression indices in VSMC (J) and EC (K) compartments in 20- to 50-μm muscularized peripheral vessels from normoxic and 1-wk hypoxic wild-type mouse lungs. Arrows indicate VSMC staining; arrowheads EC staining. Scale bar = 100 μm. Data are expressed as means ± SE (n = 4 per group). aP < 0.05 vs. wild-type normoxic control by two-tailed t-test.
Fig. 9.
Localization of ID3 expression in peripheral pulmonary vessels in wild-type and Id1 null mouse lungs. A–P: immunofluorescence for ID3 using rabbit monoclonal antibody clone 17–3 (red) and mouse monoclonal anti-α-SMA (green) in small (20- to 50-μm) muscularized peripheral pulmonary vessels in wild-type (A–D, I–L) and Id1 null mice (E–H, M–P) under normoxic conditions (A–H) and after 1 wk of hypoxia (I–P). Nuclear staining with DAPI is shown in blue. M and N: quantification of cell-specific ID3 indexes in EC (Q) and VSMC (R) compartments in peripheral vessels from normoxic and 1-wk hypoxic wild-type and Id1 null mouse lungs, as indicated. Arrows indicate nuclear staining in VSMCs; arrowheads, ECs. Scale bar = 100 μm. Values are means ± SE (n = 4 each group). aP < 0.05 vs. wild-type normoxic control by one-way ANOVA with Bonferroni correction.
Fig. 8.
Localization of ID2 expression in peripheral pulmonary vessels of wild-type and Id1 null mouse lungs. A–P: immunofluorescence staining for ID2 using rabbit monoclonal antibody clone 98–2 (red) and mouse monoclonal anti-α-SMA (green) in small (20- to 50-μm) muscularized peripheral pulmonary vessels in wild-type (A–D, I–L) and Id1 null mice (E–H, M–P) under normoxic conditions (A–H) and after 1 wk of hypoxia (I–P). Nuclear staining with DAPI is shown in blue. Q: negative staining with rabbit IgG control in wild-type normoxic lungs. R and S: quantification of cell-specific ID2 indexes in EC (R) and VSMC (S) compartments in peripheral vessels from normoxic and 1-wk hypoxic wild-type and Id1 null mouse lungs, as indicated. Arrows indicate nuclear staining in VSMCs; arrowheads, ECs. Scale bar = 100 μm. Values are means ± SE (n = 4 per group). aP < 0.05 vs. corresponding genotype normoxic control by one-way ANOVA with Bonferroni correction.
BMP-dependent hypoxic regulation of ID1 and ID3 in cultured pulmonary PASMCs.
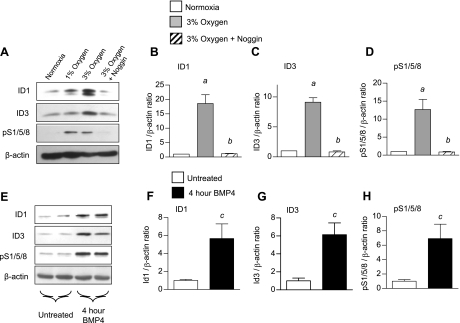
Having established that ID1 and ID3 expression are selectively upregulated in hypoxic pulmonary VSMCs in vivo, we explored whether these responses could be mediated by hypoxia-induced activation of BMP signaling. For this, we evaluated hypoxic regulation of ID1 and ID3 expression in vitro in primary PASMCs from wild-type mice. Exposure of cultured PASMCs to 1 or 3% oxygen for 24 h leads to a marked increase in expression of ID1 and ID3 (Fig. 5, A–C). Incubation with Noggin, an inhibitor of extracellular BMP ligands (42), inhibits hypoxic induction of ID1 and ID3 expression in these cells. This is associated with inhibition of hypoxia-induced SMAD1/5/8 phosphorylation (Fig. 5, A and D). Moreover, BMP-4 strongly induces ID1 and ID3 and phospho-SMAD1/5/8 expression in cultured PASMCs (Fig. 5, E–H). These findings indicate that hypoxic induction of ID1 and ID3 in cultured PASMCs is BMP dependent. In contrast, ID2 expression is not increased by hypoxia or BMP4 in these cells (Supplemental Fig. S12), indicating that ID1/3 and ID2 are differentially regulated by hypoxia and BMP signaling in PASMCs.
Fig. 5.
Hypoxic regulation of ID1 and ID3 in cultured pulmonary artery smooth muscle cells (PASMCs). A: representative immunoblot for ID1 and ID3 using rabbit monoclonal antibody clones 195–14 and 17–3, respectively, pS1/5/8, and β-actin in serum-starved mouse PASMCs maintained in normoxia, 1% oxygen, or 3% oxygen ± 200 ng/ml Noggin for 24 h, as indicated. The experiment was repeated in triplicate. B–D: densitometry of ID1 (B), ID3 (C), and pS1/5/8 (D) from A, relative to β-actin. E: representative immunoblot for ID1, ID3, pS1/5/8, and β-actin in serum-starved mouse PASMCs treated ± 10 ng/ml recombinant human bone morphogenetic protein (BMP)-4 for 4 h, as indicated. The experiment was repeated in triplicate. F–H: densitometry of ID1 (F), ID3 (G), and pS1/5/8 (H) from E, relative to β-actin. Values are means ± SE. aP < 0.05 vs. wild-type normoxic control. bP < 0.05 vs. 3% oxygen without addition of Noggin by one-way ANOVA with Bonferroni correction. cP < 0.05 vs. untreated control by two-tailed t-test.
Role of ID1 in the development of hypoxic PH.
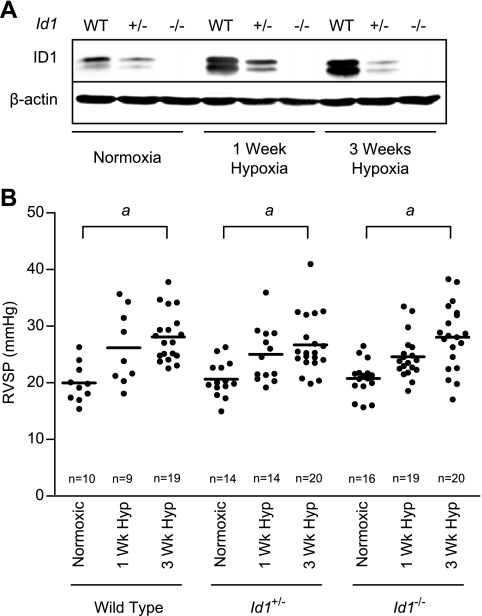
Previous studies have shown that ID1 alone is both necessary and sufficient to mediate BMP-dependent anti-proliferative responses in cultured pulmonary PASMCs (48, 49). We, therefore, reasoned that, if ID1 plays a role in regulating pulmonary vascular responses to hypoxia, loss of ID1 expression alone in Id1 null mice would have significant impact on pulmonary vascular responses to chronic hypoxia. To investigate this, we obtained mutant mice with targeted disruption of the Id1 genomic locus (46). Id1 null mice are viable, and no apparent developmental abnormalities are observed in either sex (46). Moreover, pulmonary alveolar density [mean ± SE, alveoli per mm2, wild type (n = 4): 426.5 ± 110.6 vs. Id1 null (n = 5): 467.3 ± 90.42, two-tailed t-test, P = 0.73] and peripheral vessel density [mean ± SE, vessels per 100 alveoli, wild type (n = 4): 3.28 ± 0.53 vs. Id1 null (n = 5): 3.52 ± 0.73, two-tailed t-test, P = 0.80] is indistinguishable in wild-type and Id1 null mice. These findings indicate that there is no structural abnormality in the lung or obvious developmental abnormality in the pulmonary vasculature of Id1 null mice. Immunoblot for ID1 expression confirmed that the hypoxic induction of pulmonary ID1 expression is diminished or absent in Id1+/− and Id1 null mice, respectively (Fig. 6A, the full length immunoblot using this antibody is shown in Supplemental Fig. S3A). We measured RVSP in wild-type, Id1+/−, and Id1 null mice after exposure to 1 or 3 wk of hypoxia. There is a significant increase in RVSP in response to hypoxia in wild type and Id1 mutant mice, but no difference in RVSP between wild-type and Id1-deficient mice under any of the treatment conditions (Fig. 6B and Table 1). Male mice develop more severe PH after 3 wk of hypoxia than female mice, but Id1 deficiency does not modulate this effect within either sex (Table 1). No elevation of RVDP or LVDP was observed, confirming that the increase in RVSP is not due to left-sided heart failure (J. W. Lowery, data not shown). These hemodynamic data are supported by measurements of RV hypertrophy, which also show an increase after 3 wk of hypoxia, but reveal no differences between genotypes (Table 1). Id1 mutant mice display an earlier peak in the level of hypoxia-induced increase in blood hematocrit (Table 1). However, this difference is not statistically significant. Moreover, after 3 wk of hypoxia, there is no difference in hematocrit between genotypes.
Fig. 6.
Hypoxic pulmonary hypertension (PH) in wild-type and Id1-deficient mice. A: representative immunoblot for ID1 using rabbit monoclonal antibody clone 195–14 and β-actin in normoxic and 1-wk and 3-wk hypoxic wild-type (WT), Id1+/−, and Id1 null lung lysates. B: right ventricular systolic pressures (RVSP) in wild-type, Id1+/−, and Id1 null mice exposed to normoxia or 1-wk or 3-wk hypoxia. Each dot represents the mean RVSP from a single animal; bars indicate mean values for each group. Mouse numbers are indicated (n). aP < 0.05 vs. corresponding genotype normoxic control by one-way ANOVA with Bonferroni correction.
Table 1.
Hypoxic pulmonary hypertension in wild-type and Id1-deficient mice
| Normoxia |
1-Wk Hypoxia |
3-Wk Hypoxia |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild type | Id1+/− | Id1−/− | Wild type | Id1+/− | Id1−/− | Wild type | Id1+/− | Id1−/− | |
| RVSP, mmHg | 19.96 ± 1.06 | 20.66 ± 0.84 | 20.76 ± 0.77 | 26.18 ± 2.18 | 24.15 ± 1.50 | 24.59 ± 0.88 | 28.12 ± 1.01a | 26.68 ± 1.14a | 28.04 ± 1.29a |
| n | 10 | 14 | 16 | 9 | 14 | 19 | 19 | 20 | 20 |
| Female RVSP, mmHg | 19.01 ± 1.58 | 20.91 ± 1.38 | 19.81 ± 0.83 | 23.66 ± 2.61 | 21.01 ± 1.96a | 23.16 ± 0.83a | 25.75 ± 0.84a | 24.91 ± 1.01a | 25.69 ± 1.46a |
| n | 6 | 8 | 6 | 4 | 6 | 10 | 10 | 11 | 10 |
| Male RVSP, mmHg | 21.39 ± 1.09 | 20.33 ± 0.85 | 21.33 ± 1.12 | 28.19 ± 3.27 | 26.50 ± 1.85a | 26.19 ± 1.51a | 30.75 ± 1.52a,b | 28.85 ± 2.08a | 30.39 ± 1.92a |
| n | 4 | 6 | 10 | 5 | 8 | 9 | 9 | 9 | 10 |
| RV/LV+S, mg/mg | 0.257 ± 0.008 | 0.245 ± 0.005 | 0.258 ± 0.009 | 0.291 ± 0.016 | 0.302 ± 0.011a | 0.339 ± 0.015a | 0.368 ± 0.013a | 0.362 ± 0.009a | 0.360 ± 0.007a |
| n | 10 | 20 | 15 | 5 | 14 | 13 | 17 | 22 | 21 |
| RV/body weight, mg/g | 0.186 ± 0.006 | 0.191 ± 0.004 | 0.213 ± 0.011 | 0.246 ± 0.011 | 0.250 ± 0.008a | 0.263 ± 0.01a | 0.309 ± 0.012a | 0.291 ± 0.009a | 0.288 ± 0.012a |
| n | 10 | 20 | 15 | 4 | 14 | 13 | 17 | 22 | 21 |
| Hematocrit, % | 32.50 ± 2.13 | 35.42 ± 0.92 | 36.95 ± 0.91 | 39.75 ± 1.76 | 47.36 ± 1.56a | 46.76 ± 1.21a | 46.36 ± 1.23a | 50.92 ± 1.45a | 49.33 ± 1.83a |
| n | 7 | 21 | 11 | 8 | 11 | 15 | 11 | 13 | 18 |
| Heart rate, beats/min | 421.3 ± 21.1 | 451.8 ± 21.4 | 439.1 ± 14.1 | 408.8 ± 19.5 | 415.7 ± 9.5 | 415.8 ± 10.1 | 418.5 ± 9.3 | 425.8 ± 12.9 | 398.0 ± 13.8 |
| n | 10 | 16 | 16 | 9 | 14 | 20 | 19 | 20 | 20 |
Values are mean ± SE from wild-type, Id1+/−, and Id1null mice exposed to normoxia or 1-wk or 3-wk hypoxia. Mouse numbers are indicated (n). RVSP, right ventricular systolic pressure; RV, right ventricle; LV, left ventricle; S, septal.
P < 0.05 vs. corresponding genotype normoxic control; P < 0.05 vs. female wild type 3 wk hypoxia by one-way ANOVA with Bonferroni correction.
To determine whether loss of ID1 expression is associated with alterations in hypoxia-induced pulmonary vascular remodeling, we evaluated the degree of hypoxia-induced peripheral vessel muscularization and proliferation in wild-type, Id1+/−, and Id1 null mouse lungs. Supporting our finding that ID1 is dispensable for the development of hypoxic PH, the characteristic increase in peripheral muscularization is not different between genotypes (Table 2 and Supplemental Fig. S2). Furthermore, while small, peripheral pulmonary vessels from hypoxic Id1+/− and Id1−/− mice tend to have increased numbers of PCNA-positive proliferating VSMCs, this increase is not statistically significant. There is also no difference in EC proliferation with hypoxia between genotypes (Table 2). These data indicate that ID1 expression is dispensable for the physiological events, vascular remodeling, or vascular cell proliferation associated with the development of hypoxic PH.
Table 2.
Hypoxia-induced pulmonary vascular remodeling and vascular cell proliferation in wild-type and Id1 deficient mice
| Normoxia |
1-Wk Hypoxia |
3-Wk Hypoxia |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild type | Id1+/− | Id1−/− | Wild type | Id1+/− | Id1−/− | Wild type | Id1+/− | Id1−/− | |
| Muscularized vessels, % | 20.37 ± 2.99 | 18.31 ± 4.24 | 20.35 ± 3.49 | 53.42 ± 5.18a | 57.68 ± 7.61a | 51.54 ± 5.18a | 45.17 ± 2.29a | 46.32 ± 4.14a | 48.37 ± 6.60a |
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| EC proliferation, PCNA positive/total | 0.007 ± 0.004 | 0.022 ± 0.012 | 0.004 ± 0.004 | 0.071 ± 0.015a | 0.082 ± 0.014a | 0.062 ± 0.017a | ND | ND | ND |
| n | 5 | 6 | 6 | 7 | 5 | 5 | |||
| VSMC proliferation, PCNA positive/total | 0.021 ± 0.009 | 0.018 ± 0.007 | 0.012 ± 0.004 | 0.076 ± 0.010a | 0.119 ± 0.016a | 0.113 ± 0.022a | ND | ND | ND |
| n | 5 | 6 | 6 | 7 | 5 | 5 | |||
Values are means ± SE from wild type, Id1+/−, and Id1 null mice exposed to normoxia or 1-wk or 3-wk hypoxia. Mouse numbers are indicated (n). ND, no data recorded; EC, endothelial cell; VSMC, vascular smooth muscle cell.
P < 0.05 vs. corresponding genotype normoxic control by one-way ANOVA with Bonferroni correction.
ID2 and ID3 expression and localization in the lungs of Id1-deficient mice.
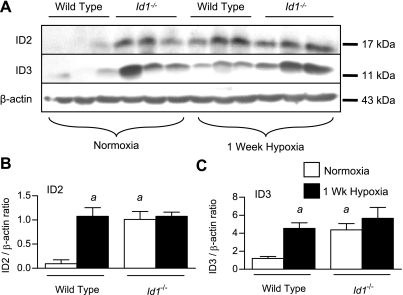
One explanation for our findings is that changes in the expression of ID2 or ID3, which share many of the same molecular targets as ID1 (31), might compensate for loss of ID1 in the intact pulmonary vasculature of Id1 null mice. To explore this possibility, we first examined expression of ID2 and ID3 in the lungs of wild-type and Id1 null mice by immunoblot. This revealed a marked increase in expression of ID2 and ID3 in normoxic Id1 null mouse lungs over wild-type littermates (Fig. 7, A–C). No further increase in ID2 or ID3 expression levels are seen in Id1 null mice compared with wild-type littermates in response to chronic hypoxia. To determine the localization of ID2 and ID3 expression in the pulmonary vasculature of Id1 null mice, we evaluated their cellular localization in wild-type and Id1 null mouse lungs under normoxic and hypoxic conditions. For this, we focused on small intrapulmonary resistance vessels, since these vessels are the main site of pulmonary vascular remodeling in hypoxic PH (26). Id1 null mice tend to have fewer ID2-positive ECs in hypoxia than wild-type mice, but these changes are not statistically significant (Fig. 8R). The proportion of ID2-positive peripheral VSMCs increases with exposure to hypoxia in both wild-type and Id1 null mice (Fig. 8S). However, there is no compensatory increase in the number of ID2-expressing cells in the VSMC compartment of these peripheral vessels in Id1 null mice under normoxic or hypoxic condition. Unlike ID2, the proportion of ID3-positive VSMCs is significantly increased under normoxic conditions in Id1 null mutant mice compared with their wild-type littermates (Fig. 9R). There is no further increase in the proportion of ID3-positive VSMCs with hypoxia in Id1 null mouse lungs (Fig. 9R) and no compensatory increase in ID3-positive cells in the EC compartment of the peripheral pulmonary vasculature of Id1 null mice under normoxic or hypoxic conditions (Fig. 9Q). These data indicate that ID3 is selectively upregulated in peripheral VSMCs in Id1 null mice and raise the possibility that ID3 might functionally compensate for loss of ID1 expression in pulmonary VSMCs from hypoxic Id1 null mice.
Fig. 7.
Expression of ID2 and ID3 in Id1 null mouse lungs. A: immunoblot for ID2 using the rabbit polyclonal Santa Cruz antibody (SC-489), ID3 using rabbit monoclonal antibody clone 17–3 and β-actin in normoxic and 1-wk hypoxic wild-type and Id1 null lung lysates. B and C: densitometry of ID2 (B) and ID3 (C) expression relative to β-actin from A. Values are means ± SE. aP < 0.05 vs. wild-type normoxic control by one-way ANOVA with Bonferroni correction.
DISCUSSION
The present study provides the first comprehensive analysis of ID family protein expression and localization in the adult mouse lung. We show that ID1 and ID3 are both upregulated in the pulmonary vasculature following prolonged exposure to hypoxia, and that this is associated with an increased proportion of ID1- and ID3-expressing VSMCs. Increased expression of ID1 and ID3 in hypoxic pulmonary VSMCs occurs throughout the pulmonary arterial tree, but is pronounced in small muscularized vessels that are distal to terminal bronchioles. This is of significance, since these peripheral vessels represent the dominant resistance vessels that undergo pulmonary vascular remodeling in the hypoxic lung (26). Moreover, while ID1 and ID3 are dominantly expressed in pulmonary ECs under normoxic conditions, EC expression of ID1 and ID3 in the pulmonary vasculature is unaffected by hypoxia. These data suggest that ID1 and ID3 could play a distinct role in regulating hypoxic VSMC responses. ID2 is also upregulated in hypoxic pulmonary VSMCs, but its expression is dominantly induced in extravascular compartments of the hypoxic mouse lung. In addition, ID4 is barely detectable in the mouse lung, and, unlike ID1–3, its expression is not increased following prolonged exposure to hypoxia. These findings are consistent with differential regulation of ID4 vs. ID1–3 in the developing embryo (13, 35, 36) and suggest that, while Id4 is a bona fide BMP target gene in some cells (20, 37), it is unlikely to play a role in regulating BMP-dependent pulmonary vascular responses to chronic hypoxia.
Earlier studies, in which antibody specificity was determined by comparing immunostaining patterns in wild-type and Id1 null mouse tissues, raised concerns about the lack of specificity of the commercial anti-ID antibodies used in a number of studies (30). We have addressed this question directly by demonstrating that two recently developed rabbit monoclonal anti-ID1 (clone 37–2) and anit-ID3 (clone 17–3) antibodies from Biocheck show overlapping staining patterns in wild-type mouse lungs, but give completely negative staining on Id1 null and Id3 null mouse lung tissue sections, respectively. This indicates that localization of ID1 and ID3 in mouse lungs using these antibodies is reliable and specific. Contrasting with these findings and consistent with previous reports (3, 8, 49), the polyclonal anti-ID1 antibody from Santa Cruz (SC-488) gave dominant cytoplasmic staining of pulmonary vascular and bronchial smooth muscle cells in wild-type mouse lungs. However, the same staining pattern was seen in Id1 null lungs, indicating that ID1 staining of pulmonary VSMCs using this antibody is unreliable. This negative finding is of importance, since a number of studies have used this antibody to identify the cellular localization of ID1 in a variety of different tissues, including the lung (1, 3, 4, 6, 8, 10, 17, 18, 30, 41, 44, 47, 52). Id2 null mice were not available for these studies, so our analyses of ID2 regulation and localization have to be interpreted with caution. Nevertheless, we show that two anti-ID2 antibodies, a rabbit polyclonal antibody from Santa Cruz (SC-489) and a new rabbit monoclonal antibody from Biocheck (clone 17–3) gave the same dominantly epithelial and occasional VSMC and EC staining in hypoxic mouse lungs. This corroborative evidence using two different antibodies raised against distinct ID2 epitopes suggests that our analysis of ID2 regulation and localization in hypoxic mouse lungs using these antibodies is also reliable.
In addition to hypoxic induction of ID1–3 in pulmonary VSMCs in vivo, we show that hypoxia increases ID1 and ID3 expression in cultured PASMCs in a BMP-dependent fashion. This is consistent with published data indicating that both Id1 and Id3 are transcriptional targets of BMP-activated SMADs in a variety of cells types (27). Furthermore, since our laboratory has previously shown that hypoxia-induced PH is dependent on intact BMP signaling (2, 8, 9), and since ID1- and ID3-positive VSMCs are also closely associated with BMP-2- and BMP-4-expressing cells in small peripheral vessels and alveoli of the hypoxic lung (2, 8), these findings are consistent with the hypothesis that hypoxia induces VSMC expression of ID1 and ID3 in the intact pulmonary vasculature in a BMP-dependent fashion. However, while Id2 is also a bona fide BMP target gene in a variety of different cell types (15, 20, 37), its expression is unaffected by hypoxia or BMP treatment in these cultured mouse PASMCs. This contrasts with the observation that both ID1 and ID2 expression are upregulated in human PASMCs following treatment with BMP-4 (51). However, there is considerable heterogeneity in BMP responsiveness among different PASMC preparations, which could account for the observed differences in BMP-induced ID2 responses between these studies. For example, while the aforementioned studies and others from the same group have shown that proliferating human PASMCs are growth inhibited by BMPs (48, 49), other studies have shown that more peripheral human pulmonary VSMCs (51) and early passage mouse PASMCs (8) proliferate in response to BMP ligands in vitro. These observations underscore the importance of evaluating these ID-dependent responses in the intact vasculature. Having said that, our in vitro findings using mouse PASMCs are consistent with the observation that ID1/3 and ID2 show distinct but overlapping expression domains in hypoxic mouse lungs and suggest that ID2 is regulated by BMP-independent signaling that is distinct from the cell autonomous, BMP-dependent ID1/3 response to hypoxia in pulmonary VSMCs. In other words, while our immunolocalization studies indicate that all three ID family members are upregulated in the VSMC compartment of the hypoxic pulmonary vasculature, these in vitro studies suggest that hypoxia regulates ID1/3 and ID2 expression in VSMCs through different mechanisms.
Previous studies have revealed complex regulation of BMP signaling in different models of PH. For example, the alkaloid toxin monocrotaline transiently increases phosphorylation of SMAD1/5/8 in cultured human ECs (32), but can lead to both increased (33) and decreased (21, 28, 50) pulmonary SMAD1/5/8 phosphorylation in rats in vivo. In response to chronic hypoxia, pulmonary SMAD1/5/8 phosphorylation is unchanged in rats (21), while it is increased in mice (2, 8, 9, 22). Our laboratory has previously shown, using a genetics-based approach, that hypoxia-induced PH, VSMC proliferation, and pulmonary vascular remodeling are dependent on an intact BMP signaling pathway in mice (2, 8, 9). Therefore, since ID1 short interfering RNA (siRNA) knockdown inhibits BMP-dependent responses in cultured pulmonary PASMCs (48, 49), we anticipated that loss of ID1 expression would modify some of these hypoxia-induced, BMP-dependent responses in the pulmonary vasculature of Id1 null mice. However, our genetic studies show that ID1 is dispensable for the development of hypoxic PH in vivo, and that Id1 null mice show the same pulmonary vascular remodeling and VSMC proliferation responses to chronic hypoxia as their wild-type littermates. One explanation for these findings is that there is compensation for the loss of ID1 expression by overexpression of other ID family members in the pulmonary vasculature of Id1 null mice. These compensatory responses may not occur in cultured PASMCs in which ID1 is transiently knocked down using sequence-specific siRNAs. Supporting this idea, both ID2 and ID3 are upregulated in the lungs of Id1 null mice. However, hypoxic regulation of ID2 is largely extravascular, and there is no compensatory increase in ID2 expression in the pulmonary vasculature of hypoxic Id1 null mice. This suggests that ID2 is unlikely to compensate for the loss of ID1 in the pulmonary vasculature. On the other hand, the expression pattern of ID3 is strikingly similar to that of ID1 in the hypoxic pulmonary vasculature, both in vivo and in vitro. Moreover, loss of ID1 expression leads to a selective increase in the proportion of pulmonary VSMCs that express ID3, suggesting that compensation for the loss of ID1 in the pulmonary vasculature occurs through increased ID3 expression. This hypothesis is consistent with the fact that ID1 and ID3 share many of the same molecular targets (31). Furthermore, while mice with single homozygous deletion of Id1 or Id3 are viable (29, 46), Id1/Id3 double null mice die with defects in angiogenesis and blood vessel stability (24). This indicates that ID1 and ID3 play a combinatorial role in regulating vascular development and suggests that ID1 and ID3 are likely to have overlapping functions in regulating pulmonary vascular responses in the adult. However, ID1 expression alone is both necessary and sufficient to mediate BMP-dependent proliferative responses in cultured PASMCs (48, 49). For this reason, evaluation of functional redundancy by further knockdown of ID3 in ID1-deficient PASMCs is unlikely to have additive effects on BMP-dependent responses in these cells. Moreover, we have been unable to achieve simultaneous siRNA-induced knockdown of both ID1 and ID3 in cultured PASMCs (J. W. Lowery and A. L. Frump, data not shown). Therefore, we feel that the combinatorial role of ID1 and ID3 in vivo cannot be addressed using isolated vascular cell culture systems, and that definitive genetic studies using Id1/Id3 compound mutant mice will be required to determine whether and how ID1 and ID3 cooperatively regulate pulmonary vascular responses to hypoxia.
Perspectives and Significance
In these studies, we provide the first comprehensive analysis of ID family protein expression and localization in the mouse pulmonary vasculature. ID1 and ID3 show overlapping expression domains in hypoxic pulmonary VSMCs and are induced by hypoxia in cultured pulmonary VSMCs in a BMP-dependent fashion. Since hypoxic PH is dependent on an intact BMP signaling pathway, these findings suggest that ID1 and ID3 could play a role in mediating these responses. However, genetic studies using Id1 null mice indicate that ID1 is dispensable for the development of hypoxic PH. Moreover there is a selective increase in ID3 expression in hypoxic pulmonary VSMCs in Id1 null mouse lungs. These findings suggest that ID1 and ID3 may play a cooperative role in regulating pulmonary VSMC responses to hypoxia. Further evaluation of their combinatorial role in regulating hypoxic PH responses will be determined from the analysis of hypoxic PH responses in compound Id1/Id3 mouse mutants. These studies advance our understanding of downstream signaling pathways that regulate BMP-dependent responses in the pulmonary vasculature and the mechanisms by which the pathogenic effects of abnormal BMPR2 signaling might promote pulmonary vascular disease in patients with HPAH.
GRANTS
J. W. Lowery is a recipient of the Mechanisms in Vascular Biology Training Grant (NIH-T32HL007751). L. Anderson is a recipient of an American Heart Association Postdoctoral Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge Robert Benezra for the Id1 mutant mice, Yuan Zhuang for the Id3 mouse mutants, the Vanderbilt University Mouse Metabolic Phenotyping Core Facility for assistance with physiology measurements, BioCheck for assistance with anti-ID1 antibody optimization, J. Joshua Smith for technical expertise, and H. Scott Baldwin for access to microscopes.
REFERENCES
- 1.Abdollahi A, Hahnfeldt P, Maercker C, Gröne HJ, Debus J, Ansorge W, Folkman J, Hlatky L, Huber PE. Endostatin's antiangiogenic signaling network. Mol Cell 13: 649–663, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Anderson L, Lowery JW, Frank DB, Novitskaya T, Jones M, Mortlock DP, Chandler RL, de Caestecker MP. Bmp2 and Bmp4 exert opposing effects in hypoxic pulmonary hypertension. Am J Physiol Regul Integr Comp Physiol 298: R833–R842, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers RC, Leoni P, Kaminski N, Laurent GJ, Heller RA. Global expression profiling of fibroblast responses to transforming growth factor-beta1 reveals the induction of inhibitor of differentiation-1 and provides evidence of smooth muscle cell phenotypic switching. Am J Pathol 162: 533–546, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darby S, Cross S, Brown N, Hamdy F, Robson C. BMP-6 over-expression in prostate cancer is associated with increased Id-1 protein and a more invasive phenotype. J Pathol 214: 394–404, 2008 [DOI] [PubMed] [Google Scholar]
- 5.de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev 15: 1–11, 2004 [DOI] [PubMed] [Google Scholar]
- 6.de Candia P, Solit DB, Giri D, Brogi E, Siegel PM, Olshen AB, Muller WJ, Rosen N, Benezra R. Angiogenesis impairment in Id-deficient mice cooperates with an Hsp90 inhibitor to completely suppress HER2/neu-dependent breast tumors. Proc Natl Acad Sci U S A 100: 12337–12342, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Bizri N, Wang L, Merklinger SL, Guignabert C, Desai T, Urashima T, Sheikh AY, Knutsen RH, Mecham RP, Mishina Y, Rabinovitch M. Smooth muscle protein 22alpha-mediated patchy deletion of Bmpr1a impairs cardiac contractility but protects against pulmonary vascular remodeling. Circ Res 102: 380–388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res 97: 496–504, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Frank DB, Lowery J, Anderson L, Brink M, Reese J, de Caestecker M. Increased susceptibility to hypoxic pulmonary hypertension in Bmpr2 mutant mice is associated with endothelial dysfunction in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 294: L98–L109, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Henke E, Perk J, Vider J, de Candia P, Chin Y, Solit DB, Ponomarev V, Cartegni L, Manova K, Rosen N, Benezra R. Peptide-conjugated antisense oligonucleotides for targeted inhibition of a transcriptional regulator in vivo. Nat Biotechnol 26: 91–100, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 118: 722–730, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iavarone A, Lasorella A. ID proteins as targets in cancer and tools in neurobiology. Trends Mol Med 12: 588–594, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Jen Y, Manova K, Benezra R. Expression patterns of Id1, Id2, and Id3 are highly related but distinct from that of Id4 during mouse embryogenesis. Dev Dyn 207: 235–252, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Kersten C, Dosen G, Myklebust JH, Sivertsen EA, Hystad ME, Smeland EB, Rian E. BMP-6 inhibits human bone marrow B lymphopoiesis–upregulation of Id1 and Id3. Exp Hematol 34: 72–81, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kowanetz M, Valcourt U, Bergstrom R, Heldin CH, Moustakas A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol Cell Biol 24: 4241–4254, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 26: 81–84, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Li AG, Wang D, Feng XH, Wang XJ. Latent TGF[beta]1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J 23: 1770–1781, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Gerald WL, Benezra R. Utilization of bone marrow-derived endothelial cell precursors in spontaneous prostate tumors varies with tumor grade. Cancer Res 64: 6137–6143, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Wang J, Kinzel B, Mueller M, Mao X, Valdez R, Liu Y, Li E. Dosage-dependent requirement of BMP type II receptor for maintenance of vascular integrity. Blood 110: 1502–1510, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Liu T, Gao Y, Sakamoto K, Minamizato T, Furukawa K, Tsukazaki T, Shibata Y, Bessho K, Komori T, Yamaguchi A. BMP-2 promotes differentiation of osteoblasts and chondroblasts in Runx2-deficient cell lines. J Cell Physiol 211: 728–735, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, Morrell NW. Altered bone morphogenetic protein and transforming growth factor-β signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation 119: 566–576, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res 98: 818–827, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Lowery JW, De Caestecker MP. BMP signaling in vascular development and disease. Cytokine Growth Factor Rev 21: 287–298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401: 670–677, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, Eickelberg O, Olschewski H, Elliott CG, Glissmeyer E, Carlquist J, Kim M, Torbicki A, Fijalkowska A, Szewczyk G, Parma J, Abramowicz MJ, Galie N, Morisaki H, Kyotani S, Nakanishi N, Morisaki T, Humbert M, Simonneau G, Sitbon O, Soubrier F, Coulet F, Morrell NW, Trembath RC. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat 27: 121–132, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Meyrick BO, Perkett EA. The sequence of cellular and hemodynamic changes of chronic pulmonary hypertension induced by hypoxia and other stimuli. Am Rev Respir Dis 140: 1486–1489, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE 2002: pe40, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, Peters DM, Dumitrascu R, Seeger W, Knaus P, Schermuly RT, Eickelberg O. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 27: 1072–1078, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Pan L, Sato S, Frederick JP, Sun XH, Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol 19: 5969–5980, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perk J, Gil-Bazo I, Chin Y, de Candia P, Chen JJ, Zhao Y, Chao S, Cheong W, Ke Y, Al-Ahmadie H, Gerald WL, Brogi E, Benezra R. Reassessment of id1 protein expression in human mammary, prostate, and bladder cancers using a monospecific rabbit monoclonal anti-id1 antibody. Cancer Res 66: 10870–10877, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer 5: 603–614, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Ramos M, Lame MW, Segall HJ, Wilson DW. Monocrotaline pyrrole induces Smad nuclear accumulation and altered signaling expression in human pulmonary arterial endothelial cells. Vascul Pharmacol 46: 439–448, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos MF, Lame MW, Segall HJ, Wilson DW. Smad signaling in the rat model of monocrotaline pulmonary hypertension. Toxicol Pathol 36: 311–320, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136: 3741–3745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riechmann V, Sablitzky F. Mutually exclusive expression of two dominant-negative helix-loop-helix (dnHLH) genes, Id4 and Id3, in the developing brain of the mouse suggests distinct regulatory roles of these dnHLH proteins during cellular proliferation and differentiation of the nervous system. Cell Growth Differ 6: 837–843, 1995 [PubMed] [Google Scholar]
- 36.Riechmann V, van Cruchten I, Sablitzky F. The expression pattern of Id4, a novel dominant negative helix-loop-helix protein, is distinct from Id1, Id2 and Id3. Nucleic Acids Res 22: 749–755, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development 131: 4131–4142, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, Loscalzo J, Zhang YY. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol 295: H677–H690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song Y, Jones JE, Beppu H, Keaney JF, Jr, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 112: 553–562, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol 211: 105–113, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Uehara N, Chou YC, Galvez J, de-Candia P, Cardiff R, Benezra R, Shyamala G. Id-1 is not expressed in the luminal epithelial cells of mammary glands. Breast Cancer Res 5: R25–R29, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umulis D, O'Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development 136: 3715–3728, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, Sideras P, ten Dijke P. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation 106: 2263–2270, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Vandeputte DAA, Troost D, Leenstra S, Ijlst-Keizers H, Ramkema M, Bosch DA, Baas F, Das NK, Aronica E. Expression and distribution of id helix-loop-helix proteins in human astrocytic tumors. Glia 38: 329–338, 2002 [DOI] [PubMed] [Google Scholar]
- 45.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 94: 1109–1114, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Yan W, Young AZ, Soares VC, Kelley R, Benezra R, Zhuang Y. High incidence of T-cell tumors in E2A-null mice and E2A/Id1 double-knockout mice. Mol Cell Biol 17: 7317–7327, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang CL, Kurczab T, Down G, Kealey T, Langlands K. Gene expression profiling of the ageing rat vibrissa follicle. Br J Dermatol 153: 22–28, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Davies RJ, Southwood M, Long L, Yang X, Sobolewski A, Upton PD, Trembath RC, Morrell NW. Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res 102: 1212–1221, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Yang J, Li X, Al-Lamki RS, Southwood M, Zhao J, Lever AM, Grimminger F, Schermuly RT, Morrell NW. Smad-dependent and Smad-independent induction of Id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo. Circ Res 107: 252–262, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Yang J, Li X, Al-Lamki RS, Southwood M, Zhao J, Lever AM, Grimminger F, Schermuly RT, Morrell NW. Smad-dependent and smad-independent induction of id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo. Circ Res 107: 252–262, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 96: 1053–1063, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Lawson WE, Polosukhin VV, Pozzi A, Blackwell TS, Litingtung Y, Chiang C. Inhibitor of differentiation 1 promotes endothelial survival in a bleomycin model of lung injury in mice. Am J Pathol 171: 1113–1126, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.