Abstract
The role of adenosine in the regulation of cardiovascular function has long been acknowledged, but only recently has its importance in angiogenesis been appreciated, most notably, through its direct regulation of the proangiogenic growth factor, VEGF. Recent work has established that proangiogenic and antiangiogenic factors, specifically VEGF and and the soluble VEGF receptor fms-like tyrosine kinase-1 (sFlt-1), are directly influenced by hypoxia in placental ischemia. While adenosine has been reported to be an important regulator of VEGF in vascular tissue, the importance of adenosine in regulating VEGF and sFlt-1 in placental tissue is unclear. Here, we have investigated the role of adenosine in the secretion of VEGF and the antiangiogenic protein sFlt-1 in placental villous explants. Under normoxic conditions (6% oxygen), the nonspecific adenosine receptor antagonist, 8-sulphophenyltheophylline (8-SPT) had no effect on either VEGF (P = 0.38) or sFlt-1 (P = 0.56) secretion. However, under hypoxic conditions (1% oxygen), 8-SPT attenuated the increase in the secretion of both VEGF and sFlt-1 (P < 0.05 and P < 0.005, respectively). Exogenous and the adenosine transporter inhibitor dipyridamole (which increases extracellular levels of adenosine) showed differential effects under normoxic conditions: sFlt-1 levels in media increased significantly (P < 0.05), whereas VEGF was unaffected (P = 0.67 and P = 0.19, respectively). These data indicate that extracellular adenosine can regulate VEGF and sFlt-1 secretion in the hypoxic placenta and could, therefore, control the balance of these competing angiogenic factors in diseases characterized by placental ischemia.
Keywords: placental ischemia, soluble vascular endothelial growth factor r1, preeclampsia, reduced uterine perfusion pressure
preeclampsia is a disorder of pregnancy-induced hypertension that affects ∼5% of all pregnancies (30). One of the initiating events in the pathophysiology of this disorder is the incomplete remodeling of placental spiral arteries due to insufficient cytotrophoblast invasion. This incomplete remodeling is thought to reduce placental perfusion, thereby creating hypoxic foci in the microenvironment of the placenta (17, 24). A central hypothesis is that placental ischemia increases circulating levels of various soluble factors that are thought to induce endothelial dysfunction along with an array of symptoms seen in preeclamptic women (25). Among these factors are thought to be antiangiogenic factors, an overproduction of which can lead to imbalances between proangiogenic and antiangiogenic factors, thus contributing to the etiology of the disease (13). One of the most well-characterized factors is sFlt-1, a soluble form of the VEGFR1 receptor, which binds free VEGF and thus neutralizes its proangiogenic actions (27). Both VEGF and sFlt-1 can be induced by hypoxia (12, 20); however, without knowledge of their differential regulation, it is not possible to predict the relative balance between these opposing angiogenic factors in the hypoxic/ischemic placenta.
The role of adenosine in the response to focal hypoxia is well characterized, as adenosine released from parenchymal cells can regulate the balance between oxygen supply and oxygen demand in tissues such as the heart (3), skeletal muscle (19), and cerebral cortex (22). More recently, adenosine has been implicated as a modulator of angiogenesis in hypoxic tissues (1). Physiological concentrations of adenosine produced under hypoxic conditions can stimulate a concentration-dependent proliferation and migration of endothelial cells obtained from large and small blood vessels. Numerous other studies have shown that adenosine or adenosine uptake inhibitors can stimulate angiogenesis in vivo (1), and it has recently been demonstrated that in preeclamptic placentas, adenosine receptors are upregulated, specifically the A2A receptor, although the physiological significance of this finding is not clear (29).
Mechanistic insights into the role of adenosine in angiogenesis have come from studies demonstrating a direct effect of adenosine signaling on the release of soluble angiogenic factors. Under normoxic conditions, several groups have demonstrated a direct effect of adenosine on VEGF secretion in vitro and in vivo in nonplacental tissue (7, 9, 11–12), but little is known about the effect of adenosine on VEGF or sFlt-1 production in the placenta. The present study was undertaken to examine the hypothesis that adenosine has an important role in hypoxia-induced regulation of VEGF and sFlt-1 in the placenta. We used placental villous explants and examined their behavior under both normoxic and hypoxic conditions in response to manipulation of adenosine signaling.
METHODS
Animals.
Timed pregnant Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN). All animal protocols were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee, and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Animals were maintained at constant temperature (23°C) with a 12:12-h light-dark cycle.
Placental explants.
Placentas were harvested on day 19 of pregnancy from normal pregnant rats and were immediately placed into cold Dulbecco's PBS (Sigma, St. Louis, MO). The decidua was carefully removed, and villous bundles from the trophospongium and labyrinth were excised. The villous explants were plated on Millicell 6-well cell culture inserts (Millipore, Billerica, MA) coated with 0.5 ml of Matrigel Matrix Basement Membrane from BD Bioscience (Bedford, MA). Explants were grown in Dulbecco's Modified Eagle's Media-Ham's F-12 supplemented with 10% FBS, 100 μg/ml streptomycin, 100 U/ml penicillin, and 25 μg/ml ascorbic acid, as previously described (4). Cells were maintained in a triple gas incubator at either 6% oxygen for normoxic conditions, or at 1% oxygen for hypoxic conditions by nitrogen purging.
Experimental protocol.
Explants were randomly assigned into control and experimental groups. Control cells were incubated in media with no supplementation. The nonspecific adenosine receptor antagonist 8-p-sulfophenyl-theophylline (8-SPT; Sigma) was used at a final concentration of 50 μM. The adenosine transporter antagonist dipyridamole (Baxter Healthcare, Deerfield, IL) was used at a final concentration of 10 μM, and exogenous adenosine (Sicor Pharmaceuticals, Irvine, CA) was utilized at 20 μM. At 24 h posttreatment, the cell culture media were removed from the explants and frozen for further analysis. A minimum of five samples was obtained for every experimental group.
Total protein concentration of the cell culture supernatants was determined by the bicinchoninic acid assay (Pierce, Rockford, IL) using BSA as a standard. Measurements of s-Flt1 and VEGF were performed by sandwich ELISAs (R&D Systems, Minneapolis, MN), according to the manufacturer's protocols. The plates were read on a Tecan GENios microplate reader, and quantitation was performed with Megellan version 4.1 software. VEGF and sFlt-1 levels were normalized to the total amount of media protein, and the results graphed with Origin Pro 8 (Microcal), which was also used for all statistical analyses. Statistical significance was determined by Student's unpaired t-test, with a significance threshold of P < 0.05.
RESULTS
Hypoxia induces VEGF and sflt-1 secretion from placental villous explants.
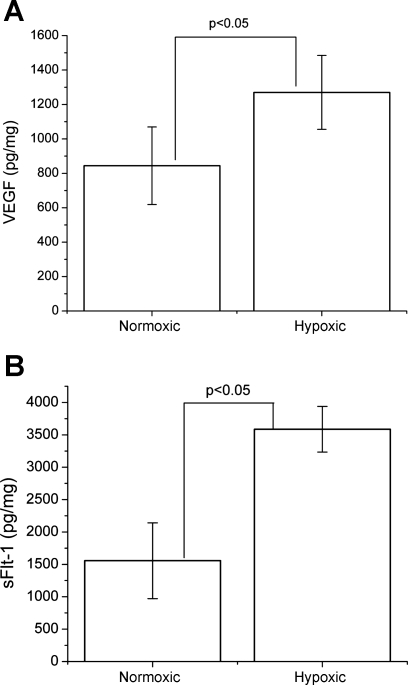
To determine the influence of hypoxia on VEGF and sFlt-1 secretion from placental villi, placental villous explants were plated on Matrigel and bathed with augmented DMEM-F12 media under normoxic (6% oxygen) or hypoxic (1% oxygen) conditions at 37°C. After 24 h of incubation under hypoxic conditions, VEGF levels in media were significantly increased by ∼1.5-fold (1,270 ± 215 pg/mg total protein; P < 0.05) compared with the normoxic control group (844 ± 225 pg/mg total protein) as seen in Fig. 1A. Hypoxia also caused an approximately twofold (P < 0.005) increase in sFlt-1 levels in media, compared with the normoxic control group. sFlt-1 levels averaged 3,586 ± 351 pg/mg total protein in hypoxic cultures compared with 1,556 ± 585 pg/mg total protein in normoxic control cultures, as shown in Fig. 1B. These data indicate that hypoxia at 1% oxygen caused significant increases in the secretion of both VEGF and sFlt-1 from placental villi.
Fig. 1.
Placental villous VEGF and soluble VEGF receptor fms-like tyrosine kinase-1 (sFlt-1) secretion is controlled by hypoxia. Placental explants were cultured in either 6% (normoxic) or 1% (hypoxic) oxygen for 24 h postharvest. Hypoxia induced a ∼1.5-fold induction of extracellular VEGF (A) and an approximately twofold increase in sFlt-1 (B), as determined by ELISA. Error bars represent means ± SD. VEGF and sFlt-1 levels were normalized to total media protein concentration. Statistically significant differences by unpaired Student's t-test are indicated with brackets and their associated P values.
Extracellular adenosine has differential effects on VEGF and sflt-1 secretion.
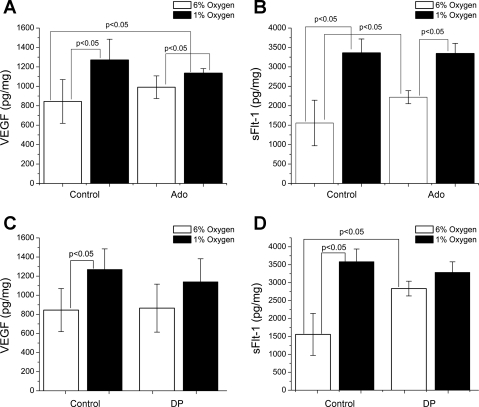
We next determined the effect of increasing exogenous levels of adenosine on VEGF and sFlt-1 secretion. As seen in Fig. 2, A and B, explants treated with adenosine (20 μM) showed significant increases in sFlt-1 secretion (control: 1,556 ± 585 pg/mg, Ado treated: 2,220 ± 168 pg/mg, P < 0.05) but not VEGF secretion (control: 844 ± 225 pg/mg, Ado treated: 991 ± 117 pg/mg, P = 0.19) under normoxic conditions. Under hypoxic conditions, exogenous adenosine had no statistically significant effect on VEGF or sFlt-1 production. We confirmed these results by increasing extracellular adenosine concentration with 10 μM dipyridamole (DP), an inhibitor of the adenosine equilibrative transporter. DP failed to elicit a rise in VEGF protein levels in media (Fig. 2C) under both normoxic and hypoxic conditions. Under normoxic conditions, VEGF levels averaged 865 ± 251 pg/mg in DP-treated cultures and 844 ± 225 pg/mg in control cultures (P = 0.67). Furthermore, treatment with DP did not enhance VEGF protein secretion under hypoxic conditions vs. hypoxic controls (1,141 ± 240 pg/mg vs. 1,270 ± 215 pg/mg total protein, respectively; P = 0.42). In contrast, in Fig. 2D, we show that DP had a marked effect on sFlt-1 levels under normoxic conditions, causing a ∼1.6-fold increase compared with control cultures (control: 1,738 ± 570 pg/mg; DP: 2,834 ± 206 pg/mg total protein; P < 0.005). However, DP did not change sFlt-1 protein levels significantly under hypoxic conditions (control: 3,586 ± 352 pg/mg; DP: 3,287 ± 293 pg/mg; P = 0.14). Therefore, these experiments suggest that dipyridamole has differential effects on the secretion of VEGF and sFlt-1 protein from placental villi under normoxic and hypoxic conditions.
Fig. 2.
Increase in extracellular adenosine has differential effects on VEGF and sFlt-1. Placental explants were cultured in 6% or 1% oxygen in the presence or absence of Ado (20 μM). Exogenous Ado had no effect on VEGF secretion under either oxygen tension (A) but significantly increased sFlt-1 release in 6% oxygen (B). Placental explants were cultured under normoxic (6% oxygen) or hypoxic (1% oxygen) conditions in the presence or absence of dipyridamole (DP). C and D: under hypoxic conditions, there was no statistically significant effect of DP on either sFlt-1 or VEGF, indicating the possibility that adenosine signaling pathways are already saturated under these conditions. Under normoxic conditions, however, there were differential effects, as sFlt-1 secretion was increased significantly, while VEGF secretion was unaffected. Error bars represent means ± SD, and relevant P values are displayed.
Adenosine signaling is important for induction of both VEGF and sflt-1 under hypoxic conditions.
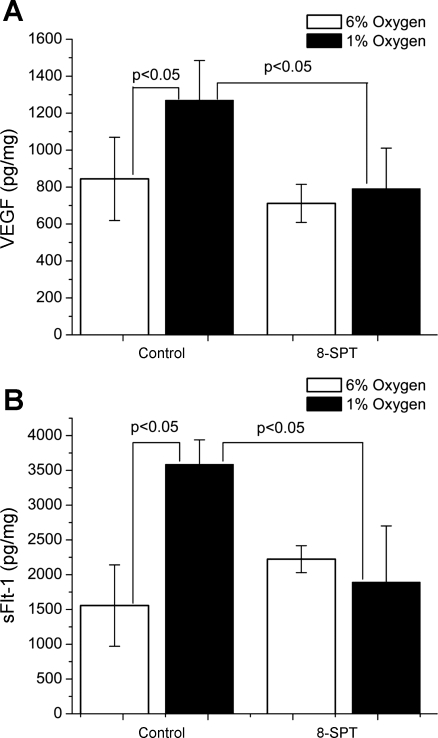
We sought to determine whether the induction of VEGF and sFlt-1 secretion in cultures exposed to a hypoxic environment was dependent on adenosine receptor activation. Placental villi were incubated in media containing 50 μM 8-SPT, a nonspecific adenosine receptor antagonist. Under normoxic conditions, treatment with 8-SPT had no significant effect on either VEGF (P = 0.38) or sFlt-1 (P = 0.056) protein levels in media, as shown in Fig. 3, A and B, respectively. However, under hypoxic conditions, treatment with 8-SPT caused a significant reduction in both VEGF (P < 0.05) and sFlt-1 (P < 0.005) protein levels in media as shown in Fig. 3, A and B. This indicates that the hypoxia-induced increases in both VEGF and sFlt-1 are heavily dependent on adenosine signaling mechanisms in placental villous explants.
Fig. 3.
The adenosine receptor antagonist 8-SPT negative regulates VEGF and sFlt-1 release under hypoxic conditions. Under normoxic conditions, there was no discernible effect on either VEGF (A) or sFlt-1 (B) secretion. Under hypoxic conditions though, both VEGF and sFlt-1 levels were significantly attenuated, returning almost to their normoxic control levels. Means ± SD are shown in error bars, and P values for significant difference are displayed above appropriate brackets.
DISCUSSION
Placental ischemia/hypoxia is increasingly believed to be a major factor in the pathophysiology of preeclampsia (10, 17). Factors proposed to be regulated by placental hypoxia include the angiogenic peptide placental growth factor (PlGF) (13, 16), AT1 autoantibodies(14), inflammatory cytokines (2, 5, 15), and reactive oxygen species (26). The release of soluble antiangiogenic factors is also believed to be a major contributor, and among these, perhaps the best characterized is sFlt-1. The regulation of sFlt-1 expression is not well understood, though recent studies on the alternate splicing of the parental Flt-1 transcript should begin to shed light on the underlying molecular mechanisms of its production (28). The role of hypoxia-inducible factor-1 (HIF-1) in the regulation of sFlt-1 has been well established (21), but we were interested in whether other pathways, like adenosine signaling, which is known to have a variety of angioregulatory functions (1) and is increased in preeclamptic women (31–32), might be important in the regulation of sFlt-1 and its target molecule, VEGF, in hypoxic placental vessels, as has been previously hypothesized (6).
The release of both of these soluble factors was increased in response to hypoxia, increasing VEGF by ∼1.5-fold and sFlt-1 by approximately twofold. This is in line with earlier studies indicating that hypoxia upregulates both molecules. (12, 20) The data from the placental explants in the present study indicate a clear role for adenosine-induced secretion of sFlt-1 in normal oxygen conditions. Both exogenous adenosine and DP, which raises the extracellular concentration of adenosine, increased sFlt-1 levels significantly, though in contrast to results obtained in other tissue types (7, 9, 11–12), increases in extracellular adenosine did not induce VEGF release, possibly indicating that any effect of adenosine in normal oxygen conditions has reached saturation or is compensated for by an alternative regulatory pathway. In contrast, the inability of adenosine or DP to elicit a further response in hypoxic villi suggests that the adenosine signaling pathways have already achieved maximal output in the secretion of both VEGF and sFlt-1 under hypoxic conditions. This differential sensitivity to adenosine is interesting, as it is the imbalance of VEGF and sFlt-1, which is hypothesized to cause many of the pathophysiological symptoms of preeclampsia. Adenosine signaling, therefore, might be a part of the explanation for this imbalance, as a lack of autonomic innervations (23) should make regulation of the placental vessel beds particularly sensitive to the extracellular environment for its regulatory cues.
In hypoxia, however, we see that adenosine has a major function in the secretion of both of these proteins, as blockade of adenosine signaling by the nonspecific adenosine receptor antagonist 8-SPT led to a marked decrease of both proteins to approximately their normoxic control levels. This argues in favor of a strong role for adenosine in the hypoxic response of placental villi to hypoxic challenge, as occurs in the preeclamptic placenta. The differential effects seen in VEGF and sFlt-1 secretion might possibly be explained by shifts in the adenosine receptor complement of the cells in a hypoxic environment vs. normal oxygen, as has been observed recently in human endothelial and smooth muscle cells. Similar to the results obtained here, both cell types exhibited no adenosine-stimulated VEGF release under normal oxygen conditions, but released VEGF in response to an adenosine receptor agonist when hypoxic, an effect attributed to differential regulation of adenosine receptor subtypes in response to hypoxia. (8) It remains to be seen whether adenosine also regulates the close VEGF homologue PlGF, the downregulation of which is also recognized as an important factor in the development of preeclampsia in humans. (13, 16, 18). Future studies examining the possibility of PlGF by adenosine will help define this role.
Perspectives and Significance
It is clear that adenosine has an important role in the regulation of VEGF and sFlt-1 release from placental villi during ischemia and hypoxia. Further investigation is warranted into the mechanism underlying this phenomenon, specifically the role of individual adenosine receptors involved in the regulation of sFlt-1 and VEGF. Given the dearth of therapeutic avenues in the treatment of preeclampsia, the adenosine system is a possible therapeutic target in the treatment of the disorder.
GRANTS
This work was supported by National Institutes of Health Grant HL-51971.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We wish to thank Dr. Sydney Murphy and Marietta Arany for technical assistance.
REFERENCES
- 1.Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol 289: R283–R296, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab 82: 1582–1588, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol 204: 317–322, 1963 [DOI] [PubMed] [Google Scholar]
- 4.Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology 138: 4977–4988, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 37: 240–249, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Escudero C, Sobrevia L. A hypothesis for preeclampsia: adenosine and inducible nitric oxide synthase in human placental microvascular endothelium. Placenta 29: 469–483, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res 90: 531–538, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Feoktistov I, Ryzhov S, Zhong H, Goldstein AE, Matafonov A, Zeng D, Biaggioni I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension 44: 649–654, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Grant MB, Tarnuzzer RW, Caballero S, Ozeck MJ, Davis MI, Spoerri PE, Feoktistov I, Biaggioni I, Shryock JC, Belardinelli L. Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res 85: 699–706, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Gu JW, Brady AL, Anand V, Moore MC, Kelly WC, Adair TH. Adenosine upregulates VEGF expression in cultured myocardial vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 277: H595–H602, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 46: 1077–1085, 2005 [DOI] [PubMed] [Google Scholar]
- 14.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 52: 1168–1172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep 9: 480–485, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Lindheimer MD, Roberts JM, Cunningham FG, Chesley LC. Chesley's Hypertensive Disorders in Pregnancy. Boston, MA: Academic, 2009, 430 p. [Google Scholar]
- 18.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA 88: 9267–9271, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall JM. Roles of adenosine in skeletal muscle during systemic hypoxia. Clin Exp Pharmacol Physiol 29: 843–849, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 145: 4838–4845, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol 291: R1085–R1093, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Regan M. Adenosine and the regulation of cerebral blood flow. Neurol Res 27: 175–181, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Reilly RD, Russell PT. Neurohistochemical evidence supporting an absence of adrenergic and cholinergic innervation in the human placenta and umbilical cord. Anat Rec 188: 277–286, 1977 [DOI] [PubMed] [Google Scholar]
- 24.Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta 23: 359–372, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 161: 1200–1204, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 9: 225–230; discussion 231, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Thomas CP, Andrews JI, Liu KZ. Intronic polyadenylation signal sequences and alternate splicing generate human soluble Flt1 variants and regulate the abundance of soluble Flt1 in the placenta. FASEB J 21: 3885–3895, 2007 [DOI] [PubMed] [Google Scholar]
- 29.von Versen-Hoynck F, Rajakumar A, Bainbridge SA, Gallaher MJ, Roberts JM, Powers RW. Human placental adenosine receptor expression is elevated in preeclampsia and hypoxia increases expression of the A2A receptor. Placenta 30: 434–442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker JJ. Pre-eclampsia. Lancet 356: 1260–1265, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Yoneyama Y, Sawa R, Suzuki S, Shin S, Power GG, Araki T. The relationship between uterine artery Doppler velocimetry and umbilical venous adenosine levels in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 174: 267–271, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Increased plasma adenosine concentrations and the severity of preeclampsia. Obstet Gynecol 100: 1266–1270, 2002 [DOI] [PubMed] [Google Scholar]