Abstract
This study presents an in-depth analysis of the effects of obesity on energy balance (EB) and fuel utilization in adult female rats, over the estrous cycle and immediately after surgical ovariectomy (OVX), to model pre- and postmenopausal states, respectively. Female Wistar rats were fed a high-fat (46%) diet for 16 wk to produce mature lean and obese animals. Stage of estrous was identified by daily vaginal lavage, while energy intake (EI), total energy expenditure (TEE), and fuel utilization were monitored in a multichamber indirect calorimeter and activity was monitored by infrared beam breaks. Metabolic monitoring studies were repeated during the 3-wk period of rapid OVX-induced weight gain. Component analysis of TEE was performed to determine the nonresting and resting portions of energy expenditure. Obesity was associated with a greater fluctuation in EB across the estrous cycle. Cycling obese rats were less active, expended more energy per movement, and oxidized more carbohydrate than lean rats. The changes in EB over the cycle in lean and obese rats were driven by changes in EI. Finally, OVX induced a large positive energy imbalance in obese and lean rats. This resulted primarily from an increase in EI in both groups, with little change in TEE following OVX. These observations reveal a dominant effect of obesity on EB, fuel utilization, and activity levels in cycling rats, which has implications for studies focused on obesity and EB in female rodents.
Keywords: energy intake, energy expenditure, indirect calorimetry, spontaneous activity, menopause
perturbations in the regulation of energy balance (EB) and fuel utilization underlie the development, progression, and recurrence of obesity (31, 36). This dysregulation in energy homeostasis has been linked to the development of many obesity-related comorbidities, including diabetes (2, 24), cardiovascular disease (24), and certain types of cancer (11, 42, 45). Understanding the impact of this dysregulation on obesity and its comorbidities has led to a greater need for accurate measurements of EB and fuel utilization in relevant disease models. This has been challenging in preclinical studies. Although measuring food intake is standard practice, simultaneous measurements of energy expenditure (EE) and fuel utilization, which provide a more complete picture of EB, are less common. Measuring these parameters becomes especially important for studies in females, because energy balance and fuel utilization are significantly affected by the estrous cycle (4, 6, 10, 25).
Many estrous cycle-related effects have been linked to the effects of estrogens on the central nervous system and peripheral tissues (4–6, 15, 23, 37, 46, 52). Rodents typically cycle over 4–5 days, and the phases of this cycle are commonly classified by histological changes in vaginal cytology, which are roughly divided into days: diestrus 1 (D1), diestrus 2/3 (D2), proestrus (P), and estrus (E) (47). We and others have reported that estradiol begins to rise in D2, peaks in P, and rapidly drops to negligible levels in E (4, 47). The latency of estrogen's suppression of food intake is ∼12–24 h and has been attributed to the time course required to alter gene expression in central and peripheral tissues (6). Consequently, food intake is generally elevated in D1 and D2, declines in P, and reaches a nadir in E (17). Less is known about the concomitant changes in EE and fuel utilization in rodents, but it is often assumed that the changes in physical activity observed across the estrous cycle are reflective of changes in total EE (TEE). Female rats, ferrets, hamsters, and cows have shown increased activity during E, whereas similar studies in monkeys did not report changes in activity over the cycle (3, 9, 16, 17, 38). Overall, the inference from these studies is that the rise in estrogens that occurs in D and early P leads to decreased energy intake (EI), increased TEE, a negative energy imbalance, and acute weight loss. In contrast, the loss of estrogens, as occurs with surgical ovariectomy (OVX), leads to a persistent positive energy imbalance and weight gain (23, 30, 33).
The cyclical changes in body weight over the cycle (D2 > P > D1 > E) provide evidence for coordinated and opposing changes in EI and TEE during the estrous cycle. However, the literature lacks confirmation with a comprehensive examination of EB and simultaneous measurements of EI and TEE at each stage of the estrous cycle. Furthermore, it is not known whether obesity alters such cycle-related changes in EB, nor have the effects of OVX on EB been fully characterized. Thus the purpose of the present study was to examine the impact of obesity on cycle-related and OVX-induced changes in EB and fuel utilization. We hypothesized that changes in metabolism across the cycle, and following OVX, would be blunted in obese compared with lean rats. These data fill a gap in metabolic research on female rodents and provide the foundation for studying the impact of EB and fuel utilization on obesity and its related comorbidities.
EXPERIMENTAL PROCEDURES
Experimental design.
Female Wistar rats (126–165 g body wt, 5 wk of age; Charles River Laboratories, Wilmington, MA) were housed in the University of Colorado Denver Center for Comparative Medicine and the Center for Human Nutrition Satellite Animal Facility (22–24°C, 12:12-h light-dark cycle) with free access to water. All procedures were approved by the Institutional Animal Care and Use Committee. Obese and lean rats were identified by their differential response to a diet high in fat (12, 33). Briefly, rats were individually housed in wire-bottom metabolic cages that limit activity (relative to group-housed animals in polycarbonate cages) and were given free access to a high-fat (HF) diet (46% kcal fat; RD D12344, Research Diets, New Brunswick, NJ). Rats were ranked by their rate of weight gain in this obesogenic environment from 10 to 18 wk of age. Those in the top tertile of weight gain were classified as obese (n = 25) and those in the lower tertile as lean (n = 23). Rats from the middle tertile were not used for this study. For all experiments, fully mature, adult lean and obese rats were studied at 20–30 wk of age, a period characterized by growth plateau (35), with ad libitum access to this HF diet. Body composition was determined by dual-energy X-ray absorptiometry (model DPX-IQ, GE Lunar, Madison, WI), with Small Animal software (version 1.0, GE Lunar) or by quantitative magnetic resonance (Echo MRI Whole Body Composition Analyzer, Echo Medical Systems, Houston, TX).
Identification of stage of estrous cycle.
Vaginal lavages were performed daily, ∼5 h prior to the onset of the dark cycle, to identify each animal's stage of the estrous cycle. Approximately 200 μl of PBS + 0.2% Brij 35 detergent (Sigma-Aldrich, St. Louis, MO) were used for each procedure, and the unstained samples were examined under a light microscope. Stage of cycle was assigned using the following criteria, as previously described (32, 47): predominantly nucleated epithelial cells in the absence of leukocytes (P), sheets of nonnucleated squamous cornified cells in the absence of leukocytes (E), equal distribution of leukocytes and cornified and nucleated epithelial cells (D1), and a mixture of epithelial cells and leukocytes, with a predominance of leukocytes, following D1 (D2). Cycle phases were assigned to the 24-h period prior to the lavage. A small number of animals failed to cycle or did not cycle consistently. It is unclear from our analysis whether this could be attributed to the age or adiposity/weight of the animals or the timing of the vaginal lavages (7, 19). Noncycling animals were excluded from the pre-OVX phase of the study, and, for this reason, not all post-OVX data points have a corresponding pre-OVX measurement.
Metabolic monitoring system.
A metabolic monitoring system (Columbus Instruments, Columbus, OH) was used to assess EB, fuel utilization, and activity in rats in the premenopausal phase and following surgical OVX. This multichamber indirect calorimetry system allows for the continuous monitoring of up to eight rats, obtaining measurements of O2 consumption (V̇o2) and CO2 production (V̇co2) from each chamber every 16 min (27, 36). The chambers also allow for the collection of daily urine, feces, and food spillage. Animals were removed from their cages ∼5 h prior to the onset of the dark cycle each day while the cages were cleaned and vaginal lavages were performed.
Animals were placed in the metabolic cages ≥2 days prior to the collection of metabolic data to allow for acclimatization to the new environment. Metabolic measurements were obtained for each animal across at least two full estrous cycles prior to OVX or sham surgery. For OVX, rats were anesthetized with isoflurane and the ovaries were surgically removed using an intra-abdominal approach. After surgery, animals were allowed to recover for 5 days, at which time they were returned to the metabolic monitoring cages. For each animal, the first day of calorimetry following OVX during which intake and expenditure were equal (±3 kcal) was identified as the EB day (OVX-EB). On average, this was the second day in the metabolic monitoring system, or 7 days post-OVX. The animals were then monitored over the subsequent 2 wk of OVX-induced weight gain (OVX-Gain). Urine was collected over each 24-h period for the estimation of protein disappearance.
EE.
Metabolic rate (MR) was calculated from gas exchange measurements acquired every 16 min over the 24-h period using the Weir equation (MR = 3.941·V̇o2 + 1.106·V̇co2 − 2.17·N, where N is urinary nitrogen) (53). In addition, the data were used to acquire resting EE (REE) and non-REE (NREE). Resting MR (RMR, cal/min) was estimated as an average MR over a 1-h period in the latter part of the light cycle, as previously described (35), and was extrapolated throughout 24 h to obtain REE. For each animal, the 1-h period was selected during a time in which MR was at a nadir and no activity was detected. It is important to note that, in these ad libitum-fed animals, RMR does not necessarily equate to basal MR. Animals were not fasted when this measurement was acquired. As such, the REE calculation may include some component of the thermic effect of food, particularly in the OVX animals that are in a large positive energy imbalance. NREE was calculated as the difference between TEE and REE. All data are expressed as kilocalories per day.
Calculations of fuel utilization.
Respiratory exchange ratio (RER) was calculated as the ratio of V̇co2 to V̇o2. Estimates of whole body substrate oxidation were calculated from V̇o2 (l/min) and V̇co2 (l/min) measurements when in EB and from measurements of N (g/min) using derivations of Weir's equation as follows: carbohydrate disappearance (g/min) = (4.57·V̇co2) − (3.23·V̇o2) − (2.6·N); lipid disappearance (g/min) = (1.69·V̇o2) − (1.69·V̇co2) − (2.03·N); protein disappearance (g/min) = 6.25·N.
While in EB, calculations of substrate disappearance are a good reflection of substrate oxidation. When out of EB, however, it is critical to also consider the relative impact of metabolite interconversions. Ketogenesis, lipogenesis, and gluconeogenesis can affect substrate disappearance, such that it becomes less reflective of oxidation alone, and this must be considered in the interpretation of the data.
Activity measurements.
Each metabolic cage was equipped with an animal activity meter (Opto-Max, Columbus Instruments, Columbus, OH), which consists of a one-dimensional series of infrared beams that, when broken by the animals' movement, allows for the measurement of total activity, as well as ambulatory and nonambulatory activity. Ambulatory and total activities were monitored continuously over the 24-h period, and subtraction of ambulatory counts from total counts was used to assess stereotypic activities, such as grooming, scratching, feeding, and other nonambulatory activities.
Plasma and urine measurements.
Tail vein blood was collected on D2 during the week prior to OVX and again at the time of death. In all cases, blood was drawn during the latter part of the light cycle, and plasma was isolated and stored at −80°C until analyzed. Estradiol was measured by enzyme immunoassay (Alpco Diagnostics, Salem, NH). Colorimetric assays were used to measure plasma free fatty acids (Wako Chemicals USA, Richmond, VA), glucose, triglycerides (TGs), and total cholesterol (TR15421, TR22321, and TR13521, respectively, Thermo Fisher Scientific, Waltham, MA). Urinary nitrogen was estimated from measurements of urea and creatine in 24-h urine collections, as previously described (34, 36).
Statistical analysis.
Data were analyzed by two-way ANOVA (obesity, cycle day, obesity-cycle day interaction), with planned comparisons between lean and obese groups, using SPSS software version 17.0. Cycling sham and pre-OVX data were combined, as there were no differences between the two groups in any of the measured parameters. For some parameters, the data were further analyzed by analysis of covariance to adjust for the variation due to specified covariates. Significance was set at P < 0.05.
RESULTS
Morphometrics.
Morphometric characteristics of intact (cycling) and OVX animals are shown in Table 1. When entering in to the study period, obese rats had a higher percentage of body fat (P < 0.001) and weighed ∼20% more than lean rats (P < 0.001). This greater body weight was due to a 35% higher fat mass and a 12% higher fat-free mass (P < 0.001 for both).
Table 1.
Morphometric and plasma characteristics
| Group |
P Value |
|||||
|---|---|---|---|---|---|---|
| Lean |
Obese |
|||||
| Variable | Cycling | OVX | Cycling | OVX | Obesity effect | OVX effect |
| Weight gain,† g/day | 0.51 ± 0.49 | 2.91 ± 0.35 | 0.44 ± 0.30 | 3.01 ± 0.41 | 0.976 | <0.001 |
| Body wt,* g | 308.8 ± 7.4 | 318.1 ± 6.1 | 380.3 ± 14.0 | 390.4 ± 9.3 | <0.001 | 0.342 |
| FFM,* g | 237.6 ± 5.1 | 246.7 ± 3.9 | 268.9 ± 9.2 | 275.7 ± 6.1 | <0.001 | 0.240 |
| FM* | ||||||
| g | 71.2 ± 5.0 | 71.5 ± 4.9 | 111.4 ± 6.0 | 114.8 ± 6.2 | <0.001 | 0.753 |
| % | 22.9 ± 1.3 | 22.3 ± 1.2 | 29.2 ± 0.9 | 29.2 ± 1.2 | <0.001 | 0.796 |
| Glucose,† mM | 9.84 ± 0.5 | 12.95 ± 0.8 | 11.05 ± 0.7 | 14.02 ± 0.7 | 0.207 | <0.001 |
| Cholesterol, mM | 2.06 ± 0.3 | 2.11 ± 0.4 | 1.88 ± 0.3 | 2.34 ± 0.3 | 0.953 | 0.459 |
| TGs,* mM | 0.93 ± 0.1 | 1.08 ± 0.2 | 1.53 ± 0.3 | 2.43 ± 0.6 | 0.008 | 0.140 |
| NEFAs,† μM | 714.0 ± 79.6 | 433.4 ± 58.0 | 650.8 ± 55.3 | 602.8 ± 66.8 | 0.546 | 0.048 |
| Estradiol,† pM | 167.2 ± 18.3 | 52.7 ± 8.6 | 187.6 ± 19.3 | 49.5 ± 5.3 | 0.886 | <0.001 |
Values are means ± SE. For rate of weight gain, all animals (n = 20–25 per group) were included in the analysis; for plasma and some morphometric measurements, a representative subset of animals (n = 10–14 per group) was used. FFM, fat-free mass; FM, fat mass; TGs, triglycerides; NEFAs, nonesterified fatty acids. Body composition was determined through a combination of dual-energy X-ray absorptiometry and quantitative magnetic resonance imaging. Plasma was collected on diestrus 2 (cycling animals) or at the time of death [ovariectomized (OVX) animals], and cholesterol, TGs, NEFAs, glucose, and estradiol were measured using standard assays. Data were examined by ANOVA, with significance set at P < 0.05:
obesity effect;
OVX effect.
In response to OVX surgery, lean and obese animals experienced a brief period of weight loss. By 5 days post-OVX, all animals began to gain weight. Using this experimental paradigm, we previously showed that OVX induces rapid weight gain for ∼3 wk; then the rate of weight gain returns to sham or pre-OVX levels (33). In the present study, animals were studied during this critical 3-wk window of rapid weight gain to determine whether OVX-induced weight gain is due to changes in EI, EE, or both. Although we did not follow the animals long enough for their total body weight to significantly surpass their pre-OVX weight, we did see a significant increase in the rate of weight gain in the lean and obese groups following OVX (P < 0.001).
EB.
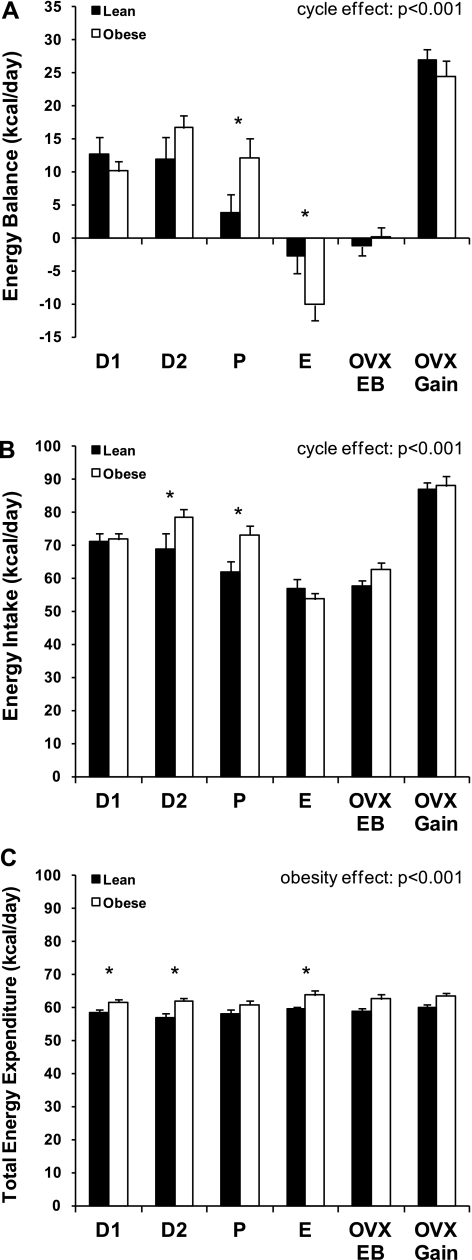
Daily EI, TEE, and EB (calculated as EI − TEE) throughout the estrous cycle and following OVX are depicted in Fig. 1. Obese and lean rats exhibited a positive energy imbalance (EI > TEE) during D1, D2, and P and shifted to negative energy imbalance during E (EI < TEE; Fig. 1A). The pattern of this cycle-dependent change in EB was significantly different in lean and obese rats (P = 0.021). Most notably, the range of EB across the cycle was much wider for the obese (from +17 to −10 kcal/day) than lean (+13 to −3 kcal/day) animals. On D1 and D2, lean and obese rats exhibited a similar positive energy imbalance. As the animals progressed to P, obese rats maintained this level of positive imbalance, while the caloric excess in the lean rats decreased significantly (P = 0.046). Finally, during E, both groups exhibited a negative energy imbalance, and this imbalance was significantly greater in the obese group (P = 0.047). Rats that underwent OVX were examined in EB, prior to any OVX-induced weight gain (OVX-EB). This allowed us to examine the impact of OVX on EB, without the complication that increased food intake and increased body weight would impart on these measurements during OVX-induced weight gain. After OVX, lean and obese animals were in significant positive imbalance (27 and 25 kcal/day excess, respectively), with no difference between groups.
Fig. 1.
Energy balance (A), energy intake (B), and total energy expenditure (C) for lean and obese rats during each phase of the estrous cycle [diestrus 1 (D1), diestrus 2 (D2), proestrus (P), and estrus (E)], immediately following surgical ovariectomy (OVX) while in energy balance (OVX-EB), and during OVX-induced rapid weight gain (OVX-Gain). Effects of obesity and cycle day and obesity-cycle day interaction were examined by ANOVA, with planned comparisons between lean and obese groups. *Significant difference between lean and obese groups (P < 0.05).
EI.
The fluctuations in EB through the cycle were driven primarily by changes in EI (Fig. 1B). In lean and obese cycling rats, EI varied significantly according to day of estrous cycle (P < 0.001), with EI reaching its peak during diestrus (D1 in lean rats and D2 in obese rats) and its lowest during E. In obese rats, EI was higher during D2 and P (P < 0.05 for both) and range of mean intakes was wider than in lean rats across the cycle (25 vs. 14 kcal/day). After OVX surgery, EI increased significantly in both groups (P < 0.001), reaching levels beyond the highest intake in cycling animals. Given that, across the estrous cycle, the average EI tended to be higher in the obese animals (P = 0.096) and that obese and lean animals did not differ in their intake following OVX, it would appear that the impact of OVX on altering food intake may be more substantial in the lean than obese animals. While this difference did not reach statistical significance, there was a trend for a greater increase in intake from pre- to post-OVX in the lean animals (P = 0.061).
TEE.
The cycle itself had no impact on TEE; however, cycling obese rats expended more energy than their lean counterparts (Fig. 1C; P < 0.001). This can be explained primarily by differences between the two groups on D1 (P = 0.037), D2 (P = 0.003), and E (P = 0.034). In general, fluctuations in TEE throughout the cycle were smaller (∼4 kcal/day range) than the fluctuation in EI (∼25 kcal/day range). Similarly, the impact of OVX on TEE (assessed in OVX-EB rats) was negligible, while EI was increased by >15 kcal/day in response to OVX.
Component analysis of TEE: REE and NREE.
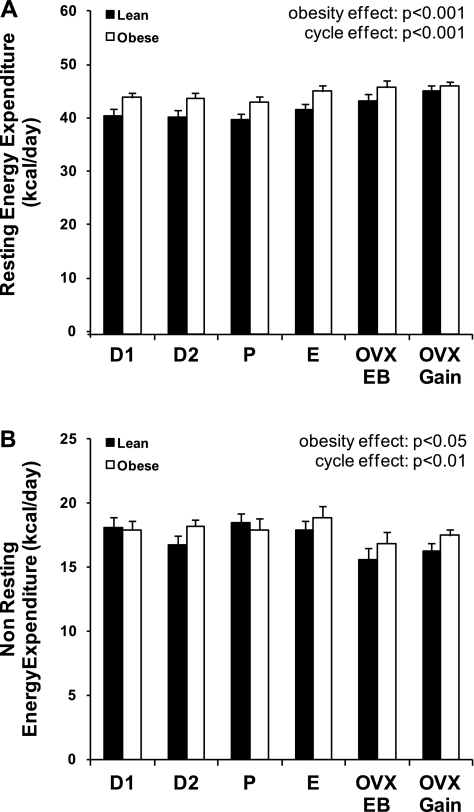
TEE was separated into its resting (REE; Fig. 2A) and nonresting (NREE; Fig. 2B) components. As expected given their higher body weight and lean body mass, REE was greater in obese rats across the cycle (P < 0.001). Differences in REE between lean and obese rats are often attributed to differences in body composition; however, the higher REE in the obese rats remained significant after statistical adjustment for fat-free mass (P < 0.001). REE did not change significantly over the days of the estrous cycle, although REE was higher during E than P (P = 0.043). After OVX, REE increased in the lean group to match REE in the obese group. In contrast to REE, NREE was similar for lean and obese rats across the estrous cycle and decreased following OVX in both groups (P = 0.002; Fig. 2B).
Fig. 2.
Component analysis of total energy expenditure (TEE). TEE was divided into its resting (A) and nonresting (B) components. Data are shown for lean and obese rats during each phase of the estrous cycle, immediately following OVX surgery while in energy balance, and during OVX-induced rapid weight gain. Effects of obesity and cycle day and obesity-cycle day interaction were examined by ANOVA.
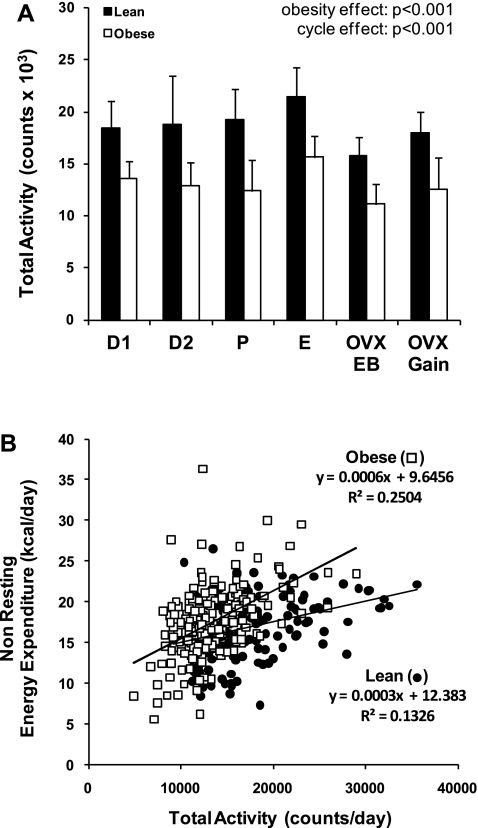
Given the greater body weight of obese rats, a similar NREE in lean and obese rats required that activity levels be lower in the obese rats. This was found to be the case, whether examined as total activity (Fig. 3A; P < 0.001) or separated into ambulatory activity or nonambulatory activity (P < 0.001 for both; data not shown). Obese rats were ∼40% less active than lean rats across the entire cycle. In both groups, activity also varied across the estrous cycle. Activity was 14% higher during E than D1, D2, and P in the lean animals (P = 0.027) and 20% higher in the obese animals (P < 0.001). After OVX, obese rats remained less active than lean rats, and activity in both groups decreased to levels below those observed across the estrous cycle.
Fig. 3.
A: total activity for lean and obese rats during each phase of the estrous cycle, immediately following OVX surgery while in energy balance, and during OVX-induced rapid weight gain. Effects of obesity and cycle day and obesity-cycle day interaction were examined by ANOVA. B: activity vs. nonresting energy expenditure for obese and lean rats. Nonresting energy expenditure positively correlated with total activity in lean (R = 0.413, P < 0.001) and obese (R = 0.510, P < 0.001) rats, and slope of the regression curve for this relationship was significantly greater in obese than lean rats (slope = 0.0006 vs. 0.0001, P < 0.001).
The fact that lean rats were more active than obese rats, with no difference in NREE, would suggest that the energetic cost of each activity movement is greater in the obese than in the lean animal. Further examination of the relationship between NREE and activity supports this notion. As shown in Fig. 3B, NREE positively correlated with activity in the lean and obese animals. Additionally, the slope of the regression curve for this relationship was significantly greater in the obese than lean animals. Not only does this indicate that the obese animals have a higher energetic cost for each unit of movement, it also demonstrates that this energetic cost increases precipitously as overall activity increases.
Nonprotein RER.
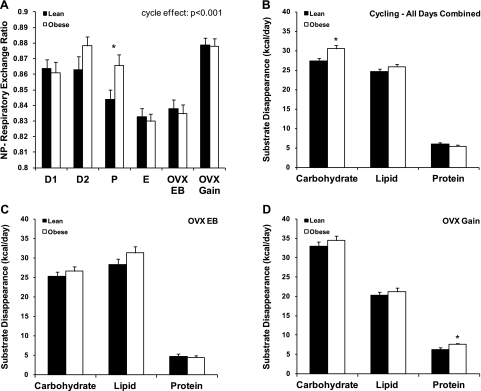
Calculations of nonprotein RER (NP-RER) are used to estimate which fuels are being oxidized, with 1.0 representing carbohydrate oxidation and 0.7 representing fat oxidation. NP-RER changed significantly over the estrous cycle (Fig. 4A; P < 0.001), reaching its highest values during D1/D2 (enhanced carbohydrate oxidation) and lowest values during E (enhanced fat oxidation). While the pattern of 24-h NP-RER was similar in lean and obese animals, the magnitude of the changes was greater in the obese rats, with a trend for higher NP-RER in the obese animals on D2 (P = 0.081) and significantly higher NP-RER on P (P = 0.031). In general, NP-RER varied primarily as a function of EB (R = 0.744, P < 0.001), but it was also inversely related to activity levels (R = −0.211, P < 0.01). Together, EB and activity levels explained 52% of the variation in NP-RER over the cycle. Notably, NP-RER was similar for lean and obese animals on E, even though the obese rats exhibited a more substantial negative energy imbalance. This provides indirect evidence that lean animals are more prone and/or have a greater capacity to utilize fat under conditions, such as negative EB, that favor the oxidation of this substrate. After OVX, 24-h NP-RER increased in lean and obese animals, with no differences between the two groups. When NP-RER was examined during the dark and light cycles, a similar pattern was observed in lean and obese animals.
Fig. 4.
A: nonprotein (NP) respiratory exchange ratio for lean and obese rats during each phase of the estrous cycle, immediately following OVX surgery while in energy balance, and during OVX-induced rapid weight gain. B–D: carbohydrate, lipid, and protein disappearance for lean and obese animals averaged across the cycle, immediately following OVX surgery while in energy balance (OVX-EB), and during OVX-induced rapid weight gain (OVX-Gain). *Significant difference between lean and obese groups (P < 0.05).
Substrate disappearance.
In EB, substrate disappearance data provide a good reflection of substrate oxidation. Substrate disappearance was averaged over the entire cycle, during which the net energy imbalance was minor (∼11 kcal/day) in lean and obese groups. Given this slight energy imbalance, it is important to make the distinction between substrate disappearance and oxidation, as there remains a possibility of metabolite interconversions and/or net retention of de novo synthesized lipid. Carbohydrate, lipid, and protein disappearance across the estrous cycle and following OVX are shown in Fig. 4. In cycling animals, the average carbohydrate disappearance was increased in obese compared with lean animals (P = 0.005). Lipid disappearance did not differ when averaged across the cycle (Fig. 4B); however, it was significantly higher in the obese animals on E (P = 0.048; data not shown). No differences in protein disappearance were identified between lean and obese cycling rats. There were also no differences in substrate disappearance immediately following OVX, when animals were in EB (OVX-EB) (Fig. 4C). In contrast, during rapid, OVX-induced weight gain, protein disappearance was significantly higher in obese than lean animals (Fig. 4D; P = 0.025). Substrate disappearance was correlated with total activity during OVX-induced weight gain. In lean animals, activity levels were correlated with lipid disappearance (R = 0.688, P < 0.001); yet, in obese animals, activity was correlated with carbohydrate disappearance (R = 0.577, P = 0.003).
Plasma measurements.
Levels of circulating glucose, free fatty acids [nonesterified fatty acids (NEFAs)], cholesterol, TGs, and estradiol are shown in Table 1. Plasma glucose levels did not vary significantly between obese and lean animals; however, after OVX, glucose levels were elevated in both groups (P < 0.001). Similarly, NEFAs did not vary significantly between lean and obese animals, but, after OVX, NEFA levels decreased in both groups (P = 0.048). TGs were the only factor that varied significantly between obese and lean animals, with higher TGs in obese than lean animals (P = 0.008). While there was a slight rise in TGs following OVX in both groups, this did not reach statistical significance. Neither obesity nor OVX altered the cholesterol levels. Finally, estradiol levels decreased significantly following OVX, as expected following removal of the ovaries, but we saw no difference between lean and obese animals at the time points measured.
DISCUSSION
This study is the first to present an in-depth analysis of the effects of obesity on EB and fuel utilization in adult female rats over the estrous cycle and during weight gain following surgical OVX. In contrast to our original hypothesis, obesity was associated with a greater positive energy imbalance during D2 and P and a more substantial negative energy imbalance during E. The majority of these fluctuations in EB over the cycle in lean and obese rats was driven by changes in EI, rather than EE. Obesity, however, was associated with lower activity levels; yet NREE in the obese animals was similar to NREE in the lean animals, suggesting a higher energetic cost for each movement in obese animals. Obese animals were also more dependent on carbohydrate over the cycle. Finally, OVX induced a large positive energy imbalance in obese and lean rats, increasing EI in the lean animals to match that of the obese animals, while reducing EE in the obese animals to match that in the lean animals. These observations reveal an effect of obesity on EB, fuel utilization, and activity levels over the estrous cycle and with loss of ovarian function, which may have implications for studies focused on obesity and EB in female rodents.
EI over the cycle: effect of obesity.
Our findings that, during E, rats decreased their EI and increased their activity are consistent with the results of several other studies (4, 9, 17, 49). These cyclical changes in EI are generally thought to be driven primarily by changes in circulating estrogens (4). There are no studies, to our knowledge, that have examined the impact of obesity on estrous-related changes in food intake. The data presented here demonstrate that the decrease in food intake commonly observed in response to rising estrogen levels is delayed in obese compared with lean animals. We measured plasma estradiol in both groups in D2, when estradiol levels are beginning to rise in preparation for E. While we saw no difference between lean and obese animals, we cannot rule out an effect of obesity on circulating estradiol levels at other stages of the cycle. It is also possible that, with the same hormonal profile, obese animals may be less sensitive to changes in estrogens over the cycle. Further studies with a more focused examination of estradiol's regulation of EB are needed to delineate the role of estrogens and estrogen sensitivity on obesity's impact on food intake over the cycle.
TEE over the cycle: effect of obesity.
Activity levels have often been used as a surrogate for EE, as measuring activity using running wheels is more logistically feasible for many research groups. The vast majority of studies demonstrate that running wheel activity increases during E (1, 9, 17), and we observed the same increase in spontaneous activity levels in the present study. The decreased activity we observed in the obese animals is also consistent with literature on the obesity-prone phenotype (40, 50). From the present observations, however, it is critical to highlight that a lower activity level in the obese animals translated to a similar level of EE for lean and obese animals. Briefly, for a given change in activity, the energetic cost is greater in the obese than lean animals. These findings suggest that, for obesity studies, lower activity levels in obese rats cannot simply be translated to lower levels of EE. However, this assertion needs to be confirmed in a system with a rapid response time and measures of true basal metabolism (in the fasted state) (8, 39).
Few studies have measured EE in rodents across the estrous cycle, and no other studies have examined the impact of obesity on E-related changes in TEE, NREE, and REE. In a study of young, rapidly growing rats (starting at 5 wk of age), TEE did not change with cycle day; however, when animals were given access to running wheels, a decrease in TEE during metestrus (equivalent to D in this study) was observed (1). Our studies did not provide the opportunity for this type of volitional activity but only monitored spontaneous activity in the home cage. It would be interesting to repeat the present study with a running wheel option, as a more substantial impact may be observed in NREE, REE, and TEE. In a separate study, REE (measured over 2 h of the light cycle) was higher in E than in D in a cohort of young adult (<300 g body wt) rats (43). We observed a similar phenomenon, but the impact of this change on overall EB was minimal. In general, TEE remained relatively stable over the cycle, and this appears to be due to the offset cycles of food intake (affecting the thermic effect of food) and physical activity (affecting activity thermogenesis).
It is important to note that our animals were fed a HF diet for most of their lives. At this level of dietary fat, we previously observed that an acute bout of HF feeding increases TEE, NREE, and REE in obesity-resistant, but not obesity-prone, rats (26), suggesting that a more pronounced effect over the cycle may have been masked by this long-term dietary regimen. Moreover, the component analysis was performed in the ad libitum-fed animal. As such, our estimate of REE is not a measurement of basal metabolism (REE measured in fasted animals); rather, it reflects the average level of EE while the animal is at rest, and usually sleeping. This deviation from basal metabolic measurements becomes particularly apparent during OVX-induced weight gain when animals are overfeeding. During this time, the rats are likely to have a substantial amount of food in their digestive tract throughout the entire day, and REE will include some component of the thermic effect of food.
EB over the cycle: effect of obesity.
When averaged across their cycle, female rodents in this study exhibited a minor positive energy imbalance. The timing of the fluctuation in EB across the estrous cycle suggests that this is an estrogen-mediated phenomenon, and there is substantial evidence to support this notion (15, 17). Whether the effect of obesity on EB is mediated through estrogens is less clear (22). While we did not see a difference in D2 estradiol levels between lean and obese rats, blood drawn across the entire cycle may have revealed an effect of obesity. Estrogens do not appear to directly alter leptin levels (44); however, their impact on leptin signaling pathways may be influenced by the reduced leptin sensitivity in obese rats (31). The signaling pathways of leptin and estrogens are known to overlap in peripheral tissues (51) and in the hypothalamus (22). It is likely that the impact of obesity on the fluctuation in EB across the cycle is the result of the interplay between estrogens, leptin, and the sensitivity of the hypothalamus (or other tissues) to these hormones.
Fuel utilization over the cycle: effect of obesity.
The primary determinant of RER is EB, which explains the higher RER in obese rats on D2 and P. However, during E, obese animals are in a much greater negative energy imbalance than lean animals, yet the RER of the two groups is approximately the same. This suggests that the obese animals may have an impaired ability to oxidize fat when faced with a metabolic state that typically ramps up the mobilization and utilization of fat for energy (a negative energy imbalance). Obesity is known to be associated with an impaired ability to regulate fat during metabolic stress, a characteristic that has been referred to as metabolic inflexibility (21). This characteristic of obesity, when overlaid on the changes in EB over the estrous cycle, may contribute to the obese phenotype. Over time, more ingested fat will be retained in adipose tissue, while carbohydrate will provide a greater portion of the substrate for energy production. This preferential use of carbohydrate for energy needs while fat is stored is an energetically efficient way of gaining weight (18, 48).
Surgical OVX: effects on EI, TEE, and EB.
Our study is consistent with overwhelming evidence that OVX induces weight gain. In general, the relative contribution of changing EI or EE to create this energy imbalance may vary with species or model, but the impact on EB is the same. While some studies explain the weight gain as an OVX-induced suppression of EE (46), others report that most of the difference is due to increased food intake (54). As stated previously, the decline in EE from reduced activity levels may be masked by an increased thermic effect of food associated with the hyperphagia and/or an increased basal energy requirement from the gained weight. These opposing effects on the components of TEE are often overlooked in the interpretation of data in the post-OVX state and may explain why the OVX-induced decline in activity is not always accompanied by a decrease in TEE.
In previous studies, we observed that the OVX-induced period of rapid weight gain lasts ∼3 wk and that feed efficiency was lower for obese rats (33). Eventually, the obese and lean rats gained a similar amount of weight, but during this transient period, it appeared that the energy imbalance and the rate of weight gain were blunted in obese rats. Obesity from forced overfeeding resulted in a similar delay in OVX-induced weight gain (41), and we tended to see the same phenomenon in the present study. It is possible to speculate that OVX-induced leptin insensitivity could play a role in this delayed weight gain in the obese animals; however, evidence for such a mechanism is equivocal (13–15). We would hypothesize that this delayed rate of weight gain reflects an impaired ability of obese animals to clear and store the excess energy (33). The higher levels of circulating nutrients may feed back through known nutrient-sensing systems to attenuate their drive to overeat. Insulin resistance is associated with an impaired ability to adjust metabolism in response to metabolic challenges, such as fasting, exercise, and overfeeding (20, 21, 28, 29). After OVX, this 3-wk period of chronic overfeeding undoubtedly represents a substantial metabolic challenge. The lack of overfeeding-induced suppression of NEFAs and higher TG levels in obese animals would suggest this as a plausible explanation, but a more thorough examination of metabolism during this time with in vivo tracers is required to characterize the response of obese rats to the metabolic challenge of OVX.
Perspectives and Significance
While the estrous cycle adds a level of complexity in conducting studies in female rodents, many research questions can be addressed only through studying female animals. The results of this study highlight the importance of awareness of the stage of the estrous cycle when designing preclinical studies in female animals, particularly where design or outcomes include EB, obesity, and physical activity. In the present study, we observed three additional aspects of the obese phenotype in females rats, which may play a relevant role in health and disease. 1) Despite lower activity levels, the obese rats essentially expended the same amount of energy as the lean rats. 2) Obese rats exhibited a more dramatic fluctuation between the extremes of EB throughout the estrous cycle. 3) Obese rats tend to have a blunted OVX-induced energy imbalance, which explains, in part, previous observations of lower feed efficiency and delayed weight gain after OVX (33, 41). We suspect that the relative contributions of EI or TEE to the changes over the cycle or in response to OVX may vary with diet, housing conditions, strain, or species or even between individuals but that the overall impact of obesity on inducing positive energy imbalance is likely to prove more consistent.
GRANTS
This research was supported by a University of Colorado Denver Thorkildsen fellowship to E. D. Giles, an American Institute for Cancer Research fellowship to E. D. Giles, and NIDDK Grant DK-038088 to P. S. MacLean.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We appreciate the assistance of the Colorado Nutrition and Obesity Research Center's Energy Balance Laboratory and Metabolic Core [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-48520].
REFERENCES
- 1.Anantharaman-Barr HG, Decombaz J. The effect of wheel running and the estrous cycle on energy expenditure in female rats. Physiol Behav 46: 259–263, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 22: 331–339, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Arney DR, Kitwood SE, Phillips CJC. The increase in activity during estrus in dairy-cows. Appl Anim Behav Sci 40: 211–218, 1994 [Google Scholar]
- 4.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 42: 461–471, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin's satiating action in ovariectomized rats. Peptides 20: 445–450, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361: 1251–1263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bouthegourd JC, Martin JC, Gripois D, Roseau S, Tome D, Even PC. Fat-depleted CLA-treated mice enter torpor after a short period of fasting. Appetite 42: 91–98, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Brobeck JR, Wheatland M, Strominger JL. Variations in regulation of energy exchange associated with estrus, diestrus and pseudopregnancy in rats. Endocrinology 40: 65–72, 1947 [DOI] [PubMed] [Google Scholar]
- 10.Butera PC. Estradiol and the control of food intake. Physiol Behav 99: 175–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4: 579–591, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Chang S, Graham B, Yakubu F, Lin D, Peters JC, Hill JO. Metabolic differences between obesity-prone and obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol 259: R1103–R1110, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Heiman ML. Chronic leptin administration promotes lipid utilization until fat mass is greatly reduced and preserves lean mass of normal female rats. Regul Pept 92: 113–119, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Heiman ML. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am J Physiol Endocrinol Metab 280: E315–E322, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 56: 1051–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Donovan BT. Wheel-running during anoestrus and oestrus in the ferret. Physiol Behav 34: 825–829, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav 70: 397–405, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Flatt JP, Tremblay A. Energy expenditure and substrate oxidation. In: Handbook of Obesity, edited by Bray GA, James WPT. New York: Decker, 1998, p. 513–537 [Google Scholar]
- 19.Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: The Physiology of Reproduction, edited by Knobil E, Neill JD. New York: Raven, 1994, p. 613–658 [Google Scholar]
- 20.Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 32Suppl 7: S109–S119, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab 295: E1009–E1017, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 294: E817–E826, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav 67: 141–147, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9: 88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry BA, Clarke IJ. Adipose tissue hormones and the regulation of food intake. J Neuroendocrinol 20: 842–849, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Jackman MR, MacLean PS, Bessesen DH. Energy expenditure in obesity-prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol 299: R1097–R1105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackman MR, Steig A, Higgins JA, Johnson GC, Fleming-Elder BK, Bessesen DH, Maclean PS. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol Regul Integr Comp Physiol 294: R1117–R1129, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kelley DE. Skeletal muscle triglycerides: an aspect of regional adiposity and insulin resistance. Ann NY Acad Sci 967: 135–145, 2002 [PubMed] [Google Scholar]
- 29.Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr 22: 325–346, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Laudenslager ML, Wilkinson CW, Carlisle HJ, Hammel HT. Energy balance in ovariectomized rats with and without estrogen replacement. Am J Physiol Regul Integr Comp Physiol 238: R400–R405, 1980 [DOI] [PubMed] [Google Scholar]
- 31.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Long JA, Evans HM. The Oestrous Cycle in the Rat and Its Associated Phenomena. Berkeley, CA: University of California Press, 1922 [Google Scholar]
- 33.MacLean PS, Giles ED, Johnson GC, McDaniel SM, Fleming-Elder BK, Gilman KA, Andrianakos AG, Jackman MR, Shroyer KR, Schedin PJ. A surprising link between the energetics of ovariectomy-induced weight gain and mammary tumor progression in obese rats. Obesity (Silver Spring) 18: 696–703, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 290: R1577–R1588, 2006 [DOI] [PubMed] [Google Scholar]
- 35.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R1306–R1315, 2004 [DOI] [PubMed] [Google Scholar]
- 36.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Peters JC, Hill JO. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R288–R297, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, Di Carlo C, Nappi C, Di Carlo R. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology 145: 3115–3121, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Moline ML, Albers HE. Response of circadian locomotor activity and the proestrous luteinizing hormone surge to phase shifts of the light-dark cycle in the hamster. Physiol Behav 43: 435–440, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Mori MA, Araujo RC, Pesquero JB. Kinin B1 receptor stimulation modulates leptin homeostasis. Evidence for an insulin-dependent mechanism. Int Immunopharmacol 8: 242–246, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Murakami DM, Horwitz BA, Fuller CA. Circadian rhythms of temperature and activity in obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol 269: R1038–R1043, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Noel R, Fleming A. Effects of prefattening on ovariectomy-induced hyperphagia and weight gain in rats. Behav Biol 19: 405–410, 1977 [DOI] [PubMed] [Google Scholar]
- 42.Osler M. Obesity and cancer. A review of epidemiological studies on the relationship of obesity to cancer of the colon, rectum, prostate, breast, ovaries, and endometrium. Dan Med Bull 34: 267–274, 1987 [PubMed] [Google Scholar]
- 43.Parker GC, McKee ME, Bishop C, Coscina DV. Whole-body metabolism varies across the estrous cycle in Sprague-Dawley rats. Physiol Behav 74: 399–403, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Pelleymounter MA, Baker MB, McCaleb M. Does estradiol mediate leptin's effects on adiposity and body weight? Am J Physiol Endocrinol Metab 276: E955–E963, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 335: 1134, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150: 2161–2168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schedin P, Mitrenga T, Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague-Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia 5: 211–225, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Schutz Y. Dietary fat, lipogenesis and energy balance. Physiol Behav 83: 557–564, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Shaw MA, Whitaker EM, Hervey E, Hervey GR. The effects of ovarian hormones on regulation of energy balance in Zucker rats. J Endocrinol 98: 165–171, 1983 [DOI] [PubMed] [Google Scholar]
- 50.Teske JA, Kotz CM. Effect of acute and chronic caloric restriction and metabolic glucoprivation on spontaneous physical activity in obesity-prone and obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol 297: R176–R184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 14: 189–206, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev 16: 235–272, 1992 [DOI] [PubMed] [Google Scholar]
- 53.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol 166: 520–528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]