Abstract
DOCA-salt treatment increases mean arterial pressure (MAP), while central infusion of benzamil attenuates this effect. The present study used c-Fos immunoreactivity to assess the role of benzamil-sensitive proteins in the brain on neural activity following chronic DOCA-salt treatment. Uninephrectomized rats were instrumented with telemetry transmitters for measurement of MAP and with an intracerebroventricular (ICV) cannula for benzamil administration. Groups included rats receiving DOCA-salt treatment alone, rats receiving DOCA-salt treatment with ICV benzamil, and appropriate controls. At study completion, MAP in vehicle-treated DOCA-salt rats reached 142 ± 4 mmHg. In contrast DOCA-salt rats receiving ICV benzamil had lower MAP (124 ± 3 mmHg). MAP in normotensive controls was 102 ± 3 mmHg. c-Fos immunoreactivity was quantified in the supraoptic nucleus (SON) and across subnuclei of the hypothalamic paraventricular nucleus (PVN), as well as other cardiovascular regulatory sites. Compared with vehicle-treated normotensive controls, c-Fos expression was increased in the SON and all subnuclei of the PVN, but not in other key autonomic nuclei, such as the rostroventrolateral medulla. Moreover, benzamil treatment decreased c-Fos immunoreactivity in the SON and in medial parvocellular and posterior magnocellular neurons of the PVN in DOCA-salt rats but not areas associated with regulation of sympathetic activity. Our results do not support the hypothesis that DOCA-salt increases neuronal activity (as indicated by c-Fos immunoreactivity) of other key regions that regulate sympathetic activity. These results suggest that ICV benzamil attenuates DOCA-salt hypertension by modulation of neuroendocrine-related PVN nuclei rather than inhibition of PVN sympathetic premotor neurons in the PVN and rostroventrolateral medulla.
Keywords: hypertension, PVN, SON, RVLM, vasopressin, sympathetic activity
high dietary salt consumption is a major environmental factor associated with hypertension and cardiovascular disease (8). High salt intake is also linked to increased sympathetic nervous activity (SNA) and changes to the function of the renin-angiotensin-aldosterone system (25, 33, 52, 63, 64).
The role of the renin-angiotensin-aldosterone system in the regulation of sodium and water balance has been extensively studied. Exogenous administration of either angiotensin II or aldosterone, accompanied by a high-salt diet, results in hypertension in experimental animals (4, 10, 17, 29, 38, 57). Chronic intracerebroventricular (ICV) infusion of the mineralocorticoid aldosterone or systemic administration of a water-soluble precursor, DOCA, also leads to hypertension in experimental animals (5, 18, 19, 31, 61). It has long been known that mineralocorticoid receptors (MRs) are located throughout the brain, including several key cardiovascular regulatory sites (2, 48, 59, 65), and DOCA-salt hypertension can be completely blocked by intracerebroventricular administration of MR antagonists (20, 30).
Changes in central neuronal activity following mineralocorticoid-salt treatment are believed to increase arterial pressure by modulation of osmosensitive pathways that regulate plasma vasopressin and peripheral sympathetic nerve activity. For example, upregulation of central vasopressin receptors (56) or increased vasopressin levels, either measured directly in plasma (49) or excreted in urine (10), have been observed following DOCA-salt treatment. Similarly, evidence for increased sympathetic nerve activity (SNA) in DOCA-salt animals has been reported by several laboratories (6, 9, 13, 37, 41, 57).
Central administration of amiloride, or its analog benzamil, has been reported to attenuate sympathetic activity and hypertension in several salt-sensitive models, including the DOCA-salt model (21, 34, 41, 61, 62). These data suggest an interaction between dietary salt, benzamil-sensitive proteins (BSPs) in the brain, and SNA; however, it remains to be shown where within autonomic pathways these BSPs are located (1). While it is still unknown which BSPs in the brain are involved in DOCA-salt hypertension, ion channels permeable to sodium, such as the epithelial sodium channel (ENaC) or the acid-sensitive ion channel (ASIC) are likely candidates (1, 27). Both the ENaC and the ASIC are members of the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. ENaCs have long been known for their role in modulating sodium transport in renal tubules and are thought to play a critical role in maintaining normal arterial pressure (7, 35). In addition, both ENaCs and ASICs may play a broad role in maintaining pressure, including in local pressure-induced vasoconstriction and in modulating the baroreceptor reflex (14). Another large group of BSPs include ion transport systems, such as the Na+/H+ exchanger, the Na+/Ca2+ exchanger, the Na+ pump, and the Ca2+ pump. These proteins may play a role in the development of certain forms of hypertension. The Na+/H+ exchanger, for instance, has been implicated in the development of hypertension in humans (51). However, while amiloride and benzamil are inhibitors of the ion transport systems, these proteins have higher affinity for other amiloride analogs, such as 3′,4′-dichlorobenzamil, 2′,4′-dimethylbenzamil, 5-(N-ethyl-N-isopropyl) amiloride, and 5-(N-methyl-N-isobutyl) amiloride (34). Benzamil is only effective at modulating activity of the ion transport systems when used at high doses, significantly higher than what is necessary to inactivate the ENaCs or ASICs.
Our current understanding of the neural pathways mediating salt-dependent forms of hypertension has been derived, at least in part, from studies employing acute osmotic stressors and extrapolating those results to long-term salt sensitivity of arterial pressure. These studies suggest a critical role for the hypothalamic paraventricular nucleus (PVN), an important brain site for the control of arterial pressure and a downstream target of the salt-sensitive forebrain circumventricular organs (32). The PVN plays a role in regulating plasma vasopressin through activity of its magnocellular neurons and lesions of the PVN attenuate several forms of hypertension (12, 24). Signals from subpopulations of parvocellular neurons in the PVN are thought to affect SNA through two pathways, either 1) by converging on sympathetic premotor neurons in the rostroventrolateral medulla (RVLM), a key brain stem site for regulation of spinal sympathetic preganglionic neurons in the intermediolateral cell column (IML) of the spinal cord, or 2) through synapses directly at the IML (26, 46, 53). With the use of c-Fos immunoreactivity as an indicator of neuronal activity, it has been reported that short-term DOCA-salt treatment (up to 8 days) increases activity of many of these key brain sites (45). However, this approach has not been used to study responses to long-term DOCA-salt treatment.
While BSPs seem to play a role in the DOCA-salt model of hypertension, the exact role of these proteins, as well as their location in specific sites within central neural pathways that regulate sympathetic activity, are unknown. Moreover, while it has been shown that the steady-state level of hypertension in DOCA-salt rats is attenuated by ICV benzamil (40), it is unknown how benzamil treatment affects the time course of DOCA-salt hypertension.
The present study was designed to test two hypotheses: 1) that chronic DOCA-salt treatment is correlated with changes in central autonomic activity as measured by c-Fos immunoreactivity that regulate fluid balance and arterial pressure, and 2) that ICV benzamil treatment will attenuate these changes in central neuronal activity and arterial pressure.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (200–225 g) were purchased from Charles River Laboratories and single housed with ad libitum access to distilled water and 0.4% NaCl chow. All procedures were approved by the University of Minnesota Animal Care and Use Committee and were conducted in accordance with the institutional and National Institutes of Health guidelines.
Protocol 1: Effect of Chronic ICV Infusion of Benzamil on the Time Course of DOCA-Salt Hypertension and Central Neuronal Activity
The purpose of this protocol was to establish the effect of ICV benzamil on the temporal pattern of DOCA-salt hypertension and on the response of central autonomic neurons during the steady-state phase of hypertension. Four groups were studied 1) Vehicle-Vehicle (n = 6), served as a control and treated with both ICV vehicle and systemic vehicle; 2) Vehicle-DOCA (n = 6), treated with ICV vehicle + 50 mg DOCA sc; 3) Benzamil-Vehicle (n = 7), treated with ICV benzamil (16 nm/day) and systemic vehicle; and 4) Benzamil-DOCA (n = 7), treated with ICV benzamil + 50 mg DOCA sc.
Experimental procedures.
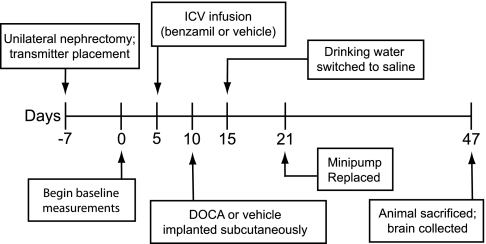
Figure 1 illustrates an overview of the experimental protocol. On study day −7, rats were anesthetized with isoflurane, and a right unilateral nephrectomy was performed as previously described with the exception that the kidney was approached ventrally rather than dorsally (29). At the same time, a radio telemetry transmitter (model TA11PA-C40; Data Sciences, St. Paul, MN) was placed for measurement of mean arterial pressure (MAP) and heart rate (HR).
Fig. 1.
Timeline of the surgical procedures and experimental protocol. ICV, intracerebroventricular.
Following a 7-day recovery period, on study day 1, control measurements of MAP, HR, and fluid ingestion were begun. The radio telemetry transmitter signal was sampled as previously described (29), and 24-h fluid intake was measured daily throughout the protocol.
On day 5, rats were anesthetized with isoflurane, and under stereotaxic guidance, a brain infusion kit (model BIK2, 3–5 mm; Alzet Osmotic Pumps, Cupertino, CA) was placed, targeting the lateral ventricle (relative to bregma: −0.15 mm posterior, 0.15 mm lateral on the right side, and lowered ventrally 0.35 mm beneath the pial surface). A catheter was connected to the brain infusion kit and tunneled caudally to a subcutaneous pocket on the dorsal surface of the rat, posterior to the scapulae. The brain infusion kit was secured to the skull with jeweler screws and dental cement (Stoelting, Woodale, IL), and the catheter was connected to an Alzet osmotic minipump (model 2004), delivering either benzamil or its vehicle at a flow rate of 0.5 μl/h. Benzamil rats received 16 nanomoles of benzamil per day, based on previous studies (28, 40) and our own preliminary experiments. Given the rate of rat cerebrospinal fluid production (3.7 ± 0.1 μl/min) (23) and the volume infused by the minipump (12 μl/day), this should result in a roughly 3 μM benzamil solution in the cerebrospinal fluid.
Five days later, on study day 10, silicone pellets containing either 0 or 50 mg DOCA were placed subcutaneously (29). On day 15 all groups were switched from ad libitum access to water to ad libitum access to 0.9% NaCl + 0.2% KCl solution. Data were collected during a 5-day baseline period and for 32 days following introduction of saline. On study day 21, rats were anesthetized with isoflurane, and the minipump was replaced with a new pump delivering the same solution, to ensure continuous infusion for the length of the study protocol. On day 47 of the protocol, animals were deeply anesthetized with isoflurane and transcardially perfused with ∼150 ml heparinized saline, followed by 500 ml 4% paraformaldehyde in 0.1 M PBS.
Protocol 2: Effect of Early Termination of ICV Benzamil on the Activity of Central Neuronal Pathways in DOCA-Salt Rats
In protocol 1, benzamil administration was initiated 5 days prior to the implantation of DOCA and 10 days prior to the start of saline ingestion. Theoretically, this is a sufficient duration for the induction of a compensatory upregulation of benzamil-insensitive proteins that indirectly may alter central autonomic activity and arterial pressure. Therefore, any group differences observed in protocol 1 could result either from the direct effects of benzamil or from upregulation of benzamil-insensitive proteins. The purpose of protocol 2 was to determine whether the continuous blockade of BSPs was necessary to explain the results of protocol 1. Therefore, a fifth group of rats, called the Benzamil-Stop group, was studied (n = 7). In this group, the experimental procedures were identical to protocol 1, with the exception that rats received ICV benzamil only until study day 21 at which point the osmotic minipumps were switched to deliver ICV vehicle. On day 47 of the protocol, animals were deeply anesthetized with isoflurane and transcardially perfused as described above.
Brain processing.
Brains were removed and stored overnight in 4% paraformaldehyde and then switched into a 30% sucrose solution and incubated for 3 days at 4°C. Brains were then blocked in optimal cutting temperature mounting media (Miles, Elkhart, IN) and stored overnight in a −80°C freezer. Brain sections (40 um) were cut using a cryostat (JUNG CM 1800; Leica). Using the atlas of Paxinos and Watson (44) as a guide, we collected brain sections containing regions of interest, and the brain sections were either used immediately for immunohistochemistry or stored in cryoprotectant (30% ethylene glycol and 20% glycerol in 0.05 M PBS) at −20°C until the time of labeling. All stored sections were washed in PBS prior to immunohistochemistry protocol.
Immunohistochemical procedures.
Immunolabeling for c-Fos and arginine vasopressin (AVP) was done as described previously (66) with minor modifications. Brain sections were washed in PBS and then blocked for 10 min in 3% H2O2 in 10% methanol. After being rinsed with PBS, sections were blocked for 1 h in 20% normal goat serum (Vector Laboratories; Burlingame, CA) at room temperature, incubated for 48 h at 4°C with rabbit anti-Fos primary antibody (Ab-5; Oncogene, San Diego, CA) diluted 1:100,000 in PBS with Triton (0.3% Triton X-100 in PBS) with 2% normal goat serum. Using a Vectastain ABC kit (Vector Laboratories), we then incubated sections for 1 h at room temperature with goat anti-rabbit biotinylated secondary antibody, followed by 1-h incubation with the avidin-biotin complex. c-Fos labeling was visualized using diaminobenzidine with nickel (Vector Laboratories), resulting in a black/purple nuclear staining. Sections from each brain containing PVN or supraoptic nucleus (SON) were then double labeled for AVP using the same procedure described for c-Fos labeling. Primary rabbit anti-AVP (1:160,000; Chemicon, Temecula, CA) antibody was visualized using diaminobenzidine without nickel, resulting in brown cytoplasmic staining. Sections were washed, mounted, and dried on glass slides, dehydrated, and coverslipped in distyrene/plasticizer/xylene (DPX) media (Sigma, St. Louis, MO) or Permount media (Fisher Scientific, Hampton, NH). Labeling for each of the antibodies was eliminated when the primary antibodies were omitted or preabsorbed with their cognate peptides (50 μg/ml, 4°C, overnight).
Quantification of c-Fos labeling.
c-Fos-positive nuclei were counted in the following regions: median preoptic nucleus (MnPO), PVN, SON, nucleus of the tractus solitarius, RVLM, dorsal motor nucleus of the vagus, and the nucleus ambiguous. Brain regions were identified based on location and morphology, following the Paxinos and Watson atlas (44). RVLM was collected immediately posterior to the caudal-most aspects of the facial nucleus, and defined as the triangular region bordered dorsally by the nucleus ambiguous, medially by the inferior olive/pyramidal tracts, and laterally by the spinal nucleus of the trigeminal nerve. For each brain nucleus or region of interest, c-Fos immunoreactivity was quantified in two tissue sections. With the exception of MnPO, areas were counted bilaterally in each tissue section; therefore, four sections of each of the nuclei were quantified blind to treatment group. Brain sections were collected from the midrostrocaudal level of the PVN that included the posterior magnocellular (pm) PVN and medial parvocellular (mp) PVN, and further caudally, which included both medial and lateral parvocellular divisions, as defined previously (53, 55). In the PVN, the parvocellular AVP-positive neurons were distinguished from the magnocellular neurons based on size and location. Cells were counted only if they were clearly located within each region, but not at the borders between subnuclei. In the more caudal PVN section, borders could not easily be visualized either with the aid of AVP staining or through cellular morphology; thus, all labeled parvocellular neurons were quantified in a single category as caudal parvocellular PVN neurons. Within the pmPVN and the SON, only double-labeled neurons were counted. In all other regions, all c-Fos-positive neurons were counted, regardless of vasopressin label. All counting was done blind to treatment group. In no cases did the individual counts differ by > 10%. Optical images were collected using a digital camera. Color in photomicrographs was auto-leveled using Adobe CS3 software.
Statistical Analyses
Data are presented as means ± SE. Statistical differences for MAP, fluid ingestion, and HR were assessed by repeated-measures ANOVAs; when ANOVA showed a significant difference within an experiment, post hoc analyses using Holms-Sidak corrections for multiple comparisons were used to identify differences between groups. Statistical assessments of c-Fos differences between groups were made for each region using two-way ANOVAs, employing Holms-Sidak corrections for multiple comparisons. Differences were considered statistically significant when the test yielded a P < 0.05.
RESULTS
Protocol 1: Effect of Chronic ICV Infusion of Benzamil on the Time Course of DOCA-Salt Hypertension and Central Neuronal Activity
Baseline values.
This study was designed to assess the effect of ICV benzamil on DOCA-salt hypertension and changes in central nervous system activity reflected by c-Fos labeling. Results are expressed as a change from baseline values for fluid ingestion, MAP, and HR. Baseline values were calculated as the mean of values collected during the 5-day baseline period for each rat. Group means ± SE are reported in Table 1. No differences between groups were observed in any of these variables during the baseline period.
Table 1.
Baseline values for mean arterial pressure (MAP), fluid ingestion, and heart rate, for all groups
| No. | MAP, mmHg | Fluid Ingestion, ml/day | HR (BPM) | |
|---|---|---|---|---|
| Vehicle-Vehicle | 6 | 100.4 ± 2.1 | 25.3 ± 0.8 | 442 ± 3 |
| Vehicle-DOCA | 6 | 100.9 ± 0.9 | 25.1 ± 0.9 | 450 ± 6 |
| Benzamil-Vehicle | 7 | 102.1 ± 0.9 | 25.3 ± 0.7 | 425 ± 4 |
| Benzamil-DOCA | 7 | 99.7 ± 0.7 | 25.5 ± 1.2 | 453 ± 3 |
| Benzamil-Stop | 7 | 101.1 ± 0.8 | 25.4 ± 0.8 | 435 ± 3 |
Arterial pressure, fluid ingestion, and HR.
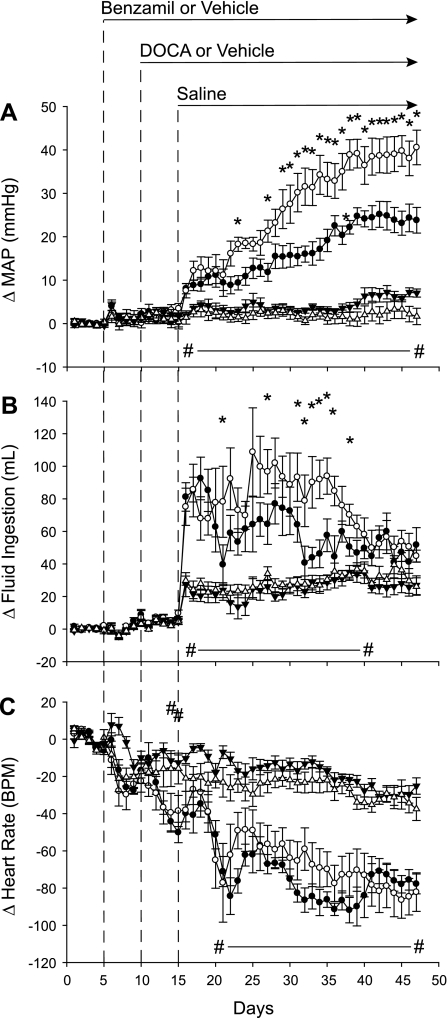
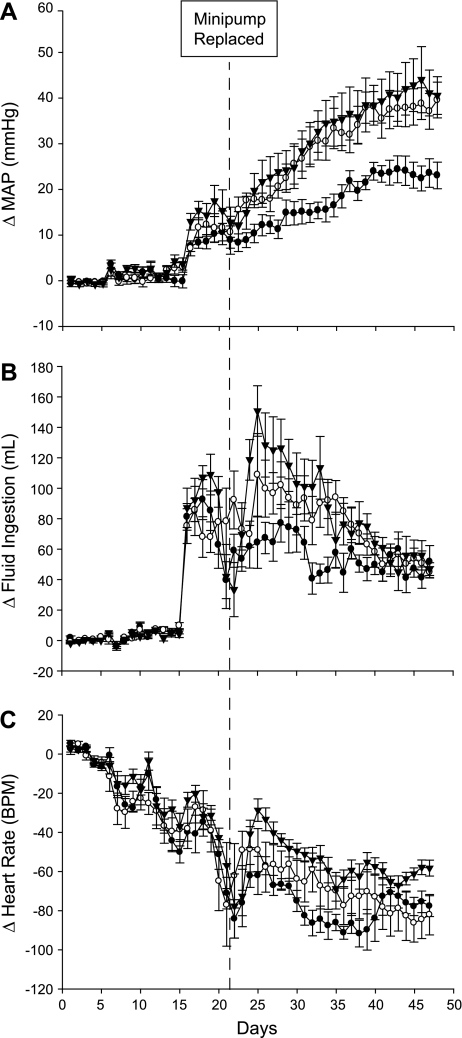
As we have reported previously (29, 42), DOCA-salt treatment increased MAP and fluid intake and decreased HR (Fig. 2). In this study, MAP (Fig. 2A) and fluid ingestion (Fig. 2B) were elevated following DOCA-salt treatment; however, ICV benzamil treatment reduced both responses. By the end of the study, MAP was reduced by nearly 50% in Benzamil-DOCA animals (increase of ∼ 25 mmHg) compared with Vehicle-DOCA animals (increase of ∼40 mmHg). While both DOCA groups increased fluid ingestion when drinking water was replaced with saline, Benzamil-DOCA rats drank less than did Vehicle-DOCA rats (Fig. 2B). HR (Fig. 2C) decreased throughout the protocol, such that all groups had significantly lower HRs than their own baselines by the end of the study. However, DOCA groups experienced greater bradycardia, with no differences observed between the two DOCA groups. Benzamil-Vehicle rats showed similar profiles to Vehicle-Vehicle rats, indicating that benzamil effects were specific to DOCA-salt animals, and not a result of a generalized effect of ICV benzamil treatment.
Fig. 2.
Changes in arterial pressure, fluid ingestion, and heart rate (HR) for Vehicle-Vehicle (n = 6, ▵), Vehicle-DOCA (n = 6, ○), Benzamil-Vehicle (n = 7, ▴), and Benzamil-DOCA (n = 7, ●) rats (24-h averages). Experimental interventions are noted by dotted lines. A: mean arterial pressure (MAP) increased in both DOCA groups compared with the Vehicle/Vehicle group, but the response of the Benzamil-DOCA group was reduced compared with the Vehicle-DOCA group. *P < 0.05, Benzamil-DOCA vs. Vehicle-DOCA rats; #P < 0.05, Vehicle-DOCA vs. Vehicle-Vehicle rats. B: fluid ingestion increased in both DOCA groups compared with the Vehicle-Vehicle group, but the response of the Benzamil-DOCA group was reduced compared with the Vehicle-DOCA group. *Days in which Benzamil-DOCA rats ingested less fluid than did Vehicle-DOCA rats; #days in which Vehicle-DOCA rats ingested more fluid than did Vehicle-Vehicle controls. C: both DOCA groups had lower HRs than did Vehicle-Vehicle rats; differences were not observed between the 2 DOCA groups; #days on which Vehicle-DOCA rats had lower HRs than did Vehicle-Vehicle controls. BPM, beats per minute.
c-Fos immunolabeling.
Experiments tested the hypothesis that chronic DOCA-salt treatment increases neural activity and that chronic ICV benzamil reduces the response. Changes in neural activity were assessed by monitoring c-Fos expression in hypothalamic and brain stem nuclei. To assess specific activation of magnocellular vasopressinergic neurons, c-Fos expression was quantified in AVP-positive neurons localized in the pmPVN (Fig. 3) and in the SON. For other PVN subnuclei (denoted in Fig. 3), only single-labeled c-Fos-positive neurons were counted.
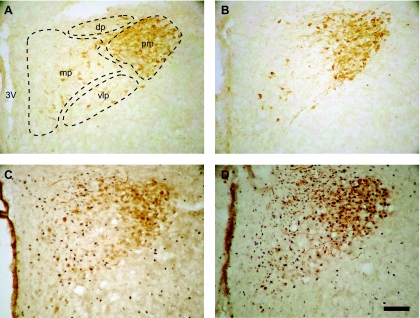
Fig. 3.
Immunolabeling for c-Fos (nuclear, black) and vasopressin (cytoplasmic, brown) in midrostral hypothalamic paraventricular nucleus (PVN) from Vehicle-Vehicle (A), Benzamil-Vehicle (B), Benzamil-DOCA (C), and Vehicle-DOCA (D) rats. Scale bar = 100 μ. 3V, third ventricle; mp, medial parvocellular neurons; dp, dorsal parvocellular neurons; vlp, ventrolateral parvocellular neurons; pm, posterior magnocellular neurons.
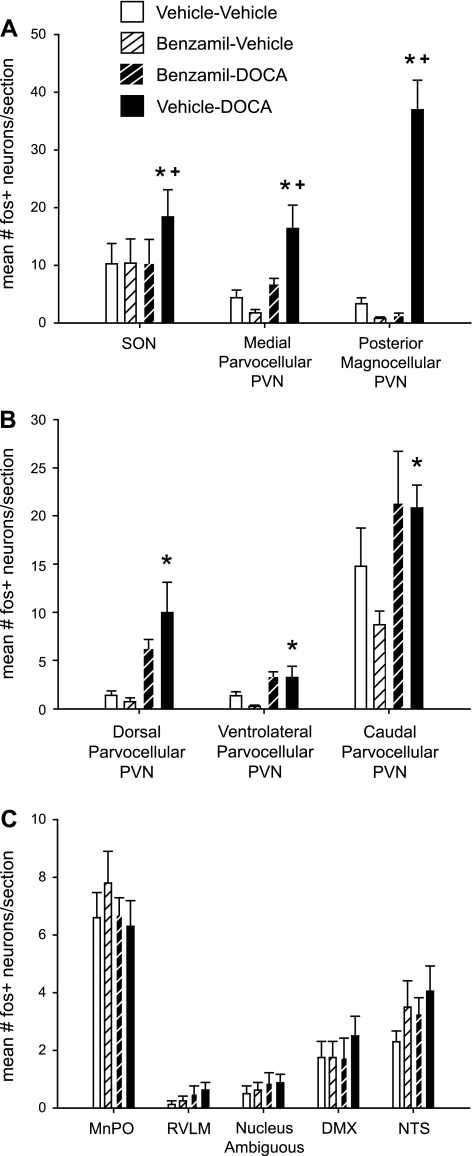
Compared with the Vehicle-Vehicle control group, c-Fos expression was increased by DOCA treatment in the SON and in all subnuclei of the PVN (Fig. 4, A and B); however, the effect of benzamil on these responses was not similar. More specifically, shown in Fig. 4A are regions in which c-Fos immunoreactivity in Benzamil-DOCA rats was statistically less than Vehicle-DOCA rats, the SON, and the pmPVN, which regulate plasma vasopressin, and the mpPVN, which regulates the anterior pituitary. In contrast, shown in Fig. 4B are regions in which Benzamil-DOCA rats were not statistically different compared with the Vehicle-DOCA group: the dorsal, ventrolateral, and caudal PVN, which are linked to regulation of sympathetic activity.
Fig. 4.
Quantification of c-Fos immunoreactivity across brain regions. A: regions showing a main effect of DOCA-salt treatment, which was significantly moderated by benzamil treatment (P < 0.05, *significant difference from Vehicle-Vehicle, +significant difference from Benzamil-DOCA). In all cases, DOCA-salt treatment increased c-Fos immunoreactivity, while benzamil treatment reduced this effect. This effect was seen most dramatically in the pmPVN neurons, but was also observed in the supraoptic nucleus (SON) and in mpPVN neurons. Note that counts in pmPVN and SON reflect only double-labeled neurons. B: regions showing a main effect of DOCA-salt treatment (P < 0.05), with no response to ICV benzamil (P < 0.05, *significant difference from Vehicle-Vehicle). DOCA-salt treatment increased c-Fos immunoreactivity in the dorsal parvocellular PVN, the ventrolateral parvocellular PVN, and in the more posterior mixed region of caudal parvocellular PVN neurons (containing both medial and lateral parvocellular neurons). C: regions that did not respond to DOCA-salt treatment with increased c-Fos immunoreactivity. We observed no group differences in c-Fos immunoreactivity within the median preoptic nucleus (MnPO), the rostroventrolateral medulla (RVLM), the nucleus ambiguous, the dorsal motor nucleus of the vagus nerve, or the nucleus of the tractus solitarius. DMX, dorsal motor nucleus of the vagus nerve; NTS, nucleus of the tractus solitarius.
c-Fos immunoreactivity was also assessed in several extra-hypothalamic nuclei associated with cardiovascular control, including the MnPO, dorsal motor nucleus of the vagus, RVLM, nucleus ambiguous, and nucleus of the tractus solitarius (Fig. 4C). These nuclei did not exhibit increases in c-Fos immunoreactivity following DOCA-salt treatment. In fact, very few c-Fos positive neurons were seen in these nuclei in any treatment group, with the exception of the MnPO area (Fig. 4C); moderate levels of neural activation of the MnPO were observed in all groups, perhaps in response to ingested saline.
Protocol 2: Effect of Early Termination of ICV Benzamil on the Activity of Central Neuronal Pathways in DOCA-Salt Rats
To determine whether continuous blockade of BSPs was necessary for the attenuation in MAP and fluid ingestion observed in protocol 1, we measured MAP and fluid ingestion in Benzamil-Stop rats, receiving benzamil only until study day 21. Despite an initial period of benzamil treatment, the Benzamil-Stop rats were most similar to the Vehicle-DOCA rats of protocol 1, not the Benzamil-DOCA group. As shown in Fig. 5A, MAP was indistinguishable between the Vehicle-DOCA rats in protocol 1 and the Benzamil-Stop rats in protocol 2. Both groups exhibited a rapid rise in MAP immediately following DOCA-salt treatment, followed by a slower increase in MAP in subsequent days. The rise in MAP observed during the entire length of the protocol was significantly greater in the Benzamil-Stop rats than in the Benzamil-DOCA animals but did not differ from the Vehicle-DOCA rats.
Fig. 5.
Effect of early cessation of benzamil treatment. Shown are 24-h means for MAP (A), fluid ingestion (B), and HR (C) for Vehicle-DOCA (n = 7, ○, animals from protocol 1), Benzamil-DOCA (n = 7, ●, animals from protocol 1), and Benzamil-Stop (n = 7, ▴, animals from protocol 2) rats. The day of the minipump replacement surgery, when Benzamil-Stop rats ceased benzamil treatment, is noted by a dotted line. P < 0.05 for all effects noted. A: 24-h averages for MAP. Benzamil-Stop rats showed identical pressure profiles to Vehicle-DOCA rats, and were significantly different from Benzamil-DOCA rats. B: while fluid ingestion initially matched between the 2 benzamil groups, when Benzamil-Stop animals stopped receiving benzamil treatment, their fluid ingestion rapidly rose to match that of the Vehicle-DOCA rats, with no significant differences observed between Vehicle-DOCA and Benzamil-Stop rats. C: all treatment groups showed similar HR decreases with no significant between group differences observed. All 3 groups showed a significant decrease in HR over the course of the study.
Fluid ingestion was calculated daily for these rats (Fig. 5B). Fluid ingestion during the first week of the protocol in the Benzamil-Stop group was similar to drinking in the Benzamil-DOCA group, but this was followed by a dramatic off response beginning 2 days after benzamil treatment was stopped. Drinking in the Benzamil-Stop group rapidly increased to match the Vehicle-DOCA group.
Changes in HR over time were similar between the three groups, with significant decreases in HR observed when comparing animals at the end of the study to their own baselines (Fig. 5C).
DISCUSSION
Effect of ICV Benzamil on the Phases of DOCA-Salt Hypertension
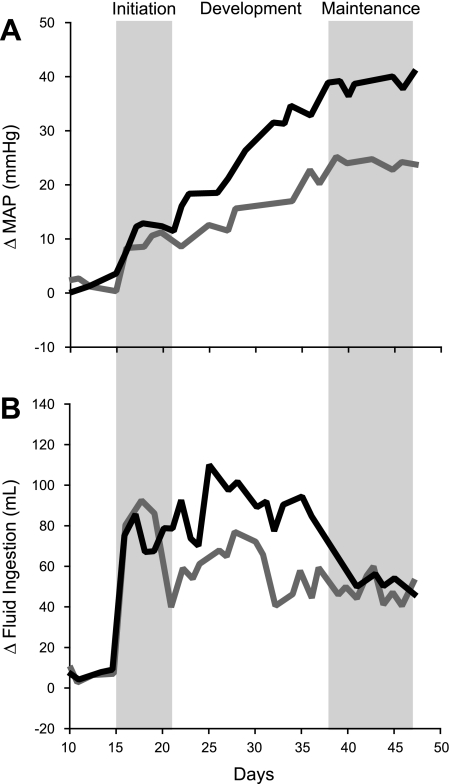
This study was designed to test the hypothesis that hypertensive effects of DOCA-salt treatment are associated with increased neuronal activity in key cardiovascular nuclei and that both hypertension and DOCA-salt induced increases in neuronal activity will be attenuated by ICV benzamil treatment. DOCA-salt treatment resulted in a robust increase in MAP, and this was attenuated by ICV benzamil treatment as previously reported (34, 40). However, in contrast with previous studies that utilized intermittent indirect measurement of arterial pressure in restrained rats over treatment periods of 7 (40) or 14 (34) days of ICV benzamil, the present study employed direct, continuous telemetric recordings of pressure in unrestrained rats 5 days before and 42 days during ICV benzamil administration. The strength of this approach is it provides much greater detail regarding each phase of DOCA-salt hypertension and the effect of ICV benzamil treatment on each phase. The effect of ICV benzamil on the temporal profile of DOCA-salt hypertension is summarized in Fig. 6. Data from Fig. 2 for Vehicle-DOCA (black line) and Benzamil-DOCA (gray line) groups are replotted in stylized form without data points and error bars for clarity. Based on 1) the rate of change of arterial pressure, 2) fluid ingestion, and 3) the effect of ICV benzamil on these variables, DOCA-salt hypertension appeared to develop in three distinct phases; an initiation phase, a developmental phase, and a maintenance phase.
Fig. 6.
Phases in the development of DOCA-salt hypertension. Data from protocol 1 are replotted to highlight putative phases in the development of DOCA-salt hypertension. Vehicle-DOCA rat are indicated by the black line and Benzamil-DOCA rats are shown by the gray line. During the initiation phase, MAP increased rapidly in DOCA-salt-treated rats, followed by a brief plateau. During this same time, fluid ingestion also showed a marked increase. During the developmental phase, Vehicle-DOCA rats exhibited a second, more gradual increase in arterial pressure and maintained high levels of fluid ingestion. In the Benzamil-DOCA animals, a shift to the developmental phase was noted when fluid ingestion declined, with significantly less saline ingested in Benzamil-DOCA rats than Vehicle-DOCA rats on the transition day. Benzamil-DOCA rats also experienced a second, more gradual increase in MAP, but this was more modest than the rise exhibited by Vehicle-DOCA rats. During the maintenance phase, both groups showed stable levels of MAP, significantly above that of control animals, and both groups showed reduced fluid ingestion.
Although we recognize that the precise time points marking the transition from one phase to the next is somewhat subjective, our rationale is the following. Comparison of the time course changes in arterial pressure and fluid ingestion (i.e., salt intake) of the two DOCA-salt groups in protocol 1 (ICV vehicle vs. ICV benzamil-treated), three phases emerge. Keeping in mind that ICV vehicle or benzamil was initiated on day 5 (not shown in Fig. 6), DOCA was implanted on day 10, and then saline was introduced on day 15, the initiation phase was characterized as an immediate rapid rise in arterial pressure during the first 2–3 days of the beginning of DOCA-salt treatment (day 15), which then stabilized for another 3–4 days. This phase was also associated with a profound increase in fluid intake compared with non-DOCA-treated rats. However, the increase in arterial pressure and fluid ingestion was similar in vehicle and benzamil-treated DOCA-salt rats during this phase. The developmental phase was marked by the more gradual rise in arterial pressure in DOCA-salt rats, and fluid ingestion remained elevated during most of this phase but began to decrease on day 35 in vehicle-treated DOCA-salt rats. In contrast with the initiation phase, ICV benzamil attenuated both the increase in arterial pressure and fluid intake during the developmental phase. Finally, the maintenance phase was characterized by a stabilization of arterial pressure and fluid intake in both vehicle and benzamil-treated DOCA-salt rats. However, as a result of the gradual decline of fluid intake in vehicle-treated DOCA-salt rats at the end of the developmental phase, and the attenuation of fluid intake in benzamil-treated DOCA-salt rats, this variable was similar in these groups during the maintenance phase. Based on this subjective phase analysis, we conclude that activation of BSPs does not contribute to the initiation phase but is critical to the developmental phase of DOCA-salt hypertension.
The observation that ICV benzamil reduced both saline intake and arterial pressure during the developmental phase suggests that at least part of the antihypertensive actions of centrally administered benzamil is secondary to the decreased sodium load. Interestingly, we observed a similar effect of renal denervation on saline intake and arterial pressure during the developmental phase of DOCA-salt hypertension (29). Indeed, the effect of renal denervation on both the magnitude and temporal pattern of arterial pressure and saline intake was essentially identical to the effect of ICV benzamil observed in the present study. In our previous study (29), we investigated the relationship between reduced saline intake and arterial pressure observed in renal-denervated rats by including a control group of intact rats in which saline intake was matched to that observed in renal-denervated rats. Restriction of saline intake in control rats resulted in the same reduction of arterial pressure during the developmental phase of DOCA-salt hypertension as that observed in renal-denervated rats. We concluded that part of the effect of renal denervation on the pathogenesis of DOCA-salt hypertension was the result of the loss of renal afferent nerves that regulate salt appetite (29). However, we have also observed that lesion of the subfornical organ, which results in more modest reductions of saline intake, compared with renal denervation or ICV benzamil, has no effect on arterial pressure during any stage of DOCA-salt hypertension (29, 42). Taken together these studies suggest that the effect of ICV benzamil on arterial pressure may be due, in part, to reduced saline intake resulting in decreased stimulation of osmosensitive pathways that drive vasopressin release and sympathetic nerve activity.
It is important to note that ICV benzamil attenuates the acute pressor response to ICV hypertonic saline as well as the slow-pressor response observed in the DOCA-salt model (1, 40, 41). Blockade of pressure responses to ICV hypertonic saline suggests that BSPs mediate this response. However, the effect of chronic ICV administration of benzamil could also be due to another protein or proteins that are upregulated in the face of long-term inactivation of BSPs. ICV benzamil treatment in protocol 1 began 5 days prior to DOCA treatment, and 10 days prior to the presentation of saline in the water bottles. This time course provides ample opportunity for the upregulation of other proteins to compensate for the loss of BSPs, potentially making benzamil blockade irrelevant to the study after the initial treatment period. Although results from protocol 1 suggested that benzamil treatment reduced MAP, fluid ingestion, and c-Fos immunoreactivity in a cell-specific manner, it was unclear whether these results were due to the direct effects of benzamil or a result of upregulation of benzamil-insensitive proteins following blockade of BSPs. Therefore, a fifth group of rats, receiving benzamil until study day 21 (i.e., during the initiation phase) and then receiving ICV vehicle for the remainder of the study, were included to assess this question. Benzamil-Stop rats quickly recovered from benzamil-induced attenuation of DOCA effects and were indistinguishable from Vehicle-DOCA rats. These results indicate that continued activity of BSPs is necessary for the DOCA-salt model to develop normally and that continuous blockade of BSPs is required for attenuation of MAP and drinking observed following ICV benzamil.
Effect of ICV Benzamil on Central Neuronal Activation in DOCA-Salt Rats
Chronic DOCA-salt hypertension resulted in increased c-Fos immunoreactivity in select nuclei, an indication of continuing high levels of neuronal activity in this model. To our knowledge, this is the first time that c-Fos has been used to demonstrate increased neuronal activity in chronic DOCA-salt hypertension. Although it may be surprising that immediate early gene activity could be used in a chronic model, this approach has been successfully employed in the chronic renal-wrap model of hypertension (11). In addition, c-Fos immunoreactivity is increased in rats following 8 days of DOCA treatment (45).
Consistent with observations that DOCA-salt treatment increases plasma vasopressin levels (39), as well as plasma norepinephrine and SNA (9, 57), we observed increased c-Fos immunoreactivity across the subnuclei of the PVN. PVN neurons in the dorsal cap (dorsal parvocellular neurons), as well as the ventrolateral portion of the PVN, have been shown to be involved in modulating SNA and are part of sympathetic pathways, either through projections to the RVLM, or through direct projections from these PVN nuclei to the IML of the spinal cord (36, 43, 53, 58). Although PVN neurons in the mp subdivision of PVN contain neurons associated with corticotrophin-releasing hormone release (50) we are not aware of any reports of increased plasma corticosteriods in DOCA-salt hypertension.
While DOCA-salt hypertension increased c-Fos immunoreactivity in a variety of brain regions, benzamil treatment reduced c-Fos immunoreactivity in a brain region-specific manner. The most dramatic effect of benzamil treatment was in the pm neurons of the PVN. The pmPVN contains both oxytocinergic and vasopressinergic cells; however, tissue was double-labeled for both c-Fos and vasopressin and only double-labeled cells were counted, indicating a role for vasopressin neurons in the benzamil-sensitive pathways involved in the DOCA-model. While we did not quantify the c-Fos immunoreactivity in pmPVN neurons that were not vasopressin-positive, almost all c-Fos immunoreactivity was expressed in double-labeled cells. This observation is consistent with published reports, showing an increase in vasopressin but not oxytocin mRNA and protein levels in PVN and SON, following chronic DOCA-salt treatment (22, 49). In addition to effects seen in the pmPVN neurons, we also observed a benzamil-induced reduction in c-Fos immunoreactivity in other neuroendocrine cell populations, such as the SON (also vasopressinergic, magnocellular) and the parvocellular mpPVN. While there are a variety of phenotypic types within the mpPVN subnucleus, this subnucleus is considered to be a neuroendocrine site because of its role in regulating the secretion of anterior pituitary hormones (3). Finally, although benzamil-treated DOCA-salt rats tended to have less c-Fos immunoreactivity in the dorsal cap of the PVN, compared with Vehicle-DOCA rats, this was not statistically significant as was the case with other regions known to regulate SNA, such as the ventrolateral and caudal parvocellular PVN.
Consistent with the idea that vasopressinergic neurons play a key role in the model, previous studies suggest that a vasopressinergic component of DOCA-salt hypertension is necessary to sustain the model and can be differentiated from sympathetic pathways activated by DOCA-salt treatment. Berecek et al. (4) showed that lesions of the anteroventral third ventricle (AV3V) region block DOCA-salt hypertension. While AV3V lesions could disrupt both sympathetic pathways and vasopressinergic pathways, in their study exogenous vasopressin was administered to AV3V-lesioned DOCA-salt animals. Following vasopressin administration, plasma vasopressin was restored to levels seen in sham-lesioned animals; arterial pressure, on the other hand, reached an intermediate level, between sham-lesioned DOCA-salt animals and lesioned DOCA-salt animals that had not received vasopressin. This result would be expected if activity of vasopressin-related pathways were restored, but SNA remained attenuated. This is consistent with our results in that DOCA-salt rats showed increased MAP and increased c-Fos immunoreactivity across all subnuclei of the PVN, while Benzamil-DOCA rats showed attenuated arterial pressure and reduction only in neuroendocrine populations of PVN neurons. Unfortunately we did not measure plasma vasopressin or sympathetic activity in this study. However, Nishimura et al. (41) reported that ICV benzamil reduces 24-h urinary vasopressin and norepinephrine excretion in DOCA-sat rats, suggesting that both plasma vasopressin and sympathetic activity are decreased by the actions of benzamil in the brain. Although this seems to contradict the fact that we did not observe a statistical effect of benzamil treatment on subnuclei of the PVN that regulate SNA, a modest decrease was seen in the dorsal parvocellular region. Finally, because certain cardiovascular regulatory regions did not show increased c-Fos immunoreactivity under any treatment conditions, (e.g., RVLM, etc.) we cannot rule out the possibility that BSPs are present and playing a role in these populations of neurons. For example, it is possible that neurons of the RVLM are benzamil-sensitive, but given the lack of c-Fos immunoreactivity, we were unable to detect this responsivity. Further studies are necessary to elucidate the precise role of BSPs in the model.
The RVLM is a critically important cardiovascular regulatory site in the central nervous system and a major brain stem site for regulation of spinal sympathetic preganglionic neurons in the IML (54). The RVLM plays an essential role in short-term modulation of IML sympathetic outflow in response to changes in arterial pressure, via the baroreflex pathway. Interestingly, the baroreceptors themselves are benzamil sensitive (15) and rely on activity of the ENaC and possibly ASIC BSPs (16, 47). It has been postulated that, in addition to its role in modulating SNA in response to acute fluctuations in MAP, the RVLM most likely also plays a role in chronic regulation of SNA. However, in the present study we did not observe increased activity of RVLM neurons of DOCA-salt rats based on c-Fos immunoreactivity. One possible explanation for the lack of activity in RVLM is that other brain sites drive SNA in this model. For example, the PVN modulates SNA via its projections to RVLM, but also has direct projections to IML. These direct PVM-spinal projections originate largely in the dorsal cap, as well as in the ventrolateral portions of the PVN. These projections may play an important role in the increased SNA observed in hypertension: while we did not observe changes in c-Fos immunoreactivity in RVLM following DOCA-salt treatment, we observed increased activity across PVN subnuclei. However, we have recently reported that blockade of spinal level V1a receptors has no effect on arterial pressure in DOCA-salt rats, suggesting that spinally projecting vasopressinergic neurons are not activated in this model (60). While data from the present study do not support a role for the RVLM in the chronic DOCA-salt model, it may be that activity of RVLM is necessary at an earlier time point or that c-Fos is not a reliable marker of increased neuronal activity in RVLM neurons.
Expression of Fra-like activity (fos family gene), another marker of neuronal activity, has been used to study responses of hypothalamic neurons to peripheral aldosterone combined with high-salt intake in rats (67). This group reported increased activity in the PVN neurons, and many of these cells were also labeled for the MR. Unfortunately, neither subregions of the PVN nor the RVLM were analyzed. In another study, Fra-like activity was measured in the PVN of rats treated with ICV vehicle or aldosterone. In agreement with the present study, this neuronal marker indicated increased activity in pm, dorsal parvocellular, and ventrolateral parvocellular PVN (68). Unfortunately, the response of mp and caudal parvocellular PVN and RVLM neurons were not studied (68). Further experiments are needed to more positively determine the role of the RVLM in driving SNA in DOCA-salt hypertensive rats.
An important question not directly addressed in this study is the molecular structure of the BSPs involved in the model. Benzamil-treated rats received an infusion of benzamil into the cerebrospinal fluid estimated to produce a concentration of ∼3 μM above the IC50 for the ENaC subunits, but at or below the IC50 for the ASIC subunits. This concentration should be well below the IC50 of benzamil for the ion exchangers, including the Na+/Ca+ exchanger, which has the highest affinity for benzamil among the exchangers (34). We therefore suggest that the benzamil-sensitive effects observed in this study are due to the action of members of the Deg/ENaC family of ion channels. However, at least one previous study suggests that the ion exchangers may be playing a critical role in DOCA-salt hypertension (34). Further studies directed at identifying the structures of the BSPs involved in the model are critical for our understanding of mineralocorticoid-induced hypertension.
While we demonstrated increased c-Fos immunoreactivity in the DOCA-salt model, and benzamil-induced reduction of this immunoreactivity, this observation was made following a relatively long period of time in which arterial pressure differed between Vehicle-DOCA and Benzamil-DOCA rats. It is therefore not possible to determine whether these changes are the cause of changes in MAP or whether they are seen only in response to changes in MAP. Observations of differences in c-Fos immunoreactivity between the groups at an earlier time point, such as the transition between the initiation phase and the development phase, would provide more compelling evidence of a causal relationship between increased neuronal function and increased MAP.
Perspectives and Significance
To our knowledge, this is the first study to show increased neural activity, as reflected by increased c-Fos immunoreactivity, following chronic DOCA-salt treatment. We also demonstrated that c-Fos immunoreactivity in neuroendocrine-type cells was reduced, following benzamil treatment. These data are consistent with a growing body of evidence, suggesting that salt-sensitive hypertension is a central nervous system disorder.
Another contribution of this study is the characterization of distinct phases in the development of DOCA-salt hypertension. Following an initiation phase in which arterial pressure increases rapidly, we observed a developmental phase, characterized by a slower rise in arterial pressure. ICV benzamil treatment was effective at attenuating MAP during the developmental phase, leading to a nearly 50% reduction in MAP during the maintenance phase of DOCA-salt hypertension. A better understanding of what drives transitions from one phase to another is critical for our understanding of the disorder.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-64176 and National Science Foundation Grant IOS0548584.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Aida Attar and Kimberly Krawczewski de Carhuatanta for technical assistance in the completion of these studies and Dr. Scott O'Grady for his insightful feedback and discussions of our experiments. We also thank Dr. Steve Davidson for his help with figure preparation and Britta Veitenheimer-Rupp for her insight in editing the manuscript. Finally, we thank Pilar Ariza Nieto and Dr. Michelle Arnhold for their time in demonstrating techniques used in these studies.
REFERENCES
- 1.Abrams JM, Osborn JW. A role for benzamil-sensitive proteins of the central nervous system in the pathogenesis of salt-dependent hypertension. Clin Exp Pharmacol Physiol 35: 687–694, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS, Leenen FH. Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am J Physiol Regul Integr Comp Physiol 289: R1787–R1797, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Antoni FA. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev 7: 351–378, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Berecek KH, Barron KW, Webb RL, Brody M. Vasopressin-central nervous system interactions in the development of DOCA hypertension. Hypertension 4: II-131–II-137, 1982 [PubMed] [Google Scholar]
- 5.Berecek KH, Bohr DF. Whole body vascular reactivity during the development of deoxycorticosterone acetate hypertension in the pig. Circ Res 42: 764–771, 1978 [DOI] [PubMed] [Google Scholar]
- 6.Berecek KH, Murray RD, Gross F. Significance of sodium, sympathetic innervation, and central adrenergic structures on renal vascular responsiveness in DOCA-treated rats. Circ Res 47: 675–683, 1980 [DOI] [PubMed] [Google Scholar]
- 7.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 362: 590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke D, Smookler HH, Barry H. Sympathetic nerve function and DOCA-NaCl induced hypertension. Life Sci 9: 1097–1108, 1970 [DOI] [PubMed] [Google Scholar]
- 10.Crofton JT, Share L, Shade RE, Lee-Kwon WJ, Manning M, Sawyer WH. The importance of vasopressin in the development and maintenance of DOC-salt hypertension in the rat. Hypertension 1: 31–38, 1979 [DOI] [PubMed] [Google Scholar]
- 11.Cunningham JT, Herrera-Rosales M, Martinez MA, Mifflin S. Identification of active central nervous system sites in renal wrap hypertensive rats. Hypertension 49: 653–658, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Darlington DN, Shinsako J, Dallman MF. Paraventricular lesions: hormonal and cardiovascular responses to hemorrhage. Brain Res 439: 289–301, 1988 [DOI] [PubMed] [Google Scholar]
- 13.De Champlain J, Eid H, Drolet G, Bouvier M, Foucart S. Peripheral neurogenic mechanisms in deoxycorticosterone acetate–salt hypertension in the rat. Can J Physiol Pharmacol 67: 1140–1145, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension 51: 1265–1271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron 21: 1435–1441, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Drummond HA, Welsh MJ, Abboud FM. ENaC subunits are molecular components of the arterial baroreceptor complex. Ann NY Acad Sci 940: 42–47, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Fink GD, Pawloski CM, Blair ML, Mangiapane ML. The area postrema in deoxycorticosterone-salt hypertension in rats. Hypertension 9, Suppl III: III206–III209, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Sanchez EP. Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology 118: 819–823, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Sanchez EP. What is the role of the central nervous system in mineralocorticoid hypertension? Am J Hypertens 4: 374–381, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Sanchez EP, Fort CM, Gomez-Sanchez CE. Intracerebroventricular infusion of RU28318 blocks aldosterone-salt hypertension. Am J Physiol Endocrinol Metab 258: E482–E484, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Sanchez EP, Gomez-Sanchez CE. Effect of central infusion of benzamil on Dahl S rat hypertension. Am J Physiol Heart Circ Physiol 269: H1044–H1047, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Grillo CA, Saravia F, Ferrini M, Piroli G, Roig P, Garcia SI, de Kloet ER, De Nicola AF. Increased expression of magnocellular vasopressin mRNA in rats with deoxycorticosterone-acetate induced salt appetite. Neuroendocrinology 68: 105–115, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Harnish PP, Samuel K. Reduced cerebrospinal fluid production in the rat and rabbit by diatrizoate. Ventriculocisternal perfusion. Invest Radiol 23: 534–536, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Herzig TC, Buchholz RA, Haywood JR. Effects of paraventricular nucleus lesions on chronic renal hypertension. Am J Physiol Heart Circ Physiol 261: H860–H867, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Holtzman E, Braley LM, Menachery A, Williams GH, Hollenberg NK. Rate of activation of renin-angiotensin-aldosterone axis and sodium intake in rats. Am J Physiol Heart Circ Physiol 256: H1311–H1315, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Hosoya Y, Sugiura Y, Okado N, Loewy AD, Kohno K. Descending input from the hypothalamic paraventricular nucleus to sympathetic preganglionic neurons in the rat. Exp Brain Res 85: 10–20, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Huang BS, Amin MS, Leenen FH. The central role of the brain in salt-sensitive hypertension. Curr Opin Cardiol 21: 295–304, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Huang BS, Leenen FH. Blockade of brain mineralocorticoid receptors or Na+ channels prevents sympathetic hyperactivity and improves cardiac function in rats post-MI. Am J Physiol Heart Circ Physiol 288: H2491–H2497, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol 289: H1519–H1528, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Janiak PC, Lewis SJ, Brody MJ. Role of central mineralocorticoid binding sites in development of hypertension. Am J Physiol Regul Integr Comp Physiol 259: R1025–R1034, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Kageyama Y, Bravo EL. Hypertensive mechanisms associated with centrally administered aldosterone in dogs. Hypertension 11: 750–753, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Kawano H, Masuko S. Synaptic contacts between nerve terminals originating from the ventrolateral medullary catecholaminergic area and median preoptic neurons projecting to the paraventricular hypothalamic nucleus. Brain Res 817: 110–116, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med 64: 193–198, 1978 [DOI] [PubMed] [Google Scholar]
- 34.Keep RF, Si X, Shakui P, Ennis SR, Betz AL. Effect of amiloride analogs on DOCA-salt-induced hypertension in rats. Am J Physiol Heart Circ Physiol 276: H2215–H2220, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Lele RD. Hypertension: molecular approach. J Assoc Physicians India 52: 53–62, 2004 [PubMed] [Google Scholar]
- 36.Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Heart Circ Physiol 281: H2328–H2336, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Mento PF, Wang HH, Sawyer WB. Contribution of vasopressin and other pressor systems to DOC-salt hypertension in rats. Proc Soc Exp Biol Med 175: 58–63, 1984 [DOI] [PubMed] [Google Scholar]
- 38.Miller AW, II, Bohr DF, Schork AM, Terris JM. Hemodynamic responses to DOCA in young pigs. Hypertension 1: 591–597, 1979 [DOI] [PubMed] [Google Scholar]
- 39.Mohring J, Mohring B, Petri M, Haack D. Vasopressor role of ADH in the pathogenesis of malignant DOC hypertension. Am J Physiol Renal Fluid Electrolyte Physiol 232: F260–F269, 1977 [DOI] [PubMed] [Google Scholar]
- 40.Nishimura M, Ohtsuka K, Iwai N, Takahashi H, Yoshimura M. Regulation of brain renin-angiotensin system by benzamil-blockable sodium channels. Am J Physiol Regul Integr Comp Physiol 276: R1416–R1424, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Nishimura M, Ohtsuka K, Nanbu A, Takahashi H, Yoshimura M. Benzamil blockade of brain Na+ channels averts Na+-induced hypertension in rats. Am J Physiol Regul Integr Comp Physiol 274: R635–R644, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Osborn JW, Jacob F, Hendel M, Collister JP, Clark L, Guzman PA. Effect of subfornical organ lesion on the development of mineralocorticoid-salt hypertension. Brain Res 1: 74–82, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the PVN by astroglial GABA. J Physiol 587: 4645–4660, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson EG. The Rat Brain in Stereotaxic Coordinates. Sydney, Australia: Academic, 1986 [Google Scholar]
- 45.Pietranera L, Saravia FE, McEwen BS, Lucas LL, Johnson AK, De Nicola AF. Changes in Fos expression in various brain regions during deoxycorticosterone acetate treatment: relation to salt appetite, vasopressin mRNA and the mineralocorticoid receptor. Neuroendocrinology 74: 396–406, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res 120: 164–172, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Sabharwal RJAW, Abboud FM, Chapleau MW. Contrasting autonomic and cardiovascular phenotypes in ASIC1a and ASIC2 deficient mice (Abstract). Experimental Biology 2008, San Diego, CA. FASEB J p. 935.911 [Google Scholar]
- 48.Rosenfeld P, van Eekelen JA, Levine S, de Kloet ER. Ontogeny of corticosteroid receptors in the brain. Cell Mol Neurobiol 13: 295–319, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Saravia FE, Grillo CA, Ferrini M, Roig P, Lima AE, de Kloet ER, De Nicola AF. Changes of hypothalamic and plasma vasopressin in rats with deoxycorticosterone-acetate induced salt appetite. J Steroid Biochem Mol Biol 70: 47–57, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Sawchenko PE, Swanson LW, Vale WW. Corticotropin-releasing factor: co-expression within distinct subsets of oxytocin-, vasopressin-, and neurotensin-immunoreactive neurons in the hypothalamus of the male rat. J Neurosci 4: 1118–1129, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siffert W, Dusing R. Sodium-proton exchange and primary hypertension. An update. Hypertension 26: 649–655, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Staessen J, Bulpitt CJ, Thijs L, Fagard R, Joossens JV, Van Hoof R, Amery A. Sympathetic tone and relation between sodium intake and blood pressure in the general population. Br Med J 299: 1502–1503, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 287: R1172–R1183, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypetension: role of rostral ventral lateral medulla. Curr Hypertens Rep 5: 262–268, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980 [DOI] [PubMed] [Google Scholar]
- 56.Swords BH, Wyss JM, Berecek KH. Central vasopressin receptors are upregulated by deoxycorticosterone acetate. Brain Res 559: 10–16, 1991 [DOI] [PubMed] [Google Scholar]
- 57.Takeda K, Bunag RD. Augmented sympathetic nerve activity and pressor responsiveness in DOCA hypertensive rats. Hypertension 2: 97–101, 1980 [DOI] [PubMed] [Google Scholar]
- 58.Takeda K, Nakata T, Takesako T, Itoh H, Hirata M, Kawasaki S, Hayashi J, Oguro M, Sasaki S, Nakagawa M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res 543: 296–300, 1991 [DOI] [PubMed] [Google Scholar]
- 59.Van Eekelen JA, Jiang W, De Kloet ER, Bohn MC. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosci Res 21: 88–94, 1988 [DOI] [PubMed] [Google Scholar]
- 60.Veitenheimer B, Osborn J. Intrathecal administration of a V1a antagonist has no effect on arterial pressure in conscious rats under acute or chronic osmotic stress. FASEB J 24: 1051.10, 2010 [Google Scholar]
- 61.Wang H, Huang BS, Leenen FH. Brain sodium channels and ouabainlike compounds mediate central aldosterone-induced hypertension. Am J Physiol Heart Circ Physiol 285: H2516–H2523, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Leenen FH. Brain sodium channels mediate increases in brain “ouabain” and blood pressure in Dahl S rats. Hypertension 40: 96–100, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Weinberger M. Salt-sensitivity of blood pressure in humans. Hypertension 27: 481–490, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Wickert L, Selbig J, Watzka M, Stoffel-Wagner B, Schramm J, Bidlingmaier F, Ludwig M. Differential mRNA expression of the two mineralocorticoid receptor splice variants within the human brain: structure analysis of their different DNA binding domains. J Neuroendocrinol 12: 867–873, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Wotus C, Arnhold MM, Engeland WC. Dehydration-induced drinking decreases Fos expression in hypothalamic paraventricular neurons expressing vasopressin but not corticotropin-releasing hormone. Am J Physiol Regul Integr Comp Physiol 292: R1349–R1358, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Xue B, Badaue-Passos D, II, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Sex differences and central protective effect of 17β-estrodiol in the development of aldosterone/NaCl-induced hypertension. Am J Physiol Heart Circ Physiol 296: H1577–H1585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008 [DOI] [PubMed] [Google Scholar]