Abstract
The trans 10, cis 12-conjugated linoleic acid (10,12-CLA) isomer reduces adiposity in several animal models. In the mouse, however, this effect is associated with adipose tissue inflammation, hyperinsulinemia and hepatic lipid accumulation. Moreover, 10,12-CLA was recently shown to promote mammary ductal hyperplasia and ErbB2/Her2-driven mammary cancer in the mouse. Reasons for detrimental effects of 10,12-CLA on the mouse mammary gland could relate to its effect on the mammary fat pad (MFP), which is essential for normal development. Accordingly, we hypothesized that mammary effects of 10,12-CLA were mediated through the MFP in a dose-dependent manner. Female FVB mice were fed 10,12-CLA at doses of 0%, 0.1%, 0.2%, or 0.5% of the diet from day 24 of age, and effects on mammary development and metabolism were measured on day 49. The 0.5% dose reduced ductal elongation and caused premature alveolar budding. These effects were associated with increased expression of inflammatory markers and genes shown to alter epithelial growth (IGF binding protein-5) and alveolar budding (TNF-α and receptor of activated NF-κB ligand). The 0.5% dose also caused hyperinsulinemia and hepatic lipid accumulation. In contrast, the 0.1% 10,12-CLA dose had no adverse effects on mammary development, metabolic events, and inflammatory responses, but remained effective in decreasing adipose weights and lipogenic gene expression. These results show that a low dose of 10,12-CLA reduces adiposity in the mouse without negative effects on mammary development, inflammation, and metabolism, and suggest that previously reported detrimental effects relate to the use of excessive doses.
Keywords: mammary fat pad, ductal growth, fatty liver, lipogenic gene expression
conjugated linoleic acids (CLA) consist of positional and geometric isomers of octadecadienoic fatty acids with a conjugated double bond. They are produced by microbial hydrogenation of polyunsaturated fatty acids in the rumen of cattle, goats, and sheep, and accordingly, they occur in trace amounts in ruminant products (<0.5% of all fatty acids). Over 70% of CLA found in animal products is accounted for by the cis 9, trans 11 isomer (9,11-CLA) and the rest by a collection of minor isomers that includes t10, c12-CLA (10,12-CLA) (25). CLA can also be produced by chemical methods, such as heat alkaline isomerization of linoleic acid. This produces a mixture of CLA isomers (20, 22, 30). Most studies have used an industrially produced CLA mixture that contains equimolar amounts of 9,11-CLA and 10,12-CLA (3, 20, 30).
Interest in CLA grew dramatically when diets containing chemically prepared CLA mixtures were shown to reduce inflammation, atherogenesis, obesity, and cancer in various animal models (3, 20, 22, 34). Later studies show that the 9,11-CLA isomer accounts for the anti-inflammatory and antiatherogenic effects of chemically prepared CLA mixtures, and 10,12-CLA for the antiobesity effect (22, 25, 34). Use of the mouse as a model to understand the mechanisms for the antiobesity effect of 10,12-CLA has been hampered by the occurrence of detrimental effects. Specifically, diets containing 10,12-CLA caused inflammation and near-complete delipidation of adipose tissue, as well as metabolic defects, such as hyperinsulinemia and hepatic lipid infiltration (7, 18, 24, 33). More surprisingly, 10,12-CLA, but not 9,11-CLA, promoted hyperplasia and distension of the mammary ductal tree, and accelerated cancer development in virgin mice overexpressing the human oncogene ErbB2/Her2 (21). These detrimental effects on the mammary gland are in complete contrast to the numerous studies in the rat model, where 10,12-CLA does not adversely affect mammary development and offers protection against chemically induced mammary cancers (19, 20).
The basis for the detrimental effects of 10,12-CLA in the mouse remain unknown. We have shown, however, that doses of 10,12-CLA, representing only 0.01 to 0.05% of the consumed diet inhibit mammary de novo lipogenesis in the lactating dairy cow by as much as 50%, without evoking negative responses, such as adipose tissue inflammation, hyperinsulinemia, lipolysis, or hepatic lipid accumulation (1, 14). This contrasts with the use of diets containing 0.25% or more 10,12-CLA in virtually all mouse studies (7, 18, 24, 33), including those focused on the mammary gland (21), thus raising the possibility that detrimental effects relate to excessive doses. To evaluate this possibility, we examined the dose-dependent effects of 10,12-CLA with a primary focus on peripubertal mammary gland development. This phase of development in the mouse is dominated by ductal growth and depends on reciprocal hormonal signaling between the proliferating epithelium and the surrounding mammary fat pad (MFP) (16, 23, 38). Ductal growth, therefore, provides an opportunity to evaluate effects of 10,12-CLA on mammary development through MFP function. In addition, we measured effects of 10,12-CLA dose on the mass and inflammatory response of adipose tissue and, on indicators of whole animal metabolism. Our results show that a low dose of 10,12-CLA is effective at decreasing adiposity with no apparent adverse effects. Moreover, adverse effects typically associated with 10,12-CLA manifest only when a high dose is used, and these are characterized by abnormal mammary gland development, inflammation, and metabolic complications.
MATERIALS AND METHODS
Animals.
All procedures were approved by the Cornell Institutional Animal Care and Use Committee. FVB mice were purchased from Taconic Farms (Hudson, NY) and the Jackson Laboratory (Bar Harbor, ME). Female mice were weaned at 21 days of age and housed individually. Mice were fed ad libitum levels of a standard rodent chow containing 22% protein and 5% fat (Harlan Teklad 8640, Madison, WI). From 24 to 49 days of age, mice were randomly allocated to 4 groups (n = 8–10 per group) receiving 10,12-CLA in amounts corresponding to 0%, 0.1%, 0.2%, or 0.5% of the daily feed intake. The daily CLA dose was administered orally in liquid form at 0900 and 2100 h and was calculated for each mouse by dividing the mass of 10,12-CLA needed (% CLA × mass of consumed diet on the previous day) by the density of the pure 10,12-CLA solution (98% pure methylester; BASF, Ludwigshafen, Germany); Control animals (0% 10,12-CLA) were dosed with water.
Mice were euthanized by CO2 asphyxiation on day 49, after the 0900 oral administration. Animals were not fasted before euthanasia. Blood was collected via cardiac puncture into a heparinized syringe, and plasma was prepared by centrifugation. Immediately after, liver, gonadal fat, and abdominal mammary glands were carefully dissected. After removing the lymph node, the left abdominal gland was frozen along with the liver and gonadal fat for subsequent analyses. The right abdominal mammary gland was used for the analysis of mammary development.
Mammary gland development.
The right abdominal gland was spread onto a glass slide for whole mount analysis. Spread glands were fixed overnight in Carnoy's solution (60% ethanol, 30% chloroform, 10% acetic acid), postfixed in 70% ethanol, rehydrated in decreasing concentrations of ethanol and stained overnight in carmine-alum (39). Slides were then dehydrated in increasing ethanol concentrations, cleared in xylene overnight, and then mounted with Permount and cover slips. Whole mounts were photographed with a digital camera mounted on a dissecting microscope. Ductal length was quantified by measuring the distance from the center of the lymph node to the furthest extending duct. The number of terminal end bud and alveolar bud structures was counted within 3 randomly selected 1 mm2 area, and the average was calculated.
Metabolic analyses.
Plasma glucose concentrations were quantified by the glucose oxidase method using reagents from Sigma (St. Louis, MO). Plasma insulin and adiponectin concentrations were measured using appropriate RIA kits (sensitive rat insulin RIA kit and mouse adiponectin RIA kit; Millipore, Billerica, MA). The analyses were performed exactly as recommended by the manufacturer. The average coefficient of variation was <4% for glucose, <2% for insulin, and <7% for adiponectin.
To determine lipid and triglyceride content, ∼100 mg of liver was homogenized with a polytron in chloroform and methanol (2:1), and total lipids were extracted according to Folch et al. (11). After evaporation of organic solvents, total extracted lipid was weighed and the lipid content of liver was calculated. Triglyceride concentration of the extracted lipid was determined by a colorimetric Hantzsch condensation method (12) using a standard curve based on glycerol trioleate (T-7140; Sigma, St. Louis, MO). The average coefficient of variation was <4%.
Gene expression in the mammary gland and gonadal fat.
Total RNA was isolated from the left abdominal mammary gland and gonadal fat using RNeasy Mini columns and on-column RNase-free DNase treatment (Qiagen, Chatsworth, CA). Quantity and integrity of RNA were determined using the RNA Nano Lab Chip Kit and BioAnalyzer (Agilent, Palo Alto, CA). Reverse transcription reactions were performed with 1–2 μg of RNA using the high-capacity cDNA reverse transcription kit with RNase inhibitor (Applied Biosystems, Foster City, CA). Real-time PCR assays were performed in duplicate in a 25-μl volume using Power SYBR Green Mix (Applied Biosystems). Reactions contained 500 nM of each primer and 25 ng of reverse-transcribed RNA [except 2.5 ng and 125 ng were used for 18S and receptor of activated NF-κβ ligand (RANKL), respectively]. Sequences of primers used for each target gene are provided in Supplemental Table S1. Data were analyzed using a relative standard curve from either mammary tissue or adipose tissue cDNA. The standard curve consisted of seven twofold dilutions covering a 64-fold difference in expression level. Unknown sample expression was determined from the standard curve. Gene expression was normalized to 18S, except for genes expressed exclusively in the mammary epithelial compartment (progesterone receptor, amphiregulin, and RANKL). For those genes, expression was normalized to the epithelial specific keratin 18 (K18), as done by others (6, 39).
Statistical analysis.
Data were analyzed by the standard least squares model using the fit model procedure of JMP (version 8, SAS Institute, Cary, NC). The statistical model accounted for the fixed effect of 10,12-CLA dose. Animal source (Taconic Farms or Jackson Laboratory) was not used in the statistical model because it did not explain any variation. Statistical significance was declared at P < 0.05. When the effect of 10,12-CLA was significant, individual means were separated by Student's t-test.
RESULTS
Induction of metabolic defects by 0.5% dietary 10,12-CLA.
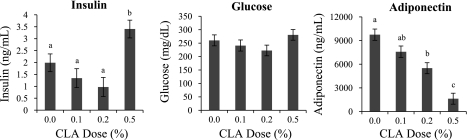
Body weight gain over the treatment period was unaffected by CLA, although all 10,12-CLA doses reduced daily feed intake by a near identical 7% (Table 1). In agreement with previous data, the 0.5% 10,12-CLA dose increased liver weight, as well as the lipid and triglyceride content of liver (Table 1). These hepatic effects were associated with a doubling of plasma insulin concentration and an 80% reduction in plasma adiponectin (Fig. 1). In contrast, the 0.1% CLA dose failed to alter any of these variables, whereas the only effect seen with the 0.2% CLA dose was a 40% reduction in plasma adiponectin. None of the CLA doses altered the plasma glucose concentration. These data show that detrimental effects of 10,12-CLA on liver and metabolic variables that are readily observed at the 0.5% dose are absent at the 0.1% dose.
Table 1.
Whole body and liver measurements of FVB mice fed either 0, 0.1%, 0.2%, or 0.5% trans 10, cis 12-conjugated linoleic acid from 24 to 49 days of age
| CLA1 |
||||||
|---|---|---|---|---|---|---|
| 0% | 0.1% | 0.2% | 0.5% | SE | P Value | |
| Intake, g/day | 4.31a | 4.04b | 4.00b | 3.98b | 0.07 | <0.01 |
| Body weight gain, g | 6.07 | 5.68 | 6.06 | 5.86 | 0.30 | NSc |
| Liver weight, g | 0.97a | 1.02a,b | 1.05b | 1.18c | 0.02 | <0.001 |
| Liver fat, % | 5.59a | 4.53a | 4.60a | 7.63b | 0.67 | <0.05 |
| Liver triglyceride, % | 2.09a | 1.85a | 2.29a | 3.46b | 0.27 | <0.01 |
Each value represents the mean of 8 to 10 mice for intakes, body weight, and liver weight. Values represent the mean of 5 mice for liver fat and triglyceride percentages.
Values with different letters are significantly different at P < 0.05.
cNot significant.
Fig. 1.
Effect of trans 10, cis 12-conjugated linoleic acid (10,12-CLA) dose on plasma concentrations of insulin, glucose, and adiponectin. Female FVB mice received 10,12-CLA at doses of 0%, 0.1%, 0.2%, or 0.5% of the diet from day 24 to 49 of age (n = 8 to 10 per treatment). Plasma was collected on day 49 of age. Each bar represents the mean ± SE of 5 to 9 mice. a,b,cBars with different letters are significantly different, P < 0.05.
Disruption of mammary ductal development by 0.5% dietary 10,12-CLA.
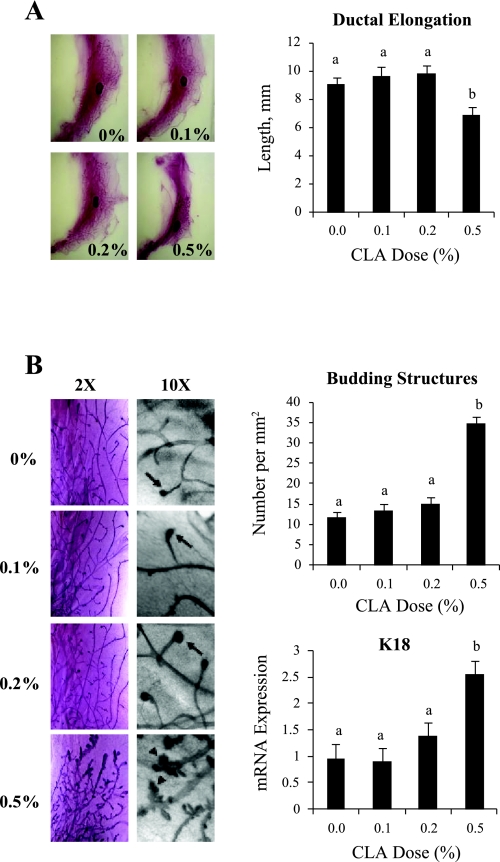
Mouse mammary development between weaning and 7 wk of age is dominated by ductal growth (16, 23, 38). Accordingly, mammary glands isolated on day 49 from control animals contained an extensive network of ducts, with a single terminal end bud (TEB) at their tips (Fig. 2, A and B). This pattern of development was impaired by the 0.5% 10,12-CLA dose as evidenced by the greater portion of the MFP devoid of ducts. Ductal elongation, measured as the distance between the lymph node and the tip of the most distal duct, was reduced by 20% in the 0.5% 10,12-CLA group (Fig. 2A). Moreover, upon magnification, many ducts were distended and decorated with numerous buds resembling alveolar bud structures (Fig. 2B). The total number of budding structures, including TEB, was increased three-fold by the 0.5% 10,12-CLA treatment. Consistent with these morphological indicators of hyperplasia, expression of the epithelial cell marker K18 was increased nearly 3-fold in the 0.5% 10,12-CLA group (Fig. 2B). None of these abnormalities were present in mice receiving the 0.1% or the 0.2% doses of 10,12-CLA. Overall, these data show that effects of 10,12-CLA on mammary development parallel the metabolic effects, with normal development at the 0.1 and 0.2% doses and abnormal development at the 0.5% 10,12-CLA dose.
Fig. 2.
Effect of 10,12-CLA dose on mammary development in FVB mice. Female FVB mice received 10,12-CLA at doses of 0, 0.1, 0.2, or 0.5% of the diet from day 24 to 49 of age (n = 8 to 10 per treatment). The right abdominal mammary gland was collected at 49 days of age. A: mammary gland was subjected to whole mount staining, and ductal length was quantified by measuring the distance from the center of the lymph node to the most distal duct. B: whole mounts were photographed at 2× and 10× magnification. In the 10× magnification panels, arrows correspond to terminal end buds, and arrowheads to alveolar-like structures. Representative images are shown. Right, top: number of budding structures was calculated for each animal as described in the materials and methods. Right, bottom: total RNA was extracted from the fourth mammary gland and analyzed by real-time PCR for keratin 18 mRNA abundance. Data were normalized to 18S expression. Each bar represents the mean ± SE of 8 to 10 mice. a,bBars with different letters are significantly different, P < 0.05.
Disruption of intramammary signals regulating ductal growth by 0.5% dietary 10,12-CLA.
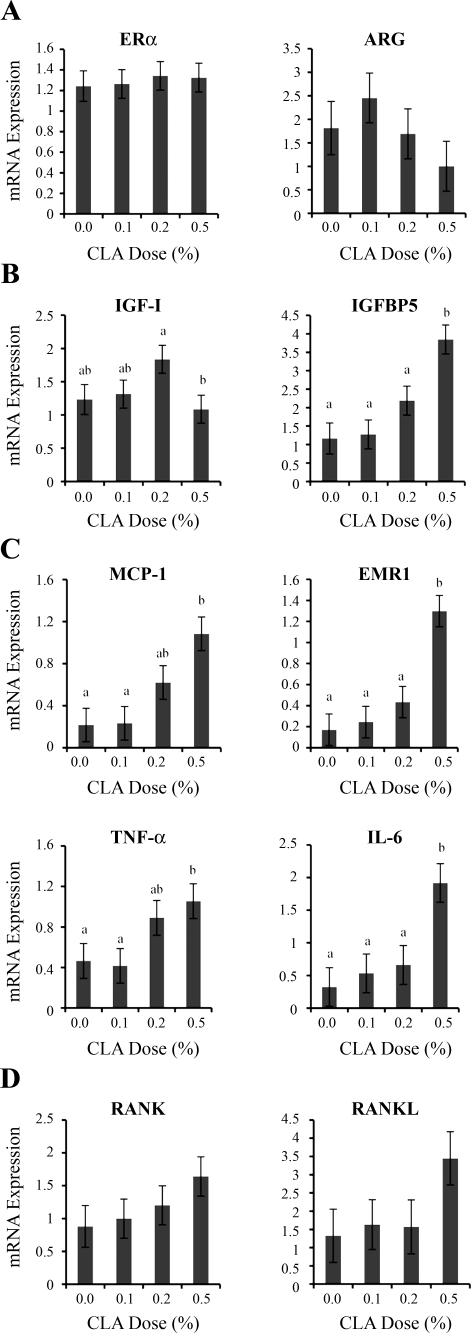
Next, we asked whether the 0.5% dose of 10,12-CLA disrupted intramammary signals driving mouse ductal growth. The various 10,12-CLA doses had no effect on the expression of estrogen receptor-α (ERα) and its dependent gene amphiregulin (ARG) (Fig. 3A). The lack of CLA effect also extended to the expression of the progesterone receptor (PR; data not shown), an ERα-dependent gene that is dispensable for ductal elongation (16, 38). Mammary ductal elongation also depends on intramammary, growth hormone-dependent production of IGF-I (23), and these actions can be opposed by local production of IGF binding protein 5 (IGFBP5) (40). The expression of IGF-I was not affected by 10,12-CLA dose, but expression of IGFBP5 was increased over three-fold in glands of mice receiving the 0.5% 10,12-CLA dose (Fig. 3B). 10,12-CLA dose also affected IGFBP3 expression (overall F test, P < 0.03), and this effect was accounted for by a 1.8-fold increase at the 0.5% dose (IGFBP3 expression, 0 vs. 0.5%, 1.3 vs. 2.4 ± 0.3, P < 0.05, Student's t-test), whereas the 0.1% and 0.2% doses did not differ from control. These data raise the possibility that the 0.5% dietary 10,12-CLA dose attenuates ductal elongation by promoting sequestration of IGF-I by IGFBP5 and/or IGFBP3, but they do not support a disruption of ERα signaling.
Fig. 3.
Effect of 10,12-CLA dose on mammary gland expression of genes regulating ductal growth. Female FVB mice received 10,12-CLA at doses of 0, 0.1, 0.2, or 0.5% of the diet from day 24 to 49 of age (n = 8 to 10 per treatment). The left abdominal mammary gland was collected at 49 days of age. Total RNA was analyzed by real-time PCR for the mRNA abundance of indicated genes. A: estrogen receptor (ERα) and amphiregulin (ARG). B: IGF-I and IGF binding protein 5 (IGFBP5). C: chemokine monocyte chemoattractant protein-1 (MCP-1) and the macrophage marker EGF-like module containing mucin-like hormone receptor-like sequence 1 (EMR1), TNF-α, and IL-6. D: receptor of activated NF-κB (RANK) and RANK ligand (RANKL). Data were normalized to 18S expression except for genes expressed predominantly in the epithelium (ARG, RANK, and RANKL). Each bar represents the mean ± SE of 8 mice. a,bBars with different letters are significantly different, P < 0.05.
The 0.5% 10,12-CLA dose has been shown previously to induce an inflammatory response in extramammary fat depots (24, 33). Moreover, mammary inflammation is associated with the presence of short and distended ducts in the fatless mouse (8). To examine whether inflammation occurs in the MFP, we measured expression of the chemokine monocyte chemoattractant protein-1 (MCP-1), the macrophage marker EGF-like module containing mucin-like hormone receptor-like sequence 1 (EMR1), and the inflammatory cytokines TNF-α and IL-6. Expression of all these genes was increased two- to seven-fold in the mammary glands of mice receiving 0.5% dietary 10,12- CLA (Fig. 3C) but remarkably, their expression was unaltered at the 0.1% dose. The 0.2% dietary 10,12-CLA treatment had few notable effects, except that it raised TNF-α and MCP-1 expression to levels that were intermediary between those seen at the 0 and 0.5% 10,12-CLA doses. Overall, these data show that the abnormal ductal development by the 0.5% 10,12-CLA dose is associated with inflammation.
Recent work has implicated NF-κB signaling via the epithelial receptor of activated NF-κβ (RANK) in the formation of ductal side-branching and alveologenesis (2, 9, 10). Administration of 10,12-CLA did not alter the expression of RANK, but it tended to increase its extracellular signal, RANKL (Fig. 3D; P = 0.17). This tendency was accounted for by a three-fold increased expression at the 0.5% dose. Thus, increased RANKL expression in the glands of the 0.5% CLA treatment group may contribute to the induction of abnormal budding structures along the ducts as observed in Fig. 2B.
10,12-CLA represses lipogenic gene expression in the mammary fat pad.
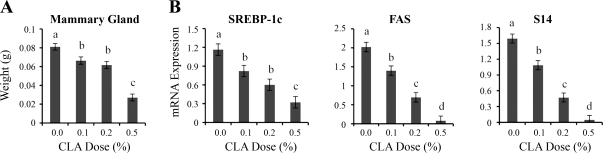
CLA caused a stepwise reduction in mammary gland weights, with the 0.1 and 0.2% doses causing an 18% reduction and the 0.5% dose causing a 65% reduction (Fig. 4A). This reduction is likely accounted for by the MFP because it represents the majority of the mammary gland mass in virgin mice. Consistent with this possibility, CLA caused a dose-dependent reduction in the expression of the master transcriptional regulator of lipogenesis, sterol regulatory element binding protein-1c (SREBP-1c) and its dependent genes fatty acid synthase (FASN) and thyroid hormone responsive spot 14 (S14). The repression achieved with the 0.5% CLA dose was 70% for SREBP-1c and over 95% for both FASN and S14 (Fig. 4B).
Fig. 4.
Effect of 10,12-CLA dose on mammary gland weight and expression of lipogenic genes. Female FVB mice received 10,12-CLA at doses of 0, 0.1, 0.2, or 0.5% of the diet from day 24 to 49 of age (n = 8 to 10 per treatment). A: mammary glands were collected from mice at 49 days of age and weighed. Each bar represents the mean ± SE of 8 to 10 mice. B: total RNA was extracted and analyzed by real-time PCR for mRNA abundance of sterol regulatory element binding protein-1c (SREBP-1c), fatty acid synthase (FASN), and thyroid hormone responsive spot 14 (S14). Data are normalized to 18S expression. Each bar represents the mean ± SE of 8 mice. a,b,c,dBars with different letters are significantly different, P < 0.05.
10,12-CLA represses lipid accretion in absence of inflammation in gonadal fat.
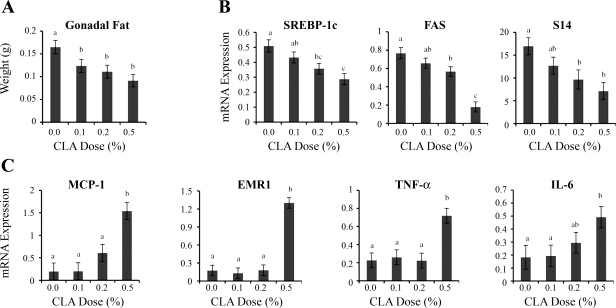
Previous work in the mouse has shown a close association between the delipidating effects of 10,12-CLA and the induction of an inflammatory response in adipose tissue (17, 24). Our data for the MFP, however, indicated that the 0.1% dose of 10,12-CLA decreased lipid accretion in the absence of any proinflammatory effects (Figs. 3C and 4A). The mass of gonadal fat was measured to determine whether this dissociation also occurred in extra-mammary fat depots. All doses of 10,12-CLA were effective in reducing gonadal fat mass, with reductions ranging from 25% for the 0.1% dietary 10,12-CLA dose to more than 40% for the 0.5% dose (Fig. 5A). There were corresponding dose-dependent reductions in the expression of SREBP-1c and its dependent genes FAS and S14, with decreases ranging from 14 to 25% at the 0.1% CLA dose (P < 0.1) and 26 to 76% at the 0.2 and 0.5% dietary doses (P < 0.05; Fig. 5B). In contrast to these graded antilipogenic responses, dietary 10,12-CLA increased expression of the inflammatory MCP-1, EMRI, TNF-α, and IL-6 genes only at the 0.5% dose (Fig. 5C). These data demonstrate that 10,12-CLA can reduce fat deposition in the absence of inflammation in the MFP and that this dissociation between adiposity and inflammatory effects extends to other fat depots.
Fig. 5.
Effect of 10,12-CLA dose on gonadal fat weight and expression of lipogenic and inflammatory genes. Female FVB mice received 10,12-CLA at doses of 0, 0.1, 0.2, or 0.5% of the diet from day 24 to 49 of age (n = 8 to 10 per treatment). Gonadal fat was collected at day 49 of age. Total RNA was extracted, and the expression of specific genes was analyzed by real-time PCR. A: mass of gonadal fat. Each bar represents the mean ± SE of 8 to 10 mice. B: expression of SREBP-1c, FASN, and S14. Data were normalized to 18S. Each bar represents the mean ± SE of 6 or 7 mice. C: expression of chemokine MCP-1, the macrophage marker EGF-like module containing EMR1, TNF-α, and IL-6. Data were normalized to 18S expression. Each bar represents the mean ± SE of 5 mice. a,b,cBars with different letters are significantly different, P < 0.05.
DISCUSSION
Starting in the late 1980s, a number of studies reported that equimolar mixtures of 9,11- and 10,12-CLA were capable of reducing inflammation, atherogenesis, obesity, and cancer (3, 20, 30). Later studies established that many of these beneficial properties were isomer-specific, with 9,11-CLA mediating the anti-inflammatory and anti-atherogenic effects, and 10,12-CLA the anti-obesity effects (18, 22, 34). However, there have also been questions regarding the safety of chronic 10,12-CLA supplementation, based primarily on data from studies with mice. Specifically, 10,12-CLA doses exceeding 0.2% of the diet not only decrease adiposity in mice, but also induce aspects of the metabolic syndrome, including increased plasma insulin, hepatic fatty infiltration, and adipose tissue inflammation (7, 17, 24, 33). Moreover, Ip et al. (21) recently showed that feeding 0.5% dietary 10,12-CLA for only 10 days was sufficient to induce mammary ductal hyperplasia in mature virgin FVB mice. These deleterious effects are apparently absent in the closely related rat fed diets containing 0.4–1.0% 10,12-CLA (13, 20, 37), suggesting that the mouse is unusually sensitive to 10,12-CLA effects. A related explanation could be the use of excessive dietary doses in most mouse studies. In dairy cattle, we have shown that 10,12-CLA inhibits mammary lipogenesis by 50% in the absence of hyperinsulinemia, insulin resistance, or hepatic fat accumulation (1). The half-maximal and maximal responses occur at dietary doses of 0.01% and 0.04%, respectively, representing only 2 and 8% of the weight-specific doses received by a mature mouse consuming a 0.5% 10,12-CLA diet. In addition, we recently observed that a 0.07% dose of dietary 10,12-CLA inhibits mammary lipogenesis in lactating mice in the absence of detrimental metabolic effects (15). The present study extends these findings by showing that 10,12-CLA can be used at effective and innocuous doses not only during lactation, but also to decrease adiposity in the growing mouse.
Mammary gland development may provide a sensitive end-point to evaluate effects of 10,12-CLA on adipose tissue function. In the newborn mouse, the mammary gland consists of a small epithelial rudiment embedded in the MFP (16, 23, 38). The rudiment remains static until weaning when it dynamically expands throughout the entire MFP. This expansion is driven by reciprocal interaction between the epithelial and stromal compartments and thus depends on a fully functional MFP. In the present study, the ductal tree nearly reached the MFP boundary in control mice, in agreement with completion of ductal growth by ∼8 wk of age (39). Ductal growth was identical in mice receiving the 0.1 and 0.2% doses of 10,12-CLA, but mice receiving the 0.5% 10,12-CLA dose had reduced ductal growth, with many ducts having an abnormal, distended appearance. Interestingly, short and distended mammary ducts are also observed in fatless A-Zip/F-1 transgenic mice devoid of a MFP (8), suggesting that a portion of the defects seen in mice receiving the 0.5% 10,12-CLA dose could relate to the 65% reduction in MFP weight. A related possibility is that the 0.5% 10,12-CLA dose alters the intra-mammary signaling that regulates ductal growth. In the mouse, ductal elongation is strictly dependent on estrogen and IGF-I signaling, with the MFP participating in both signaling loops (16, 23, 38). In the case of estrogen, activation of epithelial ERα induces production of ARG, which then stimulates stromal production of a mitogen acting on epithelial cells (6, 26, 38). The effect of CLA on MFP production of this mitogen could not be assessed because its identity is still unknown. Our data, however, show that 10,12-CLA does not disrupt the epithelial portion of this loop, as represented by ARG and PR expression. Another possibility is that the 0.5% 10,12-CLA dose may affect the MFP-produced IGF-I and/or its signaling that is responsible for ductal growth (23, 35, 42). The proliferative effect of locally produced IGF-I can be opposed by mammary-specific production of an IGFBP. This mechanism is best understood for IGFBP5, as shown by reduced epithelial growth in mice with mammary overexpression of IGFBP5, or retarded cell death at involution in mice lacking IGFBP5 (29, 40). Interestingly, the 0.5% 10,12-CLA diet increased IGFBP5 and IGFBP3 expression, raising the possibility that it reduced ductal growth by promoting IGF-I sequestration. Unfortunately, we had insufficient tissue left in this study to extend our work to the protein level, and in particular, to verify that the increases in IGFBP3 and IGFBP5 mRNA caused by the 0.5% 10,12-CLA dose were translated into corresponding changes at the protein level.
Mammary ductal growth is driven by the extensive epithelial proliferation occurring at TEB (16, 38). As expected, TEB were seen only at the tips of the ducts in control mice, as well as in mice receiving the 0.1 and 0.2% 10,12-CLA diets. In contrast, the 0.5% 10,12-CLA diet induced the emergence of buds along the entire ductal tree, and this morphological abnormality was associated with a doubling in the abundance of the marker of epithelial cell mass K18, suggesting increased proliferation. This is supported by increased Ki67 labeling in the TEB-like structures reported for the mammary glands of mature virgin mice consuming a 0.5% 10,12-CLA diet (27). The budding structures decorating the ducts in the 0.5% 10,12-CLA group resemble those seen in early pregnancy, which eventually lead to the development of the lobulo-alveolar apparatus (16, 38). This developmental process is normally driven by chronic elevation of progesterone and prolactin, which induce epithelial production of RANKL (9, 16). RANKL binds to epithelial RANK receptor and induces proliferation via a signaling cascade converging on NF-κB and cyclin D1. Accordingly, mammary alveologenesis is deficient in pregnant mice lacking RANKL but can be induced by RANKL overexpression, even in the absence of pregnancy (9, 10). Moreover, the 0.5% 10,12-CLA treatment caused a two-fold increase in TNF-α expression, a cytokine capable of stimulating proliferation of mammary epithelial cells and formation of alveolar-like structures by inducing NF-κB and cyclin D1, the same exact signaling molecules mediating RANKL effects (36, 41, 43). These observations suggest that the increase in RANKL and TNF-α expression that we observed may be functionally important in mediating the effects of 0.5% 10,12-CLA on ductal growth.
The ability of high doses of 10,12-CLA to reduce the mass of adipose tissue is well established in mice (18, 22, 24). LaRosa et al. (24) estimated that this effect was accounted for by reductions in both the size and number of adipocytes, with the former being the most important factor. Theoretically, adipocyte hypotrophy could relate to reduced triglyceride accumulation (via reductions in uptake of preformed fat, rates of de novo lipogenesis, or fatty acid esterification), increased lipid utilization (via increased oxidation) or increased lipid export (via increased lipolysis), whereas decreased cell number could reflect reduced adipogenesis or increased cell death (22). mRNA profiling studies conducted in mice receiving high 10,12-CLA doses have provided evidence to support all of these mechanisms (17, 24). In this context, McIntosh and colleagues developed a model based on in vitro effects of 10,12-CLA on human adipocytes, where inflammation plays a central role in inducing many of these mechanisms (22). Specifically, 10,12-CLA induces the production of the proinflammatory cytokines IL-6 and TNF-α, which then contribute to the delipidating effects by promoting insulin resistance, by interfering with the expression and antagonizing activity of PPAR-γ, and by inducing apoptotic signals (4, 5). Consistent with this model, we observed that mice treated with the 0.5% 10,12-CLA dose had increased adipose tissue expression of inflammatory markers, including MCP-1, EMR1, IL-6, and TNF-α, as reported by others previously (17, 24, 33).
A model involving inflammation, however, does not explain our results showing a reduction in both the MFP and gonadal adipose tissue weight at the low dose of 10,12-CLA. Indeed, our data demonstrate that inflammation is not an intrinsic component to the antiobesity effect of 10,12-CLA. Parra et al. (31, 32) recently reported reduced adiposity in the absence of inflammation in growing mice orally treated with 0.15 g/kg body wt of CLA containing equimolar levels of 9,11- and 10,12-CLA. The innocuous antiobesity effect of this treatment could not be attributed to 10,12-CLA alone, even though we estimate that it corresponded to 0.05% or less of the consumed diet. This is because 9,11-CLA exerts potent anti-inflammatory effects, leaving open the possibility that it negated proinflammatory effects of 10,12-CLA (34). Indeed, Moloney et al. (28) have shown that 9,11-CLA corrected substantially the insulin resistance of ob/ob mice by reducing WAT inflammation. Our work, on the other hand, can attribute the reduction in adipose tissue mass solely to 10,12-CLA. Absence of inflammation also suggests that the low dose reduces adipose tissue mass by evoking a narrower set of mechanisms than proposed from studies with high CLA doses. As a first step to assess this, we show that the low dose remains capable of reducing SREBP-1c, FAS, and S14, suggesting that a repression of lipogenesis is involved, as reported previously for the lactating mammary gland (14, 15). De novo lipogenesis, however, accounts for a small portion of triglyceride accumulation in mouse adipose tissue, and it will be important in future studies to determine whether low doses of 10,12-CLA alter other processes regulating triglyceride accumulation in adipocytes, as well as adipocyte cell number. Finally, it is important to determine whether the ability of 10,12-CLA to reduce fat mass in the absence of inflammation extends to other fat depots, such as the omental and perirenal depots.
Perspectives and Significance
Numerous mechanisms have been proposed to explain CLA effects on adipose tissue mass and mammary development; most often, these were identified without consideration of dose-response relationships. We show that ductal development is impaired in peripubertal mice consuming a diet containing 0.5% 10,12-CLA. This abnormal development was associated with mammary inflammation and induction of genes known to alter ductal elongation and alveologenesis, and with metabolic complications. None of these defects were seen in mice receiving 0.1% 10,12-CLA, even though they also experience a reduction in MFP mass. These data suggest that pathologies caused by the use of 10,12-CLA in the mouse, including the acceleration of mammary cancers driven by the human oncogene ErbB2/Her2 (21), relate to the use of excessive CLA doses. Establishing dose-response relationships is often overlooked, but from this, we were able to clearly show that the antiobesity effects of 10,12-CLA are not dependent on a proinflammatory response. It remains to be seen whether the proinflammatory responses that occur at higher doses are essential components of the antiobesity effects of CLA or merely correlative responses observed when excessive doses of 10,12-CLA are used.
GRANTS
This project was supported in part by Cornell University Center for Vertebrate Genomics Seed Grant (to Y. R. Boisclair), Agriculture and Food Research Initiative Competitive Grant 2010-65206-20723 from the U.S. Department of Agriculture National Institute of Food and Agriculture (to D. E. Bauman), and the American Lung Association, Senior Research Training Fellowship (to M. R. Foote).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Susanne Pelton and Ramona Ehrhardt for excellent technical assistance.
REFERENCES
- 1.Bauman DE, Perfield JW, 2nd, Harvatine KJ, Baumgard LH. Regulation of fat synthesis by conjugated linoleic acid: lactation and the ruminant model. J Nutr 138: 403–409, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA 107: 2989–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem 17: 789–810, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, Boysen MS, Chung S, Fabiyi O, Morrison RF, Mandrup S, McIntosh MK. Conjugated linoleic acid induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J Biol Chem 279: 26735–26747, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung S, Brown JM, Provo JN, Hopkins R, McIntosh MK. Conjugated linoleic acid promotes human adipocyte insulin resistance through NF-κB-dependent cytokine production. J Biol Chem 280: 38445–38456, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci USA 104: 5455–5460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clement L, Poirier H, Niot I, Bocher V, Guerre-Millo M, Krief S, Staels B, Besnard P. Dietary trans-10,cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res 43: 1400–1409, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, Green J. Adipose tissue: a vital in vivo role in mammary gland development but not differentiation. Dev Dyn 223: 459–468, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103: 41–50, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Valdivia R, Mukherjee A, Ying Y, Li J, Paquet M, DeMayo FJ, Lydon JP. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol 328: 127–139, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Folch J, Lees MSloane, Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 12.Foster LB, Dunn RT. Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clin Chem 19: 338–340, 1973 [PubMed] [Google Scholar]
- 13.Gudbrandsen OA, Rodriguez E, Wergedahl H, Mork S, Reseland JE, Skorve J, Palou A, Berge RK. Trans-10, cis-12-conjugated linoleic acid reduces the hepatic triacylglycerol content and the leptin mRNA level in adipose tissue in obese Zucker fa/fa rats. Br J Nutr 102: 803–815, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Harvatine KJ, Bauman DE. SREBP1 and thyroid hormone responsive spot 14 (S14) are involved in the regulation of bovine mammary lipid synthesis during diet-induced milk fat depression and treatment with CLA. J Nutr 136: 2468–2474, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Harvatine KJ, Robblee MM, Boisclair YR, Bauman DE. Trans-10, cis-12 conjugated linoleic acid (CLA) induces a dose-dependent reduction in milk fat synthesis in C57BL6J mice. J Dairy Sci 91E-Suppl 1: 567, 2008 [Google Scholar]
- 16.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol 6: 715–725, 2005 [DOI] [PubMed] [Google Scholar]
- 17.House RL, Cassady JP, Eisen EJ, Eling TE, Collins JB, Grissom SF, Odle J. Functional genomic characterization of delipidation elicited by trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) in a polygenic obese line of mice. Physiol Genomics 21: 351–361, 2005 [DOI] [PubMed] [Google Scholar]
- 18.House RL, Cassady JP, Eisen EJ, McIntosh MK, Odle J. Conjugated linoleic acid evokes de-lipidation through the regulation of genes controlling lipid metabolism in adipose and liver tissue. Obes Rev 6: 247–258, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Ip C, Dong Y, Ip MM, Banni S, Carta G, Angioni E, Murru E, Spada S, Melis MP, Saebo A. Conjugated linoleic acid isomers and mammary cancer prevention. Nutr Cancer 43: 52–58, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Ip MM, Masso-Welch PA, Ip C. Prevention of mammary cancer with conjugated linoleic acid: role of the stroma and the epithelium. J Mammary Gland Biol Neoplasia 8: 103–118, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Ip MM, McGee SO, Masso-Welch PA, Ip C, Meng X, Ou L, Shoemaker SF. The t10,c12 isomer of conjugated linoleic acid stimulates mammary tumorigenesis in transgenic mice over-expressing erbB2 in the mammary epithelium. Carcinogenesis 28: 1269–1276, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy A, Martinez K, Schmidt S, Mandrup S, LaPoint K, McIntosh M. Antiobesity mechanisms of action of conjugated linoleic acid. J Nutr Biochem 21: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinberg DL, Wood TL, Furth PA, Lee AV. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev 30: 51–74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaRosa PC, Miner J, Xia Y, Zhou Y, Kachman S, Fromm ME. Trans-10, cis-12 conjugated linoleic acid causes inflammation and delipidation of white adipose tissue in mice: a microarray and histological analysis. Physiol Genomics 27: 282–294, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lock AL, Bauman DE. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 39: 1197–1206, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA 103: 2196–2201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng X, Shoemaker SF, McGee SO, Ip MM. t10,c12-Conjugated linoleic acid stimulates mammary tumor progression in Her2/ErbB2 mice through activation of both proliferative and survival pathways. Carcinogenesis 29: 1013–1021, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moloney F, Toomey S, Noone E, Nugent A, Allan B, Loscher CE, Roche HM. Antidiabetic effects of cis-9, trans-11-conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue. Diabetes 56: 574–582, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Ning Y, Hoang B, Schuller AG, Cominski TP, Hsu MS, Wood TL, Pintar JE. Delayed mammary gland involution in mice with mutation of the insulin-like growth factor binding protein 5 gene. Endocrinology 148: 2138–2147, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Pariza MW, Park Y, Cook ME. The biologically active isomers of conjugated linoleic acid. Prog Lipid Res 40: 283–298, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Parra P, Palou A, Serra F. Moderate doses of conjugated linoleic acid reduce fat gain, maintain insulin sensitivity without impairing inflammatory adipose tissue status in mice fed a high-fat diet. Nutr Metab (Lond) 7: 5, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parra P, Serra F, Palou A. Moderate doses of conjugated linoleic acid isomers mix contribute to lowering body fat content maintaining insulin sensitivity and a noninflammatory pattern in adipose tissue in mice. J Nutr Biochem 21: 107–115, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Poirier H, Shapiro JS, Kim RJ, Lazar MA. Nutritional supplementation with trans-10, cis-12-conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes 55: 1634–1641, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Reynolds CM, Roche HM. Conjugated linoleic acid and inflammatory cell signalling. Prostaglandins Leukot Essent Fatty Acids 82: 199–204, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Richards RG, Klotz DM, Walker MP, Diaugustine RP. Mammary gland branching morphogenesis is diminished in mice with a deficiency of insulin-like growth factor-I (IGF-I), but not in mice with a liver-specific deletion of IGF-I. Endocrinology 145: 3106–3110, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Rivas MA, Carnevale RP, Proietti CJ, Rosemblit C, Beguelin W, Salatino M, Charreau EH, Frahm I, Sapia S, Brouckaert P, Elizalde PV, Schillaci R. TNFα acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt, and NF-κB-dependent pathways. Exp Cell Res 314: 509–529, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Sisk MB, Hausman DB, Martin RJ, Azain MJ. Dietary conjugated linoleic acid reduces adiposity in lean but not obese Zucker rats. J Nutr 131: 1668–1674, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 8: 201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorn SR, Giesy SL, Myers MG, Jr, Boisclair YR. Mammary ductal growth is impaired in mice lacking leptin-dependent signal transducer and activator of transcription 3. Signal Endocrinol 151: 3985–3995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonner E, Barber MC, Allan GJ, Beattie J, Webster J, Whitelaw CB, Flint DJ. Insulin-like growth factor binding protein-5 (IGFBP-5) induces premature cell death in the mammary glands of transgenic mice. Development 129: 4547–4557, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Varela LM, Stangle-Castor NC, Shoemaker SF, Shea-Eaton WK, Ip MM. TNFα induces NF-κB/p50 in association with the growth and morphogenesis of normal and transformed rat mammary epithelial cells. J Cell Physiol 188: 120–131, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Walden PD, Ruan W, Feldman M, Kleinberg DL. Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology 139: 659–662, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Warren MA, Shoemaker SF, Ip MM. NFκB1/p50 is not required for tumor necrosis factor-stimulated growth of primary mammary epithelial cells: implications for NF-κB2/p52 and RelB. Endocrinology 148: 268–278, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.