Abstract
Sympathetic nerve discharge (SND) responses to hyperthermia are attenuated in aged rats without heart failure (HF) and in young HF (YHF) rats, demonstrating that individually aging and HF alter SND regulation. However, the combined effects of aging and HF on SND regulation to heat stress are unknown, despite the high prevalence of HF in aged individuals. We hypothesized that SND responses to heating would be additive when aging and HF are combined, demonstrated by marked reductions in SND and mean arterial pressure (MAP) responses to heating in aged HF (AHF) compared with aged sham HF (ASHAM) rats, and in AHF compared with YHF rats. Renal and splenic SND responses to hyperthermia (colonic temperature increased to 41.5°C) were determined in anesthetized YHF, young sham (YSHAM), AHF, and ASHAM Fischer rats. HF was induced by myocardial infarction and documented using echocardiographic, invasive, and postmortem measures. The severity of HF was similar in YHF and AHF rats. SND responses to heating were attenuated in YHF compared with YSHAM rats, demonstrating an effect of HF on SND regulation in young rats. In contrast, AHF and ASHAM rats demonstrated similar SND responses to heating, suggesting a prominent influence of age on SND regulation in AHF rats. Splenic SND and MAP responses to heating were similar in YHF, AHF, and ASHAM rats, indicating that the imposition of HF in young rats changes the regulatory status of these variables to one consistent with aged rats. These data suggest that the effect of HF on SND regulation to hyperthermia is age dependent.
Keywords: sympathetic nerve discharge, sympathetic nervous system, acute heat stress
heart failure (HF) is an important clinical syndrome and a major cause of morbidity and mortality in industrialized nations. An estimated five million Americans have congestive HF, and the aged are at an especially high risk (13, 17, 33, 37, 41, 53). The prevalence of HF increases from <1% in individuals younger than 40 years to >10% in individuals older than 80 years (33). The combined impact of an aging population and the age-related distribution of HF has increased the incidence of this condition to epidemic levels.
The sympathetic nervous system plays a crucial role in the regulation of physiological homeostasis under basal conditions and in response to acute and chronic stressors. Sympathetic dysregulation is considered a hallmark of both HF and aging (18, 43, 55), although, as discussed by Kaye and Esler (18), the regional pattern of basal sympathetic activation differs in humans with healthy aging and in human congestive HF patients. Renal sympathetic nerve outflow (as estimated using norepinephrine kinetic analyses) is increased in HF but not aging, splanchnic and hepatic sympathetic nerve outflows are increased in aging but not HF, and epinephrine secretion is reduced with aging but remains unchanged or marginally elevated in HF (18). Differentiation in regulation of regional sympathetic nerve outflow in human subjects suggests diversity in mechanisms controlling basal sympathetic nerve discharge (SND) in the aging and HF syndromes. However, little information is known regarding SND regulation to acute stress in aged subjects with HF. In the absence of specific studies involving aged HF (AHF) rats, information aimed at understanding the effects of HF on mechanisms mediating SND responses to acute stress has been extrapolated from studies using young HF (YHF) rats, a glaring omission based on the combined impact of the aging population and the age-related distribution of HF.
Hyperthermia is an environmental stressor that produces marked changes in the level of SND in young subjects. Acute heating increases muscle SND in conscious humans (4, 39), splanchnic SND in conscious rats (32), and renal, splanchnic, splenic, and lumbar SND in anesthetized rats (10, 12, 20, 21, 23–25). Visceral SND responses to hyperthermia are reduced in aged compared with young Fischer (F344) rats (24), and renal SND responses to hyperthermia are attenuated in YHF compared with young sham HF (YSHAM) rats (25), demonstrating that individually both advanced age and HF substantially modulate sympathetic nervous system regulation to acute heating. However, not a single study has determined SND responses to acute heat stress in AHF rats. We previously hypothesized that the attenuated SND responses to acute heating in aged rats and in YHF rats may be a function of an altered neural strategy that actively suppresses SND in response to increased internal body temperature (Tc) (24, 25). Therefore, we suspected that mechanisms mediating the attenuated SND responsivity to acute heating may be additive when aging and HF are combined, providing the basis for predicting that progressive hyperthermia would produce marked reductions in SND and mean arterial blood pressure (MAP) in AHF compared with aged sham HF (ASHAM) rats, and in AHF compared with YHF rats.
In the present study, renal and splenic SND responses to hyperthermia were determined in YHF, YSHAM, AHF, and ASHAM rats before and during progressive hyperthermia that increased Tc from 38.0 to 41.5°C. HF was induced by myocardial infarction and documented using a combination of transthoracic echocardiographic, invasive, and postmortem measures.
METHODS
The experimental procedures and protocols used in the present investigation were performed in accordance with the American Physiological Society's guiding principles for research involving animals and approved by the Institutional Animal Care and Use Committee at Kansas State University.
Surgically induced myocardial infarction.
Young (5–6 mo old) and aged (24–25 mo) male F344 rats were anesthetized with isoflurane (induction, 5%; maintenance, 2.0–3.0%), intubated, and connected to a rodent respirator. After a left side thoracotomy between the 5th and 6th ribs, the pericardial sac was opened, the heart was exteriorized, and the left main coronary artery was ligated between the pulmonary artery and the left atrium (7, 25, 36). Sham operations were completed using the same surgical procedures with the exception that the coronary artery was not ligated. The lungs were hyperinflated, the ribs were approximated, and the thoracotomy was closed using surgical suture. The skin incision was closed, and topically administered antibacterial and analgesic agents were applied. Anesthesia was withdrawn, and the animals were extubated. Each rat received ampicillin (25 mg/kg sc) one time per day for 10 days. Experiments were initiated 5–7 wk after the myocardial infarction (or sham) procedure.
Assessment of cardiac function.
Transthoracic echocardiography under isoflurane (1.5–2.5%) anesthesia was performed with the sonographer blinded to the group (myocardial infarcted or sham, young, or aged). Rats were placed in left lateral recumbency, and studies were performed from the right parasternal location. A Vivid 7 Dimension Cardiovascular Ultrasound System BT '06 (GE Healthcare, Milwaukee, WI) equipped with a 13-MHz linear array transducer was used for image acquisition. Echocardiographic data were collected simultaneously with single-lead ECG, and images were digitally stored. A minimum of seven consecutive heart cycles was stored for each view. Measurements are reported as the average of three to five heart cycles. Only measurements obtained from sinus beats were considered for further analyses. Real-time, gray-scale two-dimensional (2D) cineloops were generated from short-axis views at the level of the papillary muscles, mitral valve, and aortic root. From the same views, M-mode tracings were also obtained with direct 2D guidance. From these views, the following 2D and M-mode parameters were measured: left ventricular (LV) internal diameter in diastole (LVIDd), LV internal diameter in systole (LVIDs), left atrial diameter (LA), and aortic root diameter (Ao), from which the LA-to-Ao ratio was calculated. 2D and M-mode fractional shortening (FS) were also calculated. 2D images were also obtained from the right parasternal long-axis four-chamber view and the right parasternal long-axis five-chamber view. From these images, color-Doppler interrogation of the mitral and aortic valve was performed.
After collection of echocardiographic data, rats were maintained on isoflurane anesthesia and supplemented with α-chloralose (80 mg/kg ip) and urethane (800 mg/kg ip) anesthesia. The right carotid artery was isolated and cannulated with a 2-Fr Millar catheter-tip pressure manometer, and the micromanometer was advanced in the left ventricle in a retrograde fashion for measuring left ventricular end diastolic pressure (LVEDP) (7, 25).
General procedures and sympathetic nerve recordings.
Following the measurement of LVEDP, the 2-Fr Millar catheter was replaced with a cannula for monitoring carotid arterial pressure. A cannula was placed in the femoral vein for the intravenous administration of maintenance doses of α-chloralose (35–45 mg·kg−1·h−1). Maintenance doses of urethane (200 mg/kg every 4 h) were administered intraperitoneally. Carotid arterial pressure was monitored using a pressure transducer connected to a blood pressure analyzer. Heart rate (HR) was derived from the pulsatile arterial pressure output of the blood pressure analyzer. The trachea was cannulated; rats were paralyzed with gallamine triethiodide (5–10 mg/kg iv, initial dose; 10–15 mg·kg−1·h−1, maintenance dose) and artificially ventilated; and isoflurane anesthesia was withdrawn. Tc was measured with a thermistor probe inserted ∼5–6 cm in the colon and was kept at 38.0°C during surgery by a temperature-controlled table.
SND was recorded biphasically with a platinum bipolar electrode after capacity-coupled preamplification (bandpass 30–3,000 Hz) from the central end of cut or distally crushed renal and splenic sympathetic nerves (12). Nerves were isolated retroperitoneally, and nerve-electrode preparations were covered with silicone gel. Sympathetic nerve potentials were full wave rectified and integrated (time constant 10 ms). Total power in splenic SND and renal SND was quantified as volts times seconds, and SND recordings were corrected for background noise after administration of the ganglionic blocker chlorisondamine (5 mg/kg iv) (12).
The adequacy of anesthesia during the myocardial infarction, transthoracic echocardiographic, and initial surgical (arterial and venous cannulations, establishment of SND recordings) procedures was indicated by the absence of a withdrawal reflex in response to mechanical stimulation of the tail or hind limb. The adequacy of anesthesia after the establishment of SND recordings and following initiation of neuromuscular blockade was indicated by an inability of mechanical stimulation of the hind limb or tail to increase SND or MAP.
Experimental protocol.
After completion of surgical procedures, rats were allowed to stabilize for 60 min. Tc was maintained at 38°C during the stabilization period. At the end of this period, Tc was increased at a rate of 0.08–0.09°C/min from 38.0 to 41.5°C using a heat lamp. Heating experiments were completed on 16 YSHAM, 11 YHF, 10 ASHAM, and 11 AHF rats. Six YHF, three ASHAM, and two AHF rats died either before initiation of the acute heating protocol or during progressive heating and were not included in data analyses. SND (renal and splenic), MAP, and HR were recorded continuously before and during progressive increases in Tc.
Postmortem data.
At the conclusion of each experiment, rats were given an overdose of methohexital sodium (Brevital, 150 mg/kg iv). The thorax was opened, and placement of the carotid artery catheter in the aortic arch was confirmed. The lungs were excised and weighed. The heart was removed, the right ventricle was separated from the left ventricle and septum, and both tissues were weighed. To determine left ventricle infarct size, an incision was made through the interventricular septum, from the base to the apex of the left ventricle, and a digital photograph of the endocardium was taken (9). The image was printed, and endocardial infarct surface area was determined by planimetry (9).
Data and statistical analyses.
Congestive HF was identified using a combination of echocardiographic, invasive, and postmortem data. Echocardiographic indexes included LV dyskinesia/hypokinesia/akinesia (LVIDs), atrial enlargement, LV eccentric hypertrophy (increased LVIDd), and changes in LV FS. Left atrial enlargement was defined as an LA/Ao ratio of >1.7. This relatively strict criterion was adopted to optimize the specificity (i.e., low false positives) in the identification of congestive HF. Obviously, this criterion has intrinsic limitations in terms of sensitivity (i.e., low false negatives), but, for the purpose of this study, a higher specificity would give better internal validity to the data. LV dysfunction was documented by measurement of LVEDP, lung congestion was estimated based on changes in the lung weight-to-body weight ratio (LW/BW), and right ventricular hypertrophy was indicated by examining the right ventricle weight-to-body weight ratio (RV/BW). Left ventricle endocardial infarct surface area was expressed as the percentage of the left ventricle endocardial circumference.
ANOVA techniques followed by Student-Newman-Keuls post hoc tests were performed on SND, MAP, HR, FS, LA/Ao ratio, LVIDd, LVIDs, LVEDP, LW/BW, and RV/BW data. Responses were compared within groups of rats of similar ages with and without HF and between groups of rats of different ages with and without HF. The overall level of significance was set at P < 0.05.
RESULTS
Indexes of HF.
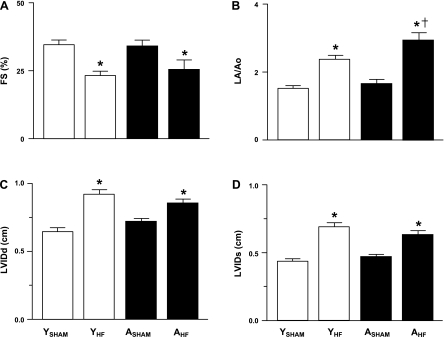
Transthoracic echocardiographic procedures were completed on 10 YSHAM, 11 YHF, 7 ASHAM, and 8 AHF rats, and variable degrees of LV dyskinesia, hypokinesia, or akinesia were identified in all YHF and AHF rats. LV FS at the level of the apical portion of the papillary muscles from the right parasternal short-axis view was significantly reduced in YHF compared with YSHAM rats and in AHF compared with ASHAM rats (Fig. 1A). Left atrial dimensions, reported as the LA/Ao ratio, were significantly increased in YHF compared with YSHAM rats, AHF compared with ASHAM rats, and AHF compared with YHF rats (Fig. 1B). LVIDd and LVIDs were significantly increased in YHF and AHF rats compared with YSHAM and ASHAM rats, respectively (Fig. 1, C and D).
Fig. 1.
Echocardiographic data [fractional shortening (FS) (A), ratio of left atrial diameter (LA) and aortic root diameter (Ao) (LA/Ao) (B), left ventricular internal diameter in diastole (LVIDd) (C), and left ventricular internal diameter in systole (LVIDs) (D)] from young sham heart failure (YSHAM), young heart failure (YHF), aged sham heart failure (ASHAM), and aged heart failure (AHF) rats. P < 0.05 vs. age-matched sham groups (*) and vs. young condition-matched groups (†).
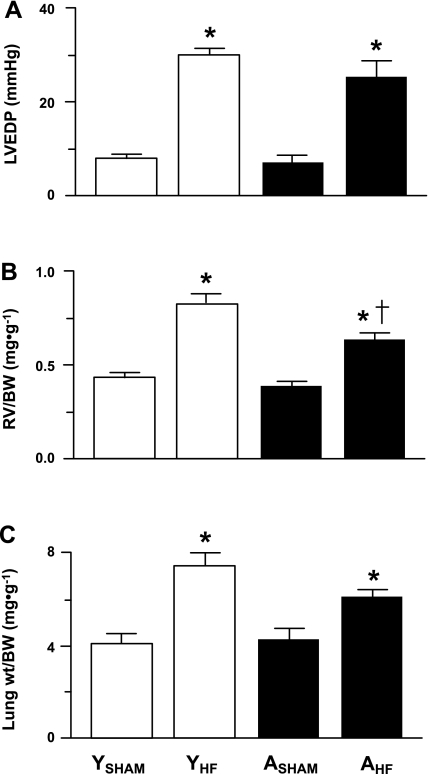
Invasive (LVEDP) and/or postmortem (RV/BW and LW/BW ratios) data were obtained on 16 YSHAM, 11 YHF, 10 ASHAM, and 11 AHF rats. LVEDP (Fig. 2A) and the RV/BW (Fig. 2B) and LW/BW (Fig. 2C) ratios were significantly increased in YHF compared with YSHAM rats and in AHF compared with ASHAM rats. The RV/BW ratio was significantly reduced in AHF compared with YHF rats (Fig. 2B). Left ventricle endocardial infarct surface area was significantly reduced in AHF (26 ± 1%) compared with YHF (36 ± 2%) rats.
Fig. 2.
Invasive [left ventricular end-diastolic pressure (LVEDP) (A)] and morphometric [right ventricle weight-to-body weight ratio (RV/BW) (B) and lung weight-to-body weight ratio (LW/BW) (C)] data from YSHAM, YHF, ASHAM, and AHF rats. *P < 0.05 vs. age-matched sham groups (*) and vs. young condition-matched groups (†).
SND and cardiovascular responses to acute heat stress.
Heating experiments were completed on 16 YSHAM, 11 YHF, 10 ASHAM, and 11 AHF rats. Body weights did not differ between groups (Table 1). Basal MAP was significantly lower in ASHAM compared with YSHAM rats, but otherwise did not differ between groups (Table 1). Basal HR was significantly lower in YHF compared with YSHAM rats, AHF compared with ASHAM rats, and ASHAM compared with YSHAM rats (Table 1).
Table 1.
Basal body weight and hemodynamic measures for young and aged rats with HF and for young and aged sham HF rats
| YSHAM (n = 16) | YHF (n = 11) | ASHAM (n = 10) | AHF (n = 11) | |
|---|---|---|---|---|
| Body wt, g | 368 ± 8 | 383 ± 9 | 385 ± 7 | 389 ± 8 |
| MAP, mmHg | 108 ± 4 | 100 ± 2 | 85 ± 3† | 92 ± 4 |
| HR, beats/min | 390 ± 6 | 347 ± 11* | 359 ± 11† | 330 ± 9* |
Values are means ± SE; n, no. of rats. YSHAM and ASHAM, young and aged rats without heart failure (HF), respectively; YHF and AHF, young and aged rats with HF, respectively; MAP, mean arterial pressure; HR, heart rate. P < 0.05, HF vs. age-matched sham groups (*) and aged vs. young condition-matched groups (†).
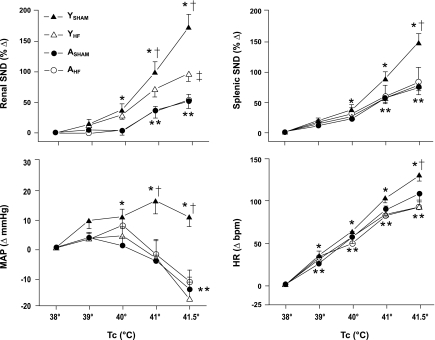
Figure 3 summarizes changes in renal SND, splenic SND, MAP, and HR from control (38°C) during progressive increases in Tc from 38 to 41.5°C. Renal and splenic SND were significantly increased from control during heating in YSHAM, ASHAM, YHF, and AHF groups. Renal SND responses to heating were significantly higher in YSHAM compared with ASHAM rats, YSHAM compared with YHF rats, and YHF compared with AHF rats but were similar in ASHAM compared with AHF rats. Splenic SND responses to heating were significantly higher in YSHAM compared with both ASHAM and YHF rats but were similar in YHF compared with AHF rats and in ASHAM compared with AHF rats. Compared with control levels, MAP was significantly increased during heating in YSHAM rats but significantly decreased in ASHAM, YHF, and AHF groups. MAP responses to heating were significantly higher in YSHAM compared with both YHF and ASHAM rats. In contrast, MAP responses to heating did not differ in AHF compared with ASHAM rats or in YHF compared with AHF rats. HR was significantly increased from control during heating in each experimental group. Heating-induced increases in HR were significantly higher in YSHAM compared with both YHF and ASHAM rats. In contrast, HR responses to heating did not differ in AHF compared with ASHAM rats, or in AHF compared with YHF rats.
Fig. 3.
Changes in renal sympathetic nerve discharge (SND), splenic SND, mean arterial pressure (MAP), and heart rate (HR) during increases in internal body temperature (Tc) from 38 to 41.5°C in YSHAM (▴), YHF (▵), ASHAM (●), and AHF (○) rats. P < 0.05, YSHAM vs. control (38°C) values (*), AHF, ASHAM, and YHF vs. control (38°C) values (**), YSHAM vs. YHF and ASHAM groups (†), and YHF vs. AHF (‡).
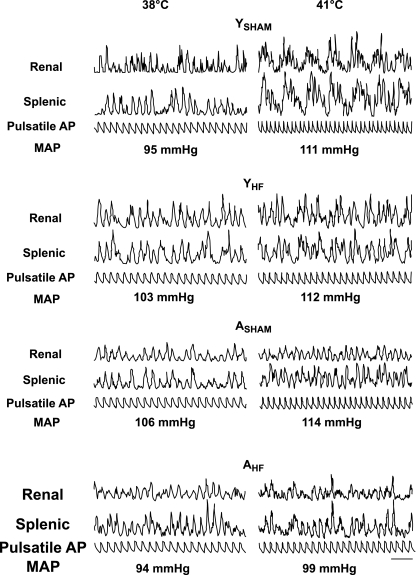
Figure 4 shows traces of simultaneously recorded renal and splenic SND bursts and pulsatile arterial blood pressure during control (38°C; Fig. 4, left) and after increasing Tc to 41.0°C (Fig. 4, right) in representative YSHAM, YHF, ASHAM, and AHF rats. Levels of MAP recorded during each period are shown below the pulsatile arterial blood pressure traces. During control, the majority of renal and splenic SND bursts was coupled to the arterial pulse in both HF and sham HF rats, regardless of age. After increasing Tc to 41°C, the SND bursting pattern in the YSHAM rat was transformed to a pattern dominated by the presence of high-amplitude, low-frequency bursts. In contrast, the sympathetic nerve signals recorded at 41°C in the YHF, ASHAM, and AHF rats were not dominated by the uniform presence of high-amplitude, low-frequency bursts, rather, they were characterized by a mixture of cardiac-related and low-frequency bursts (YHF and AHF rats) or the presence of mainly cardiac-related bursts (ASHAM rat). MAP was increased from control levels during heating in each experiment shown in Fig. 4. SND pattern transformation during heating is considered the signature of an activated sympathetic state because the cardiac-related rhythmicity of SND bursts is reduced at a time when arterial blood pressure is increased (21), a stimulus that would be expected to enhance the coupling of SND bursts to the arterial pulse. Therefore, heating-induced changes in the SND bursting pattern from cardiac-related to low-frequency bursts was examined in experiments only in which MAP was at or above control values after Tc had been increased to 41°C. This included 14 YSHAM, 6 YHF, 5 ASHAM, and 6 AHF rats. The SND bursting pattern during heating was dominated by the presence of low-frequency, high-amplitude bursts in 13 YSHAM rats and contained a mixture of cardiac-related and low-frequency bursts in one YSHAM rat. In contrast, the presence of a marked heating-induced SND activation state was observed much less frequently in YHF, ASHAM, and AHF rats. Specifically, the SND bursting pattern during heating was characterized by the presence of uniform low-frequency, high-amplitude bursts in only 1/6 YHF, 0/5 ASHAM, and 1/6 AHF rats, whereas the SND bursting pattern contained a mixture of cardiac-related and low-frequency bursts in 3/6 YHF, 2/5 ASHAM, and 2/6 AHF rats and maintained a signal dominated by the presence of cardiac-related bursts in 2/6 YHF, 3/5 ASHAM, and 3/6 AHF rats.
Fig. 4.
Traces of integrated SND bursts (renal and splenic) and pulsatile arterial pressure (AP) recorded during control (38°C) and after increasing Tc to 41°C in YSHAM, YHF, ASHAM, and AHF rats. MAP values for each period are shown below the pulsatile AP traces. Horizontal calibration is 500 ms.
DISCUSSION
Because relevant data are lacking, an evidence-based approach to understanding sympathetic regulation to acute stress in aged rats with HF has not been available. The present study is the first to determine the effect of combined aging and HF on SND regulation to acute heat stress in F344 rats. As expected (24, 25), SND and cardiovascular responses to progressive hyperthermia were significantly attenuated in ASHAM compared with YSHAM rats, indicating that aging alters SND regulation to heat stress, and in YHF compared with YSHAM rats, demonstrating a marked effect of HF on sympathetic neural and cardiovascular regulation to hyperthermia in young rats. However, and contrary to our hypothesis, AHF and ASHAM rats demonstrated similar SND (renal and splenic), arterial blood pressure, and HR responses to heating, suggesting a prominent influence of age and not HF on sympathetic nervous system and cardiovascular responsiveness to progressive hyperthermia in older rats. The responsivity of renal SND to heating was reduced in AHF compared with YHF rats; however, splenic SND and cardiovascular responses to increased Tc were similar in YHF, AHF, and ASHAM rats, suggesting that the imposition of HF in young rats changes the regulatory status of these variables during heating to one consistent with that observed in ASHAM and AHF rats.
We have previously reported diminished SND responses to heating in aged rats (24) and in YHF rats (25), demonstrating that individually both aging and HF alter SND regulation to acute heat stress. We hypothesized that the attenuated SND responses to acute heating in these animals may be a function of an altered neural strategy that actively suppresses SND responses to increased Tc (24, 25). We suspected that mechanisms mediating the attenuated SND responsivity to acute heating may be additive when aging and HF are combined, leading to profound reductions in SND and arterial blood pressure during progressive hyperthermia in AHF rats. This was not the case under the current experimental conditions, since SND (renal and splenic) and cardiovascular responses to progressive heating were similar in ASHAM and AHF rats. One possible explanation is that ASHAM and AHF rats demonstrate similar cardiac phenotypes; however, echocardiographic (FS, LA/Ao ratio, LVIDd, and LVIDs), invasive (LVEDP), and postmortem (RV/BW and LW/BW ratios) indexes of HF differed significantly in AHF compared with ASHAM rats, indicating that the aged rats who received a surgically induced myocardial infarction were in HF.
HF-induced changes in the majority of echocardiographic (FS, LVIDd, LVIDs), invasive (LVEDP), and postmortem (LW/BW ratio) parameters were similar in YHF and AHF rats, suggesting an equivalent severity of HF in the aged and young animals. However, the influence of HF on SND and cardiovascular regulation to acute heating was strongly age-dependent. Splenic SND and cardiovascular responses to progressive hyperthermia were similar in YHF, AHF, and ASHAM rats, indicating that, from a regulatory perspective, HF effectively transformed young rats to aged rats with regard to these variables. In contrast, the imposition of HF in aged rats did not alter SND and cardiovascular changes to heating, since AHF and ASHAM rats demonstrated similar responses. These findings provide experimental support for two important considerations. First, most if not all studies in rats demonstrating HF-induced alterations in sympathetic nerve outflow (direct SND recordings) have used YHF rats as the model of choice, despite the fact that the majority of HF patients are aged. The current results provide a degree of experimental validation for this approach because splenic SND and cardiovascular responses to progressive hyperthermia were similar in YHF and AHF rats. However, the responsivity of renal SND to heating was reduced in AHF compared with YHF rats, indicating that HF-related changes in SND regulation to progressive hyperthermia in young rats may be nerve specific. Second, the fact that AHF and ASHAM rats demonstrated similar SND and cardiovascular responses to acute heating suggests that aging, and not HF, plays a critical regulatory role in response to this stimulus. Mechanisms mediating this profound effect of aging on SND regulation to heat stress remain to be determined. Although a role for age-related changes in SND regulation has been considered (8, 15, 16, 49), studies have not been completed in a thorough manner. In fact, so few investigations have utilized central neural microinjection techniques along with peripheral SND recordings in aged animals that it is difficult to develop an evidence-based framework regarding the effects of age on central SND regulation.
Heating-induced activation of visceral SND plays a critical role in mediating cardiovascular responses to hyperthermia in young mammals. Kregel and Gisolfi (31) reported that celiac ganglionectomy abolished increases in mesenteric resistance to progressive hyperthermia, and loss of splanchnic vasoconstriction adversely affects cardiovascular responses to heat stroke. Kenney et al. (21) reported that blockade of autonomic ganglionic neural transmission at peak hyperthermia reduces arterial blood pressure to values less than those produced by ganglionic blockade under normothermic conditions, suggesting that activation of sympathetic nerve outflow is important for counteracting vasodilatory influences during hyperthermia. In the current study, arterial blood pressure responses to acute heating were significantly lower in YHF, AHF, and ASHAM rats compared with YSHAM rats, suggesting an important functional consequence for the diminished SND responses to heating in YHF, AHF, and ASHAM rats.
One possible contributing factor to the altered sympathetic neural and arterial blood pressure responses to heat stress in YHF, AHF, and ASHAM rats is that these animals may not be able to activate thermoregulatory responses, possibly secondary to a stimulus deficit. This is unlikely, since renal SND, splenic SND, and HR were progressively and significantly increased from control levels during heating in YHF, AHF, and ASHAM rats, demonstrating that these animals are capable of activating thermoregulatory effectors in response to acute heating. In the present study, basal levels of HR were significantly reduced in YHF compared with YSHAM rats and in AHF compared with ASHAM rats, indicating that, under the current experimental conditions, HF produced basal bradycardia. These results are consistent with those of Behnke et al. (3) who reported significant reductions in basal HR in young and aged HF rats compared with young and aged sham HF rats. However, the results of several studies indicate that basal HR is significantly increased or tends to be higher in YHF compared with YSHAM rats (35, 50, 54), whereas other studies have reported similar levels of basal HR in YHF compared with YSHAM rats (25, 28, 51, 52). Thus it appears that a substantial amount of variability exists in the published literature concerning the effect of HF on regulation of basal HR in studies using rats as an experimental model.
The absolute level of activity recorded in multifiber SND preparations is influenced by several experimental factors, including the use of bipolar recording techniques, the proximity of the recording electrodes to active nerve fibers, the number of active nerve fibers, and specific recording conditions. Because of these methodological considerations, the interpretation of the absolute level of SND is problematic; therefore, SND responsiveness is typically reported as percent change from baseline for each experimental animal, which raises concern when comparing SND responses between groups of animals. With this in mind, it must be considered that the attenuated responsiveness of SND to hyperthermia in YHF, AHF, and ASHAM rats may be in part due to a ceiling effect, that is, levels of SND recorded at peak hyperthermia in these animals may represent the physiological maximum. This is likely not the case because application of a short bout of asphyxia (10–15 s) in our previous investigations (YSHAM rats, middle-aged rats, ASHAM rats, and YHF rats) (24) and in select experiments in the current study (YSHAM, YHF, AHF, and ASHAM rats, data not shown) produces, without exception, additional activation of SND above levels recorded at peak hyperthermia. However, additional sympathoexcitation in response to asphyxia does not eliminate the possibility of a SND ceiling effect to hyperthermia, that is, maximum levels of sympathetic excitation may be stimulus specific. In addition to changes in the level of activity, central sympathetic neural circuits are capable of generating a complex array of output patterns (2), and the SND bursting pattern is altered in response to a variety of experimental interventions (19–22, 24, 29, 30). We have previously reported that SND pattern transformation from cardiac-related to low-frequency bursts is a consistent feature of SND responses to acute heating in young but not aged rats (24) and is considered the signature of an activated sympathetic state. The heating-induced low-frequency bursts contain more activity than cardiac-related bursts, establishing pattern transformation as an important strategy for increasing the level of sympathetic nerve outflow (21). In the current study, the SND bursting pattern during heating in the YHF, AHF, and ASHAM rats was less likely to be transformed to a signal dominated by low-frequency bursts than in the YSHAM rats. These data support the hypothesis that HF and aging alter the response characteristics of sympathetic neural circuits during increased Tc.
Transthoracic echocardiography is a widely available noninvasive technology that allows for the anatomic and functional evaluation of the cardiovascular apparatus in the clinical and research arena. The adoption of a high-frequency transducer makes this diagnostic technique applicable to small mammals with optimal feasibility. The linear transducer used in this study allowed us to obtain good-quality short-axis views from the right parasternal location. However, as a consequence of the relatively long footprint, right parasternal long-axis images and left apical images were not consistently of diagnostic quality; therefore, results from those insonating planes, in particular LV ejection fraction, were not included in the final analysis.
Despite the fact that left ventricle endocardial infarct surface area was reduced in AHF compared with YHF rats, the present data suggest an equivalent severity of HF in the aged and young animals because HF-induced changes in the majority of echocardiographic, invasive, and postmortem parameters were similar in YHF and AHF rats. These observations indicate that a composite of anatomical and functional variables must be considered when determining the pathophysiological status of HF rats. The sympathetic nervous system is capable of producing selective changes in efferent SND directed to specific target organs; therefore, the present findings are applicable to renal and splenic SND only. The present results provide insight regarding SND regulation to a specific experimental intervention (whole body heating) using an in vivo, anesthetized preparation; therefore, they cannot be directly applied to other interventions or experimental preparations. Anesthesia can affect basal levels of sympathetic nerve activity (44) and alter SND responses to various stimuli (38, 40); therefore, it must be considered that the present findings may be influenced by the anesthetic state. However, despite this potential limitation, we chose to complete the current study using anesthetized rats for the following reasons. SND responses to heat stress are similar in conscious and anesthetized preparations, since acute heating increases SND in conscious humans (4, 39), conscious rats (32), and young, anesthetized rats (10, 12, 20, 21, 23–25), whereas SND remains unchanged during acute heating in conscious (45) and anesthetized (24) aged F344 rats. Because acute heating in young rats increases the level of activity in sympathetic nerves innervating diverse targets, it was important in the current study to record the discharges in regionally selective nerves. The simultaneous recording of discharges in multiple sympathetic nerves is a methodology that, at least in our hands, is typically completed in anesthetized preparations. Finally, physiological responses to acute heating can be influenced by behavior modifications; therefore, we chose to study SND regulation to heating in anesthetized rats to eliminate this influence.
Although mechanisms mediating the marked suppression of SND and cardiovascular responses to heating in YHF, AHF, and ASHAM rats remain to be determined, one central neural site that may contribute is the rostral ventral lateral medulla (RVLM). The RVLM plays a critical role in basal and reflex regulation of SND (1, 5, 6, 34, 46–48), and it is well established that regulation of SND involves a complex balance between RVLM excitatory and inhibitory systems (5, 6, 11, 14, 27, 42). Recently, we demonstrated that a substantial amount of the sympathetic activation during peak hyperthermia in young rats is dependent on the integrity of RVLM neural circuits (12). SND activation in response to progressive hyperthermia likely involves multiple receptor systems that interact in a dynamic and coordinated manner to augment RVLM synaptic excitation and attenuate synaptic inhibition. It may be that the imposition of HF in young rats as well as advanced age (with and without HF) shift the balance of RVLM regulation during heating to a state characterized by enhanced synaptic inhibition and reduced synaptic excitation.
Perspectives and Significance
The acute responsivity of the sympathetic nervous system is considered a hallmark of physiological responses to acute physical stress. Increasing the level of activity in sympathetic nerves innervating visceral organs and changing the pattern of SND bursts are primary strategies used by healthy, young rats to respond to heat stress (20, 21, 23–26). However, SND responses to hyperthermia are attenuated in aged compared with young F344 rats (24) and in YHF compared with YSHAM rats (25), demonstrating that individually both advanced age and HF affect sympathetic nervous system responsivity to acute heating. However, a dearth of information exists regarding SND regulation to acute heating in aged subjects with HF, despite the high prevalence of HF in aged individuals. The present results suggest a marked attenuation in the responsiveness of selected SND and cardiovascular indexes to heating in YHF, AHF, and ASHAM rats and support the notion that HF-induced effects on SND regulatory strategies to progressive hyperthermia are age-dependent. Because the incidence of many chronic disease states increases with age (43), the present results encourage the use of aged animals in studies aimed at determining the effect of specific pathophysiological syndromes/conditions on sympathetic nervous system regulation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-091342 (M. J. Kenney).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Barman SM. Brainstem control of cardiovascular function. In: Brainstem Mechanisms of Behavior, edited by Klemm WR, Vertes RP. New York: Wiley, 1990 [Google Scholar]
- 2.Barman SM, Kenney MJ. Methods of analysis and physiological relevance of rhythms in sympathetic nerve discharge. Clin Exp Pharmacol Physiol 34: 350–355, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Behnke B, Delp M, Poole D, Musch T. Aging potentiates the effect of congestive heart failure on muscle microvascular oxygenation. J Appl Physiol 103: 1757–1763, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 277: H2348–H2352, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand 177: 209–218, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Eklund KE, Hageman KS, Poole DC, Musch TI. Impact of aging on muscle blood flow in chronic heart failure. J Appl Physiol 99: 505–514, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Esler M, Hastings J, Lambert G, Kaye D, Jennings G, Seals DR. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol 282: R909–R916, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 188: 3–13, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gisolfi C, Matthes R, Kregel KC, Oppliger R. Splanchnic sympathetic nerve activity and circulating catecholamines in the hyperthermic rat. J Appl Physiol 70: 1821–1826, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Horiuchi J, Killinger S, Dampney RA. Contribution to sympathetic vasomotor tone of tonic glutamatergic inputs to neurons in the RVLM. Am J Physiol Regul Integr Comp Physiol 287: R1335–R1343, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Hosking KG, Fels RJ, Kenney MJ. Inhibition of RVLM synaptic activation at peak hyperthermia reduces visceral sympathetic nerve discharge. Auton Neurosci 150: 104–110, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huynh BC, Rovner A, Rich MW. Long-term survival in elderly patients hospitalized for heart failure: 14-year follow-up from a prospective randomized trial. Arch Intern Med 166: 1892–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ito S, Sved AF. Tonic glutamate-mediated control of rostral ventrolateral medulla and sympathetic vasomotor tone. Am J Physiol Regul Integr Comp Physiol 273: R487–R494, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Itoh H, Bunag R. Catecholaminergic nucleus tractus solitarius lesions in anesthetized rats alter baroreflexes differently with age. Mech Ageing Dev 64: 69–84, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Itoh H, Bunag RD. Aging reduces cardiovascular and sympathetic responses to NTS injections of serotonin in rats. Exp Gerontol 27: 309–320, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev 5: 167–173, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Kaye D, Esler M. Sympathetic neuronal regulation of the heart in aging and heart failure. Cardiovasc Res 66: 256–264, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kenney MJ. Frequency characteristics of sympathetic nerve discharge in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 267: R830–R840, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Kenney MJ, Barney CC, Hirai T, Gisolfi CV. Sympathetic nerve responses to hyperthermia in the anesthetized rat. J Appl Physiol 78: 881–889, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Kenney MJ, Claassen DE, Bishop MR, Fels RJ. Regulation of the sympathetic nerve discharge bursting pattern during heat stress. Am J Physiol Regul Integr Comp Physiol 275: R1992–R2001, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Kenney MJ, Claassen DE, Fels RJ, Saindon CS. Cold stress alters characteristics of sympathetic nerve discharge bursts. J Appl Physiol 87: 732–742, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Kenney MJ, Fels RJ. Forebrain and brain stem neural circuits contribute to altered sympathetic responses to heating in senescent rats. J Appl Physiol 95: 1986–1993, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kenney MJ, Fels RJ. Sympathetic nerve regulation to heating is altered in senescent rats. Am J Physiol Regul Integr Comp Physiol 283: R513–R520, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Kenney MJ, Musch TI, Weiss ML. Renal sympathetic nerve regulation to heating is altered in rats with heart failure. Am J Physiol Heart Circ Physiol 280: H2868–H2875, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kenney MJ, Pickar JG, Weiss ML, Saindon CS, Fels RJ. Effects of midbrain and spinal cord transections on sympathetic nerve responses to heating. Am J Physiol Regul Integr Comp Physiol 278: R1329–R1338, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Kiely JM, Gordon FJ. Role of rostral ventrolateral medulla in centrally mediated pressor responses. Am J Physiol Heart Circ Physiol 267: H1549–H1556, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kocsis B, Fedina L, Gyimesi-Pelczer K, Ladocsi T, Pasztor E. Differential sympathetic reactions during cerebral ischaemia in cats: the role of desynchronized nerve discharge. J Physiol 469: 37–50, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kocsis B, Fedina L, Pasztor E. Two-phase change of sympathetic rhythms in brain ischemia, Cushing reaction, and asphyxia. Am J Physiol Regul Integr Comp Physiol 256: R120–R132, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Kregel KC, Gisolfi CV. Circulatory responses to heat after celiac ganglionectomy or adrenal demedullation. J Appl Physiol 66: 1359–1363, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Kregel KC, Stauss H, Unger T. Modulation of autonomic nervous system adjustments to heat stress by central ANG II receptor antagonism. Am J Physiol Regul Integr Comp Physiol 266: R1985–R1991, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215 [DOI] [PubMed] [Google Scholar]
- 34.Loewy A. Central autonomic pathways. In: Central Regulation of Autonomic Functions, edited by Loewy A, Spyer K. New York, NY: Oxford Univ Press, 1990, p. 28–43 [Google Scholar]
- 35.Machackova J, Sanganalmath SK, Barta J, Dhalla KS, Dhalla NS. Amelioration of cardiac remodeling in congestive heart failure by beta-adrenoceptor blockade is associated with depression in sympathetic activity. Cardiovasc Toxicol 10: 9–16, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Musch T, Terrell J. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992. [DOI] [PubMed] [Google Scholar]
- 37.National Institutes of Health Disease statistics. In: National Heart, Lung, and Blood Institute Fact Book. Bethesda, MD: National Institutes of Health, 2005, p. 37–55 [Google Scholar]
- 38.Ng CW, De Matteo R, Badoer E. Effect of muscimol and l-NAME in the PVN on the RSNA response to volume expansion in conscious rabbits. Am J Physiol Renal Physiol 287: F739–F746, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst 63: 61–67, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Ramchandra R, Barrett CJ, Guild SJ, Malpas SC. Evidence of differential control of renal and lumbar sympathetic nerve activity in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 290: R701–R708, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Rich MW. Heart failure in older adults. Med Clin North Am 90: 863–885, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Sakima A, Yamazato M, Sesoko S, Muratani H, Fukiyama K. Cardiovascular and sympathetic effects of l-glutamate and glycine injected into the rostral ventrolateral medulla of conscious rats. Hypertens Res 23: 633–641, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol 528: 407–417, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimokawa A, Kunitake T, Takasaki M, Kannan H. Differential effects of anesthetics on sympathetic nerve activity and arterial baroreceptor reflex in chronically instrumented rats. J Auton Nerv Syst 72: 46–54, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Stauss HM, Morgan DA, Anderson KE, Massett MP, Kregel KC. Modulation of baroreflex sensitivity and spectral power of blood pressure by heat stress and aging. Am J Physiol Heart Circ Physiol 272: H776–H784, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491: 156–162, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol 47: 157–233, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Sun MK. Pharmacology of reticulospinal vasomotor neurons in cardiovascular regulation. Pharmacol Rev 48: 465–494, 1996 [PubMed] [Google Scholar]
- 49.Tanabe S, Bunag R. Aging escalates baroreceptor reflex suppression by the posterior hypothalamus in rats. Hypertension 17: 80–90, 1991 [DOI] [PubMed] [Google Scholar]
- 50.Wang RJ, Zeng QH, Wang WZ, Wang W. GABA(A) and GABA(B) receptor-mediated inhibition of sympathetic outflow in the paraventricular nucleus is blunted in chronic heart failure. Clin Exp Pharmacol Physiol 36: 516–522, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am J Physiol Heart Circ Physiol 295: H1216–H1226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Tonic glutamatergic input in the rostral ventrolateral medulla is increased in rats with chronic heart failure. Hypertension 53: 370–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young JB. The global epidemiology of heart failure. Med Clin North Am 88: 1135–1143, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol 297: R1364–R1374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zucker IH, Pliquett RU. Novel mechanisms of sympatho-excitation in chronic heart failure. Heart Fail Monit 3: 2–7, 2002 [PubMed] [Google Scholar]